Summary
We sought to assess the feasibility of using thermosensitive chitosan/β-glycerophosphate for embolotherapy. The renal arteries in nine rabbits were embolized with chitosan/β-glycerophosphate. The animals were studied angiographically and sacrificed at one week (n = 3), four weeks (n = 3), and eight weeks (n = 3) after embolotherapy. Histology was obtained at these three time points. Delivery of chitosan/β-glycerophosphate was successful in all cases. Complete occlusion was achieved in all cases. No recanalization was observed in the follow-up angiograms. No untoward inflammatory reactions were observed in the target renal arteries and infarcted kidneys during the histological examinations. Our preliminary feasibility evaluation in rabbit renal arteries indicates that C/GP is a satisfactory embolization agent.
Key words: chitosan/β-glycerophosphate, liquid embolic agent, embolization, in vivo
Introduction
Chitosan is a modified natural carbohydrate polymer obtained by the alkaline deacetylation of chitin. The main sources of chitin are shrimps and crabs. In 2000, Chenite et al. reported that solutions containing chitosan/β-glycerophosphate (C/GP) could transition from solution into gel at 37°C 1. From then on, the researchers focused on drug delivery and tissue engineering. Considering the thermosensitive characteristics of C/GP, we aimed to examine the feasibility of using C/GP as an embolic material. Based on our previous study, we hypothesized that C/GP has the potential to be a liquid embolic agent in vitro. Here, we conduct an in vivo assessment of C/GP as a liquid embolic agent.
Materials and Methods
Materials
Nine New zealand rabbits weighing 2.5-3.0 kg were provided by the experimental Animal Center of Jilin university. Chitosan (medium viscosity, medium molecular weight, 90.3% deacetylation) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shenyang, China). β-Glycerophosphate (β-GP) was purchased from Fluka (Switzerland).
Methods
Preparation of Thermosensitive Hydrogels
The chitosan solution was prepared by adding 5 g chitosan in a 250 ml HCI solution (0.1 M). The solution was stirred thoroughly until the chitosan was completely dissolved and then sterilized at 120°C for 15 minutes. β-GP (28 g) was dissolved in 50 ml of distilled water and sterilized by filtration. Both solutions were stored at 4°C. The β-GP solution (0.5 ml) was added to 3.5 ml of the sterilized chitosan solution drop by drop to prepare 4 ml of the C/GP solution. The C/GP solution was mixed with 1 g of tantalum powder in an ice bath to achieve the appropriate radio-opacity prior to the embolization operation. At all times, the C/GP solution was stored and transferred in an ice box.
Embolization Procedures
All animal experiments were approved by the Animal Research Committee of Jilin University and in accordance with the Chinese National Animal Law on the use of laboratory animals. After an overnight fast, anesthesia was induced with an intramuscular injection of a mixture of ketamine hydrochloride (50 mg/kg body weight) and xylazine hydrochloride (4 mg/kg body weight).
The right femoral artery was surgically exposed and distally ligated under sterile conditions. A 4F sheath was introduced into the right femoral artery. Selective angiography was performed with a 4F angiographic catheter (Terumo Corporation, Tokyo, Japan). After affirming the normal vascularity of the bilateral kidneys, the right or left renal artery was catheterized with a 4F catheter, and renal angiograms were obtained by using 2 ml contrast medium (Ultravist 300; Bayer Schering Pharmaceutical Co., Ltd., Guangzhou, China). Then 1-1.5 ml of C/ GP solution was slowly injected into the renal artery to embolize it. The injection time was controlled to last between 1.8 and 2 min.
Selective angiography was performed with the other 4F angiographic catheter after the injection was complete to ensure that the renal artery was completely occluded.
The right femoral artery was ligated after the sheath removal at the end of the procedure.
Follow-up Angiography and Histopathological Analysis
We divided the rabbits into three different groups according to the follow-up period with three rabbits in each group. The follow-up angiography was performed at one week (group 1), four weeks (group 2), or eight weeks (group 3) after embolization. The animals were euthanized by an overdose injection of pentobarbital after the follow-up angiography. Then the embolized kidney including the renal artery was removed surgically. The harvested organ was macroscopically examined and fixed in 10% formaldehyde. Sections of the embolized kidney and renal artery were prepared and stained with hematoxylin and eosin by an experienced pathologist to evaluate histopathological changes in the embolized kidney.
Results
Angiographic Findings
The renal artery was successfully embolized with 1-1.5 ml of C/GP solution in all cases. Catheter adhesion was not observed in any of the cases. The C/GP solution was easily handled between the injection procedures, and no occlusion of the catheter by the material was observed. Prior to embolization, the angiogram clearly showed the renal artery and its peripheral branches.
The angiogram obtained immediately after embolization showed full occlusion of the target renal artery in all cases. The tantalum powder allowed the C/GP to be clearly observed in the angiographic images, and the renal artery and most of its peripheral branches were embolized with the C/GP gel. No recanalization was observed in the follow-up angiograms for up to eight weeks (Figure 1).
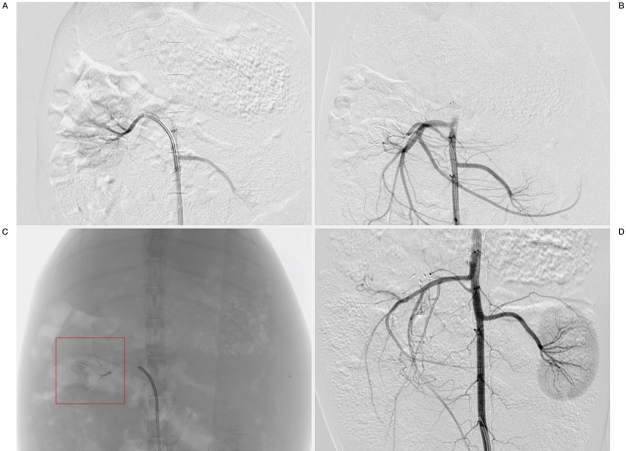
Figure 1.
Angiographic findings. A) Digital subtraction angiography (DSA), before embolization, right renal artery injection, anteroposterior projection. The bilateral renal artery and its peripheral branches are shown. B) DSA, immediately after embolization, abdominal aorta injection, anteroposterior projection. The right renal artery is completely occluded. The left renal artery and its peripheral branches are clearly shown. C) Plain radiography, anteroposterior projection, demonstrating the embolic material cast in the right renal artery and its peripheral branches. D) DSA, eight weeks after embolization, abdominal aorta injection, anteroposterior projection. The right renal artery is still completely occluded, and the embolic material can be observed. The left renal artery and its peripheral branches are clearly shown.
Histopathological Findings
The injection of C/GP into the renal artery aroused the blood clot and granulation tissue in the lumen, which led to the completely occlusion of the renal artery (Figure 2A). The occlusion led to the infarction of the kidney. No evidence of untoward inflammatory reaction was observed in the target renal artery and the infarction kidney (Figure 2B,C). The color of the embolized kidney was pale, and it was considerably smaller than the control kidney (Figure 2D).
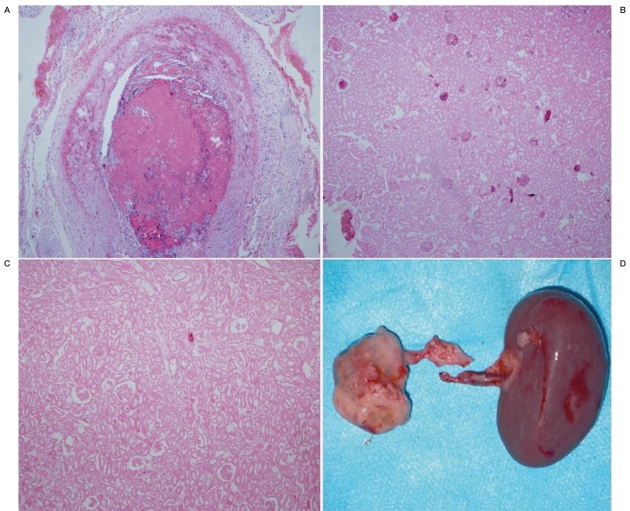
Figure 2.
Histopathological findings. A) Right renal artery, one week after embolization, elastica van Gieson, ×100. Complete occlusion of the renal artery by embolic material with blood clot and granulation tissue. B) right kidney, four weeks after embolization, elastica van Gieson, ×100. Most of the normal glomerular were infarcted. C) right kidney, eight weeks after embolization, elastica van Gieson, ×100. The normal glomerulars have completely disappeared. D) Kidneys, eight weeks after embolization, macroscopic findings. The right kidney is pale and considerably smaller than the left kidney.
Discussion
Artery embolization is a minimally invasive procedure that is increasingly being used to treat a wide range of conditions, such as vascular malformations, aneurysms, and tumors. The International Subarachnoid aneurysm trial provided evidence that coil embolization results in superior clinical outcomes for ruptured aneurysms than surgical clipping 2,3. Moreover, liquid embolic agents have been widely adopted in the field of intravascular embolization. The most commonly used liquid embolic agents are N-butyl-2 cyanoacrylate (NBCA) and ethylene vinyl alcohol copolymer (EVoH). These agents were approved by the U.S. Food and Drug Administration (FDA) for cerebral arteriovenous malformation (AVM) embolization in 2000 and 2005, respectively. NBCA works instantly to permanently and completely occlude vessels. However, during slow injection periods, gluing of the catheter within the vascular pedicle has been reported (REF). EVOH is nonadhesive, but vascular toxicity caused by dimethyl sulfoxide has been reported in some studies (REFS). In order to overcome these weaknesses, scientists have made efforts to search for safer liquid embolic agents. Recently, temperature-responsive, sol-gel polymers have attracted the attention of investigators. Wang et al. performed renal embolization in two cases with renal carcinoma by using N-isopropylacrylamide 4. Their results suggested that this temperature-responsive polymer could serve as a new liquid embolic material for the endovascular treatment of renal carcinoma and vascular diseases. Thermosensitive polymer solutions will gel at special temperatures, and the transition temperature can be adjusted by altering the composition of the polymer. C/GP has these thermosensitive characteristics. Chenite et al. Observed that C/GP solutions had a physiological pH and could be stored as liquids below room temperature, but when injected in vivo, the liquid formulations turned into gel implants in situ 1. Our experiment demonstrates that the C/GP solutions remained liquid at temperatures lower than the sol-gel transition temperature (Figure 3A,B), and they became solid gels at temperatures higher than the transition temperature (Figure 3C). Ruel-Gariepy et al. hypothesized that the addition of β-GP to a chitosan solution would promote the protective hydration of the chitosan chains 5. Therefore, low temperatures prevent the association of the chitosan chains to form a gel, even at a neutral pH. Raising the temperature increases the chitosan-chitosan hydrophobic interactions, which play a major role in the gelation of C/GP solutions. Zhen et al. mixed 2% (w/v) chitosan solutions with 56% (w/v) β-GP solutions in different ratios and measured the pH, colloidization time at 37°C, and mechanic strength of the solutions 6. They believed the best ratio of chitosan to β-GP solutions was 7:1.
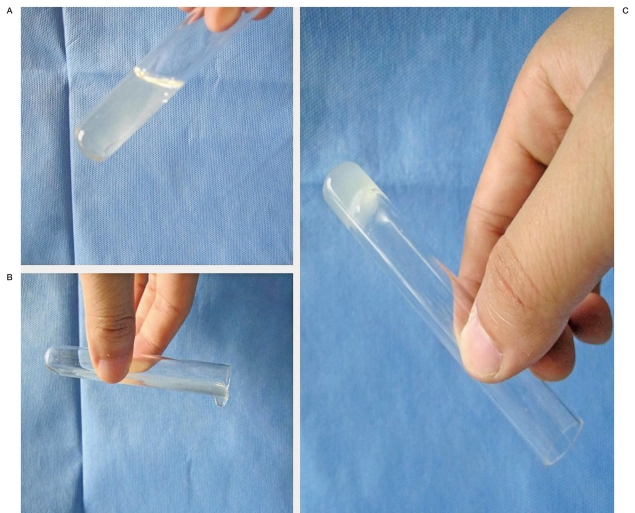
Figure 3.
Sol-gel Transition. A,B) Liquid solutions at a temperature lower than the sol-gel transition temperature. C) Solid gel at a temperature higher than the sol-gel transition temperature.
The C/GP gelation process requires approximately 2 min. Therefore, the injection was completed in 1.8-2 min to prevent the gel from being washed away from the injection site by the blood flow and to prevent catheter occlusion. In our study, all embolizations were performed successfully with no clinical complications (such as catheter adhesion, catheter occlusion and ectopic embolization), and no recanalization was observed. In our opinion, C/GP possesses a number of advantages as a new embolic material for intravascular treatments.
First, C/GP has advantageous physical properties. C/GP is a solidifying liquid gel at 37°C, which is malleable when it is solid. It is radiopaque when tantalum powder is added to the solution. The C/GP solution requires no organic solvents in its preparation or application. Moreover, we observed no evidence of gluing the catheter within the vascular pedicle. Second, one of the characteristics of C/GP is that it is safe. Chitosan is widely regarded as being a non-toxic, biologically compatible polymer. It has been approved for dietary applications in Japan, Italy, and Finland, and it has been approved by the FDA for use in wound dressings. In 2004, Nakai et al. assessed the safety of another type of polymer based on chitosan, which is called photocrosslinkable chitosan, as an embolization material for aneurysms. Ultraviolet irradiation was applied to an aqueous photocrosslinkable chitosan solution to make the material insoluble. This study indicated that photocrosslinkable chitosan was safe 7. Moreover, glycerophosphate is an organic compound that is naturally found in the body. Its veinal administration has been approved by the FDA.
Third, the cost of C/GP is reasonable, and the materials are easy to obtain. Metal coils are more expensive for patients, particularly in developing countries, such as China. Intravascular treatments will be more widely utilized and adopted if there are reasonable operational costs. Lastly, further studies are necessary to evaluate the long-term effects and behavior of the C/GP in the artery.
Conclusion
C/GP was a satisfactory embolization agent in this preliminary feasibility evaluation in rabbit renal arteries. C/GP is a potential candidate for use as an embolic agent to treat cerebral arteriovenous malformations and hypervascular tumors.
References
- 1.Chenite A, Chaput C, Wang D, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21(21):2155–2161. doi: 10.1016/s0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux A, Kerr R, Stratton I, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAt) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Pang Q, Liu Z, et al. A new liquid agent for endovascular embolization: initial clinical experience. ASAIO J. 2009;55(5):494–497. doi: 10.1097/MAT.0b013e3181b4b5b7. [DOI] [PubMed] [Google Scholar]
- 5.Ruel-Gariepy E, Chenite A, Chaput C, et al. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int J Pharm. 2000;203(1-2):89–98. doi: 10.1016/s0378-5173(00)00428-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhen Y, Xu K, Chen XS, et al. Embolization of aneurysm by chitosan-glycerophosphate-fibroblast tissue hydrogel, a tissue engineering material: experiment with rabbits. Zhonghua Yi Xue Za Zhi. 2009;89(11):727–731. [PubMed] [Google Scholar]
- 7.Nakai K, Kojima T, Hattori K, et al. Feasibility of photocrosslinkable chitosan as an embolization material for aneurysms. Biological reaction after aneurysm embolization. Interventional Neuroradiology. 2004;10(Suppl 2):95–100. doi: 10.1177/15910199040100S217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crompton KE, Goud JD, Bellamkonda RV, et al. Polylysine-functionalised thermoresponsive chitosan hydrogel for neural tissue engineering. Biomaterials. 2007;28(3):441–449. doi: 10.1016/j.biomaterials.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Zheng X, Wei X, et al. A novel composite drug delivery system: honokiol nanoparticles in thermosensitive hydrogel based on chitosan. J Nanosci Nanotechnol. 2009;9(8):4586–4592. doi: 10.1166/jnn.2009.217. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya S, Tozer. KR, Chen. J. An overview of embolic agents. Semin Intervent Radiol. 2008;25:204–215. doi: 10.1055/s-0028-1085930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niimi Y, Berenstein A, Setton A. Complications and their management during NBCA embolization of craniospinal lesions. Interventional Neuroradiology. 2003;9(Suppl 1):157–164. doi: 10.1177/15910199030090S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debrun GM, Aletich V, Ausman JI, et al. Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery. 1997;40(1):112–120. discussion 20-21. [PubMed] [Google Scholar]
- 13.Duffner F, Ritz R, Bornemann A, et al. Combined therapy of cerebral arteriovenous malformations: histological differences between a non-adhesive liquid embolic agent and n-butyl 2-cyanoacrylate (NBCA) Clin Neuropathol. 2002;21(1):13–17. [PubMed] [Google Scholar]
- 14.Murayama Y, Vinuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery. 1998;43(5):1164–1175. doi: 10.1097/00006123-199811000-00081. [DOI] [PubMed] [Google Scholar]
- 15.Dai F, Tang L, Yang J, et al. Fast thermoresponsive BAB-type HEMA/NIPAAm triblock copolymer solutions for embolization of abnormal blood vessels. J Mater Sci Mater Med. 2009;20(4):967–974. doi: 10.1007/s10856-008-3632-x. [DOI] [PubMed] [Google Scholar]
- 16.Takao H, Murayama Y, Ebara M, et al. New thermoreversible liquid embolic agent for embolotherapy: technical report. Neuroradiology. 2009;51(2):95–98. doi: 10.1007/s00234-008-0469-4. [DOI] [PubMed] [Google Scholar]
- 17.Takao H, Murayama Y, Yuki I, et al. Endovascular treatment of experimental aneurysms using a combination of thermoreversible gelation polymer and protection devices: feasibility study. Neurosurgery. 2009;65(3):601–609. doi: 10.1227/01.NEU.0000350929.31743.C2. discussion 609. [DOI] [PubMed] [Google Scholar]
- 18.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15(9):1326–1331. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- 20.Wedmore I, McManus JG, Pusateri AE, et al. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60(3):655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Su ZG, Ma GH. A thermo- and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int J Pharm. 2006;315(1-2):1–11. doi: 10.1016/j.ijpharm.2006.01.045. [DOI] [PubMed] [Google Scholar]