Summary
We describe the occurrence of the trigeminocardiac reflex (TCR) during DMSO pre-flushing of the microcatheter in preparation for Onyx embolization via the internal maxillary artery. TCR has not been previously associated with embolization of extradural entities. Familiarity with this clinical reflex and its proper management may help in planning neurointerventional procedures involving DMSO injection in the trigeminal territory.
Key words: juvenile nasal angiofibroma, embolization, onyx, trigeminocardiac reflex
Introduction
We describe a case of reflex sinus bradycardia as a component of the trigemino-cardiac reflex (TCR) in a child during injection of dimethyl sulfoxide (DMSO) prior to planned embolization with onyx.
Previous case reports of this reflex with surgical manipulation of the cranial dura and more recently with creation of an Onyx cast have been reported, but in our case the reflex resulted from the DMSO solvent itself, and is the first to report TCR in the treatment of an extradural entity.
TCR is a physiological reflex manifesting with sinus bradycardia, systemic hypotension, apnea, and gastric hypermotility due stimulation of any of the sensory branches of the trigeminal nerve.
Case Report
Institutional Review Board approval was obtained for review of cases involving extracranial embolization with onyx. The patient was a ten-year-old Chilean boy with a two-year history of epistaxis from the left nostril and CT scan evidence of a left nasopharyngeal mass. He underwent attempted excision, aborted due to intraoperative bleeding. Pathology was consistent with juvenile nasopharyngeal angiofibroma. He was referred to the Children's Hospital Boston. Further imaging demonstrated a left nasopharyngeal enhancing mass extending into the left masticator space and left sphenoid sinus, associated with osseous destruction of the left sphenoid bone, and soft tissue extension into the left foramen magnum. He was referred for pre-operative embolization, to decrease the intra-operative blood loss, as is the standard care for these richly vascular tumors at most high-volume centers. Intraprocedurally, the patient was monitored with pulse oximetry, a noninvasive blood pressure cuff, electrocardiography, and a temperature probe. The child weighed 28.6 kg. The baseline parameters were a pulse of 80 and blood pressure of 100/70 mm Hg. Anesthesia was induced with sevoflurane and nitrous oxide, and maintained using sevoflurane.
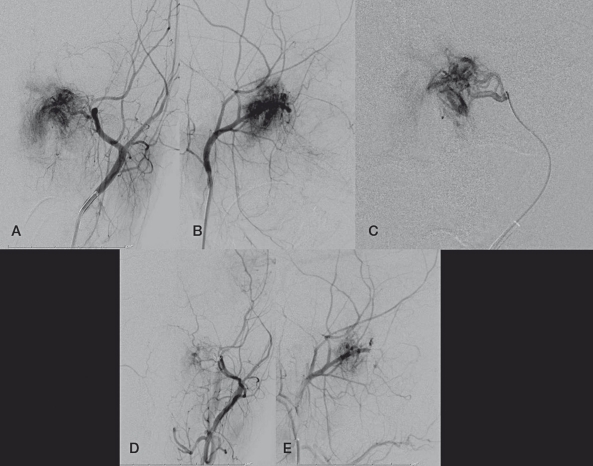
A 5 Fr envoy guide catheter (Cordis, Miami Lakes, FL, USA) was positioned in the left external carotid artery distal to the facial artery origin, and angiography was performed (Figure 1A, B). An echelon 14 (ev3, Irvine, CA, USA) microcatheter was advanced over a 0.014" Transcend (Boston Scientific, Natick, MA, USA) microglidewire into the distal left internal maxillary artery supplying the tumor (Figure 1C). Given our success with the use of Onyx for embolization of extracranial head and neck arteriovenous malformations 1, and the potential benefits in terms of increased visualization of onyx relative to particulate agents (thereby lowering the risk of inadvertent external carotid to internal carotid embolization), embolization with Onyx (ev3, Irvine, CA, USA) was planned. However while flushing the dead space of the microcatheter with DMSO there was a sudden onset of bradycardia to the low 40's. After rapid administration of 0.4 mg of atropine by the anesthesiologist, the patient's heart rate normalized; there was no evidence of other physiologic instability. The Echelon 14 (Boston Scientific, Natick, MA, USA) microcatheter was removed from the left internal maxillary artery, and replaced by an Excelsior SL-10 microcatheter over a 0.014" Transend microglidewire. Embolization was performed using polyvinyl alcohol particles and a detachable coil. There were no further episodes of bradycardia. The patient was extubated and was found to be at his baseline.
Figure 1.
A, B) Frontal and lateral views of a left external carotid artery injection demonstrating the hypervascular nasopharyngeal JNA. C) Frontal view of a microcatheter injection of the left internal maxillary artery. D,E) Frontal and lateral views of the left external carotid artery after the embolization, demonstrating devascularization of the mass.
Discussion
The occurrence of TCR has been described previously during craniofacial operations, especially LeFort I osteotomy 2,3, operations in the cerebellopontine angle 4-6 and skull base 7, transnasal sphenoidal procedures 8-10, and trigeminal sensory root rhizotomy 11,12.
To the best of our knowledge, all prior reports of TCR during embolization occurred during the treatment of intradural or dural pathology. One recent case report of TCR was described during embolization of a tentorial dural arteriovenous fistula with onyx 13. The authors in that case postulated that the TCR was due to direct compression of the nervus spinosus by the onyx cast in the dilated middle meningeal artery in the foramen spinosum. A recent series of nine patients with dural AVF or carotid cavernous fistula reported bradycardia in four cases and asystole in two 14. In a recent case series reviewing dural fistulae embolized with Onyx, 7.7% of those treated intra-arterially and 33.3% of those treated intravenously were found to have a drop in heart rate of 50% or greater 15. The high incidence of TCR during transvenous embolization renders the direct middle meningeal artery compression explanation unlikely, as does our case, as onyx was never injected, and thus no cast was formed.
In our case, where the targeted pathology was extradural, the TCR likely resulted from chemical stimulation of the sensory nerve endings of the trigeminal nerve by DMSO. Interestingly, the reflex occurred during injection of the dead space of the microcatheter with DMSO rather than during the initial injection of Onyx. This strongly suggests that there is not insignificant DMSO escape during the dead space flush.
Sensory nerve endings of the trigeminal nerve project to the sensory nucleus of the trigeminal nerve via the gasserian ganglion 11,16 and connect to the motor nucleus of the vagus via the reticular formation, with cardio-inhibitory fibers from the motor nucleus of the vagus nerve terminating in the myocardium to complete the reflex arc 17.
It is important to recognize TCR, as this reflex manifesting in the setting of underlying heart disease may potentially pose a greater hazard than in healthy patients 18. Most often, cessation of the offending manipulation leads to normalization of heart rate and blood pressure, although vagolytics and possibly sympathomimetics may be needed. Recognition of this reflex and its potential stimulation via DMSO can help in expedient management. Particular predisposing risk factors for TCR include a light general anesthesia, hypoxia, pediatric patients with high resting vagal tone, and prior administration of potent narcotics such as sufetanil and alfentanil 19,20, beta blockers, or calcium channel antagonists 21. The sensory field of the trigeminal nerve extends from the scalp to the face, including the mucosa of the nose and mouth, and thus embolics delivered via several external carotid branches may theoretically result in the reflex, as may internal carotid - external carotid anastomoses.
References
- 1.Thiex R, Wu I, Mulliken JB, et al. Safety and clinical efficacy of onyx for embolization of extracranial head and neck vascular anomalies. Am J Neuroradiol. 2011;32(6) doi: 10.3174/ajnr.A2439. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts RS, Best JA, Shapiro RD. Trigeminocardiac reflex during temporomandibular joint arthroscopy: report of a case. J oral Maxillofac Surg. 1999;57(7):854–856. doi: 10.1016/s0278-2391(99)90829-7. [DOI] [PubMed] [Google Scholar]
- 3.Bohluli B, Ashtiani AK, Khayampoor A, et al. Trigeminocardiac reflex: a MaxFax literature review. Oral Surg oral Med oral Pathol oral Radiol Endod. 2009;108(2):184–188. doi: 10.1016/j.tripleo.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Gharabaghi A, Acioly de Sousa MA, Tatagiba M. Detection and prevention of the trigeminocardiac reflex during cerebellopontine angle surgery. Acta Neurochir (Wien) 2006;148(11):1223. doi: 10.1007/s00701-006-0894-2. [DOI] [PubMed] [Google Scholar]
- 5.Gharabaghi A, Koerbel A, Samii A, et al. The impact of hypotension due to the trigeminocardiac reflex on auditory function in vestibular schwannoma surgery. J Neurosurg. 2006;104(3):369–375. doi: 10.3171/jns.2006.104.3.369. [DOI] [PubMed] [Google Scholar]
- 6.Prabhakar H, Anand N, Chouhan RS, et al. Sudden asystole during surgery in the cerebellopontine angle. Acta Neurochir (Wien) 2006;148(6):699–700. doi: 10.1007/s00701-005-0712-2. discussion 700. [DOI] [PubMed] [Google Scholar]
- 7.Koerbel A, Gharabaghi A, Samii A, et al. Trigeminocardiac reflex during skull base surgery: mechanism and management. Acta Neurochir (Wien) 2005;147(7):727–732. doi: 10.1007/s00701-005-0535-1. discussion 732-733. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Zeid AH, Davis JR, Kearney T, et al. Transient asystole during endoscopic transsphenoidal surgery for acromegaly: an example of trigeminocardiac reflex. Pituitary. 2009;12(4):373–374. doi: 10.1007/s11102-008-0118-2. [DOI] [PubMed] [Google Scholar]
- 9.Filis A, Schaller B, Buchfelder M. [Trigeminocardiac reflex in pituitary surgery. A prospective pilot study] Nervenarzt. 2008;79(6):669–675. doi: 10.1007/s00115-007-2380-3. [DOI] [PubMed] [Google Scholar]
- 10.Schaller B, Sandu N, Ottoviani G, et al. Transient asystole during endoscopic transsphenoidal surgery: an example of trigeminocardiac reflex. Pituitary. 2009;12(3):271–272. doi: 10.1007/s11102-008-0132-4. [DOI] [PubMed] [Google Scholar]
- 11.Cha ST, Eby JB, Katzen JT, et al. Trigeminocardiac reflex: a unique case of recurrent asystole during bilateral trigeminal sensory root rhizotomy. J Craniomaxillofac Surg. 2002;30(2):108–111. doi: 10.1054/jcms.2001.0264. [DOI] [PubMed] [Google Scholar]
- 12.Fowler SJ, Featherston M. Recurrent atrial tachyarrhythmia triggered by percutaneous balloon rhizotomy of the trigeminal nerve. Anaesth Intensive Care. 2004;32(3):410–412. doi: 10.1177/0310057X0403200318. [DOI] [PubMed] [Google Scholar]
- 13.Lv X, Li Y, Lv M, et al. Trigeminocardiac reflex in embolization of intracranial dural arteriovenous fistula. Am J Neuroradiol. 2007;28(9):1769–1770. doi: 10.3174/ajnr.A0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiridze NS, Darwish RS. Hemodynamic instability during treatment of intracranial dural arteriovenous fistula and carotid cavernous fistula with Onyx: preliminary results and anesthesia considerations. J Neuroint Surg. 2009;1(1):146–150. doi: 10.1136/jnis.2009.000042. [DOI] [PubMed] [Google Scholar]
- 15.Lv X, Li Y, Jiang C, et al. The incidence of trigeminocardiac reflex in endovascular treatment of dural arteriovenous fistula with onyx. Interv neuroradiol. 2010;16(1):59–63. doi: 10.1177/159101991001600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaller B, Probst R, Strebel S, et al. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg. 1999;90(2):215–220. doi: 10.3171/jns.1999.90.2.0215. [DOI] [PubMed] [Google Scholar]
- 17.Lang S, Lanigan DT, van der Wal M. Trigeminocardiac reflexes: maxillary and mandibular variants of the oculocardiac reflex. Can J Anaesth. 1991;38(6):757–760. doi: 10.1007/BF03008454. [DOI] [PubMed] [Google Scholar]
- 18.Fayon M, Gauthier M, Blanc VF, et al. Intraoperative cardiac arrest due to the oculocardiac reflex and subsequent death in a child with occult Epstein-Barr virus myocarditis. Anesthesiology. 1995;83(3):622–624. doi: 10.1097/00000542-199509000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Rivard JC, Lebowitz PW. Bradycardia after alfentanil-succinylcholine. Anesth Analg. 1988;67(9):907. doi: 10.1213/00000539-198809000-00031. [DOI] [PubMed] [Google Scholar]
- 20.Starr NJ, Sethna DH, Estafanous FG. Bradycardia and asystole following the rapid administration of sufentanil with vecuronium. Anesthesiology. 1986;64(4):521–523. doi: 10.1097/00000542-198604000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Schmeling WT, Kampine JP, Warltier DC. Negative chronotropic actions of sufentanil and vecuronium in chronically instrumented dogs pretreated with pro-pranolol and/or diltiazem. Anesth Analg. 1989;69(1):4–14. [PubMed] [Google Scholar]