Abstract
The POTE gene family encodes very closely related proteins that are highly expressed in testis and in many cancers. Recent studies indicate that the POTE proteins have a pro-apoptotic function. To examine if POTE is associated with cells that are undergoing apoptosis in testis, we determined the cellular location of POTE and of cleaved caspase-3 in testicular tissues from 26 azoospermic men. We found intense expression of POTE in round spermatids that are undergoing apoptosis, which are positive for cleaved caspase-3. This study suggests POTE may have a role in apoptosis in the human testis.
Keywords: POTE gene family, spermatids, apoptosis, Cleaved Caspase-3
1. Introduction
Cancer testis (CT)2 antigens are proteins that are expressed in testis and many cancers. Because of their pattern of expression, CT antigens are very attractive candidates for immuno-based therapies of cancer. The POTE gene family is unusual in that it encodes primate-specific CT antigens [1]. The POTE family is large and in humans consists of 13 highly homologous variants dispersed among 8 different chromosomes: 2, 8, 13, 14, 15, 18, 21 and 22. Seven of these variants are expressed in testis with POTE 15 being the most highly expressed [2]. The POTE proteins are made up of three distinctive regions, suggesting that they can function as scaffold or adaptor proteins. The amino terminal region contains cysteine-rich repeats (CRRs) of 37 amino acids each; the central region contains ankyrin repeat motifs of 33 amino acids each; the C-terminal region has spectrin like α helices [1]. Each paralog has a different number of CRRs and ankyrin repeats [3]. There is also an actin retroposon insertion at the carboxy terminus of one of the ancestral POTE paralogs that appeared before the divergence of Old World monkeys and apes [4].
Like many other CT antigens, the physiological function of the POTE genes is not clear, although there is evidence that they play a role in apoptosis, since over-expression of POTE in HeLa cells is a potent inducer of apoptosis and POTE levels are elevated in cells undergoing Fas receptor-dependent apoptosis [5]. It has been reported that many of the more advanced, post-meiotic testicular germ cells undergo apoptosis during germ cell development, and that Fas and Fas ligand, potent inducers of apoptosis, are expressed in testis [6]. To determine if there is any association of POTE with cells undergoing apoptosis, we have used immunohistochemistry with an anti-POTE antibody and an antibody that detects apoptotic cells to examine if cells in the testis that express POTE are undergoing apoptosis.
2. Methods
2.1. Testicular tissue source
Human tissue specimens were available from 26 infertile adult men who had undergone testicular biopsy as part of a routine clinical evaluation for azoospermia at the Clinical Center of the National Institutes of Health during 1971–1978. Biopsies had been performed to differentiate between obstructive and non-obstructive etiologies. All of the tissues had been preserved in Bouin’s solution to avoid the shrinkage artifact commonly found with formalin-based fixation; and paraffin imbedded. All patients at the time of the biopsy were engaged in IRB approved protocols or biopsies were obtained for clinically relevant diagnostic purposes only. Prior to performing the biochemical studies on these previously preserved testicular tissues, we were provided an exemption for new IRB approval from the Office of Human Subjects Research based on: 1) the biopsies were obtained originally more than 30 years prior to the proposed study and the biopsy material already existed; 2) the material was available usually as part of a prior IRB approved protocol; 3) no patient-identifiers were to be used in our study; 4) the results would not be sent back to the patient’s non-NIH provider; and 5) there are no conflicts of interest by any of the involved NIH employees.
2.2. Immunohistochemistry
Paraffin blocks were sectioned at 4-μm and consecutive sections were applied to three separate glass slides set aside for immunohistochemical staining of 1) POTE protein (PG5) [7], 2) Cleaved Caspase-3 (rabbit polyclonal antibody; Cell Signaling Technology, Beverley, MA) [8], and 3) a second antibody control. The sections were deparaffinized and rehydrated through graded ethanol into phosphate buffered saline, and then subjected to microwave antigen retrieval in 10 mM citrate buffer pH 6.0 under pressure (Histoserv, Germantown, MD). Endogenous peroxidases were degraded by immersion of the sections in 0.3% hydrogen peroxide in methanol. Sections were blocked with blocking solution (CSA-Kit K1500; Dakocytomation, Carpenteria, CA) and incubated with either PG5 antibody at 60 ng/ml for POTE or rabbit polyclonal antibody against Cleaved Caspase-3 at 1:200 dilution [8] overnight at 4°C. The primary antibody was detected by a second reagent using diaminobenzidine as chromogen. All biopsy slides were counter-stained with hematoxylin to facilitate identification of the various specific spermatogenic cell types. The 26 biopsies fell into three distinct diagnostic categories: 1) complete spermatogenesis (n=3); 2) hypospermatogenesis (most spermatogenic cell types present, but in reduced numbers; n=20); or 3) germinal aplasia (only Sertoli cells present, with no evidence of any spermatogenic cells; n=3).
3. Results and Discussion
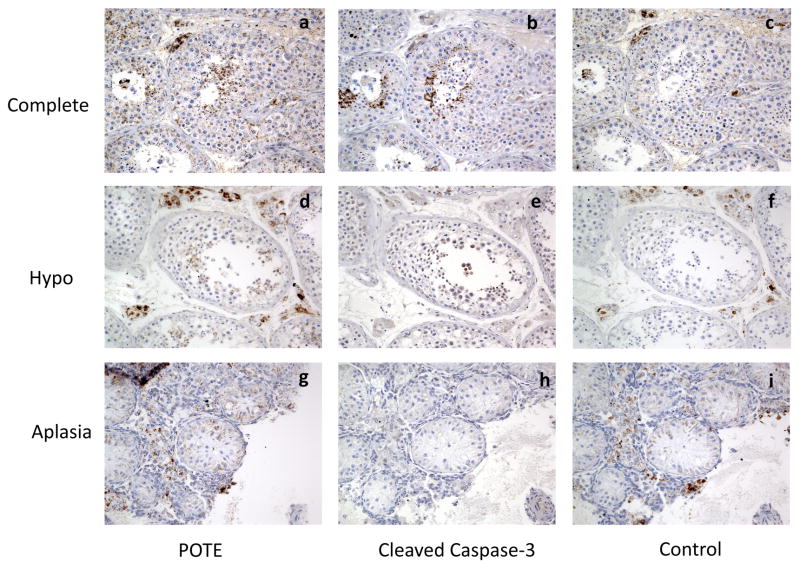
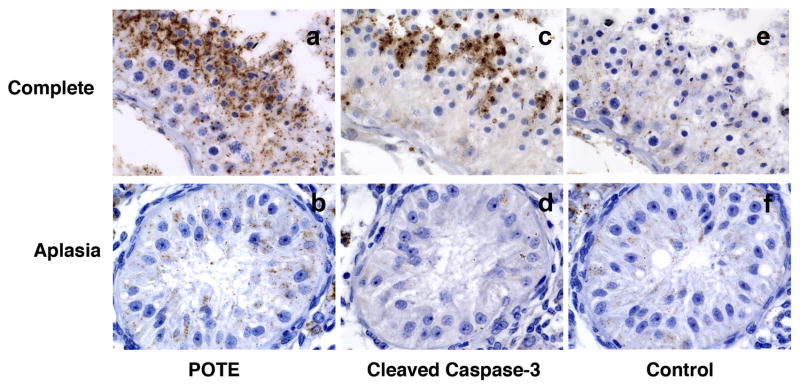
We performed an immunohistochemical analysis using the anti-POTE monoclonal antibody PG5 in human testicular tissues from 26 azoospermic men. As shown in Figure 1a, in tissue samples with complete spermatogenesis, POTE staining occurs in small clusters of late germ cells in the adluminal seminiferous tubule compartment; and intense staining of the POTE protein is present mostly in round spermatids and some elongating spermatids (Fig. 2a). POTE protein is also seen occasionally in secondary spermatocytes; though much less frequently (Fig. 2b). POTE staining was never seen in early germ cells (spermatogonia or primary spermatocytes) or Sertoli cells. A similar staining pattern was also observed in testicular tissues with hypospermatogenesis although the spermatogenic cells are much less abundant in these samples (Fig. 1d) and the staining is completely absent in tissues with germinal aplasia (Fig. 1g & 2b).
Fig. 1.
Representative photomicrographs (200X) showing localization of Cleaved Caspase-3 and POTE protein in human testis. Serial tissue sections from a biopsy with complete spermatogenesis (a, b, c); hypospermatogenesis (d, e, f); or germinal aplasia (g, h, i) are shown for POTE protein (a, d, g); Cleaved Caspase-3 (b, e, h) stained cells identified only in clusters of germ cells in the adluminal seminiferous tubule compartment; and not in the earlier germinal cells in the basal compartment. Control panel (c, f, i) showing tissues stained with second antibody only.
Fig. 2.
Photomicrographs (630X) showing localization of POTE and Cleaved Caspase-3 in specific cell types in testis. Tissue sections from a biopsy with complete spermatogenesis (a, c, e) or germinal aplasia (b, d, f) are shown stained for POTE protein (a, b), Cleaved Caspase-3 (c, d) and control (e, f); showing intense localization of staining in round and elongating spermatids; but none is seen in Sertoli cells.
To identify the specific cell types that are undergoing apoptosis, we stained the adjacent serial sections with antibodies against Cleaved Caspase-3, a well-characterized apoptosis marker. As shown in Figures 1b and 2c, scattered pockets of intense staining are evident in spermatids in testicular tissues with complete and hypo-spermatogenesis; but staining is absent in tissues with germinal aplasia (Fig. 1h and 2d). From the serial sectioned tissues, it is evident that Cleaved Caspase-3 stained cells are adjacent to the POTE stained cells; but some areas of tubules heavily stained for POTE protein are associated with cells less stained for Cleaved Caspase-3 and vice versa. Some areas of tubules heavily stained for Cleaved Caspase-3 are associated with cells less stained for POTE; occasionally both proteins are localized in the same cells (data not shown). Some nonspecific staining was observed when secondary antibody alone was used in all three tissues (Fig. 1c, f & i). The results from POTE and Cleaved Caspase-3 staining in 26 testis biopsies are summarized in Table 1.
Table 1.
Summary of cellular localization of POTE and Cleaved Caspase-3 protein in human testicular tissue
| Tissue Type | Sertoli Cell | Spermatogonia | Primary Spermatocyte | Secondary Spermatocyte | Round Spermatid | Elongated Spermatid | Sperm |
|---|---|---|---|---|---|---|---|
| Complete Spermatogenesis n=3 | |||||||
| Cleaved Caspase-3 | − | − | − | + | ++++ | ++ | − |
| POTE | + | − | − | + | ++++ | ++ | − |
| CSA Control | + | − | − | − | − | − | − |
| Hypospermatogenesis n=20 | |||||||
| Cleaved Caspase-3 POTE | |||||||
| CSA Control | + | − | − | − | − | − | − |
| Germinal Aplasia n=3 | |||||||
| Cleaved Caspase-3 | − | − | − | − | − | − | − |
| POTE | + | − | − | − | − | − | − |
| CSA Control | + | − | − | − | − | − | − |
CSA, catalyzed signal amplification; +, weak; ++, moderate; ++++, strong; −, no expression
All of the testicular tissue from men with complete spermatogenesis, as well as from those having varying degrees of hypospermatogenesis, consistently show scattered pockets of intense localization of Cleaved Caspase-3 and POTE protein only in late germinal elements; round spermatids and some elongated spermatids (Fig. 1 and 2, Table 1). Because serial tissue sections were utilized for histochemical staining, Cleaved Caspase-3 and POTE protein are always seen in adjacent cells; and at times also identified in the same cells. Among tissue sections showing complete spermatogenesis, where spermatogenic cells are considerably more abundant, localization of both proteins (Cleaved Caspase-3 and POTE) could be seen in an occasional secondary spermatocyte. However, no histochemical localization of Cleaved Caspase-3 or POTE protein was identified in earlier germinal elements (basal spermatogonia or primary spermatocytes) or the supporting Sertoli cells in germinal aplasiaI, where only Sertoli cells are present in the seminiferous tubules. Controls using catalyzed signal amplification (CSA) blocking reagent show heavily stained interstitial cells with spill onto Sertoli cells in a few cases (most likely nonspecific); and accounts for the heavy interstitial staining and occasional Sertoli cell staining seen on some POTE slides. No interstitial staining on Cleaved Caspase-3 slides was seen.
POTE is a primate-specific gene family which entered the primate genome about 30 million years ago and is still under strong selection pressure as most of the paralogs in the family are expressed as intact open reading frames. All POTE proteins are made up of three distinct domains including ankyrin and spectrin motifs that are known to interact with other regulatory proteins. Some POTE paralogs are fused with an in frame actin cDNA producing POTE-actin fusion protein. Our recent finding shows that the over-expression of POTE induces apoptosis in HeLa cells, and the process is dependent on BAK expression. The expression of POTE is highest in testis compared to other normal tissues. Our data are consistent with the speculation that POTE protein is involved in apoptosis of testicular germ cells. A highly active apoptotic process is known to occur during spermatogenesis where there is a high level of attrition of spermatogenic cells, particularly with the appearance of haploid spermatids following meiosis [9–12]. We have demonstrated the association of POTE protein in cells that are adjacent to cells that highly express Cleaved Caspase-3 and occasionally in the same cells expressing Cleaved Caspase-3. However, the role of POTE protein is currently uncertain and it has not been determined if the protein has a structural or operational role, or acts as a chaperone. It is most intriguing that testicular POTE paralogs have been identified only among primate genomes; we are unaware of any differences in spermatogenesis among mammalian species other than temporal intervals involved in the spermatogenic cycle.
Highlights.
We showed POTE protein expression in round and elongating spermatids.
POTE expression is more intense in tissues with complete spermatogenesis.
POTE expression co-localized with Cleaved caspase 3 expressing round and elongating spermatids.
POTE may have a role in apoptosis in the human testis.
Acknowledgments
TKB, DAW, and IP’s research was supported by the Intramural Research Program of the NIH, National Cancer Institute; RJS’s research was supported by the National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Abbreviations CRRs, cysteine-rich repeats; CSA, catalyzed signal amplification; CT, cancer testis
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tapan K Bera, Email: BeraT@mail.nih.gov.
Dawn A Walker, Email: walkerd@mail.nih.gov.
Richard J Sherins, Email: rsherins@comcast.net.
Ira Pastan, Email: pastani@mail.nih.gov.
References
- 1.Bera TK, Zimonjic DB, Popescu NC, Sathyanarayana SK, Kumar V, Lee BK, Pastan I. POTE, a highly homologous gene family located on numerous chromosomes and expressed in prostate, ovary, testis, placenta and prostate cancer. Proc Natl Acad Sci USA. 2002;99:16975–16980. doi: 10.1073/pnas.262655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bera TK, Saint Fleur A, Lee Y, Kydd A, Hahn Y, Popescu NC, Zimonjic DB, Lee B, Pastan I. POTE paralogs are induced and differentially expressed in many cancers. Cancer Res. 2006;66:52–56. doi: 10.1158/0008-5472.CAN-05-3014. [DOI] [PubMed] [Google Scholar]
- 3.Bera TK, Huynh N, Maeda H, Sathyanarayana BK, Lee BK, Pastan I. Five POTE paralogs and their splice variants are expressed in human prostate and encode proteins of different lengths. Gene. 2004;337:45–53. doi: 10.1016/j.gene.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Ise T, Ha D, Saint Fleur A, Hahn Y, Liu XF, Nagata S, Lee B, Bera TK, Pastan I. Evolution and expression of chimeric POTE-actin genes in the human genome. Proc Natl Acad Sci USA. 2006;103:17885–17890. doi: 10.1073/pnas.0608344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu XF, Bera TK, Liu LJ, Pastan I. A primate-specific POTE-actin fusion protein plays a role in apoptosis. Apoptosis. 2009;14:1237–1244. doi: 10.1007/s10495-009-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francavilla S, D'Abrizio P, Rucci N, Silvano G, Properzi G, Straface E, Cordeschei G, Necozione S, Gnessi L, Arizzi M, Ulisse S. Fas and Fas ligand expression in fetal and adult human testis with normal or deranged spermatogenesis. J Clin Endocrinol Metab. 2000;85:2692–2700. doi: 10.1210/jcem.85.8.6723. [DOI] [PubMed] [Google Scholar]
- 7.Ise T, Das S, Nagatas S, Maeda H, Lee Y, Onda M, Anver MR, Bera TK, Pastan I. Expression of POTE protein in human testis detected by novel monoclonal antibodies. Biochem Biophy Res Commun. 2008;365:603–608. doi: 10.1016/j.bbrc.2007.10.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem. 2002;50:449–454. doi: 10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- 9.Print CG, Loveland L. Germ cell suicide: new insights into apoptosis during spermatogenesis. BioEssays. 2000;22:423–430. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Oldereid NB, DeAngelis P, Wiger R, Clausen OPF. Expression of Bcl-2 proteins and spontaneous apoptosis in normal human testis. Mol Human Reprod. 2001;7:403–408. doi: 10.1093/molehr/7.5.403. [DOI] [PubMed] [Google Scholar]
- 11.Hikim APS, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- 12.Richburg JH. The relevance of spontaneous- and chemically-induced alterations in testicular germ cell apoptosis to toxicology. Toxicol Lett. 2000;112–113:79–86. doi: 10.1016/s0378-4274(99)00253-2. [DOI] [PubMed] [Google Scholar]