Summary
Long-term follow-up studies after endovascular treatment for intracranial aneurysm are still rare and inconclusive. The aim of this study was to assess long-term clinical and angiographic outcome of patients with endovascularly treated aneurysms.
The Clinical outcome of all 185 patients with endovascularly treated aneurysms were analyzed and 77 out of 122 surviving patients were examined with MRI and MRA nine to 16 years (mean 11 years) after the initial endovascular treatment.
Sixty-three patients were deceased at the time of follow-up. The cause of death was aneurysm- related in 34 (54%) patients. The annual re- bleeding rate from the treated aneurysms was 1.3% in the ruptured group and 0.1% in the unruptured group. In long-term follow-up MRA 18 aneurysms (53%) were graded as complete, 11 aneurysms (32%) had neck remnants and five aneurysms (15%) were incompletely occluded in the ruptured group. Occlusion grade was lower in the unruptured group with 20 an- eurysms (41%) graded as complete, 11 (22%) had neck remnants and 18 (37%) were incomplete. However, only three aneurysms were unstable during the follow-up period and needed retreatment.
Endovascular treatment of unruptured aneu- rysms showed incomplete angiographic outcome in 37% of cases. However, annual bleeding rate was as low as 0.1%. Endovascular treatment of ruptured aneurysms showed incomplete angiographic outcome in 15% of cases and the annual rebleeding rate was 1.3%.
Keywords: intracranial aneurysm, angiographic follow-up, endovascular treatment
Introduction
Endovascular coiling has been increasingly used for intracranial aneurysm occlusion since its introduction in 1991. Our hospital started endovascular treatment in 1989 and the first coiling with Guglielmi detachable coils (GDCs) was done in 1992. Nowadays in our institution 70% of aneurysms are coiled. While short and mid-term results are encouraging for endovas- cular procedures, there are still only few long- term follow-up studies 1-4. In approximately 20% of patients, the coiled aneurysm reopens in follow-up 2,5-8. Thus, delayed bleeding rate after endovascular coiling is slightly higher than after surgical clipping 1,2,9,10. The aim of this study was to assess the long-term angiographic results after endovascular coiling and to ascertain rebleeding rate for embolized aneurysms.
Materials and Methods
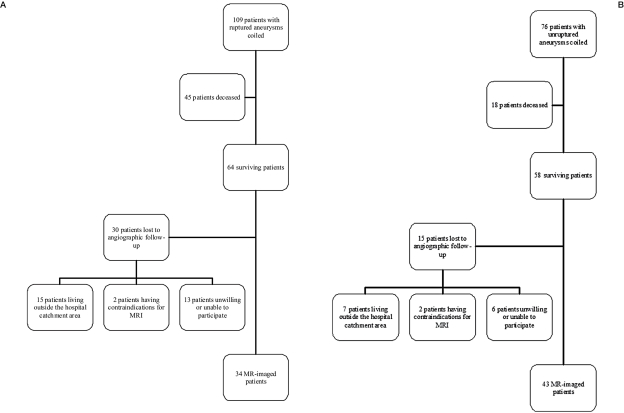
Patient population: A total of 617 patients with 513 ruptured and 189 unruptured intracra- nial aneurysms were treated between 1992 and 1999. Of these, 185 patients with 200 aneurysms were treated with endovascular coiling. Coiling was done for 109 patients with ruptured aneu- rysms and 76 patients with unruptured aneu- rysms (Figure 1). Endovascular procedures were carried out in cases where the aneurysm seemed to be treatable in the estimation of the endovascular radiologist and neurosurgeon. Aneurysms were embolized with platinum Guglielmi detachable coils (GDC, Target Therapeutics, Fremont California, USA) and peri- procedural medication was 5000 IU unfraction- ated heparin intravenously before coiling in unruptured cases and 5000 IU unfractionated heparin intravenously after two coils in ruptured cases. Intravenous nimodipine was used routinely. A total of 63 patients were deceased at the time of follow-up. Clinical outcome could be evaluated from 116 (95%) out of 122 surviving patients. Surviving patients, except 26 patients excluded were contacted by letter proposing an MRI investigation. Patients were excluded if they lived outside our hospital catchment area or had contraindications to MRI. Seventy-seven patients were examined with MRI and MRA approximately 11 years after initial coiling (range 9-16 years). Follow-up time was 792 patient-years in the unruptured group and 688 patient-years in the ruptured group.
Figure 1.
A,B) Flow chart summarizing the included and excluded patients in ruptured (A) group and unruptured (B) group.
Data collection: All hospital records, cerebral images and angiograms were evaluated retrospectively. Death certificates were examined to ascertain the date and cause of death. Surviving patients were contacted by letter proposing an MRI investigation. A total of 77 patients were examined neurologically and studied with MRI and MRA (magnetic resonance angiography) between the years 2007 and 2008. The clinical outcome evaluation was done with the Glasgow Outcome Score (GOS). Surviving patients not imaged by MRI were interviewed by phone and GOS was assessed and possible rebleeding episodes were elicited. The study was approved by the Hospital Ethics Committee and all patients gave their informed consent to the study.
Imaging protocol: The follow-up MR imaging was performed with a 1.5 T unit (GE Signa HD, Milwaukee, USA) with a one channel head coil. The MRA was supplemented with cross- sectional imaging including FLAIR, T1 and T2* sequences mainly to detect ischemic parenchyma, size of the CSF spaces and superficial siderosis. The imaging parameters for the non-contrast 3D time-of-flight angiography were: TR 30, TE 2.5, FOV 22×16.5 cm, slice thickness 1.0 mm/interpolated to 0.5 mm, matrix 320×224. Magnetization transfer contrast and flow compensation were included.
Imaging assessment: The diagnostic, procedural and follow-up angiograms were reviewed independently by a neuroradiologist, an inter- ventional radiologist and a neurosurgeon. Thereafter the results were reviewed and a consensus statement for each study was made. Aneurysm neck and fundus sizes were measured and dome-neck ratio was calculated. The degree of aneurysm occlusion was classified according to modified Raymond's classification2 as follows: complete occlusion, neck remnant and incomplete occlusion. Treatment result was also classified as loose or dense, the former meaning that there was opacification between coils and the latter meaning no contrast media was detected between coils.
Statistical analysis: The patient population was divided into two different groups: ruptured and unruptured aneurysms. Statistical analysis was performed using NCSS (NCSS, Kaysville, Utah, USA) statistical software. Categorical variables were compared using Fisher's exact two-tailed test. Continuous variables between groups were compared using the Mann-Whitney U-test or Student T-test. The level of significance was set at p<0.05. Correlation between variables was analyzed with Spearman's rank correlation test.
Results
Part I
Outcome of all endovascularly treated patients
Population characteristics: A total of 109 patients with ruptured aneurysms and 76 patients with unruptured aneurysms were treated with endovascular coiling. Unruptured aneurysms were detected because of previous SAH (sub- arachnoid hemorrhage) in 14 patients (18%). Nine (12%) were studied because of a family history of SAH. Forty-two (55%) were detected incidentally and 11 (14%) presented with cranial nerve dysfunction. The anterior communicating artery was the most frequent location in the ruptured group and internal carotid artery in the unruptured group. The ruptured group had more posterior circulation aneurysms than the unruptured group and this difference was statistically significant (p=0.02). Aneurysm mean size was 4.7 mm in the ruptured group and 6.3 mm in the unruptured group. Size varied more in the unruptured group and aneu- rysms tended to be bigger and have higher fun- dus-neck ratio than ruptured aneurysms but the difference was not statistically significant (p=0.16). Primary occlusion grade did not differ between ruptured and unruptured aneurysms (p=0.7). However, packing density differed significantly between the groups (p=0.04) being denser in the ruptured group. Angiographic occlusion grade and packing density were not related to the locations of aneurysms (p>0.7). There was a weak negative correlation between aneurysm size and occlusion grade (rho -0.3, p<0.03). Aneurysms, with fundi less than 5 mm, were totally occluded in 46% of cases whereas aneurysms over 10 mm wide were completely occluded in only 29% of cases.
Retreatment: Retreatment was provided for 27 patients with ruptured aneurysms (25%) and for 16 (21%) patients with unruptured an- eurysms. Retreatment within 6 months after initial coiling was done for 15 (56%) patients with ruptured aneurysms and for eight (50%) patients with unruptured aneurysms. Nineteen (70%) patients with ruptured aneurysms were retreated once, three (11%) were retreated twice, three (11%) patients were retreated three times and one (4%) patient five times and one (4%) patient six times. Ten (63%) patients with unruptured aneurysms were retreated once, five (30%) patients were retreated twice and one (6%) patient was retreated six times. Retreated aneurysms were larger than others: mean fundus diameter was 7.8 mm compared to 4.8 mm in aneurysms treated only once. The aneurysm neck was also wider in retreated aneurysms than in once treated aneu- rysms (mean 3.6 mm compared to 2.8 mm).
Rebleedings: There were ten postprocedural bleedings in all 185 endovascularly treated patients. Nine out of 109 SAH patients and one out of 75 patients with unruptured aneurysms bled after coiling procedure (Odds ratio 6.7, 95% CI 0.9 to 295.8, p=.05). One (1.3%) large unruptured aneurysm bled seven years after incomplete coiling. The annual bleeding rate was 0.1% in the unruptured group. Two patients were lost to follow-up and the bleeding rate, assuming these had rebleedings, was 3.9% (0.4% annual bleeding rate).
Nine ruptured aneurysms rebled (8.3%). Four rebleedings (3.7%) occurred within five weeks after coiling and five aneurysms (4.6%) rebled more than four years after initial treatment. One aneurysm was completely occluded, three had neck remnant and five were incompletely occluded in the post-procedural angiogram. Only three aneurysms were monitored after treatment. The reason for this was advanced patient age or poor clinical outcome after initial treatment. All but one patient died from rebleeding. The annual rebleeding rate was 1.3%. Four patients were lost to follow-up and bleeding rate, assuming all those had rebleedings, was 11.9% (annual bleeding rate 1.9%).
Mortality: Thirty-four (54%) out of 63 deceased patients died due to aneurysmal disease (Table 1). Eighteen patients with ruptured an- eurysms died from acute SAH, one patient died from treatment complications, one patient from brain infarcts caused by vasospasm and two patients survived many months in poor clinical outcome and the cause of death was primary SAH. Three (5%) patients died from bleeding of aneurysms that were left untreated before. Nine (14%) patients died from rebleeding. Twenty-nine (46%) out of 63 deceased patients died from diseases not related to aneurysm.
Table 1.
Cause of death of the 63 deceased patients
| Patients with SAH | Patients with unruptured aneurysms |
|
|---|---|---|
| Aneurysmal disease | 32 | 2 |
| Other cerebral diseases | 4 | 2 |
| Other cardiovascular diseases | 9 | 3 |
| Cancer | 4 | 2 |
| Respiratory | 1 | 0 |
| Trauma | 1 | 0 |
| Other | 0 | 3 |
| Total | 51 | 12 |
Clinical outcome of all patients: Most surviving SAH patients were in good clinical condition after follow-up: 34 (57%) patients had a GOS score of 5, and 12 (20%) patients had a GOS score of 4. Thirteen patients (22%) were dependent (GOS score of 3) and one (2%) patient was vegetative. Four patients were lost to clinical outcome. A total of 31 (55%) patients with unruptured aneurysms had a GOS score of 5 and 16 (29%) patients had a GOS score of 4. Nine patients (16%) were dependent after 11 years of follow-up. Two patients with unrup- tured aneurysms were lost to clinical follow-up. The poor outcome in patients with unruptured aneurysms was related to aneurysm in three patients only.
Part II
A subgroup analysis of 77 patients studied with MRI
Follow-up angiographic results: MR imaging was done for 34 patients with ruptured aneu- rysms and 43 patients with unruptured aneu- rysms (Table 2) more than nine years after the coiling procedure. MRA showed complete occlusion in 18 aneurysms (53%) in the ruptured group and 20 aneurysms in the unruptured group (40%). Angiographic results could not be evaluated from one patient because of artifacts in MRI. Eleven ruptured aneurysms (32%) and 11 (22%) unruptured aneurysms had neck remnants in MRA. Five aneurysms (15%) in the ruptured group and 18 (38%) in the unruptured group were graded as incomplete.
Table 2.
Baseline characteristics of the 77 included patients
| Patients with ruptured aneurysms n=34 |
Patients with unruptured aneurysms n=43 |
|
|---|---|---|
| Gender | ||
| Male | 15 | 15 |
| Female | 19 | 28 |
| Mean age (range) | 54 years (34-73) | 50 years (21-79) |
| Number of aneurysms | ||
| 1 | 26 | 29 |
| 2 | 6 | 10 |
| 3 | 2 | 4 |
| Number of aneurysms coiled | 34 | 43 |
| Reason for initial angiography | ||
| SAH | 34 (100%) | 7 (16%) |
| family history of SAH | 4 (9%) | |
| cranial nerve dysfunction | 4 (9%) | |
| incidental | 28 (65%) | |
| Hunt & Hess at the time of diagnosis | ||
| I | 12 | |
| II | 12 | |
| III | 6 | |
| IV | 4 | |
| V | 0 | |
| Fisher | 10 primary CT scans were missing | |
| 1 | 3 | |
| 2 | 1 | |
| 3 | 9 | |
| 4 | 9 | |
| Intraventricular hemorrhage | 2 | |
| Intracerebral hemorrhage | 9 | |
| Hydrocephalus in primary CT | 10 | |
| Aneurysm location | ||
| Anterior circulation | ||
| ICA | 2 (6%) | 20 (40%) |
| MCA | 3 (9%) | 14 (28%) |
| ACoA | 13 (38%) | 5 (10%) |
| ACoP | 3 (9%) | 1 (2%) |
| ACA/pericallosa | 2 (6%) | 5 (10%) |
| Vertebrobasilar circulation | 11 (32%) | 5 (10%) |
| Aneurysm size | ||
| ≤5mm | 23 (68%) | 29 (58%) |
| 6-10mm | 9 (26%) | 16 (32%) |
| >10mm | 2 (6%) | 5 (10%) |
| Initial angiographic result | p=0.7 | |
| complete | 14 (41%) | 24 (48%) |
| neck remnant | 9 (26%) | 10 (20%) |
| incomplete | 11 (32%) | 16 (32%) |
| Packing density | ||
| densely occluded | 22 (65%) | 19 (38%) |
| Retreatment | 13 (38%) | 12 (28%) |
| GOS at follow-up | ||
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 3 | 6 |
| 4 | 8 | 13 |
| 5 | 23 | 24 |
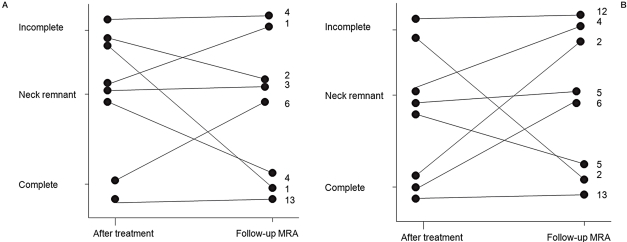
A change to a better occlusion grade (i.e. progression) was detected in seven (21%) ruptured aneurysms, 20 (59%) were stable, six (18%) had remnant growth and one (3%) had recurrence in the follow-up MR images (Figure 2). In the unruptured group, seven (14%) aneurysms progressed, 30 (61%) were stable, six (12%) had remnant growth and six (12%) had recurrence. Although incomplete occlusion was seen in 23 aneurysms (27%), only three had poorer occlusion grade than in previous control images. Twenty incompletely occluded aneu- rysms were monitored for many years after the initial treatment. If the occlusion grade was stable, follow-up images were discontinued. Four of these incompletely coiled aneurysms are still being followed-up.
Figure 2.
A,B) Changes in occlusion grades per aneurysm in the ruptured group (A) and in the unruptured group (B). Number on the right indicates number of cases.
Unruptured aneurysms were more incompletely occluded than ruptured ones and this difference reached statistical significance (p=0.03). Incompletely occluded aneurysms had wider necks and fundi than better occluded aneurysms. No de-novo aneurysms were detected in this series.
Only nine (36%) retreated aneurysms were graded as complete in MRA compared to 82 (51%) of once-treated aneurysms. Neck remnant was detected in eight (32%) and occlusion grade was incomplete in eight (32%) of retreated aneurysms.
Discussion
Our institution started coil embolization in 1992. In the early years of endovascular treatment, coiling was mainly used for patients with high surgical risk 11. However, in our institution endovascular treatment was approved for standard treatment at an early stage. Our aim was to examine all surviving patients with follow-up MRI, but because of the voluntary nature of the study, we could reach only 79% of included patients. Those patients we could not reach may be in poorer clinical condition and unable or unwilling to participate. However, this selection bias is unlikely to influence the angiographic occlusion grades. To the best of our knowledge, this series has the longest follow-up period published with most of the treated patients included (792 patient-years in the unruptured group and 688 patient-years in the ruptured group).
We conducted this long-term follow-up study with MRI because it is noninvasive and cost- effective compared to DSA (digital subtraction angiography) 12,13. The sensitivity of MRI has been demonstrated [14-16]. We studied patients with 1.5T rather than with 3.0T because they had previously been studied with 1.5T MRI as well in a study that ascertained the feasibility of MRA in our material 14. The follow-up images were thus comparable with each other.
Endovascular treatment is less frequently complete than surgical clipping of aneurysms. Furthermore, recurrences are more frequent. It has been shown that complete or near complete occlusion of the aneurysm sac is sufficient to prevent rebleeding in 98% of cases treated in the acute phase of SAH 17. Conversely, the presence of a residual aneurysm is associated with subsequent bleeding 17,18. Neck remnant obliteration may be sufficient to protect against rebleeding after SAH, but the recurrence risk is higher, and longer follow-up is needed.
Several Authors have discussed a wide variety in occlusion grades in earlier reports because anatomic results are subjective and difficult to standardize. Complete obliteration has previously been reported in 26%-88% of ruptured aneurysms, neck remnant in 18% to 50% and incomplete occlusion in 2% to 26% 5,8,9,17-20. In our study 27% of aneurysms were incompletely occluded and many of our angiographic results reported here would not be accepted today in our clinical practice. The aneurysms in our series were treated before the advent of newer techniques such as balloon-assisted remodeling, three-dimensional GDC or stents. These new techniques may decrease recurrence rates.
Gallas et al. 21 found total or subtotal occlusion in 97% of ruptured and unruptured aneu- rysms. This is the best angiographic outcome published and better than in our series or in earlier published series 2,3. Complete occlusion or neck remnant was found in 63% of unruptured aneurysms in our series and this is less than previously reported 3,19. Regardless of unfavorable angiographic occlusion grades, most of these were stable in follow-up. Only three aneurysms out of 23 incompletely occluded cases were unstable in follow-up and were retreated after this study. No recurrences occurred in completely occluded ruptured aneu- rysms in our series. These results are comparable to those of earlier reports 2-4,21.
Although the initial angiographic occlusion grade was similar in the ruptured and unrup- tured groups, unruptured aneurysms had lower packing density. In the early years it was thought that change in flow would prevent bleeding. This is probably the reason why unruptured aneurysms were less well occluded in the follow-up angiogram than ruptured aneu- rysms. Retreatment was performed for 43 an- eurysms (23%) which is higher than previously reported 5,8,9,17,18,20,21
Earlier studies have reported rebleeding rates after coiled, ruptured aneurysms to be between 0% and 6.5% 5,11,21,22. The annual rebleeding rate in earlier reports is between 0% and 3.5% 5,13,21,22. In our series annual rebleeding rate was I.3% and concurs with earlier studies.
Only a few studies have reported delayed bleeding rates for coiled unruptured aneurysms11,23-25. Rates have varied from 0% to 2.4% 19,23,25,26. The annual rupture rate in our series was 0.1%. The ISUIA study 27 reported annual rupture rate of 0.1% in patients with unrup- tured aneurysms of less than 7 mm in diameter who had not had a previous SAH. The rupture risk is so low that the overall treatment risks outweigh the treatment benefits. In our series 56% of unruptured aneurysms were less than 7 mm raising a question if the treatment of these was pointless or even harmful to patients. However, it is difficult to believe that coiling increases the risk for delayed bleeding in unruptured aneurysms. In our series 81% of ruptured aneu- rysms were less than 7 mm and thus beside an- eurysm size, inflammation of the aneurysm wall also seems to play important role in aneurysm degeneration and rupture28,29.
Conclusions
Endovascular treatment of unruptured aneu- rysms had incomplete angiographic outcome in 37% of cases. However, the annual bleeding rate was as low as 0.1%. Endovascular treatment of ruptured aneurysms had incomplete angiographic outcome in 15% of cases and the annual rebleeding rate was 1.3%.
Acknowledgement
This research was supported by EVO-fund- ing of Pirkanmaa Hospital District and Maire Taponen Foundation.
References
- 1.Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid hemorrhage, death, or dependence and standardized mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (ISAT): Long-term follow- up. Lancet Neurol. 2009;8:427–433. doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 3.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 4.Sprengers ME, Schaafsma J, van Rooij WJ, et al. Stability of intracranial aneurysms adequately occluded 6 months after coiling: a 3T MR angiography multicenter long-term follow-up study. Am J Neuroradiol. 2008;29:1768–1774. doi: 10.3174/ajnr.A1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:348–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 6.Piotin M, Spelle L, Mounayer C, et al. Intracranial aneurysms: treatment with bare platinum coils-aneurysm packing, complex coils, and angiographic recurrence. Radiology. 2007;243:500–508. doi: 10.1148/radiol.2431060006. [DOI] [PubMed] [Google Scholar]
- 7.Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Aneurysm Trial (ISAT) Stroke. 2007;38:1538–1544. doi: 10.1161/STROKEAHA.106.466987. [DOI] [PubMed] [Google Scholar]
- 8.Ferns SP, Sprengers ME, van Rooij WJ, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009;40:523–529. doi: 10.1161/STROKEAHA.109.553099. [DOI] [PubMed] [Google Scholar]
- 9.Byrne JV, Sohn MJ, Molyneux AJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656–663. doi: 10.3171/jns.1999.90.4.0656. [DOI] [PubMed] [Google Scholar]
- 10.Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid hemorrhage, death, or dependence and standardized mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433. doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng P, Khangure MS, Phatouros CC, et al. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke. 2002;33:210–217. doi: 10.1161/hs0102.100486. [DOI] [PubMed] [Google Scholar]
- 12.Ringer A J, Lanzino G, Veznedaroglu E, et al. Does angiographic surveillance pose a risk in the management of coiled intracranial aneurysms? A multicenter study of 2243 patients. Neurosurgery. 2008;63:845–849. doi: 10.1227/01.NEU.0000333261.63818.9C. [DOI] [PubMed] [Google Scholar]
- 13.Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522–528. doi: 10.1148/radiol.2272012071. [DOI] [PubMed] [Google Scholar]
- 14.Kähärä VJ, Seppänen SK, Ryymin PS, et al. MR angiography with three-dimensional time-of-flight and targeted maximum-intensity-projection reconstructions in the follow-up of intracranial aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1999;20:1470–1475. [PMC free article] [PubMed] [Google Scholar]
- 15.Urbach H, Dorenbeck U, von Falkenhausen M, et al. Three-dimensional time-of-flight MR angiography at 3T compared to digital subtraction angiography in the follow-up of ruptured and coiled intracranial aneurysms: a prospective study. Neuroradiology. 2008;50:383–389. doi: 10.1007/s00234-007-0355-5. [DOI] [PubMed] [Google Scholar]
- 16.Brunereau L, Cottier JP, Sonier CB, et al. Prospective evaluation of time-of-flight MR angiography in the follow-up of intracranial saccular aneurysms treated with Guglielmi detachable coils. J Comput Assist Tomogr. 1999;23:216–223. doi: 10.1097/00004728-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997;41:1235–1245. doi: 10.1097/00006123-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery. 1998;43:1016–1025. doi: 10.1097/00006123-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Murayama Y, Viñuela F, Duckwiler GR, et al. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg. 1999;90:207–214. doi: 10.3171/jns.1999.90.2.0207. [DOI] [PubMed] [Google Scholar]
- 20.Solander S, Ulhoa A, Viñuela F, et al. Endovascular treatment of multiple intracranial aneurysms by using Guglielmi detachable coils. J Neurosurg. 1999;90:857–864. doi: 10.3171/jns.1999.90.5.0857. [DOI] [PubMed] [Google Scholar]
- 21.Gallas S, Januel AC, Pasco A, et al. Long-term follow- up of 1036 cerebral aneurysms treated by bare coils: a multicentric cohort treated between 1998 and 2003. Am J Neuroradiol. 2009;30:1986–1992. doi: 10.3174/ajnr.A1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999;45:793–803. doi: 10.1097/00006123-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Ries T, Siemonsen S, Thomalla G, et al. Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. Am J Neuroradiol. 2007;28:1755–1761. doi: 10.3174/ajnr.A0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brilstra EH, Rinkel GJ, van der Graaf Y, et al. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999;30:470–476. doi: 10.1161/01.str.30.2.470. [DOI] [PubMed] [Google Scholar]
- 25.Lanterna LA, Tredici G, Dimitrov BD, et al. Treatment of unruptured cerebral aneurysms by embolization with Guglielmi detachable coils: case-fatality, morbidity, and effectiveness in preventing bleeding-a systematic review of the literature. Neurosurgery. 2004;55:767–778. doi: 10.1227/01.neu.0000137653.93173.1c. [DOI] [PubMed] [Google Scholar]
- 26.Thornton J, Debrun GM, Aletich VA, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002;50:239–249. doi: 10.1097/00006123-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 27.International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 28.Frösen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture - histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 29.Tulamo R, Frosen J, Junnikkala S, et al. Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery. 2006;59:1069–1076. doi: 10.1227/01.NEU.0000245598.84698.26. [DOI] [PubMed] [Google Scholar]