Summary
Direct surgical clipping proves to be difficult and dangerous for intracranial aneurysms associated with moyamoya disease (MMD) or moyamoya syndrome (MMS). This study presents our clinical experience of endovascular embolization of intracranial aneurysms associated with these diseases. A total of 13 cases of intracranial aneurysms associated with MMD or MMS were treated by endovascular embolization between January 2001 and January 2009. Patients were divided into two groups: a saccular aneurysm group (n=10) and a pseudoaneurysm group (n=3). Different endovascular therapeutic strategies were employed for each type of case. Of the 13 cases, 11 received successful endovascular embolization and had an uneventful postoperative course during one to two years of follow-up. However, endovascular embolization failed in the other two cases, of whom one died from rebleeding after the five-month follow-up, while the other was conservatively treated and experienced no rebleeding during the two-year follow-up. A favorable prognosis may be secured through careful selection of endovascular treatment regimens for patients with intracranial aneurysms associated with MMD or MMS according to the site of intracranial aneurysms.
Key words: moyamoya disease, moyamoya syndrome, intracranial aneurysms, endovascular treatment
Introduction
Moyamoya disease (MMD) is a chronic obstructive vascular disorder that progressively involves bilateral internal carotid arteries and is characterized by the formation of abnormal moyamoya vessels at the skull base due to terminal occlusion of internal carotid arteries 1. When moyamoya-like vascular changes occur in the unilateral internal carotid artery, it is known as moyamoya syndrome (MMS) (i.e., unilateral moyamoya phenomenon) 2,3. MMD or MMS are commonly seen in association with intracranial aneurysms, and most of these cases present with intracranial hemorrhage caused by aneurysm rupture. Previous studies have reported that about 15% of hemorrhagic incidents in patients with MMD resulted from the associated aneurysms 4,5. However, the incidence of MMS associated with intracranial aneurysm remains unclear 6,7. Intracranial aneurysms seen in MMD or MMS are characterized by abnormally developed blood vessels and the presence of a collateral circulation, which makes direct surgical clipping difficult and causes high disability rates 5,8. In recent years, with improving endovascular techniques, endovascular embolization is emerging as an efficient procedure for treatment of these aneurysms 9,10. However, few studies have been conducted regarding the methods and prognosis of endovascular treatment. Therefore, the present study reports our clinical experience of treating 13 cases of MMD and MMS associated with intracranial aneurysms by endovascular treatment.
Materials and Methods
1. Baseline Data
The study population consisted of 13 patients (eight males and five females) aged 27 to 67 years (mean: 46.8 years). All patients presented with intracranial hemorrhage, including nine cases of subarachnoid hemorrhage (SAH), one case of SAH and intraventricular hemorrhage (IVH), two cases of IVH, and one case of intracerebral hemorrhage (ICH). The time from symptom onset to treatment was five days or less. All patients had Hunt-Hess scale grades of I to III.
2. Patient Grouping
All patients were diagnosed as having intracranial hemorrhage by head computed tomography (CT) examination, and were subsequently determined as having MMD or MMS associated with intracranial aneurysm by head CT angiography (CTA) or digital subtraction angiography (DSA). The patients were divided into two groups based on the location and shape of the associated aneurysm in CTA or DSA: the saccular aneurysm group (n=10) and the pseudoaneurysm group (n=3). Saccular aneurysms were further categorized into anterior circulation aneurysm and posterior circulation aneurysm.
2.1 Saccular aneurysm group (n=10)
2.1.1 Anterior circulation aneurysm (n=5)
There were three cases of anterior communicating artery aneurysm, one case of left ophthalmic artery aneurysm, and one case of right posterior communicating artery aneurysm; all cases were diagnosed as MMS by DSA; and the ophthalmic and posterior communicating artery aneurysms were located contralateral to the side affected by MMS. In addition, four cases of SAH and one case of SAH and IVH were found in this group.
2.1.2 Posterior circulation aneurysm (n=5)
There was one case of left posterior inferior cerebellar artery aneurysm, two cases of basilar tip aneurysm, one case of pontine artery aneurysm of the basilar artery, and one case of right posterior cerebral artery aneurysm; all cases were diagnosed as MMD by DSA and presented with SAH. There was one case of wide-necked aneurysm in the saccular aneurysm group.
2. 2 Pseudoaneurysm group (n=3)
There was one case of left distal middle cerebral artery aneurysm, one case of perforating artery aneurysm of the left cerebral middle artery, and one case of left anterior choroidal artery aneurysm. DSA examination revealed two cases of MMD and one case of MMS. Two cases of IVH and one case of ICH were found in this group.
3. Neurosurgical management
3.1 Preoperative management
Nimotop was intravenously administered at a rate of 4 mL/h one to three days before surgery to prevent vasospasm. For the one case of wide-necked aneurysm, 75 mg of clopidogrel and 100 mg of aspirin (enteric-coated tablet) were orally administered three days before surgery (once daily for both drugs).
3.2 Endovascular procedure
All surgery was performed under general endotracheal anesthesia. Three-diminsional (3D)-DSA was performed before surgery. The anatomical relationship between aneurysms and peripheral vessels was evaluated by using 3D reconstruction of aneurysms. Subsequently, a suitable operating angle was selected for endovascular embolization.
Embolization was performed with electrically detachable coils or hydrocoils (including two-dimensional and 3D coils with various degrees of flexibility) in the saccular aneurysm group.
In addition, Neuroform stent-assisted embolization was performed for the wide-neck posterior inferior cerebellar aneurysm. For patients with pseudoaneurysm, endovascular parent artery occlusion was performed with Onyx (ev3, Irvine, CA, USA).
3.3 Postoperative management
For patients with SAH alone or combined with hemorrhage at another site, lumbar cistern drainage was performed for at least seven consecutive days after surgery, and hemorrhagic cerebrospinal fluid was drained by an external ventricular drainage system to reduce intracranial pressure and improve cerebral vasospasm. Meanwhile, changes of intracranial pressure were closely monitored according to the liquid height of the external ventricular drainage tube in order to bring the intracranial pressure under 200 mm H2O 11. Nimodipine (4 mL/h) was continuously administered for three weeks after surgery to prevent or alleviate vasospasm. For patients with significantly increased intracranial pressure, a combination of mannitol, furosemide, and albumin was used to lower intracranial pressure. The details of the patients' clinical features are summarized in Table 1.
Table 1.
Clinical features of the patients
| Saccular aneurysm group |
Case | Sex/Age (y) |
Intracranial hemorrhage |
Aneurysm location | DSA |
Wide neck |
Modes of embolization |
| 1 | F/43 | SAH | Anterior communicating artery |
Right MMS | No | Coil | |
| 2 | M/57 | SAH | Anterior communicating artery |
Right MMS | Yes | Coil | |
| 3 | M/67 | SAH | Anterior communicating artery |
Left MMS | No | Coil | |
| 4 | M/27 | SAH | Left ophthalmic artery | Right MMS | No | Coil | |
| 5 | M/61 | SAH+IVH | Right posterior communicating artery |
Right MMS | No | Coil | |
| 6 | F/44 | SAH | Left posterior inferior cerebellar artery |
MMD | Yes | Stent+Coil | |
| 7 | F/47 | SAH | Basilar artery tip | MMD | No | Coil | |
| 8 | M/32 | SAH | Basilar artery tip | MMD | No | Coil | |
| 9 | M/38 | SAH | Pontine branch of the basilar artery |
MMD | No | Unsuccessful | |
| 10 | M/55 | SAH | Right posterior cerebral artery |
MMD | No | Coil | |
| Pseudo- aneurysm group |
1 | F/39 | IVH | Left anterior choroidal artery |
MMD | No | Parent artery occlusion |
| 2 | M/51 | IVH | Left distal middle cerebral artery |
Right MMS | No | Parent artery occlusion |
|
| 3 | F/47 | ICH | Perforating branch of left middle cerebral artery |
MMD | No | Unsuccessful | |
|
F: female, M: male, SAH: subarachnoid hemorrhage, IVH: intraventricular hemorrhage, ICH: intracerebral hemorrhage, MMS: moyamoya syndrome, MMD: moyamoya disease. | |||||||
Results
Following adequate preoperative preparation, all cases underwent endovascular embolization of aneurysms within one week after onset.
1. Successful embolization (n=11)
The procedure was successful in 11 of 13 patients. Re-examination by DSA six months later revealed complete embolization of aneurysms and no recurrence in the 11 patients who also showed favorable prognosis after one to two years of follow-up (Figures 1-3).
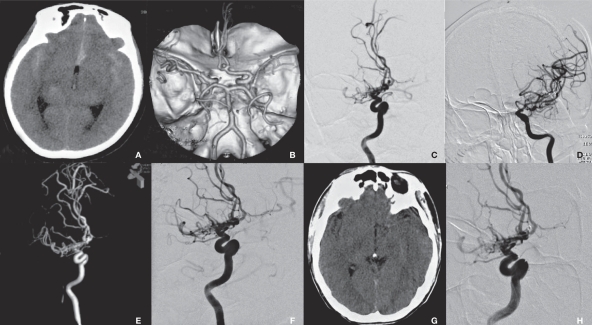
Figure 1.
Radiological imaging of MMS associated with the anterior communicating artery aneurysm in the saccular group (case 1). A) CT shows SAH. B) CTA shows an anterior communicating artery aneurysm and right MMS. C,D) DSA shows an anterior communicating artery aneurysm and right-side MMS. E) 3D-DSA provides an optimal view of aneurysm embolization. F) DSA shows a well-developed left carotid artery (arrow). G) Repeated DSA shows no recurrence of aneurysm (arrow).
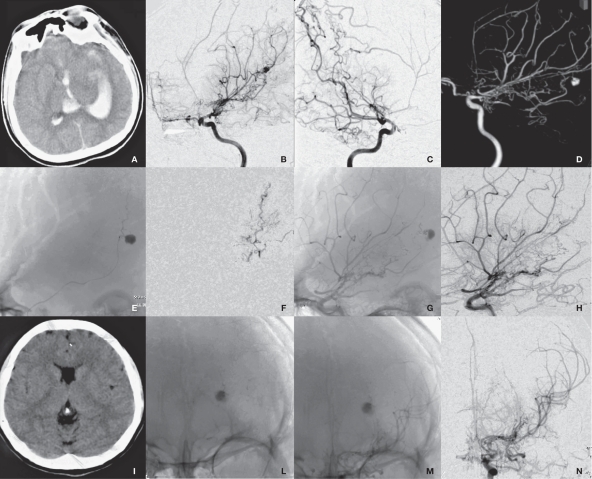
Figure 2.
Radiological imaging of MMD associated with basilar tip aneurysm in the saccular aneurysm group (case 7). A) SAH is shown. B) CTA shows MMD and basilar tip aneurysm. C,D) DSA shows the aneurysm (arrow) and extended blood supply from posterior circulation (ellipse). E) DSA shows basilar tip aneurysm (arrow). E) aneurysm embolization is shown. F) Repeated CT scan six months after surgery reveals radial artifacts. G) Repeated DSA shows no recurrence of aneurysm.
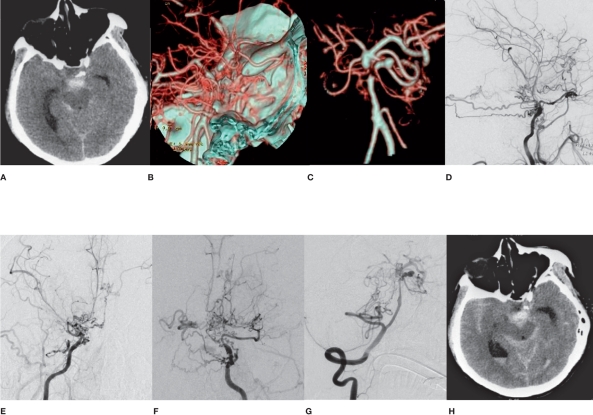
Figure 3.
Radiological imaging of MMD associated with left anterior choroidal artery aneurysm in the pseudoaneurysm group (case 1). A) CT shows IVH. B-D) DSA shows a anterior choroidal artery aneurysm and MMD. E) Microcatheter superselective angiography. F,G) DSA shows the parent artery and the embolized aneurysm. H-I) Repeated DSA shows no recurrence of aneurysm.
2. Unsuccessful embolization (n=2).
2.1 Saccular aneurysm group (n=1)
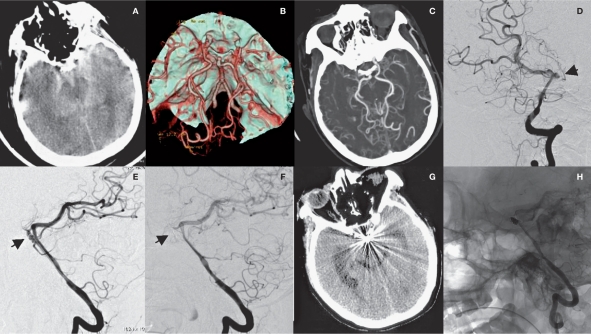
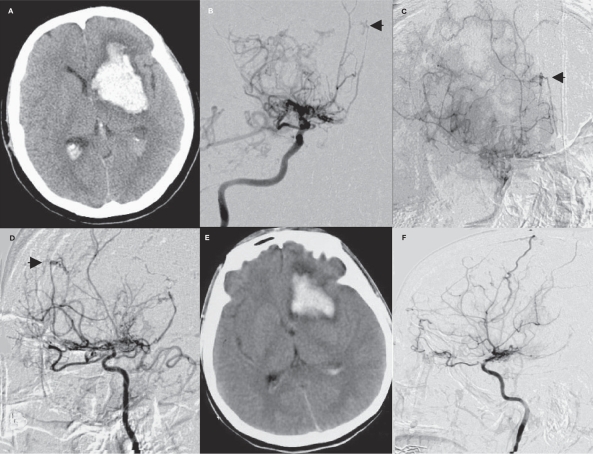
One patient had an aneurysm located at the starting portion of the pontine branch of the basilar artery. As a result of the communication of the aneurysm with the basilar artery through small arteries, as well as the presence of a stenotic upper segment in the basilar artery that was too narrow to allow a guidewire to pass through, conservative treatment was adopted. After five months of follow-up, the patient died of intracranial hemorrhage at the primary site (Figure 4).
Figure 4.
A case in the saccular aneurysm group who died after receiving unsuccessful endovascular embolization (case 9). A) CT shows SAH in the interpeduncular cistern. B) CTA shows pontine aneurysm of the basilar artery. C-F) DSA shows MMD and pontine aneurysm of the basilar artery. G) The patient died of aneurysm rupture and rebleeding five months after surgery.
2.2 Pseudoaneurysm group (n=1)
The patient had a perforating artery aneurysm of the left middle cerebral artery. Endovascular embolization was abandoned for the patient because of the failure to introduce the guidewire into the tortuous starting portion of the parent artery despite repeated efforts. Follow-up DSA examination one week later showed the disappearance of the aneurysm. No rupture of the aneurysm occurred during the two-year follow-up. The patient is still being monitored (Figure 5).
Figure 5.
A case in the pseudoaneurysm group with favorable prognosis after unsuccessful endovascular embolization (case 3). A) CT shows basal ganglia hemorrhage. B-D) Bilateral carotid artery angiography shows MMD and pseudoaneurysm (arrow). E) Repeated CT shows hematoma being absorbed one week after attempted embolization. F) DSA shows that the aneurysm disappeared one week after surgery.
Discussion
Given that MMS shares many similar angiographic manifestations and clinical characteristics with MMD, both disorders are usually regarded as tightly correlated disease entities in the literature 3,7. This report retrospectively analyzed the efficacy of endovascular treatment for patients with MMD or MMS associated with intracranial aneurysm. At present, various classification systems have been proposed for MMD or MMS associated with intracranial aneurysm in the literature. For example, intracranial aneurysm can be divided into pseudoaneurysm, saccular (true) aneurysm, and dissecting aneurysm, according to the morphology and histology of the aneurysm 12-14. Alternatively, Kodama et al. provided the classification of aneurysms arising from the circle of Willis and those from the abnormal vessel network 15. In fact, major artery aneurysms from the circle of Willis are almost saccular (true) aneurysms. They are related to high flow of the main trunk artery in MMD and MMS. However, aneurysms from the distal segments of the peripheral cerebral arteries or moyamoya vessels are pseudoaneurysms that come from dissection rupture or are a result of moyamoya vessel rupture. These aneurysms are formed due to increased stress on the vessel wall from the high flow imposed by occlusion of the anterior circulation 12-16. Because in our study all patients presented with intracranial hemorrhage, we could not distinguish the origin of pseudoaneurysms between dissection rupture and moyamoya vessel rupture. So it is reasonable to divide the aneurysms presenting with intracranial hemorrhage in MMD and MMS into saccular (true) aneurysm group and pseudoaneurysm group.
Treatment strategies for intracranial aneurysms associated with MMD or MMS include surgical clipping and endovascular embolization 8-10. However, thorough surgical clipping of these saccular aneurysms arising from the circle of Willis often present substantial challenges mainly derived from considerable dysplastic blood vessels and stiff arteries without needed plasticity, thus causing great difficulty in the localization and exposure of aneurysms. Meanwhile, the destruction of the previously established collateral circulation during micro-operation also accounts for increased risk and difficulty associated with this procedure 17,18. Furthermore, accurate localization of pseudoaneurysms from the distal segments of the peripheral cerebral arteries or moyamoya vessels is also extremely challenging during surgical clipping procedures 8. Since the first application of endovascular embolization in the occlusion of aneurysms associated with MMD or MMS using electrically detachable coils, there have been several reports concerning the use of this procedure 10,18,19. However, most of these studies are limited to only a few cases, lacking a systematic perspective. We therefore performed a systematic retrospective analysis of our experience in treating 13 cases of MMD or MMS associated with aneurysm using endovascular embolization during the last decade in our hospital.
In the saccular aneurysm group, five cases of the anterior circulation aneurysm had MMS. DSA examination showed no significant stenosis in the vertebral-basilar artery and contralateral carotid artery except moyamoya-like changes in the unilateral carotid artery. The aneurysms in the five cases were successfully embolized with endovascular coils. Moreover, the procedures were not different from those for cases without MMS and also showed a favorable prognosis, indicating that unilateral moya-moya changes do not have a severe effect on the hemodynamics of the whole brain in cases of MMS. Therefore, for patients with MMS associated with anterior circulation saccular aneurysms, endovascular treatment and prognosis are expected to be similar to those cases of common intracranial aneurysms.
Another five cases of the posterior circulation saccular aneurysm suffered MMD. The vertebral-basilar artery is the only artery supplying the whole brain in MMD patients. Endovascular embolization thus poses greater difficulty in these patients than in MMS patients or in patients with no MMD. Massoud et al. reported the application of endovascular embolization in two MMD patients with basilar tip aneurysms, of whom one died of complications [10]. This suggests that great care must be taken in the treatment of these aneurysms; even the slightest mistake may lead to serious adverse consequences. Of the five cases in this group, four cases underwent successful endovascular embolization and had a favorable prognosis, whereas endovascular embolization was not achieved in one patient who died of cerebral hemorrhage five months after surgery. Aneurysms from the posterior circulation sustain higher blood pressure in the presence of MMD than in MMS or in the absence of MMD. Therefore, patients with MMD associated with these saccular aneurysms are more susceptible to bleeding 10,18. Aggressive efforts are required for the treatment of MMD associated with saccular aneurysms in posterior circulation, since conservative therapy is linked with substantial risk of aneurysm rupture. Moreover, much care must be taken while performing endovascular embolization in order to prevent complications.
In the pseudoaneurysm group, three cases were included (two had MMD and one MMS). Pseudoaneurysms in these cases localized either in the distal segments of the peripheral cerebral arteries or in the moyamoya vessels. Inherent vascular fragility of collateral vessels and altered hemodynamics should be considered the major responsible cause [19]. Previous studies suggest that these aneurysms preferentially localized on the ventricular wall or within the hematoma cavity and accounted for 39% cases of MMD or MMS associated with intracranial aneurysms 20,21. In this group two pseudoaneurysms were situated within the hematoma cavity and one on the ventricular wall presenting with IVH. At present the natural history of pseudoaneurysm in MMD and MMS remains a controversial issue. Kawaguchi et al. 17showed that such aneurysms disappeared after conservative treatment, while Hamada et al. 22reported that recurrent bleeding occurred during conservative treatment of these aneurysms. Consequently, we recommend active treatment for patients with an unclear natural history of pseudoaneurysms. Because the psuedoaneurysms in this group localized at the distal segments of unusual branch vessels or moyamoya vessels, aneurysm resection or parent artery occlusion should be feasible to prevent rebleeding and complications. Although the successful resection of pseudoaneurysms has been reported 21, accurate localization of these aneurysms is very difficult in practice.
Meanwhile aneurysmectomy carries a risk of additional brain damage, because these aneurysms are usually deeply located. In addition, because collateral circulation is important in patients with MMD, surgical injury of these vessels can produce additional ischemic brain damage 19.
Nevertheless, endovascular embolization provides a feasible solution to this problem. Of the three patients in this group, two cases were embolized with Onyx by inserting a microcatheter into the proximal parent artery. Both patients showed a good prognosis.
The aneurysm in the remaining one case was situated in the distal perforating branch of the middle cerebral artery. Because the starting part of the parent artery is too tortuous to allow catheter entry, conservative treatment was administered to this patient who was later followed up for two years and experienced no aneurysm rupture.
Hence, we recommend that endovascular embolization of the parent artery may prove to be a safe option for patients with an unclear natural history of pseudoaneurysms that localized at the distal segments of unusual branch vessels or moyamoya vessels.
Meanwhile, temporary conservative treatment with close follow-up should be also feasible for those patients experiencing unsuccessful endovascular procedure. However, preoperative aneurysm localization should be performed and endovascular embolization of the parent artery is suggested for pseudoaneurysms found at the terminal portion of cerebral arteries in order to avoid postoperative complications.
Conclusions
In MMD or MMS associated with intracranial aneurysms, coil embolization was performed for saccular aneurysms whereas endovascular parent artery occlusion with Onyx was conducted for pseudoaneurysms. Most of the patients have a good prognosis. Strategies of endovascular treatment need to vary depending on the localization and type of aneurysms so as to ensure satisfactory efficacy of endovascular treatment.
References
- 1.Nishimoto A. Moyamoya disease (author's transl) Neurol Med Chir (Tokyo) 1979;19(3):221–228. doi: 10.2176/nmc.19.221. [DOI] [PubMed] [Google Scholar]
- 2.Kelly ME, Bell-Stephens TE, Marks MP, et al. Progression of unilateral moyamoya disease: A clinical series. Cerebrovasc Dis. 2006;22(2-3):109–15. doi: 10.1159/000093238. [DOI] [PubMed] [Google Scholar]
- 3.Natori Y, Ikezaki K, Matsushima T, et al. ‘Angiographic moyamoya’ its definition, classification, and therapy. Clin Neurol Neurosurg. 1997;99(Suppl 2):S168–72. doi: 10.1016/s0303-8467(97)00052-8. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi S, Sakaki T, Kakizaki T, et al. Clinical features of the haemorrhage type moyamoya disease based on 31 cases. Acta Neurochir (Wien) 1996;138(10):1200–10. doi: 10.1007/BF01809751. [DOI] [PubMed] [Google Scholar]
- 5.Iwama T, Todaka T, Hashimoto N. Direct surgery for major artery aneurysm associated with Moyamoya disease. Clin Neurol Neurosurg. 1997;99:191–193. doi: 10.1016/s0303-8467(97)00081-4. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Ogashiwa M, Takeuchi K. The moyamoya phenomenon with accompanying intracranial aneurysm. Neuroradiology. 1978;16:289–90. doi: 10.1007/BF00395276. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama K, Mishima K, Saito N, et al. Radiation-induced aneurysm and moyamoya vessels presenting with subarachnoid haemorrhage. Acta Neurochir (Wien) 2000;142(2):139–43. doi: 10.1007/s007010050016. [DOI] [PubMed] [Google Scholar]
- 8.Muizelaar JP. Early operation of ruptured basilar artery aneurysm associated with bilateral carotid occlusion (moyamoya disease) Clin Neurol Neurosurg. 1988;90(4):349–55. doi: 10.1016/0303-8467(88)90009-1. [DOI] [PubMed] [Google Scholar]
- 9.Arita K, Kurisu K, Ohba S, et al. Endovascular treatment of basilar tip aneurysms associated with moyamoya disease. Neuroradiology. 2003;45(7):441–4. doi: 10.1007/s00234-003-0997-x. [DOI] [PubMed] [Google Scholar]
- 10.Massoud TF, Guglielmi G, Viñuela F, et al. Saccular aneurysms in moyamoya disease: endovascular treatment using electrically detachable coils. Surg Neurol. 1994 Jun;41(6):462–7. doi: 10.1016/0090-3019(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 11.Klimo P, Jr, Kestle JR, MacDonald JD, et al. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 2004;100:215–224. doi: 10.3171/jns.2004.100.2.0215. [DOI] [PubMed] [Google Scholar]
- 12.Borota L, Marinkovic S, Bajic R, et al. Intracranial aneurysms associated with moyamoya disease. Neurol Med Chir (Tokyo) 1996;36(12):860–4. doi: 10.2176/nmc.36.860. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Jr, Kassell NF, Wisoff HS, et al. Intracranial saccular aneurysm and moyamoya disease. Stroke. 1979;10(2):4–9. doi: 10.1161/01.str.10.2.174. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita M, Tanaka K, Matsuo T, et al. Cerebral dissecting aneurysms in patients with moyamoya disease. Report of two cases. J Neurosurg. 1983;58(1):120–5. doi: 10.3171/jns.1983.58.1.0120. [DOI] [PubMed] [Google Scholar]
- 15.Kodama N, Sato M, Sasaki T. Treatment of ruptured cerebral aneurysm in moyamoya disease. Surg Neurol. 1996;46(1):62–6. doi: 10.1016/0090-3019(96)00043-2. [DOI] [PubMed] [Google Scholar]
- 16.Nagata S, Matsushima T, Morioka T, et al. Unilaterally symptomatic moyamoya disease in children: long-term follow-up of 20 patients. Neurosurgery. 2006;59(4):830–6. doi: 10.1227/01.NEU.0000227527.69766.43. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi S, Sakaki T, Morimoto T, et al. Characteristics of intracranial aneurysms associated with moyamoya disease. A review of 111 cases. Acta Neurochir (Wien) 1996;138(11):1287–94. doi: 10.1007/BF01411057. [DOI] [PubMed] [Google Scholar]
- 18.Irie K, Kawanishi M, Nagao S. Endovascular treatment of basilar tip aneurysm associated with moyamoya disease-case report. Neurol Med Chir (Tokyo) 2000;40(10):515–8. doi: 10.2176/nmc.40.515. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Kwon OK, Jung CK, et al. Endovascular treatment of ruptured aneurysms or pseudoaneurysms on the collateral vessels in patients with moyamoya disease. Neurosurgery. 2009;65(5):1000–4. doi: 10.1227/01.NEU.0000345648.46096.CE. [DOI] [PubMed] [Google Scholar]
- 20.Waga S, Tochio H. Intracranial aneurysm associated with moyamoya disease in childhood. Surg Neurol. 1985;23(3):237–43. doi: 10.1016/0090-3019(85)90088-6. [DOI] [PubMed] [Google Scholar]
- 21.Ali MJ, Bendok BR, Getch CC, et al. Surgical management of a ruptured posterior choroidal intraventricular aneurysm associated with moyamoya disease using frameless stereotaxy: case report and review of the literature. Neurosurgery. 2004;54(4):1019–24. doi: 10.1227/01.neu.0000115676.99378.9c. [DOI] [PubMed] [Google Scholar]
- 22.Hamada J, Hashimoto N, Tsukahara T. Moyamoya disease with repeated intraventricular hemorrhage due to aneurysm rupture. Report of two cases. J Neurosurg. 1994;80(2):28–31. doi: 10.3171/jns.1994.80.2.0328. [DOI] [PubMed] [Google Scholar]