Summary
This work presents a unique single center experience with intra-arterial delivery of tissue plasminogen activator (t-PA) doses as high as 100mg for thrombolysis. Hemorrhage volumes, hemorrhage rates, clinical outcomes and radiographic outcomes were assessed.
Prospectively collected angiographic, clinical and laboratory information on 67 consecutive patients with acute ischemic stroke involving either the m1 segment of the middle cerebral artery, the intracranial internal carotid artery or the basilar artery were retrospectively analyzed. Patients who received more than 50 mg t-PA were compared with those patients receiving 50 mg or less. Outcome measures included: symptomatic hemorrhage, significant hemorrhage volume (greater than 25 ml), hemorrhage rate, change in National Institutes of Health stroke scale score at 24 hours and at hospital discharge, modified Rankin score at 90 days, in-hospital deaths, death within 90 days, reperfusion rate, and infarct volume.
Multivariate logistic regression analysis demonstrated that t-PA dose over 50 mg was associated with higher rates of hemorrhage and larger hemorrhages. Poor pial collateral formation, poor reperfusion (less than 50% of the territory involved), and platelet count below 200 K/µL influenced hemorrhage. Limiting t-PA dose to 100mg rather than 50mg improved documented reperfusion rates from 37% to 61%.
Restricting intra-arterial t-PA administration to 100mg rather than 50mg, is associated with higher overall reperfusion rates and improves overall outcomes, however, the hemorrhage rate is also elevated. Poor pial collateral formation and platelet count less than 200 K/µL may be reasons to curtail the use of higher t-PA dose to reduce hemorrhage rate.
Key words: thrombolytic therapy, intracerebral hemorrhage, acute ischemic stroke
Abbreviation Key
ΔNIHSSS = change in NIHSSS from the time of ictus
Δ24hrNIHSSS = change in NIHSSS from the time of ictus until 24 -36 hours
ΔdcNIHSSS = change in NIHSSS from the time of ictus until time of hospital discharge
24h NIHSSS = NIHSSS 24-36 hours following ictus
ATLANTIS = acute noninterventional therapy in ischaemic stroke
CT = computed tomography
dcNIHSSS = NIHSSS at the time of hospital discharge
ECASS = European Cooperative Acute Stroke Study
HT = hemorrhagic transformation
IA = intra-arterial
IAT = intra-arterial thrombolysis
ICA = internal carotid artery
ICH = intracerebral hemorrhage
IV = intravenous
IVT = intravenous thrombolysis
MCA = middle cerebral artery
mRankin = modified Rankin scale score
NIHSS = National Institutes of Health stroke scale
NIHSSS = National Institutes of Health stroke scale score
NINDS = National Institute of Neurological Disorders and Stroke
PROACT = prolyse in acute cerebral thromboembolism
Prob = probability
SHT = symptomatic hemorrhagic transformation
t-PA = tissue plasminogen activator
UK = urokinase
Introduction
Results from the National Institute of Neurological Disorders and Stroke (NINDS) rt-PA stroke study 1 indicated that 0.9mg/kg intravenous (IV) tissue plasminogen activator (t-PA) administered within three hours of symptom onset of acute ischemic stroke led to better neurologic outcomes. Intra-arterial (IA) thrombolysis (IAT) using fibribolytic agents within 6 hours of symptom onset of acute ischemic stroke has also been shown to improve outcomes 2-6. Intracerebral hemorrhage (ICH) is considered the most important complication of thrombolytic therapy regardless of route of administration. There is no direct evidence limiting IA t-PA dose to less than that used in IV thrombolysis (IVT), however, all published studies have consistently limited IA t-PA dose below 60 mg and often down to 20 mg 6. It has been unclear whether continued IA t-PA infusion beyond these doses results in higher reperfusion rates or is a futile effort predominantly met with increased hemorrhage rates. This study assesses clinical and radiographic outcomes, hemorrhage rate and hemorrhage volume associated with the use of up to 100 mg tPA for IAT.
Methods
This study was approved by the local institutional review committee. Data was collected from the review of consecutive patients who underwent IAT with t-PA at a single institution. Patients seen within six hours of symptoms of a carotid or vertebrobasilar stroke who were considered thrombolytic candidates using clinical, laboratory, computed tomography (CT) and angiographic criteria derived from the Prolyse in Acute Cerebral Thromboembolism (PROACT) study underwent screening cerebral angiography 3,4. Unlike the PROACT study, this study also included patients with ischemic stroke involving territories other than the middle cerebral artery (MCA), and patients over the age of 85. Only patients with occlusions of the supraclinoid internal carotid artery (ICA), m1 segment of the MCA and the basilar artery were included. Patients with more distal occlusions were not included. All patients seen between zero and three hours were offered IV t-PA as an alternative to IA t-PA. Patients underwent angiography and thrombolytic treatment using local anesthesia, aseptic technique and a biplane angiographic suite (Siemens Neurostar). Sedatives were avoided where possible. Microcatheter manipulation was at the discretion of the operator but the microcatheter was embedded within the thrombus. No thrombectomy device was used in this series of patients. Patients not eligible for thrombolytic treatment were offered embolectomy without thrombolytics if they were not candidates for t-PA (i.e. patient on anticoagulants, or presentation after 360 minutes). T-PA was delivered at 1 mg per minute. Treatment end points were considered to be: 1) angiographic signs of hemorrhage; 2) complete reperfusion with complete recanalization; 3) if infusion was begun prior to six hours but the patient did not recanalize by six hours, continued infusion of t-PA was left at the discretion of the physicians involved. Patients receiving t-PA as a rule were not to be anticoagulated following treatment. Presentation NIHSS score (NIHSSS), NIHSSS 24-36 hours following ictus (24h NIHSSS), NIHSSS at the time of hospital discharge (dcNIHSSS), modified Rankin scale score (mRankin) at three months, presentation laboratory values (platelet count, glucose level), systolic blood pressure, length of hospital stay, time to treatment, age, and sex were all recorded prospectively. Change in NIHSSS (δNIHSSS) from the time of ictus was calculated at 24 -36 hours δ24h NIHSSS) and at the time of hospital discharge δdcNIHSSS). NIHSSS and mRankin were determined by a stroke neurologist (AS, YM).
Angiograms of all patients were reviewed for occlusion site, pial collateral formation and reperfusion by an interventional neuroradiologist (GAC) who was blinded to all clinical information during this review. Both pial collaterals and reperfusion have been shown to be associated with improved outcomes following thrombolytic treatment 7-20. Pial collaterals were graded as good or poor based on anatomic extent as defined elsewhere 17. Good pial collaterals were equivalent to grades 3 and 4 described by Higashida and Furlan 20. Reperfusion was assessed as a percentage of the affected vascular territory that was revascularized. Re-perufusion involving less than 50% of the vascular territory involved was considered poor reperfusion. In this study complete reperfusion was considered to be complete recanalization with complete reperfusion 21. Acute infarction on 24-48 hour CT was defined as a new hypodense region relative to pretreatment CT.
Hemorrhagic transformation (HT) was defined as a new hyperdense region identified on 24-48 follow-up CT scan. It is recognized that petechial blood products may be isodense and ill defined relative to gray, however, identification of this type of hemorrhage is rather subjective on a 24-48 hour CT scan. In order to optimize reproducibility only hyperdense foci were used to define hemorrhage in this study. Infarcted regions and foci of hemorrhage were traced out on axial cross-sectional images by a CAQ certified neuroradiologist (GAC) and areas were calculated using IMPAX image analysis software (Agfa Corporation, Ridgefield Park, NJ). Infarct and hemorrhage volumes were then determined by multiplying cross-sectional areas by slice thickness and adding them up. A large hemorrhage was defined as one greater than 25 ml volume 30. Symptomatic hemorrhagic transformation (SHT) was defined as any HT associated with increase in Δ24h NIHSSS by four or more.
T-PA dose was categorized as low if less than or equal to 50 mg and high if greater than 50 mg. Presentation clinical and angiographic information as well as outcome measures were analyzed for significant differences between the two groups using pairwise comparisons. P-values were calculated with Pearson's χ2 test for ordinal and nominal data. Because continuous data was not normally distributed, the non-parametric 1-way Wilcox on rank sums test was used to determine statistical significance for continuous data; Bonferroni's correction was applied for multiple pairwise comparisons. Comparisons involving three month mRankin included only those patients who had a baseline (immediately prior to ictus) mRankin of 0. Multivariate logistic regression analyses were performed for large hemorrhage (greater than 25ml) and presence of hemorrhage, adjusting for: t-pA dose >50, age >65, platelet counts <200 K/μL, glucose >200, presenting NIHSS, time to treatment, poor reperfusion, and poor pial collateral formation. All risk factors with p <0.10, were entered into the final model as predictors of clinical outcome using backward selection. Only variables that have been previously shown to influence outcomes or hemorrhage rates were included in this analysis 1,18,22-28-30.
Kaplan-Meier analysis was employed to demonstrate efficacy of t-PA dose using complete reperfusion as a well defined end point. Because reperfusion was monitored throughout the procedure, time is given in minutes. It is noteworkthy that t-PA was infused at the predetermined rate and in that respect equivalent to a time variable. It is also true that stopping at a lower dose could have resulted in eventual recanalization if no further thrombolytic was delivered. Other assumptions for Kaplan-Meier analysis include a well defined starting point (time t-PA administration begins) and that censorhip and secular trends do not influence the outcome variable all of which were considered to be satisfied 31. Therefore, calculated reperfusion rates presented in this plot only apply if continued infusion is always possible and should be interpreted with that in mind.
Results
A total of 67 patients, who fulfilled the entry criteria, underwent intra-arterial thrombolytic treatment between April 1999 and April 2007.
Thirty-five patients did not completely recanalize. Eight of these patients received 100 mg tPA; in two patients t-PA administration was terminated because they displayed angiographic signs of hemorrhage (one after 2 mg and one after 20 mg t-PA) and in 27 patients in whom t-PA delivery began prior to the six hour limit, delivery was terminated prior to reaching the full 100 mg because the time since symptom onset exceeded six hours during the infusion. Tables 1 and 2 display presenting variables and outcomes dichotomized by high vs. low dose tPA. Lack of statistically significant differences does not exclude type II error. The strength of independence can be inferred from χ2 analysis. A substantial difference in time to treatment between the two groups can be explained by the fact that t-PA delivery was stopped if the time to treatment exceeded six hours.
Table 1.
Clinical information on presentation, and angiographic data dichotomized by t-PA dose.
| P value | χ2 | t-PA ≤50 mg | t-PA >50 mg | Total | ||
|---|---|---|---|---|---|---|
| N | 39 | 28 | 67 | |||
| Age | 0.208 * | 1.59 | 64 (55-73) | 70 (60-76) | 68 (55 -75) | |
| Female sex | 0.347 † | 0.884 | 15 (38%) | 14 (50%) | 29 (43%) | |
| Diabetes | 0.200 † | 1.64 | 5 (13%) | 7 (25%) | 12 (18%) | |
| Time to treatment | 0.0278 * | 4.84 | 301 (223-350) | 260 (202-300) | 285 (210-330) | |
| Presenting NIHSS score | 0.402 * | 4.21 | 16 (12-20) | 18 (16 - 22) | 17 (13-21) | |
| Systolic blood pressure (mm Hg) | 0.407 * | 0.686 | 142 (129-165) | 146 (135-170) | 144 (131-166) | |
| Platelet count (K/µL) | 0.689 * | 0.160 | 234(187-278) | 226 (188-264) | 229 (187-268) | |
| Glucose (mg/dl) | 0.553 * | 0.457 | 119 (99-141) | 128 (104-156) | 121 (102-150) | |
| Basilar a | 0.318 † | 2.29 | 5 (13%) | 1 (3.6%) | 6 (9.0%) | |
| Occlusion site Internal carotid a. | 5 (13%) | 6 (21%) | 11 (16%) | |||
| Middle cerebral a. | 29 (74%) | 21 (75.0%) | 50 (75%) | |||
| Good pial collaterals | 0.231 † | 1.44 | 27 (69%) | 23 (82%) | 50 (75%) | |
| Median dose (mg) | 30 (20.0-45.0) | 88 (65-90) | 50 (25-75) | |||
| 25-75% quartiles presented in parentheses for continuous data and percentages presented in parentheses for nominal/ordinal data. NIHSS scores were treated as continuous data. Wilcoxon Rank Sums 1-way test (continuous variables); † Pearson χ2(nominal/ordinal variables); Bonferroni correction for multiple tests requires p < .00263 for significance. | ||||||
Table 2.
Outcomes dichotomized by t-PA dose
| P value | χ2 | T-PA ≤ 50 mg | t-PA >50 mg | Total | |
|---|---|---|---|---|---|
| N | 39 | 28 | 67 | ||
| Reperfusion ≥ 50% | 0.564† | 0.332 | 25 (64%) | 16 (57%) | 41 (61%) |
| Complete reperfusion | 0.464† | 0.496 | 20 (51%) | 12 (43%) | 32 (48%) |
| Median hemorrhage volume (ml)* | 0.120 § | 2.41 | 7.6 (2.0-37) | 22 (7.8-114) | 8.8 (4.5-49.9) |
| Presence of hemorrhage | 0.258 >† | 1.28 | 9 (23%) | 8 (36%) | 19 (28%) |
| Symptomatic hemorrhage | 0.306 † | 4.68 | 1 (2.6%) | 5 (18%) | 6 (9.1%) |
| Hemorrhage >25 ml | 0.206† | 1.60 | 3 (7.7%) | 5 (18%) | 8 (12%) |
| Infarct volume (ml) | 0.689§ | 0.160 | 44 (12-109) | 47 (11-147) | 47 (11-134) |
| Modified Rankin ≤2 | 0.756† | 0.097 | 21 (54%) | 14 (50%) | 35 (52%) |
| ΔNIHSS by discharge | 0.541§ | 0.373 | -8.0 (-11 − +2.0) | -5.0 (-12 − +3.5) | - 7.0 (-11 − 0) |
| ΔNIHSS by 24 hours | 0.449§ | 0.574 | -5.0 (-10 − 0) | -3.5 (-10 − +2.0) | -4.0 (-10- +1.0) |
| Death during hospitalization | 0.326† | 0.568 | 5 (13%) | 5 (18%) | 10 (15%) |
| Death by 3 months | 0.664† | 0.189 | 8 (20%) | 7 (25%) | 15 (22%) |
| *in patients who hemorrhaged (n=18); 25-75% quartiles presented in parentheses for continuous data and percentages presented in parentheses for nominal/ordinal data. ΔNIHSS scores were treated as continuous data. §Wilcoxon Rank Sums 1-way test (continuous variables); †Pearson χ2 (nominal/ordinal variables); Bonferroni correction for multiple tests requires p < .005 for significance. ΔNIHSS = change in NIHSS. | |||||
The final logistic regression analyses models for large hemorrhage and presence of hemorrhage are presented in Tables 3 and 4. They indicate that both hemorrhage rate and hemorrhage volume are associated with high doses of t-PA. Significant predictors for hemorrhagic transformation identified on logistic regression were analyzed relative to t-PA dose and presented in Table 5. These suggest that high t-PA doses have an additive effect toward hemorrhage rate and hemorrhage volume when pial collateral formation and platelet counts are considered.
Table 3.
Logistic regression analysis for hemorrhage volume greater than 25 ml.
| Term | Estimate | Std Error | χ2 | Odds Ratio (95% C.I.) | Prob >χ2 |
|---|---|---|---|---|---|
| Intercept | -2.28 | 1.38 | 2.73 | 0.0984 | |
| t-PA dose >50 mg | 3.31 | 1.58 | 4.36 | 27.3 (2.01 - 1340) | 0.0369 |
| Poor pial collaterals | 3.94 | 1.52 | 6.71 | 51.5 (4.22 − 2290) | 0.0096 |
| Platelet count < 200K/µL | 3.07 | 1.39 | 4.89 | 21.5 (2.11 - 709) | 0.0270 |
| Reperfusion < 50% | 1.61 | 0.82 | 3.83 | 25.0 (1.81 - 1630) | 0.0500 |
| (r2= 0.573; Prob> χ2 <0.0001; n=67) | |||||
Table 4.
Logistic regression analysis for presence of hemorrhage.
| Term | Estimate | Std Error | χ2 | Odds Ratio (95% C.I.) | Prob > χ2 |
|---|---|---|---|---|---|
| Intercept | -1.63 | 0.848 | 3.71 | 0.0542 | |
| Poor pial collaterals | 2.40 | 0.776 | 9.58 | 11.1 (2.64-58.7) | 0.0020 |
| Time to treatment >270 min | 0.851 | 0.369 | 5.33 | 5.49 (1.42-26.9) | 0.0209 |
| Diabetes | 0.788 | 0.430 | 3.36 | 4.84 (0.933-29.2) | 0.0669 |
| t-PA dose >50 mg | 1.21 | 0.719 | 2.85 | 3.37 (0.864-15.3) | 0.0913 |
| (r2= 0.277; Prob>χ2 < 0.0001; n=67) | |||||
Table 5.
Additive effects of t-PA dose and other factors on hemorrhage.
| t-PA | n | No hemor- rhage |
0-25 ml | >25 ml | p | ||
|---|---|---|---|---|---|---|---|
| N | 48 | 10 | 8 | ||||
| Platelet count |
t-PA dose >50mg | Platelet count <200K/µL | 9 | 4 (44%) | 2 (22%) | 3 (33%) | 0.255 |
| Platelet count ≥200K/µL | 19 | 14 (74%) | 3 (16%) | 2 (10%) | |||
| t-PA dose ≤ 50mg | Platelet count <200K/µL | 14 | 10 (71 %) | 1 (7.1%) | 3 (21.4%) | 0.065 | |
| Platelet count ≥200K/µL | 25 | 20 (80%) | 5 (20%) | 0 | |||
| Pial collateral |
t-PA dose > 50mg | Poor pial collaterals | 5 | 0 | 2 (40%) | 3 (60%) | 0.0030 |
| Good pial collaterals | 23 | 18 (78%) | 3 (13%) | 2 (8.7%) | |||
| t-PA dose ≤ 50mg | Poor pial collaterals | 12 | 7 (58%) | 2 (17%) | 3 (25%) | 0.023 | |
| Good pial collaterals | 27 | 23 (85%) | 4 (15%) | 0 | |||
| Reperfusion | t-PA dose >50mg | Poor reperfusion | 12 | 7 (58%) | 1 (8.3%) | 4 (33%) | 0.135 |
| Good reperfusion | 16 | 11 (69%) | 4 (25%) | 1 (6.2%) | |||
| t-PA dose ≤ 50mg | Poor reperfusion | 14 | 8 (57%) | 3 (21%) | 3 (21%) | 0.030. | |
| Good reperfusion | 25 | 22 (88%) | 3 (12%) | 0 | |||
| * Pearson χ2; Bonferroni correction for multiple tests requires p < .0083 for significance | |||||||
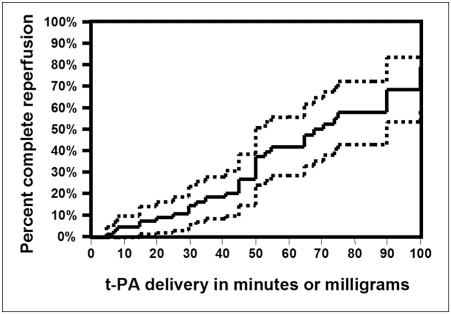
The Kaplan Meier plot (Figure 1) shows rates of complete reperfusion based on delivered dose with 95% confidence intervals. Cumulative rates for complete reperfusion were 37.5% (standard error 0.0680) if up to 50 mg was delivered and 79.0% (standard error = 0.100) if up to 100mg was delivered. Kaplan-Meier plots use a censor to estimate complete reperfusion rates had t-PA been continued in all cases up until recanalization and should be interpreted with this in mind.
Figure 1.
Kaplan Meier plot of complete reperfusion relative to t-PA delivery time. The dotted lines represent 95% confidence intervals. Note: because t-PA dose is delivered at a specified rate, it is time dependant and can be substituted here for time (see text). This plot suggests that if t-PA infusion is continued beyond 50mg, reperfusion occurs at a rate similar to lower doses. Calculated reperfusion rates presented in this plot only apply if continued infusion is always possible (see text).
Discussion
This study looks at recanalization and hemorrhage when administering IA t-PA doses similar to those given for IV t-PA. Not all patients received the maximum dose. Those who did not receive the maximum dose, either recanalized, exceeded the six hour time window or showed angiographic signs of active bleeding during the infusion. Based on Medline and PubMed search, the study presented here is unique because no other study in the literature has examined the use of IA t-PA doses up to 100 mg. Presumably reperfusion helps improve outcomes. By increasing the maximum dose to 100 mg, 28 patients received more than 50 mg t-PA. Sixteen of these 28 patients (57%) reperfused more than 50% of the involved territory and 5 (18%) had a large hemorrhage (over 25 ml). Thus the estimated overall reperfusion rate increased from 37% to 61% and the estimated overall rate of large hemorrhage increased from 7.7% to 12%. Indeed, cumulative plot for complete reperfusion (figure 1) indicates that a continued reperfusion benefit results as higher doses of t-PA are delivered. Outcomes detailed in Table 2 demonstrate a clinical benefit at 24 hours, at the time of discharge and at three months similar to patients who re-quired less than 50 mg t-PA despite the higher hemorrhage rate. Had extra t-PA not been delivered in the patients who received more than 50 mg t-PA, their reperfusion outcomes would be suggested to have been worse. Because t-PA has a short half-life, any additional recanalization that may have resulted if no more t-PA was delivered, would be suggested to occur at a lower rate than that seen with patients receiving more that 50 mg t-PA in this study.
The rate of poor clinical outcomes based on death, discharge NIHSS score and three month modified Rankin score was not worse in patients receiving more than 50 mg t-PA. Regression analyses in Tables 3 and 4 identify risk factors for hemorrhage. Subgroup analysis in Table 4 suggests that patients receiving higher doses of t-PA are more prone to hemorrhage if patients have added risk factors. Although this type of analysis is prone to type II error, some information can be deduced. Patients with poor pial collateral formation appear to be especially prone to significant hemorrhage. Therefore, continuation to higher doses of t-PA in patients with additional risk factors should be taken with caution.
The risk of developing HT has been shown to increase as the dose of IV t-PA reaches 100 mg 7,8. However, del Zoppo et al. 20 were not able to demonstrate that a relationship existed between dose of t-PA and rate of HT. Previous investigations have shown that IAT for acute is chemic stroke results in a higher local concentration of thrombolytic agent relative to the intravenous route and is effective in the treatment of ischemic stroke 2-6,23. Approximately 47% of patients receiving IV t-PA within three hours of symptom onset have shown clinical improvement of 4 points in the NIHSS scale, with a risk of symptomatic HT of 6.4% 1,11. Results from the PROACT study show that patients receiving IAT within six hours of symptom onset have neurological improvement at follow-up 3-6. The overall median improvement in NIHSS score was four points in this study, which is similar to patients treated within three hours mentioned above. IA thrombolytic studies differ from IVT studies in that the angiographic data, available before and after IAT can provide more definitive information regarding presence of occlusion at the time of thrombolytic administration, site of occlusion, and extent of reperfusion which can be used to exclude patients. For example, a candidate for IAT may have a normal angiogram despite symptoms 24. Furthermore, two endpoints resulting in the termination of thrombolytic agent via the IA route are not possible by the IV route. These are: 1) the angiographic demonstration of complete reperfusion and 2) angiographic demonstration intra-procedural hemorrhage. This can hypothetically serve to avoid potential hemorrhage by stopping the delivery of thrombolytics after reperfusion and curtail hemorrhage volumes by stopping drug delivery with angiographic signs of hemorrhage. Therefore, outcomes comparisons between IAT versus IVT are inherently biased.
Hemorrhage rates in this study do not differ from those in large trials. In the cohort of patients presented here, who underwent IAT, rate of SHT was 9.1% which is similar to the 9-10.9% SHT rate reported by other IAT studies 2-6. These rates are slightly higher than the 5.9-8.8% rates reported by trials infusing IV t-PA, 1,10,22. The higher SHT rate in the present study and in the PROACT study could be attributed to higher presentation NIHSS scores and differing inclusion criteria, such as longer time to treatment. Presenting NIHSS score has been shown to be associated with SHT 1,2,6-16,18,23,28-30,32. The median presenting NIHSS scores in this study and in the PROACT study were 17, whereas in the European Cooperative Acute Stroke Study (ECASS) it was 11, in the NINDS t-pA trial it was 14, and in the Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischaemic Stroke (ATLANTIS) study it was 11 1,3-5,10,12,22,25. Many studies have demonstrated that earlier thrombolytic treatments for acute is chemic is more beneficial 1,11,12,22,26. In the ECASS IV t-PA study risk of SHT increased to 19.4% among patients treated within six hours of symptom onset 12,22. Since the completion of this study, intravenous tPA delivered between three hours and 4.5 hours in the setting of acute ischemic stroke has been shown to improve outcomes based on primary outcome of disability at 90 days with an odds ratio of 1.28 compared to placebo 33.
Past investigations have identified important predictors of HT in patients receiving thrombolytic therapy with use of IV t-PA, rt-PA, IA pro-urokinase (UK), combined IV-IA t-PA (up to 22 mg), IV streptokinase, and IA UK 1-11,26. In previous IV t-PA studies, it was determined that presenting NIHSS score, age, and elevated serum glucose were found to be risk factors for HT 1,7-10,12-16,28,30. Among IAT studies, baseline NIHSS score, lower platelet count, longer time to treatment, and elevated serum glucose were significant predictors for the presence of HT 2,4,5,26. Poor pial collateral supply to an ischemic region has been reported to predict hemorrhagic transformation 16,18,19,30. Even though all these factors were taken into account in this study not all were shown to influence hemorrhage on regression analysis.
The current study indicates that 50-100 mg intra-arterial t-PA doses delivered in the setting of acute ischemic stroke can be beneficial. Because measures predictive of outcome (see table 1) were similar between low and high dose groups, outcomes cannot be readily attributed to selection bias. Although subgroup analysis shown in Table 5 carries a large risk for type II error, one can infer that patients receiving higher t-PA doses with poor pial collateral formation, or possibly platelet levels less than 200 K/µL may be at higher risk for hemorrhage. No patients with platelet counts greater than 250 developed any hemorrhage which may lend some credence to a hypothesis that the administration of platelets after intra-arterial thrombolysis may be protective from hemorrhage.
Complete reperfusion rate increased with tPA dose based on Kaplan-Meier analysis (figure 1). The results of this plot should be interpreted with caution (see methods section). This analysis takes into account patients without complete reperfusion but with early termination of t-PA administration and assumes that complete reperfusion rates in this group of patients would not differ from the rest had t-PA administration been continued. Furthermore, the Kaplan-Meier analysis incorporates cumulative censorship. In essence the complete reperfusion rates censor for patients who did not receive the full dose of t-PA due to time limitations or early signs of hemorrhage. The graph shows no indication that complete reperfusion rates diminish with t-PA dose. Indeed, the rate of complete reperfusion did not differ between the high and low dose group (Table 2). Comparing complete reperfusion rate on Kaplan-Meier analysis, there was an 86.3% improvement by using the higher dose. Results from the Kaplan-Meier analysis cannot be extrapolated directly. The rate of complete reperfusion derived from this analysis is based on the assumption that continued infusion of t-PA is always possible which is not practical. Simply stated the Kaplan-Meier analysis can only be used to imply that there is potential for continued increased in recanalization rate if additional t-PA can be safely delivered. To determine reperfusion rates, it is more practical to compare favorable reperfusion rates (greater than 50% of the involved territory) assuming that little recanalization would continue within the next few hours, had t-PA delivery been discontinued at a lower dose. Had t-PA dose been limited to 50 mg the documented reperfusion rate would have been 37.5% versus reperfusion of 61% for the entire group one can see that there is a substantial reperfusion benefit towards using the higher dose. The assumption that documented recanalization would increase further in the lower dose group if an angiogram was performed one hour later also applies to the higher dose group, however, it is also possible that some of the patients would reocclude.
The differences in undocumented theoretical recanalization between the two groups in the hours following thrombolytic delivery would be expected to be negligible and the estimated increase in reperfusion rate is valid. Alteplase is a recombinant form of a naturally occurring serine protease with a plasma half-life of four to six minutes 34-36. TPA primarily acts by converting of plasminogen to plasmin which is a rate limiting step for fibrinolysis. Plasmin in turn degrades fibrin, fibrinogen and other plasma proteins. Plasmin is inactivated immediately by antiplasmin. In vitro studies indicate that alteplase unlike other forms of recombinant tPA primarily binds to the surface of the clot relative to other forms of tPA. Alteplase binds more readily to the fibrin-plasminogen complex in clot rather than circulating plasminogen. This lowers its systemic fibrinolytic effect, but also lowers its ability to penetrate into the clot. Thrombolytic activity may continue up to four hours following administration of alteplase via degradation products. Fibrin degradation products are known to be much lower with tPA versus urokinase for example. The risk of bleeding associated with thrombolytic therapy is probably more dependant on reperfusion injury rather than the fibrin degradation products 34,35. The concentration of active tPA in blood is regulated by the rate of secretion of tPA, the inhibition of tPA by plasminogen activator inhibitor and other inhibitors such as neuroserpin, astrocytic clearance of tPA, and the hepatic clearance of tPA. Furthermore the relative concentration of fibrin within a clotvaries depending on whether clot was cardiogenic or represented artery to artery embolization.. As a result the action of fibrinolytic agents can vary from patient to patient 34-36. We conjecture that the lesser ability of alteplase to recanalize large clots demonstrated by Tomsick et al. 37 is due to its tendency to bind primarily to the surface of the clot and not penetrate as readily. Intra-arterial delivery has the potential to distribute the tPA within the clot. Dosing regimens for intravenous alteplase are a modification from dosing regimens used in myocardial infarction 36.
In the current study, there are several limitations of note. Retrospective review could result in misclassification and selection bias. These were controlled by using a consecutive series, blinding the investigators and using pre-established definitions to categorize patients. A larger prospective study can confirm the stated results. The reasoning that it is consistent with previous findings supports the regression model's validity. A larger cohort of patients may be required to derive statistically significant hemorrhage rates on pairwise comparisons. For example, assuming a 10% incidence of large hemorrhages with the low dose group, in order to detect a 10% difference, between the high dose and the low dose group with a power of 80% and α of 0.05 a cohort of 195 patients would be required 29. In this study only one concentration of t-PA used (1 mg/ml). It is certainly possible that lower concentrations of t-PA administered at the same volume rate may result in similar recanalization rates with lower t-PA doses.
Conclusion
Data presented in a consecutive series of patients, suggests that judicious increase in the maximum dose for IA t-PA to 100mg for thrombolysis in acute ischemic stroke, may improvereperfusion rates. However, the rate of significant hemorrhage may also increase with higher doses. Presence of a small penumbra zone in the form suggested by poor pial collateral formation and possibly platelet counts lower than 200 K/µL are suggested relative contraindications to doses higher than 50 mg t-PA.
Acknowledgements
The authors wish to acknowledge Peggy Notestine B.S. and Hoda Jradi, M.S. for their help.
References
- 1.The National Institute of Neurological Disorders and Stroke (NINDS) Trial. NEJM. 1995;33:1581–1587. [Google Scholar]
- 2.Kidwell CS, Saver JL, Carnado J, et al. Predictors of hemorrhagic transformation in patients receiving intraarterial thrombolysis. Stroke. 2002;33:717. doi: 10.1161/hs0302.104110. [DOI] [PubMed] [Google Scholar]
- 3.Furlan A, Higashida R, Weschler L, et al. Intra-arterial prourokinase for acute ischemic stroke:the PROACT II study. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 4.Del Zoppo G, Higashida R, Furlan A, et al. PROACT: A phase ii randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Kase C, Furlan AJ, Weschler L, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke:The PROACT II Trial. Neurology. 2001;57:1603–1610. doi: 10.1212/wnl.57.9.1603. [DOI] [PubMed] [Google Scholar]
- 6.Lisboa RC, Javanovic BD, Alberts MJ. Analysis of the safety and efficacy of intra-arterial thrombolytic therapy in ischemic stroke. Stroke. 2002;33:2866–2871. doi: 10.1161/01.str.0000038987.62325.14. [DOI] [PubMed] [Google Scholar]
- 7.Von Kummer R, Hacke W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke. 1992;23:646–652. doi: 10.1161/01.str.23.5.646. [DOI] [PubMed] [Google Scholar]
- 8.Levy D, Brott T, Haley C, et al. Factors related to intracranial hematoma formation in patients receiving tissue-type plasminogen activator for acute ischemic stroke. Stroke. 1994;25:291–297. doi: 10.1161/01.str.25.2.291. [DOI] [PubMed] [Google Scholar]
- 9.Del Zoppo G, Poeck K, Pessin M, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 10.Clark WM, Wissman S, Albers GW, et al. Recombinant tissue-type plasminogen activator (alpteplase) for ischemic stroke 3 to 5 hours after symptom onset: The ATLANTIS Study. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 11.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 12.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 13.Jaillard A, Cornu C, Durieux A. Hemorrhagic transformation in acute ischemic stroke: the MAST-E study. Stroke. 1999;30:1326–1332. doi: 10.1161/01.str.30.7.1326. [DOI] [PubMed] [Google Scholar]
- 14.Larrue V, von Kummer R, del Zoppo G, et al. Hemorrhagic transformation in acute ischemic stroke potential contributing factors in the European Cooperative Acute Stroke Study. Stroke. 1997;28:957–960. doi: 10.1161/01.str.28.5.957. [DOI] [PubMed] [Google Scholar]
- 15.Demchuk A, Morgenstern L, Krieger D, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–39. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- 16.Selim M, Fink JN, Kumar S, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33:2047–2052. doi: 10.1161/01.str.0000023577.65990.4e. [DOI] [PubMed] [Google Scholar]
- 17.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. Am. J. Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 18.Theron J, Coskun O, Huet H, et al. Local intraarterial thrombolysis in the carotid territory. Interventional Neuroradiology. 1996;2:111–126. doi: 10.1177/159101999600200204. [DOI] [PubMed] [Google Scholar]
- 19.Liebeskind DS. Collaterals in acute stroke: Beyond the clot. Neuroimaging Clin N Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Higashida RT, Furlan AJ. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:109–137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 21.Khatri P, Neff J, Broderick JP, et al. and for the IMS-I Investigators. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;35:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 22.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;353:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 23.Suarez J, Sunshine J, Tarr R, et al. Predictors of clinical improvement, angiographic recanalization, and intracranial hemorrhage after intra-arterial thrombolysis for acute ischemic stroke. Stroke. 1999;30:2094–2100. doi: 10.1161/01.str.30.10.2094. [DOI] [PubMed] [Google Scholar]
- 24.Slivka A, Christoforidis G, Bourekas E, et al. Clinical and imaging outcomes after stroke with normal angiograms. AJNR. 2005;26:242–245. [PMC free article] [PubMed] [Google Scholar]
- 25.Albers GW, Clark WM, Madden KP, et al. ATLANTIS Trial: For patients treated within 3 hours stroke onset. Stroke. 2002;33:493–496. doi: 10.1161/hs0202.102599. [DOI] [PubMed] [Google Scholar]
- 26.Suarez J, Sunshine J, Tarr R, et al. Predictors of clinical improvement, angiographic recanalization, and intracranial hemorrhage after intra-arterial thrombolysis for acute ischemic stroke. Stroke. 1999;30:2094–2100. doi: 10.1161/01.str.30.10.2094. [DOI] [PubMed] [Google Scholar]
- 27.Baron J, von Kummer R, del Zoppo G. Treatment of acute ischemic stroke: challenging the concept of a rigid and universal time window. Stroke. 1995;26:2219–2221. doi: 10.1161/01.str.26.12.2219. [DOI] [PubMed] [Google Scholar]
- 28.Weir C, Murray G, Dyker A, et al. Is hyperglycemia an independent predictor of poor outcome after acute stroke: Results of a long term follow up study. BMJ. 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christoforidis GA, Karakasis C, Mohammad YM, et al. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: the role of pial collateral formation. Am J Neuroradiol. 2009;30:165–170. doi: 10.3174/ajnr.A1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christoforidis GA, Slivka A, Mohammad Y, et al. Size matters: hemorrhage volume as an objective measure to define significant intracranial hemorrhage associated with thrombolysis. Stroke. 2007;38:1799–1804. doi: 10.1161/STROKEAHA.106.472282. [DOI] [PubMed] [Google Scholar]
- 31.Norman GR, Streiner DL. Biostatistics: the bare essentials. Hamilton, Ontario: B.C. Decker Inc.; 2000. [Google Scholar]
- 32.Alsop DC, Makovetskaya E, Kumar S, et al. Markedly reduced apparent blood volume on bolus contrast magnetic resonance imaging as a predictor of hemorrhage after thrombolytic therapy for acute ischemic stroke. Stroke. 2005;36:746–750. doi: 10.1161/01.STR.0000158913.91058.93. [DOI] [PubMed] [Google Scholar]
- 33.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 34.Fischer S, Kohnert U. Major mechanistic differences explain the higher clot lysis potency of reteplase over alteplase: lack of fibrin binding is an advantage for bolus application of fibrin-specific thrombolytics. Fibrinolysis Proteolysis. 1997;11:129–135. [Google Scholar]
- 35. Alteplase, recombinant package insert (Activase, Genentech-US), Rev 11/87, Rec 12/87; Rev 6/96, Rec 6/96.
- 36.Kurz MA. Thrombus and stroke. New York, NY: Informa Health Care U.S.A. Inc.; 2008. Pharmacology and clot: immediate and chronic interventions; pp. 103–122. In: Eds. Wakhloo AK, Gounis MJ, Lieber BB, et al. [Google Scholar]
- 37.Tomsick T, Barsan W, Brott T, et al. Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. Am J Neuroradiol. 1996;17:79–85. [PMC free article] [PubMed] [Google Scholar]