Summary
Intra-arterial infusion of urokinase (UK) has been widely used. However, the optimal infusion rate of the reagent has never been determined. This was investigated in the acute stage of middle cerebral artery (MCA) embolism in the present study.
Sprague Dawley male rats (n=43) were randomly divided into sham-operation and five ischemic groups with urokinase administration at different infusion rates or without urokinase administration. Ischemia was induced with MCA embolism. Two hours after embolism, total urokinase (urokinase, 170,000U/kg) was given in groups A,B,C and D (n=8 each) at different rates: 1,000 U (0.03 ml/min) per minute, 4,000U (0.12 ml/min), 10,000U (0.30 ml/min), and 16,000U (0.48 ml/min), respectively. Group E received normal saline at a rate of 0.48 ml/ min. The sham-operation group (no embolism) received urokinase at (170,000U/kg, 1.5 ml, 16,000 U/min). During ischemia and thrombolysis, regional cerebral blood flow (CBF) was monitored by laser Doppler flowmetry. The neurological deficits, infarct volumes and mortalities in each group were determined.
The CBF in ischemic hemisphere were significantly (p<0.05) decreased after embolism in groups A~E at similar levels (27.32±8.20% to 34.71 ±6.84%). After different treatments, in group B 4,000U/min infusion of UK induced the best reperfusion, the least neurological deficits and infarct volume, as well as the least mortality and lowest incidence of hemorrhage.
The effect of intra-artery thrombolysis of urokinase was related to the infusion rate. Our study demonstrated an optimal infusion rate at 4,000U/min, suggesting relatively low levels of infusion are better able to improve brain reperfusion and reduce brain injury after stroke.
Key words: autologous blood clot, embolism, urokinase, intra-artery thrombolysis, infusion rate
Introduction
Ischemic cerebrovascular disease has become one of most life-threatening diseases. Antiplatlet drug and thrombolysis are the main treatments of this disease. Thrombolysis includes intravenous thrombolysis (IVT), intra-arterial thrombolysis (IAT), and combined intravenous and intra-arterial thrombolysis 1,2. Many experimental and clinical studies have proved the efficacy of intra-arterial thrombolysis 3-7. Clinical studies PROACT 8 and PROACT II 9 reported a high recanalization rate in intra-arterial thrombolysis (IAT) and a good outcome in ischemic cerebrovascular disease. However, the high recanalization rate was associated with intracranial hemorrhage which is the most worrying problem due to the increased mortality 9-11. Urokinase was commonly used in thrombolytic treatment in developing countries because of the low price and good pharmacodynamic action. In IAT treatment, the dosage and infusion rate of the drug may influence the effective and the hemorrhage incidence of thrombolysis. Although the urokinase dose of 10,000 U/kg has been widely used in a clinical setting, there is no strong experimental and clinical evidence to support this usage. Some research on the normal relationship between the urokinase dose and the effect in thrombolysis was not convincing because of missing control group 12. In the present research, we used a stable and reversible model to elucidate the effect of the intraarterial infusion rate of urokinase administration on thrombolysis in acute stroke and determine an optimal rate. We used a middle cerebral artery (MCA) embolism stroke model in rat 13. We infused the same dose of urokinase at the same time point at different rates during different periods, and we evaluated the outcome of ischemic rats with different treatments.
Experimental Procedures
Subjects
Male Sprague Dawley (SD) rats (280~350 g, n=43) were used. Animals were housed in the vivarium, with free access to water, but food 12 hours before surgery. The animals were randomly divided into five experimental groups (A~E, n=8×5) and a sham-operation group (S, n=3).
Preparation of the Embolus
In our study, the embolus was prepared with a modified method described previously by Zhang et al 13. Briefly, blood in each rat was collected from the femoral artery using a 20 cm of PE-50 tubing and retained in the tube for 2 h at room temperature. Blood clot formed in the tubing was subsequently retained for 22 h at 4°C. A 5 cm of the PE-50 tube containing clot was cut and attached at each end to a 20 cm PE 10 tubing interconnected by a syringe filled with saline. The clot was shifted by continuous alternating movement from one syringe to the other for 5 min. A single clot was removed to a modified PE-50 catheter with a 0.35 mm outer diameter filled with saline.
Animal Stroke Mode
Animals were anesthetized with 4.0% halothane. The anesthesia was maintained with 1.5% halothane in 70% N2O and 30% O2 using a tracheal intubation. Rectal temperature was maintained at 37±0.50C throughout the surgical procedure using a feedback regulated heating system. Under the operating microscope (Carl Zeiss Inc.) the right common carotid arteries (CCA), the right external carotid artery (ECA), and the internal carotid artery (ICA) were isolated via a midline incision. A modified PE-50 catheter with a 0.35 mm outer diameter filled with a clot with 1.5 mm diameter, which was attached with a 1ml Hamilton syringe, was introduced into the ECA lumen. A 13~14 mm length of catheter was gently advanced from the ECA into the lumen of the ICA. The clot along with 5 ul of saline in the catheter was injected into the ICA over ten seconds. The catheter was withdrawn from the right ECA five minutes after injection. The right ECA was ligated. Heparin was not administered to any animal. In the sham group, the rats received the same operation without injection of the clot.
Intra-Arterial Thrombolysis with Urokinase
In clinical settings, the acute ischemic stroke patients receive a dosage of urokinase in the range from 300,000 to 1,500,000 Units intraarterially. Mori et al. 14 reported that the hemorrhage incidence may rise if the dosage exceeds 1,000,000 U. We considered that the patient received the dosage 17,000 U/kg (1,000,000 U/60 kg). In previous work, drug dosage in humans was effective with only 10% of dosage in rat 6,15. Since there is no evidence showing the relation in dosage of urokinase between human and animal administrations, based on our previous experience, the dosage of 170,000 U was applied in the present study. The rats received the dosage 170,000 U, which mixed with normal saline (normal sodium) to 1.5 ml. The urokinase perfusion rate in each experimental group is listed in Table 1.
Table 1.
Clinical information on presentation, and angiographic data dichotomized by t-PA dose.
| group | drug | dosage | injecting rate |
|---|---|---|---|
| A n=8 | urokinase | 1.5 ml, 170,000 U/kg | 0.12 ml/min, 1,000 U/min |
| B n=8 | urokinase | 1.5 ml, 170,000 U/kg | 0.12 ml/min, 4,000 U/min |
| C n=8 | urokinase | 1.5 ml, 170,000 U/kg | 0.30 ml/min, 10,000 U/min |
| D n=8 | urokinase | 1.5 ml, 170,000 U/kg | 0.48 ml/min, 16,000 U/min |
| E n=8 | normal saline | 1.5 ml | 0.48 ml/min |
Blood Analysis
Arterial blood gas (pH, pCO2, and pO2) was measured before clot injection and after the IAT.
Monitoring of Regional CBF by LDF
Regional CBF was measured using laser Doppler flowmetry (LDF). A 1.5 mm diameter burr hole was placed at 3.0 mm posterior and 5.0 mm lateral to the bregma in the ipsilateral hemisphere.
The LDF probe was placed in the hole. We tested the CBF before and after clot injection at 0,30,60,90 and 120 min and after injection of urokinase or normal saline. Regional CBF was expressed as a percentage of pre-ischemic baseline values.
Neurologic Deficits
Neurologic examination was performed before the operation and at 2 h (I2hR0h), 24 h (I2hR22h), and 48 h (I2hR46h) after clot injection. The deficits were scored on a modified scoring system developed by Longa et al. 16, as follows: 0, no deficits; 1, unable to extend the contralateral forelimb; 2, flexion of contralateral forelimb; 3, mild circling to the contralateral side; 4, severe circling; and 5, falling to the contralateral side. Animals that did not have severe neurological deficits (score -3) during MCA occlusion were excluded from further analysis.
Ischemic Volume
At 46 hours after the IAT, animals, deeply anesthetized with 10% chloral hydrate, were killed.
Their brains were cut into 2 mm thick coronal section, and reacted with tetrazolium chloride (TTC) at 37° for 10 min. We calculated the ischemic volume using Image-Pro Plus Analysis Software System. The infarct volume was presented as a percentage of the volume of the contralateral hemisphere.
Statistical Analysis
We used SPSS 11.5 to perform the statistical analysis. Results were presented as mean values ± one standard deviation. Differences in the percentage of CBF, infarct volume and behavior assessment score between different treated groups were examined with a one-way analysis. P value of 0.05 was used to determine statistical significance.
Results
Physiological Parameters
The arterial blood gas values showed the blood pH of rats in every group before and after surgery was in the normal range (7.35-7.45), and there was no difference in the pH and the PO2 among every group and there was also no difference between before and after surgery. The mean arterial blood pressures (MABP) and rectal temperature were in a normal physiological range prior to injection of a clot and after injection of urokinase or normal saline.
Mortalities
The mortalities of group A~E at 22 h after thrombolysis were 37.5% (group A), 25.0% (B), 50% (C), 62.5% (D), and 42.9% (E), respectively. At 46 h after thrombolysis, the mortalities were 62.5% (A), 37.5% (B), 62.5% (C), 62.5% (D), and 57.1% (E), respectively. It is obvious that ischemic rats with urokinase thrombolysis at 4,000U/min have the lowest mortality rate.
Neurological Deficits
Neurologic deficits were determined in groups A~E (n=8 each) before embolism, at the time the animals were awake after operation, and after thrombolysis at 22 and 46 hours. The neurologic deficits were scored as described by Longa et al. 16. After the operation, one animal in group D died from subarachnoid hemorrhage, but the dead animal's CBF decreased rapidly during urokinase infusion, thus we thought that this phenomenon may have been related to the urokinase infusion rate. In group A with 1,000U/minute, the score was 4.29±0.76 immediately after reperfusion, 3.40±0.55 at 22h after reperfusion, and 3.33±0.58 at 46h, respectively. In group B with 4,000U/ minute, score was 3.38±0.74 immediately after reperfusion, 2.83±0.75 at 22h, and 1.80±0.45 at 46h, respectively. In group C with 10,000U/ minute, the score was 4.00±0.58 right after reperfusion, 4.25±0.50 at 22h, and 2.33±0.58 at 46h, respectively. In group D with 16,000U/ minute, the score was 4.29±0.76 immediately after reperfusion, 4.00±1.73 at 22h, and 4.00±1.73% at 46h. In group E with normal saline, the score was 3.86±0.38 right after reperfusion, 3.75±0.50 at 22h and 2.67±0.58% at 46h. Taken together, the animals exhibited moderate to severe neurological deficits after the operation. When the animals had awakened after thrombolysis, animals with 4,000U/minute infusion rate (group B) showed a lower neurological deficit score than groups A and D (F=3.109, P=0.040), but no significant difference from those of groups C (P=0.193) and E (P=0.640). However, animals with different infusion rates had different neurological deficits score at 22h after thrombolysis (F=2.182, P=0.115). At 46h after thrombolysis, ANOVA analysis demonstrated that animals with 4,000 U/minute infusion rate (group B) showed a significant further reduction in their deficit compared to the deficit at the time as the animals were awake and 22h after thrombolysis (F= 8.15 P=0.004). These scores were better than the deficits scores of 16,000 U/minute infusion rate (droup D) at this time (p=0.028). In addition, the high mortalities in groups C, D and E also indicated the poor outcome of the treatment (Figure 1).
Figure 1.
The neurological deficits score of the rats in group B was better than that of group D p<0.05 at the time of 46h after IAT waking, but there were no significant differences between control treatment group with our group with different infusion rates p>0.05). The high mortalities in groups C, D and E may show the poor outcome of the treatment.
Ischemic Volume and Hemorrhage
At 46 after thrombolysis, the brains were cut into 2 mm thick coronal sections and stained with tetrazolium chloride (TTC) at 370C for 10 min. The ischemic volumes in groups A-E were 35.70±8.70 (A, n=3), 27.10±7.88% (B, n=4), 35.34±7.19% (C, n=3), 58.69±1.73% (D, n=3) and 56.63±6.19% (E, n=3). Obviously, ischemic rats with urokinase infusion at 4,000U/min had the least infarct volume, which is significantly different from those in groups D and E (F=13.74, p<0.05).
We reviewed all brains of rats including the dead rats, and found that one in group C, and three in group D had hemorrhage. One case in group D was serious, leading to a severe brain edema (Figure 2).
Figure 2.
Brain coronal section and the hemorrhagic focus: the gray part was the normal brain tissue, the white part was the ischemic tissue, the brown part (the arrow) was the hemorrhagic focus.
Cerebral Blood Flow
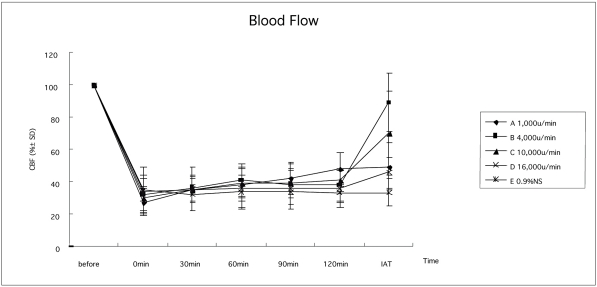
As measured by LDF, the CBF in all rats (A-E) after clot injection was significantly (p<0.05) decreased to ischemic level (27.32±8.20% 32.05±11.39% 29.59±7.66% 34.37±14.50%, and 34.71±6.84%, respectively). This reduced CBF retained the same low level until thrombolytic treatments were applied. We determined CBF in groups A~E after thrombolysis with urokinase at different infusion rates or saline infusion. We found at 15 min after thrombolysis, ischemic rats with urokinase infusion at 4,000U/minute had significantly (F=13.081 and p <0.05) better brain reperfusion with 88.87±18.11% CBF, as compared to 46.37±14.69%, 69.88± 25.91%, 45.45±9.52%, and 33.25±8.58% in groups A,C,D and E, respectively (Figure 3).
Figure 3.
CBF changes in each group. When the clot was injected, the CBF decreased to ischemic level (groups A-E, 27.32±8.2O% 32.05±11.39% 29.59±7.66% 34.37±14.50%, and 34.7l±6.84%), which all obviously differed from the CBF before the injection p 0.05). This reduced CBF maintained the same low level until thrombolytic treatments were applied p 0.05). After thrombolysis, ischemic rats with urokinase infusion at 4,000U/minute had a significantly better brain reperfusion p 0.05).
Discussion
Intravenous thrombolysis (IVT) and intraarterial thrombolysis (IAT) are the most important treatments for acute ischemic stroke, and recombinant tissue plasminogen activator (rt-PA) is widely used in western countries. In China and some Asian countries, urokinase is widely used as a thrombolytic agent for treatments in acute ischemic stroke 21. Intra-arterial thrombolysis (IAT) can induce high drug concentrations at local artery level, therefore less dosage is needed, but it appears that a high-risk complication during the operation, such as hemorrhage, influences its application 8,9.
Although the neurotoxicity of urokinase has not been verified, the relationship between the dosage and the hemorrhage of urokinase was studied by Mori et al., and the upper limit of urokinase dosage should be considered at less than 1,000,000IU. In clinical practice, the incidence of intracranial hemorrhage was not related to the total dosage of urokinase. Some studies 3,15,17 reported that thrombolysis degrading effect, hemorrhage incidence and mortality are increased with the dosage of thrombolytic drug. However, interventionists usually undertake angiography during the IAT. As soon as the trunk of the occluded artery was re-canalized, intra-arterial infusion of urokinase would be stopped immediately. So the dosage of thrombolytic drug can be well-controlled, that means the dosage might not be the key factor for hemorrhage. We consider that the infusion rate was the main factor inducing hemorrhage during and after IAT. On the other hand, the thrombolytic efficiency could be decreased with a very low infusion rate of urokinase, prolonging the ischemia time to the penumbra. In order to obtain the best thrombolytic effect, such as highest recanalization rate, best neuroprotection and lowest hemorrhage rate, the optimal infusion rate of urokinase needs to be established. In our research, we used the model invented by Zhang et al 13. We observed IAT thrombolytic treatment with different rates of urokinase. We found that the outcome in group B (4000U/min) was better than in the other groups, with the highest CBF after IAT, the most reduction in their deficit, the lowest mortality, and lowest incidence of hemorrhage. In the operation, we found that the CBF was significantly decreased to ischemic level and retained the same low level until thrombolytic treatments were applied. After the treatment, the CBF of some rats increased, however, the averages of CBF in each group differes with the different injecting rates of urokinase: in our study, the rats of group B had the highest CBF than the rates in other groups, and we also found the same phenomenon in neurologic deficits and mortality rate. In the group A (1000U/min), the CBF, neurologic deficits, and mortality rate had no obvious differences compared to those in groups D (16000U/ min) and E (normal sodium), which as a letter "U" with the rates of urokinase changes. This phenomenon might have occurred because of the pharmacologic action of urokinase which degraded the fibronolytic protein, and the blood might play an important part in the process. Several laboratories have shown that the neuronal injury after an acute arterial occlusion is not abrupt and complete at the onset but actually evolves over time. This has been shown in rodents and primate models of focal ischemia 18. By contrast, the region most distant from the occlusion may show injury within minutes of arterial occlusion, other regions in the same distribution may be non-functional but viable and potentially reversible hours after the insult. This ''penumbra'' could be reversed if the cerebrum is ''neuroprotected'' until the artery opens up spontaneously, or if the artery is opened with thrombolytic therapy. In group A, the concentration of urokinase at the proximal end of the thrombus was too low because of the low injecting rate to destroy the thrombus, leading to the poor outcome of IAT, and the longer duration of IAT in group A. The longer time of ischemia may also be another important factor in the poor outcome.
The midrange injecting rate of urokinase, such as 4,000U/min, can well combine the blood and urokinase, as well as the thrombus, so the urokinase can desolve the thrombus and recanalize the artery. When the infusion rate was increased, the urokinase aggregated quickly at the proximal end of the thrombus in the artery which raised pressure. Thus, the blood flow could not reach the surface of the thrombus, and the blood and the urokinase could not combine thoroughly. Otherwise, the high infusion rate of urokinase might induce the high local concentration of urokinase which might up-regulate some molecules, such as matrix metalloproteinase-9 (MMP-9) to injure the blood-brain barrier like tPA 19. It is suggested that the relatively decreased infusion rate of thrombolytic drug induced a better outcome. However, if the rate is too slow to achieve the effective concentration, the effect might not be good either. Therefore, the pressure at proximal end of thrombus was the important factor in IAT thrombolysis.
Conclusion
The effect of intra-artery thrombolysis was related to the infusion rate of urokinase. The relatively low rate induced the high CBF after IAT, reduction in neurologic deficit, and low mortality, as well as lower incidence of hemorrhage. Our study suggested that the optimal rate of urokinase infusion was 4000U/min in our rodent stroke model, suggesting a lower rate for patients.
References
- 1.Shaltoni HM, Albright KC, Gonzales NR, et al. Is intra-arterial thrombolysis safe after full-dose intravenous recombinant tissue plasminogen activator for acute ischemic stroke? Stroke. 2007;38:80–84. doi: 10.1161/01.STR.0000251720.25337.b0. [DOI] [PubMed] [Google Scholar]
- 2.Zaidat OO, Suarez JI, Santillan C, et al. Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke. 2002;33:1821–1827. doi: 10.1161/01.str.0000020363.23725.67. [DOI] [PubMed] [Google Scholar]
- 3.Shuaib A, Yang Y, Siddiqui MM, et al. Intraarterial urokinase produces significant attenuation of infarction volume in an embolic focal ischemia model. Experiment Neurol. 1998;154:330–335. doi: 10.1006/exnr.1998.6925. [DOI] [PubMed] [Google Scholar]
- 4.Busch E, Krüger K, Allegrini PR, et al. Reperfusion after thrombolytic therapy of embolic stroke in the rat: magnetic resonance and biochemical imaging. J Cereb Blood Flow Metabol. 1998;18:407–418. doi: 10.1097/00004647-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Neissen F, Hilger T, Hoehn M, et al. Thrombolytic treatment of clot embolism in rat: comparison of intraarterial and intravenous application of recombinant tissue plasminogen activator. Stroke. 2002;33(12):2999–3005. doi: 10.1161/01.str.0000038096.60932.f4. [DOI] [PubMed] [Google Scholar]
- 6.Busch E, Krüger K, Hossmann KA. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res. 1997;778:16–24. doi: 10.1016/s0006-8993(97)01008-1. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa A, Mori E, Minematsu K, et al. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke. Stroke. 2007;38:2633–2639. doi: 10.1161/STROKEAHA.107.488551. [DOI] [PubMed] [Google Scholar]
- 8.del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke: PROACT Investigators: prolyse in acute cerebral thromboembolism. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Furlan AJ, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. JAMA, 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 10.Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: The PROACT II trial. Neurology. 2001;57(9):1603–1610. doi: 10.1212/wnl.57.9.1603. [DOI] [PubMed] [Google Scholar]
- 11.Meguro T, Higashi H, Nishmoto K. Acute subdural hematoma after intra-arterial thrombolysis for acute ischemic stroke-case report. Neurol Med Chir (Tokyo) 2005;45(12):627–630. doi: 10.2176/nmc.45.627. [DOI] [PubMed] [Google Scholar]
- 12.Yizhi L, Caifang N, Xiaoli Z. An experimental investigation on the rate of selective infusion of urokinase in super acute cerebral stroke in rabbit. China Radiol J. 1997;31(3):196–198. [Google Scholar]
- 13.Zhang RL, Chopp M, Zhang ZG, et al. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 14.Mori E, Tabuchi M, Yoshida T, et al. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke. 1988;19:802–812. doi: 10.1161/01.str.19.7.802. [DOI] [PubMed] [Google Scholar]
- 15.Burggraf D, Martens HK, Dichgans M, et al. rt-PA causes a dose-dependent increase in the extravasation of cellular and non-cellular blood elements after focal cerebral ischemia. Brain Res. 2007;1164:55–62. doi: 10.1016/j.brainres.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 16.Longa EZ, Weinstein PR, Carlson S. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Gautier S, Petrault O, Gele P, et al. Involvement of thrombolysis in recombinant tissue plasminogen activator-induced cerebral hemorrhages and effect on infarct volume and postischemic endothelial function. Stroke. 2003;34:2975–2979. doi: 10.1161/01.STR.0000101914.62066.7B. [DOI] [PubMed] [Google Scholar]
- 18.Obrenovich TP. The ischemic penumbra: twenty years on. Cerebr Brain Metab Rev. 1995;7:297–323. [PubMed] [Google Scholar]
- 19.Kelly MA, Shuaib A, Todd KG. Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Experiment Neurol. 2006;200:38–49. doi: 10.1016/j.expneurol.2006.01.032. [DOI] [PubMed] [Google Scholar]