Summary
Despite the expression of antigens by tumor cells, spontaneous immune-mediated rejection of cancer seems to be a rare event. T-cell receptor engagement by peptide/major histocompatibility complexes constitutes the main signal for the activation of naive T cells but is not sufficient to initiate a productive generation and maintenance of effector cells. Full activation of T cells requires additional signals driven by costimulatory molecules present on activated antigen-presenting cells but rarely on tumors. Following the discovery of B7-1 (CD80), several other costimulatory molecules have been shown to contribute to T-cell activation and have relevance for improving anti-tumor immunity. Moreover, increasing understanding of coinhibitory receptors has highlighted key additional pathways that can dominantly inhibit anti-tumor T-cell function. Improving positive costimulation, and interfering with negative regulation, continues to represent an attractive immunotherapeutic approach for the treatment of cancer. This review focuses upon those pathways with the highest potential for clinical application in human cancer patients.
Keywords: T cells, costimulation, tumor immunity, T-cell regulation, tolerance/suppression/anergy
Introduction
The last two decades of research in the field of cancer immunology have revealed that the majority of cancer cells express antigens that can be recognized by T cells of the host immune system. However, despite the expression of antigens, spontaneous immune-mediated rejection of cancer seems to be a rare event. Failure of spontaneous immune-mediated tumor elimination could generally be explained by poor T-cell priming or inadequate execution of the effector phase of the immune response. In each of these contexts, a potential role of T-cell costimulatory and coinhibitory receptors has been implicated. Most tumors lack the expression of positive costimulatory molecules such as B7-1 (CD80) and B7-2 (CD86), and in some models the antigen-presenting cells from the tumor microenvironment have been shown to be tolerogenic rather than immunostimulatory (1). Several murine tumor systems have demonstrated abortive proliferation of tumor antigen-specific T-cell receptor (TCR) transgenic T cells associated with subsequent T-cell hyporesponsiveness (2), a situation that mirrors observations made in other models of peripheral tolerance. However, in some mouse tumor models and human cancer patients, brisk spontaneous T-cell responses against tumor antigens have been observed, which in some cases have been found to be associated with trafficking of effector cells into metastatic tumor sites (3). However, ex vivo analysis of tumor-infiltrating lymphocytes has generally demonstrated a dysfunctional state (4), which can be reversed upon culture in vitro (5, 6). At the effector phase as well, lack of positive costimulatory ligands or presence of inhibitory ligands, has been suggested to contribute to poor anti-tumor T-cell efficacy. Fortunately, the characterization of these important ligand/receptor interactions has pointed towards opportunity for therapeutic intervention to augment and/or restore the function of tumor antigen-specific T cells in vivo. This review focuses upon costimulatory and coinhibitory pathways with the highest current potential impact on clinical translation in human cancer patients.
Positive costimulatory pathways and anti-tumor immunity
B7-1, B7-2/CD28, and the concept of anergy
T-cell activation occurs through the recognition of a specific peptide presented on the major histocompatibility complex (MHC) I or II of antigen presenting cells (APCs), such as dendritic cells (DCs), to the TCR of CD8+ or CD4+ T cells. TCR engagement constitutes the main signal for the activation of naive T cells but is not sufficient to initiate an efficient immune response. Full activation of T cells requires additional signals driven by costimulatory molecules present on the APCs. In the late 1980s, the Schwartz laboratory (7) characterized in vitro anergy using CD4+ T helper type 1 (Th1) cell clones showing that TCR engagement was not sufficient for T-cell activation. They found that antigen presented by fixed APCs induced a subsequent hyporesponsive state characterized by diminished proliferation and interleukin-2 (IL-2) production by T cells compared to initial stimulation with live APCs. Around the same time period, the CD28 receptor was cloned as a T-cell-specific protein (8). B7-1 (CD80) was cloned as a B-cell activation antigen (9) and was subsequently found to be the first identified ligand for CD28 in 1990 (10). The role played in vitro by the engagement of CD28 by B7-1 as a costimulatory signal able to enhance proliferation and IL-2 secretion by T cells was rapidly discovered (11–13). Anti-CD28 monoclonal antibodies (mAbs) were additionally observed to prevent the induction of anergy in T-cell clones, thus linking the two phenomena of costimulation and anergy prevention (14). The observation that APCs from B7-1-deficient mice retained costimulatory function led to the identification of B7-2 (CD86), a second ligand for CD28 (15, 16).
The B7-1/B7-2/CD28 pathway has been shown to constitute the primary and strongest costimulatory signal delivered by APCs to amplify T-cell activation (17). The biologic consequences of CD28 costimulation include increased cytokine gene expression (11), stabilization of cytokine messenger RNA (mRNA) (18), augmentation of glucose uptake and utilization (19), promotion of T-cell survival (20), and maintenance of T-cell responsiveness upon subsequent restimulation (21). The intracellular signals induced following CD28 ligation have been reviewed recently by Song and colleagues (22).
TCR engagement in the absence of costimulation can result in a hyporesponsive state characterized as anergy (23). Signaling events based on in vitro anergy model systems suggest that anergy arises upon the disproportionate overactivation of calcium/nuclear factor of activated T cells (NFAT) signaling compared with other biochemical signaling intermediates, such as the Ras/mitogen-activated protein (MAP) kinase (24). Recent advances in the understanding of molecular regulation of T-cell anergy have confirmed a crucial role for defective Ras activation in mediating this state (25, 26). This diminished Ras activation is mediated, in part, by upregulated expression of diacylglycerol kinases (DGKs) and of other negative regulators, including the transcription factors early growth response-2 (EGR-2) and EGR-3 and the E3 ubiquitin ligases gene related to anergy in lymphocytes (GRAIL) and Casitas B-cell lymphoma-b (Cbl-b) (23, 25–27). The characterization of these inhibitory signaling molecules in anergic T cells has raised the possibility that pharmacologic approaches might be developed in the future to counter inhibitory signaling and improve or restore optimal T-cell function in settings of unwanted peripheral tolerance in vivo.
Since many tumors lack expression of positive costimulatory molecules, in particular B7-1 and B7-2, but conserve the ability to present antigens on MHC I, it was hypothesized that T cells in the tumor microenvironment may receive chronic TCR stimulation without costimulation, and therefore lead to T-cell anergy rather than productive activation. To test the hypothesis that forced B7 expression on tumor cells may enable better T-cell priming and hence tumor rejection, tumor cell transfection experiments were performed. Introduction of B7-1 on tumor cells was sufficient to induce CD8+ T-cell-mediated rejection in several models, as well as a memory response able to protect the mice against rechallenge with the wildtype tumor (28–31). It was also clear that B7 expression was not sufficient to mediate the rejection of non-immunogenic tumors (32), arguing that expression of tumor antigens and perhaps other biologic properties of the tumors were critical for this approach to be effective. In some settings, B7-1 was found to be superior to B7-2 in promoting the greatest tumor protection (33).
Although introduction of B7 molecules into tumors prior to their implantation in vivo often led to spontaneous tumor rejection, integration of B7 costimulation into therapeutic settings with pre-established tumors was less efficacious. For example, immunization with irradiated B7-1 transfectants as a vaccine could protect against subsequent challenge with wildtype P815 tumors but did not induce rejection of established tumors (34). These were some of the first results to suggest that inadequate costimulation might not provide a complete picture of the complex nature of immune resistance by pre-established tumors.
While the initial hypothesis was that expression of B7 on tumors cells would promote more effective T-cell priming, the observation that activation of class I-restricted CD8+ T cells by tumors occurred through cross-presentation by host APCs (rather than directly by tumor cells) (33) called this hypothesis into question. Spontaneous rejection of immunogenic tumors was found to depend upon B7 ligands of the host (35), supporting the notion that costimulation via APCs was critical. Several experimental systems suggested that the major beneficial effect of B7-1 expression by tumor cells was through improvement of the effector phase of the anti-tumor immune response rather than via increased T-cell priming (36). These careful observations have implications for how to optimally integrate improved B7 costimulation into clinical/translational studies of cancer immunotherapy.
The striking findings in early murine studies triggered rapid translation of B7 biology into clinical studies in cancer patients. A phase I trial of vaccination with autologous tumor cells engineered to express B7-1 in combination with systemic IL-2 was performed in metastatic renal cell carcinoma patients. Two patients with a partial response (PR) and two with stable disease (SD) were observed out of 15 treated subjects (37). A recent phase II trial extending these results in 39 patients showed one patient with a complete response (CR), two with a PR, and 24 with SD at 12 weeks. The overall response rate of 7.7% was not better than what has been reported with IL-2 alone (38). In an independent phase I trial in non-small cell lung cancer, 19 patients were vaccinated with a gene-modified allogeneic adenocarcinoma line expressing B7-1 and human leukocyte antigen-A1 (HLA-A1) or A2. Following three sets of immunizations, all but one patient had measurable induction of CD8+ T cells, and PR and 5 patients with SD were reported (39).
B7-1 was also integrated into other vaccination strategies with defined antigens. A non-replicating canarypox virus vector constructed to express both B7-1 and human carcinoembryonic antigen (CEA) was used to vaccinate patients with CEA-expressing adenocarcinomas. CEA-specific immune responses were induced and a few patients (three out of 16 in the first study and eight out of 30 in the second) experienced a stabilization of disease (40, 41). More recently, the same B7-1 and CEA-expressing ALVAC virus was combined with chemotherapy in a phase-2 trial for metastatic colorectal cancer. An overall objective response rate of 40.4% was observed (42), which is however similar to the objective response rate reported for chemotherapy alone (43).
A next generation of vaccines utilizing a fowlpox-based viral vector encoding B7-1 as well as 2 other costimulatory molecules [intercellular adhesion molecule 1 (ICAM-1) and lymphocyte function-associated antigen 3 (LFA-3)] in combination with the antigens CEA, Mucin 1 cell surface associated (MUC-1), or prostate-specific antigen (PSA) was subsequently developed. This vector showed encouraging results in preclinical studies and has been under evaluation in a series of early phase clinical trials (44). Overall, published results have confirmed that these vaccines are safe and can generate significant specific immune responses against the specific antigen with clinical benefit in some patients (45–49).
In order to provide augmented B7 costimulation at the effector phase of the anti-tumor immune response, intratumoral injection of B7-encoding vectors has been pursued. A recombinant vaccinia virus expressing B7-1 was tested in a phase I trial by injection into accessible melanoma lesions. An increased frequency of specific T cells against gp100 and melanoma-associated antigen recognized by T cells-1 (MART-1) antigen was observed by enzyme-linked immunosorbent spot (ELISPOT). Although clinical response was not a primary endpoint, two injected lesions (18%) underwent an objective partial response and another three lesions (27.3%) were stable and were correlated to a prolonged survival (50).
Overall, the inclusion of B7-1 during the priming phase in various vaccine strategies has consistently demonstrated improved induction of tumor antigen-specific T-cell responses. However, clinical benefit has been limited, despite augmentation in systemic immunity. It is likely that multiple layers of immune resistance at the level of the tumor microenvironment contribute to tumor escape from anti-tumor T-cell responses, a concept which will be addressed in more detail below and which has been recently reviewed (51).
4-1BB/4-1BBL
4-1BB (CD137) is another costimulatory receptor expressed on activated T cells (CD4+, CD8+, and NKT, activated NK cells, DCs, eosinophils, and mast cells. 4-1BB is a member of the tumor necrosis factor (TNF) receptor superfamily that is inducibly expressed on T cells following stimulation through the TCR complex (52–54).
The ligand of 4-1BB (4-1BBL or CD137L), a member of the TNF family, is expressed on activated macrophages, DCs, and B cells (55, 56). When coupled to a strong signal through the TCR, engagement of 4-1BB can induce IL-2 production independently of CD28 ligation (57). Early studies demonstrated also that ligation of 4-1BB by either cell surface 4-1BBL or specific antibodies provide a costimulatory signal particularly to CD8+ T cells, enhancing proliferation, cytokine production, and particularly survival (58–60). More recent data point to the role of 4-1BB engagement in augmenting rather than initiating T-cell response and in sustaining effector functions (61).
In vivo, the first use of anti-4-1BB antibodies as an anti-tumor therapy was reported by Melero et al. (62), who demonstrated the therapeutic potential of this strategy to promote rejection of P815 mastocytoma and Ag104A sarcoma tumors (62). Further studies using either 4-1BB agonistic antibodies or gene-modified tumor cells expressing 4-1BBL confirmed their efficacy to expand tumor-reactive T cells, suppress tumor growth, and in some cases induce regression of pre-established tumor in different tumor models (63–65). CD8+ T cells constitute the main effector subset involved in tumor rejection following treatment by 4-1BB mAb since CD4+ T cells, NK, and NKT cells were not required for the antitumor effects (66). Interestingly, several reports in rodent models have shown synergistic effects of agonistic anti-4-1BB mAb in combination with anti-tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and CD40 mAbs (67), with intratumoral introduction of the IL-12 gene (68), or with chemotherapy or radiotherapy (69, 70). These observations provide a guide for developing combination studies in the clinic.
An unusual feature of anti-4-1BB mAb therapy is the almost paradoxical observation that, while improved immunity is seen in tumor settings, diminished pathology has been observed in autoimmune models. Fu and colleagues (71) showed that same agonist antibody to 4-1BB used to promote anti-tumor immunity resulted in ameliorating both the incidence and the severity of experimental autoimmune encephalomyelitis (EAE). Many other studies have confirmed the beneficial effects of anti-4-1BB mAB in various autoimmune disease models including rheumatoid arthritis, EAE and systemic lupus erythematosis (72). In a transplantation model, anti-4-1BB also was shown to inhibit rejection of intestinal allografts in mice (73). Although a complete understanding of these differential effects is lacking, the promotion of regulatory T cells (Tregs) development and activity is believed to play a role (74). Additional potential explanations for immune suppression in some settings include the apparent ability to delete CD4+ T cells, retard B-cell function, and upregulate indoleamine-2,3-dioxygenase (IDO) and interferon-γ (IFN-γ) (65, 72). Together these results suggest that the possibility of both immune potentiating and immune suppressive effects of 4-1BB engagement should be taken into consideration and monitored in clinical/translational studies in patients.
A human anti-human 4-1BB mAB (BMS-663513) is currently under investigation by Bristol-Myers Squibb in a number of phase I and II trials in patients with metastatic or locally advanced solid tumors (64, 75).
OX-40/OX-4OL
OX40 also known as CD134 is another costimulatory molecule of the TNF receptor superfamily that was identified in 1987 (76) and that is expressed mainly on activated CD4+ but also CD8+ T cells. Its ligand OX40L is mainly expressed on professional APCs such as DCs, B cells, and macrophages, particularly after stimulation with TLR ligands or CD40L (77–79). OX40L also has been observed on T cells and endothelial cells (80, 81). OX40 functions as a late costimulatory receptor and artificial engagement by OX40L stimulates proliferation, cytokine secretion, and survival of T cells, in part by increasing the expression of anti-apoptotic molecules of the Bcl-2 family (82). Several systems have shown that OX40 expression is not dependant of CD28 but that CD28 can augment the level of OX40 expression (82, 83), suggesting that the two molecules cooperate in a sequential manner. Therefore, a model was proposed whereby OX40 signals act in a temporal manner after CD28 signals, supporting continued survival and proliferation of effector (84). Interestingly, recent data have also shown that OX40 ligation may suppress the function and generation of CD4+Foxp3+ Tregs (85, 86).
In vivo, OX40L transduction of tumor cells and engagement of OX40 by an agonist Ab or an OX40-immunoglobulin (Ig) chimeric protein has been shown to increase tumor immunity against different murine tumor model including B16 melanoma and hepatic colon metastases (63). Interestingly, it was shown that agonistic anti-OX40 mAb given in combination with agonistic anti-4-1BB and intratumoral injection of an adenovirus expressing IL-12 showed a synergistic effect to induce long-term survival of mice bearing large chemically-induced colon carcinoma (87).
The first phase I clinical trial using a murine anti-human OX40 mAb has been initiated in patients with advanced cancer. This trial is essentially a proof of concept, as neutralizing human anti-mouse antibodies are expected to be limiting, and a humanized or fully human antibody should be pursued. A humanized OX40 agonist was recently developed that could be tested in future clinical studies (88).
HVEM/LIGHT
The herpesvirus entry mediator (HVEM) is another member of the TNF receptor superfamily member, but in contrast to others is expressed both by resting and by activated T cells. The principal ligand for HVEM is the TNF superfamily member 14 (LIGHT/TNFSF14), and ligation by LIGHT acts as a positive costimulatory signal. LIGHT expression is induced on activated T cells themselves, but also by NK cells, monocytes, and DCs (63). In addition to its binding to HVEM, LIGHT also can bind the lymphotoxin-β receptor, which can be expressed by stromal cell populations in lymph nodes and other tissues, including the tumor microenvironment. Evidence supporting the costimulatory effect of LIGHT came from experiments showing that blocking of LIGHT inhibits early T-cell proliferation and cytokine secretion in an allogeneic mixed lymphocyte reaction (89–91). However, in vivo experiments suggest that a major impact of LIGHT is through induction of chemokine production by stromal cells that results in recruitment of T cells and DCs, leading to the generation of lymph node-like structures. Transgenic mice expressing LIGHT driven by the CD2 promoter show increased number of activated T cells, higher proportions of effector and memory T cells, and signs of autoimmunity (92, 93). Transgenic mice expressing LIGHT driven by the rat insulin promoter develop autoimmune diabetes mellitus, with pancreatic islets showing a lymph node-like morphology (94). LIGHT knockout mice also have been generated. These mice show defective expansion of superantigen-reactive CD8+ T cells and defective cytotoxic lymphocyte (CTL) generation after peptide priming in vivo (95, 96), supporting a positive immunoregulatory role.
The attractive properties of LIGHT to serve as both a costimulatory ligand for T cells, and a stromal cell activator to induce chemokine production and lymphoid recruitment, made it attractive to consider for intratumoral application. Introduction of a LIGHT cDNA into P815 tumor cells induced regression of established tumors with the maximal therapeutic effect requiring both CD4+ and CD8+ T cells. The anti-tumor effect of LIGHT was shown to be CD28-independent in this model (97). Introduction of LIGHT in Ag104 sarcoma cells expressing the alloantigen Ld has been shown to promote direct antigen presentation, in vivo chemokine production, T-cell recruitment, and tumor rejection (98). LIGHT also appears to promote recruitment of NK cells into tumor sites, which may participate in a bridge to adaptive immunity (99).
There is growing interest to bring therapies involving LIGHT forward into the clinic. As the maximal impact is expected to be at the level of altering the tumor microenvironment, strategies for intratumoral targeting would be desirable. Along these lines, an adenoviral vector encoding LIGHT injected intratumorally was shown to promote T cell recruitment, control of the injected tumor, and elimination of non-injected metastases in mice (100). Another potential strategy for clinical translation would be in vivo electoporation directly into tumor metastases, as has recently been reported using an IL-12 cDNA in patients with melanoma (101).
ICOS, GITR and CD27/CD70
Other receptors that have been described as having positive costimulatory functions and could be attractive to consider for augmenting anti-tumor immunity include inducible costimulator (ICOS), a member of the B7 family, and glucocorticoid-induced tumor necrosis factor receptor (GITR), and CD27, members of the TNF receptor superfamily. ICOS and GITR are mainly expressed by activated CD4+ and CD8+ T cells, but constitutive expression by CD25+CD4+Foxp3+ Tregs has also been demonstrated (102). CD27 is expressed on naive CD4+ and CD8+ T cells, and also by NK cells. The ligands for these receptors, GITR ligand (GITRL), CD27 ligand (CD70) and ICOS ligand (B7h or LICOS), are predominantly expressed on populations of APCs (B cells, macrophages, and DCs) (63, 84). Interestingly, recent studies have confirmed the critical role for CD70 expression on activated DCs to promote optimal T-cell activation in vivo. Transgenic expression of CD70 on DCs enabled T-cell priming in vivo in the absence of adjuvant (103), arguing that it is perhaps one of the most critical of the costimulatory ligands for adaptive immunity.
Animal models targeting these additional costimulatory pathways in the tumor context are under evaluation but earlier in their development. B7h expression on tumor cells was demonstrated to promote regression in the Sa1/N fibrosarcoma and J558 plasmocytoma models via a CD8-dependent mechanism (104, 105). Like for B7-1 and B7-2, this strategy was ineffective when weakly immunogenic tumors were investigated (106). Other studies have shown that CD27/CD70 can enhance tumor rejection, with CD70 transfection enhancing both NK-dependent and T cell-dependent mechanisms of tumor elimination (107–109). The administration of an anti-GITR agonistic antibody (clone DTA-1) also has been shown to induce the rejection of several murine syngeneic tumors through an IFN-γ-dependant mechanism and without obvious autoimmune manifestations (110, 111). Anti-GITR mAb promoted the activation of effector CD4+ and CD8+ T cells, and altered the intratumoral ratio of Tregs/T effectors. More recently, observations made by Zhou et al. (112) suggested that the anti-tumor effects of anti-GITR antibody were mainly driven by its positive costimulatory signal in effector CD4+ T cells rather than by its possible effect to abrogate the suppressive functions of Tregs.
Costimulation of APCs: CD40/CD40L
In addition to providing costimulatory signals directly to T cells, it also is attractive to promote activation of APCs that are likely cross-presenting tumor antigen so that increased costimulatory ligand expression is achieved in situ. The most attractive of these strategies is arguably through CD40 engagement. CD40 belongs to the TNF receptor superfamily and is crucial for the function of B cells and of DCs that express it constitutively. CD40 ligand (CD40L or CD154) is principally expressed on activated T helper cells but can also be found on NK cells, platelets and plasmacytoid DCs (113). Ligation of CD40 has been shown to license APCs and enable them to drive effector CTL responses, in part through the induction of IL-12 secretion but also through upregulation of B7 family members (114, 115). CD40/CD40L costimulation has been shown to be important for induction effective anti-tumor T-cell responses, since blocking CD40L with a neutralizing mAb blocked the generation of a protective anti-tumor immune response following vaccination with granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing B16 melanoma cells (116). Moreover, incorporation of CD40L into tumor cell-based vaccines was shown to significantly enhance immune responses to poorly immunogenic tumors in mice (117). The combination of CD40L expression with other immunomodulators such as GM-CSF, IFN-γ or IL-2 has also shown improved immunity in several tumor models (118).
Perhaps the most straightforward approach for CD40 engagement that is translatable to the clinic is through the use of agonistic anti-CD40 mAbs. While anti-CD40 mAb has been explored as a direct cancer therapeutic for CD40-expressing malignancies (119), it also has been investigated as an APC activator to promote anti-tumor T-cell responses (120). In the latter case, the anti-tumor effect of anti-CD40 mAb clearly requires a functional immune system and therefore acts indirectly via the host (121).
A fully human agonistic anti-CD40 mAb has been developed for clinical investigation by Pfizer (CP-870,893). A phase I clinical trial was completed in patients with advanced solid tumors. Of the 29 patients enrolled, four patients with melanoma (14% of all and 27% of melanoma patients) had objective partial response (122). Other strategies to target CD40 clinically have included the administration of soluble CD40L (123), the expression of CD40L and GM-CSF by tumor cells as a vaccine (124), and the use of CD40L to prime DCs ex vivo prior to administration as a vaccine in vivo. The encouraging results seen with many of these studies have prompted continued exploration of modulation of the CD40 pathway as a cancer therapeutic.
Coinhibitory pathways and anti-tumor immunity
While a significant amount of effort has been invested studying the provision of costimulatory ligands to augment anti-tumor immune responses and promote better immune-mediated tumor elimination, more recent evidence has argued that negative regulatory pathways that serve to impede ongoing immune responses may dominate in the tumor context. Thus, interfering with these immune inhibitory checkpoints has gained in interest as a complementary strategy for cancer therapy. Several of these inhibitory mechanisms involve coinhibitory receptors and ligands related to the CD28/B7 system and will be discussed here.
CTLA-4
CTLA-4 (cytotoxic T lymphocyte antigen-4, CD152), a CD28 homologue and member of the immunoglobulin superfamily, was discovered in 1987 through differential screening of a murine cyotixic T cell cDNA library (125). Sequence homology between the human CD28 and CTLA-4 proteins was demonstrated, particularly in the juxtamembrane and cytoplasmic regions (126), suggesting a possible functional overlap of these two molecules. In contrast to CD28 that is constitutively expressed on the cell surface of T cells, CTLA-4 is inducibly expressed, both via new transcription and through regulated trafficking to the cell surface (127) with maximal expression occurring two to three days following T-cell activation(128, 129). CTLA-4 was subsequently shown to bind to B7.1 and B7.2 with an approximately 10–20-fold higher affinity than CD28 (16, 130). The development of anti-CTLA-4 antibodies made a functional analysis of the CTLA-4 protein possible (128, 129, 131). In contrast to the costimulatory signal provided by binding of CD28 to B7.1 and B7.2, several lines of evidence pointed to a negative regulatory effect of CTLA-4 on T-cell function, both in vitro and in vivo (128, 129, 132).
The definitive role of CTLA-4 as a major negative regulator of T-cell activation was established with the description of CTLA-4−/− mice in 1995 (133, 134). CTLA-4−/− mice succumbed at three-to-four weeks of age from massive lymphoproliferation within the spleen and lymph nodes and end-organ infiltration by activated lymphocytes. The phenotype of CTLA-4−/− mice was completely dependent on CD4+ T cells (135). The development CTLA-4−/− mice both clarified the inhibitory function of CTLA-4 and suggested an important role in the regulation of peripheral tolerance to self.
In addition to a direct inhibitory function on effector T cells, a second potential negative regulatory role of CTLA-4 was suggested when it was shown that natural Tregs constitutively expressed high levels of CTLA-4 on the cell surface in a Foxp3-dependent manner (136, 137). As Tregs are capable of suppressing anti-tumor T-cell responses (138), it was speculated that Treg-mediated suppression might in part be regulated through CTLA-4. This was definitively shown to be the case following the development of mice with a conditional deletion of CTLA-4 in Tregs (CKO mice) (139). CKO mice developed a spontaneous lymphoproliferation syndrome reminiscent to that seen in CTLA-4−/− mice, although delayed in onset and of milder severity. Thus, part of the inhibitory function of CTLA-4 is through modulation of Tregs. CTLA-4 on Tregs was also shown to be relevant for suppressing anti-tumor immune responses in this study.
Because of the contribution of CTLA-4 to the maintenance of peripheral T-cell tolerance, it was hypothesized that blockade of CTLA-4 might augment anti-tumor effector T-cell responses and promote improved tumor control in vivo. The laboratory of James Allison (140) showed that wildtype mice challenged with syngenic colon carcinoma or fiborsarcoma cells and treated with several doses of anti-CTLA-4 antibody demonstrated markedly improved tumor rejection compared to mice treated with an isotype control antibody. The CTLA-4 effect was seen in both the prophylactic and therapeutic tumor settings, and was accompanied with a long-lived memory response in which cured mice were frequently protected from a second tumor challenge. The efficacy of CTLA-4 blockade in tumor immunity was confirmed by other groups (141), and its efficacy correlated directly with the stage of the tumor prior to the initiation of anti-CLTA-4 antibody administration (142), as well as with the inherent immunogenicity of the tumor cells (143). Whereas CTLA-4 blockade was quite effective when given when tumors were small (144), it could not reverse tumor-induced T-cell tolerance in the setting of more advanced tumors (145).
While the efficacy of CTLA-4 blockade alone was impressive, it was usually insufficient to cure large, established, and poorly immunogenic tumors. Thus, several groups tested anti-CTLA-4 mAb in combination with other immunotherapeutic approaches (143, 146–148). For example, anti-CTLA-4 was combined with a vaccine consisting of granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing irradiated SM1 tumor cells. Growth of SM1, a poorly immunogenic mammary cancer cell line, was completely resistant to CTLA-4 blockade alone (140, 143). However, the combination of CLTA-4 blockade and active immunization with the irradiated cellular vaccine led to rejection of short-term, pre-established SM1 tumors in wildtype mice. Similar results were observed in the B16 melanoma model (147). The same group later reported improved rejection of B16 melanoma when CD25+ T-cell depletion was combined with both anti-CTLA-4 therapy and vaccination. Depletion of Tregs in combination with CTLA-4 blockade and vaccination was associated with a greater expansion of functional tyrosinase-related protein-2 (TRP-2)-specific CD8+ T cells when compared to cohorts treated with CTLA-4 blockade and vaccination alone (148).
These exciting preclinical results led to the simultaneous development of two fully human anti-CTLA-4 antibodies, ipilimumab (MDX-101; Medarex/Bristol-Myers Squibb) and tremelimumab (CP-675,206; Pfizer), which are currently being investigated in clinical trials. In phase I studies in patients with metastatic melanoma and ovarian cancer, ipilimumab treatment was associated with several PRs, and tumor necrosis was seen in a larger number of patients (149). Ipilimumab was subsequently tested in several phase II studies for patients with metastatic melanoma either alone or in combination with peptide vaccination, and objective response rates varied between 6% and 21%, and disease-control rates were in the 30% range (150–154). Toxicity following ipilimumab treatment in phase II studies included occasionally severe autoimmune colitis, dermatitis, uveitis, hypophysitis, and hepatitis. Development of autoimmunity appeared to correlate with response (152). Phase III clinical trials of Dacarbazine (DTIC) alone versus DTIC plus ipilimumab have been completed in patients with metastatic melanoma and mature results should be reported in the near future.
Tremelimumab has also demonstrated activity in metastatic melanoma. In a phase I, dose-escalation study, tremelimumab treatment led to two CR, two PR, while four patients experienced SD in 34 patients with melanoma (155). Another phase I/II study confirmed objective response rates of approximately 10% with SD occurring in approximately 30% of melanoma patients (156, 157). Autoimmune-like sequelae were also seen in patients treated with tremelimumab. A phase III study of tremelimumab in which untreated patients with advanced melanoma were randomized to receive the anti-CTLA-4 antibody either alone or in combination with DTIC or temozolomide chemotherapy, was recently discontinued as overall survival in both patient cohorts was similar (158).
Clearly, anti-CTLA-4 mAbs are active in patients with melanoma. As the overall response rates are around 10%, emphasis is being placed on biomarker discovery in an attempt to enrich for the patient population most likely to benefit from this treatment. Rationally designed combination therapies also should be considered. Perhaps most importantly, these Abs have established blockade of negative immunoregulatory pathways as an important paradigm for immune potentiation and cancer therapeutics.
PD-1
Originally isolated from activated T cells undergoing apoptosis (159), Programmed cell death-1 (PD-1; CD279) is a negative costimulatory receptor of the immunoglobulin gene superfamily. PD-1 can be expressed on the cell surface of activated T cells, B cells NKT cells, activated monocytes, and DCs. T cells, in particular, do not express PD-1 on their cell surface at rest, but can do so following activation (160). Upon binding to its ligands PD-L1 (also called B7-H1 and CD274) and PD-L2 (also called B7-DC and CD273), PD-1 becomes phosphorylated on intracellular tyrosine residues within its immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) (161). Subsequently, protein phosphatases, such as SHP-2 are recruited to bind to the ITSM, become activated and inhibit proximal TCR signaling events (162).
The role of PD-1 in regulation of peripheral self-tolerance became evident following the discovery that PD-1−/− mice developed strain-specific autoimmunity. PD-1−/− mice on the C57BL/6 background develop a lupus-like arthritis, while BALB/c mice develop a cardiomyopathy secondary to production of an autoantibody directed against cardiac troponin (163, 164). The introduction of a PD-1 null mutation into the 2C TCR transgenic strain resulted in a disorder resembling graft-versus-host disease in mice bred onto the H-2b/d background (164).
The PD-1 receptor has two known ligands, PD-L1/B7-H1 and PD-L2/B7-DC. PD-L1 was originally discovered in 1999 as B7-H1 (165), and subsequently in 2000 as PD-L1 (166) by two separate groups. PD-L1 is an inhibitory B7 family member with significant amino acid sequence homology to B7-1 and B7-2 (165). PD-L1 mRNA expression is present in multiple peripheral tissues, while PD-L1 protein can be expressed on the cell surface of activated T cells, B cells, monocytes, DCs, and vascular endothelial cells (165–167). PD-L1 expression can be augmented following exposure to type I or type II interferons (166, 168, 169). Initial reports were conflicting with respect to the costimulatory role of PD-L1. One group demonstrated that stimulation of purified human T cells with anti-CD3 in the presence of a B7-H1-Ig fusion protein resulted in increased cellular proliferation and secretion of IL-10, and to a lesser extent, IFN-γ (165). On the other hand, opposite results were seen in similar experiments using a PD-L1-Ig fusion protein (166). Blocking DC-expressed PD-L1 with mAb resulted in enhanced T-cell proliferation and cytokine secretion. Taken together, these results suggested that PD-1/PD-L1 interactions generally result in inhibition of T-cell function.
PD-L1 is also expressed on many tumors, including glioblastoma and melanoma, as well as cancers of the head and neck, lung, ovary, colon, stomach, kidney, and breast (170, 171). In renal cell cancer, a high expression level of PD-L1 on tumor cells, tumor infiltrating lymphocytes or both correlated with aggressive tumor behavior and was associated with a 4.5-fold higher risk of cancer-related death (172). PD-L1 expression on ovarian epithelial tumor cells also correlated with a decreased overall patient survival (173). Interestingly, expression level of PD-L1 on tumor cells was found to correlate inversely with numbers of ovarian intraepithelial CD8+ T cells, the presence of which was associated with improved patient outcomes. (173).
The mechanism underlying PD-L1 upregulation by tumor cells in vivo is not known. In vitro, PD-L1 expression is upregulated by exposure to type I and II interferons, and to a lesser extent, by TNF-α. Our data and those of others suggest that PD-L1 expression is greater on tumor cells freshly analyzed ex vivo than on in vitro cultured cell lines (170, 174) (J. K., unpublished observations), suggesting that the cytokine milieu within the tumor microenvironment may be contribute to PD-L1 expression. It is also interesting to speculate that cytokines, such as IFN-γ and TNF-α generally thought of as promoting tumor immunity by causing upregulation of MHC-expression and DC maturation, might at the same time hinder anti-tumor T-cell responses via upregulation of PD-L1. Whether PD-L1 upregulation is a driving force of tumor aggressiveness or simply a marker of it remains to be demonstrated.
The immunohistochemical analyses of PD-L1 expression led to investigation of PD-L1 blockade as cancer immunotherapy. To test whether forced expression of PD-L1 on tumor cells impaired anti-tumor immunity, murine immunogenic P815 cells were tansfected to express PD-L1. P815.PD-L1-expressing tumor cells were relatively resistant to in vitro cytolysis and compared to control P815 cells, and failed to be rejected when inoculated into wildtype, syngeneic mice (175). When mice were challenged with P815.PD-L1 cells, treatment with anti-PD-L1 mAb led to markedly improved tumor control compared to treatment with control antibody. The effect of PD-L1 expression appeared to be dependent on T cells, as growth rates of control and PD-L1-expressing P815 cells were similarly rapid in nude mice. In a different model, the effect of forced expression of PD-L1 on the murine squamous cell cancer cell line SCCVII resulted in diminished immune-mediated control that was restored upon PD-L1 blockade (169). Tumor outgrowth of the naturally PD-L1-expressing J558L myeloma cell line was controlled in syngeneic PD-1−/− mice and in wildtype mice treated with anti-PD-L1 mAb (175). However, poorly immunogenic tumors not expressing PD-L1, such as B16 melanoma, grew similarly in PD-1+/+ and PD-1−/− mice (175). Adoptive transfer of PD-1−/− 2C TCR transgenic T cells also showed superior rejection of P1.HTR tumors in vivo compared to either wildtype or CTLA-4−/− 2C T cells (174).
In addition to a direct effect by PD-L1 expressed on tumor cells, PD-L1 expressed on DCs may also inhibit anti-tumor T-cell responses. Myeloid DCs (MDCs) generated from peripheral blood of ovarian cancer patients expressed high levels of PD-L1, which could be augmented following exposure to IL-10 and vascular endothelial growth factor (VEGF) in vitro (176). MDCs expressing PD-L1 were poor stimulators of allogeneic T cells, and promoted increased T-cell expression of IL-10. When human ovarian carcinomas were established in non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) mice, T cells stimulated in the presence of autologous tumor MDCs and anti-PD-L1 mAb generated augmented T-cell effector function and led to improved control of tumor growth (176). Studies in renal cell carcinoma also have suggested that PD-L1 blockade can improve anti-tumor T-cell responses in vitro (177).
PD-L1 expression may form a `molecular shield' preventing T-cell-mediated lysis of tumor cells (138, 178). Control P815 cells or those expressing PD-L1 were combined with activated tumor-specific P1A CTL. After a 12 hour incubation, P1A-specific T cells lysed control P815 cells normally, but were less able to lyse PD-L1-expressing P815 cells, suggesting rather than inducing T-cell anergy or tolerance, expression of PD-L1 on tumor cells simply rendered them resistant to killing by activated T cells (178).
There is also evidence to suggest that in animals with chronic viral infections, PD-1 is expressed by `exhausted' viral antigen-specific T cells. The functional impairment of such cells can be reversed through PD-L1 blockade (179). While there is no clear evidence that exhaustion of T-cells expressing PD-1 is a potential mechanism of T-cell inhibition by PD-L1 in the tumor setting, it is interesting to hypothesize that a similar inflammatory state similar to that seen in chronic viral infections may also be operational in at least a subset of patients with cancer.
Based on these preclinical observations, two humanized anti-PD-1 antibodies have been developed for clinical investigation (CT-011, Cure Tech and MDX-1106, Medarex). In a single-dose phase I study of CT-011, 17 patients with advanced hematological malignancies were treated with CT-011 at doses ranging from 0.2 to 6.0 mg/kg. No serious adverse events were described, and no maximum tolerated dose (MTD) of CT-011 was defined. Clinical benefit was documented in 33% of patients, including one CR. Development of autoimmunity was not reported (180). Brahmer (181) conducted a single-dose phase I study of MDX-1106 in patients with advanced solid cancers. Thirty nine patients received doses of MDX-1106 of 0.3 to 10mg/kg. Administration of MDX-1106 was safe, and one partial tumor responses was seen in 36 evaluable patients. One patient developed severe colitis, and two experienced polyarthritis, all requiring treatment with corticosteroids (181). Currently, the efficacy of PD-1 antibodies is currently being tested in phase II trials for various tumor subtypes.
PD-L2 (B7-DC) was discovered as a second ligand for PD-1 simultaneously by two groups in 2001 (182, 183). PD-L2 mRNA expression is confined largely to DCs, but in humans, PD-L2 mRNA expression can also be seen in heart, placenta, liver, and pancreas (182, 183). Murine PD-L2 mRNA expression on normal tissues is more tightly regulated. PD-L2 expression was also found in several murine tumor cell lines (182). Whether expression of PD-L2 at the protein level occurs commonly in tumors is not known. Using a PD-L2-Ig fusion protein, the ability of PD-L2 to costimulate T cells was examined. One group demonstrated a T-cell inhibitory effect for PD-L2 (182), while PD-L2 was found to stimulate T cells by another (183). These conflicting results with regard to ability of PD-L2 to costimulate and/or inhibit T-cell responses may be explained by the existence of a putative second receptor distinct from PD-1 that may have a positive costimulatory function analogous to CD28 (184, 185).
The in vivo role of PD-L2 was examined more definitively using PD-L2−/− mice (186). In vivo responses of adoptively-transferred OVA peptide-specific CD4+ T cells were diminished in PD-L2−/− mice following vaccination with OVA peptide-pulsed bone marrow derived DCs (BMDCs) (186). Following establishment of hepatic metastases in wildtype or PD-L2−/− mice by splenic injection of CT26 murine colon cancer cells, tumors grew more rapidly, and survival was decreased, in PD-L2−/− mice. Collectively, these data suggest that a significant component of the function of PD-L2 in vivo is likely stimulatory. In contrast to PD-L1, it seems unlikely that neutralizing anti-PD-L2 mAbs would lead to improvement of anti-tumor immunity.
B7-H4/B7.x
The discovery of this negative costimulatory ligand was simultaneously reported by three groups in 2003 (187–189). B7-H4 (B7S1, B7x) was found to be 20–30% homologous to other B7 family members at the amino acid level, but was unique as it is believed to be anchored to the cell membrane via a glycosyl phosphatidylinositol (GPI) linkage (187), as opposed to other B7 family members which are all type I transmembrane proteins. B7-H4 is expressed broadly in peripheral tissues at the mRNA level, but protein expression of B7-H4 was seen only on activated B cells, T cells, and monocytes (187, 188). A B7-H4-Ig fusion protein potently inhibited T-cell responses, and blockade with anti-B7-H4 mAb augmented in vitro T-cell responses and exacerbated EAE in vivo (187–189). Thus, B7-H4 was defined as a negative costimulatory ligand. Consistent with in vitro data, Th1 responses were slightly augmented and Leishmania major infection was better controlled in B7-H4−/− mice (190). However, other immune responses remained intact. Thus, B7-H4 likely plays a negative, but minor role in vivo, and may serve along with other costimulatory/coinhibitory ligands to finely tune T-cell responses against cognate antigens.
The identity of the cognate receptor on T cells for B7-H4 remains controversial, although it was reported that BTLA-4, a recently described CD28 family, may be a receptor (189, 191). B7-H4 mRNA has been detected in murine tumor cell lines, including those of originating from prostate, colon, breast, ovarian, and lung (189). Immunohistochemically, B7-H4 could be detected with regularity on freshly isolated ovarian and lung cancer (192) specimens. Simon and colleagues identified B7-H4 cDNA to be highly overexpressed in breast and ovarian cancer tissues compared with healthy breast and ovarian tissue (193). Using novel B7-H4 antibodies, they confirmed overexpression of B7-H4 in lysates of human ovarian cancer cell lines and in cancer tissues. An enzyme-linked immunosorbent assay (ELISA) technique was developed to analyze serum, ascites, and tumor lysates from ovarian cancer patients, and B7-H4 was found to be expressed almost uniquely in ovarian tumor tissues. B7-H4 levels were also elevated in the serum of ovarian cancer patients, and B7-H4 was similarly useful to identify ovarian cancer as was CA-125, a standard marker of ovarian cancer. When used in combination, B7-H4 and CA-125 improved the sensitivity and specificity of detection compared with either marker alone (193). These results confirmed that B7-H4 was expressed by ovarian tumor tissue, and that it may be useful in the future as a screening or monitoring marker, although larger, confirmatory studies are needed to substantiate these findings. B7-H4 is also expressed on approximately 60% of renal cancers as well as on adjacent endothelial tissue (194). B7-H4 expression was associated with advanced tumor stage and grade. Patients with B7-H4-expressing tumors were three times more likely to die from their cancer (194). In our own laboratory, we have not detected B7-H4 in metastatic melanomas (Gajewski et al., unpublished observations).
The suppressive mechanism through which B7-H4 operates has been clarified recently when it was demonstrated in the setting of ovarian cancer, a subset of cell surface B7-H4-expressing tumor associated macrophages (TAM), but not its intracellular expression on purified ovarian cancer cells, could suppress tumor-antigen specific T-cell responses (195). B7-H4 expression in TAM was positively upregulated by high concentrations of IL-6 and IL-10 present in the tumor microenvironment, while its expression was inhibited by GM-CSF and IL-4 (195). Blockade of B7-H4 expression with antisense oligonucleotides reversed the suppressive effects of TAM and restored T cell-mediated tumor immunity in vivo. Induced expression of B7-H4 on TAM restored their suppressive capabilities (195). Treg-stimulated production of IL-10 by APCs was also shown to contribute to upregulation of B7-H4, conferring a suppressive phenotype (196). Thus, a model has emerged in which APCs present in the ovarian cancer microenvironment are licensed to produce IL-6 and IL-10 by Tregs, which in turn leads to B7-H4 upregulation in an autocrine fashion. Consequently, B7-H4-expressing APCs are capable of inhibiting effective anti-tumor T-cell responses in ovarian cancer patients (197). Development of reagents to block of the B7-H4/B7.x pathway is thus attractive to consider for a subset of human malignancies.
LAG-3
The lymphocyte activation gene 3 (LAG-3, CD223) was characterized from activated T cell and NK cells in 1990 (198). The LAG-3 protein is a member of the Ig superfamily containing four extracellular Ig domains, and is closely related to CD4. The ligand for the LAG-3 receptor was subsequently determined to be MHC class II (199) with higher affinity than CD4 itself (200). LAG-3 is not expressed in resting T cells, but is upregulated following T-cell stimulation and appears to be associated with the CD3/TCR complex (201, 202). When LAG-3 on activated T cells crosslinked with a specific mAb, potent inhibition of T-cell proliferation and cytokine production was seen that was reversed by exogenous IL-2. LAG-3 crosslinking also led to downmodulation of the CD3/TCR complex and CD3-mediated calcium flux in activated T cells in vitro (202).
In vivo, DNA-based vaccination of Balb-neuT mice, which develop spontaneous mammary cancers, along with administration of a LAG-3-Ig fusion protein protected the majority of mice from tumor development, and was associated with augmented B- and T-cell responses when compared with vaccination alone (203). Furthermore, when PBMC from melanoma patients stimulated in vitro in the presence of LAG-3-Ig resulted in sustained generation and expansion of tumor antigen-specific CD8+ T cells (204).
Additional evidence for an immune inhibitory function comes from the analysis of LAG-3−/− mice. CD8+ T cells from LAG-3−/− mice proliferated more rapidly than those from wildtype mice when transferred into lymphopenic hosts (205). This effect could be abrogated upon forced expression of a wildtype form, but not a signaling-deficient form, of the LAG-3 gene. Furthermore, blockade of LAG-3 with mAb or adoptive transfer of LAG-3−/− hemagglutinin (HA)-specific CD8+ T cells led to augmented anti-tumor immune responses against HA-expressing tumors in TRAMP (Trf4p, Air1p or Air2p, and Mtr4p complex) mice (206).
LAG-3 may also play an important role in the suppressive capabilities of Tregs. Using a differential gene array analysis in TCR transgenic CD4+ T cells, it was shown that LAG-3 was expressed at higher levels in induced Tregs responding to a self-expressed transgene (HA) (207). When LAG-3 was blocked in vitro or in vivo, Treg-mediated suppression was significantly diminished, and ectopic expression of LAG-3 was sufficient to induce regulatory properties upon conventional CD4+ transgenic T cells. In tumor samples from patients with Hodgkin's Disease (HD), LAG-3 expression was induced on tumor-infiltrating lymphocytes, and LAG-3 was highly expressed on circulating Tregs in patients with active HD but not on those in remission (208). Endogenous CD8+ T cells from HD patients specific for Epstein-Barr virus epitopes were hyporesponsive, and function correlated with the level of LAG-3 expression present in tumor-infiltrating lymphocytes (208). Together, these data suggest that in vivo expression of LAG-3 on conventional T cells and Treg confers a hyporesponsive or suppressive phenotype, and that LAG-3 is a negative regulatory T-cell receptor which could be targeted using a mAb approach in cancer patients, both to inhibit Treg-mediated suppression and to restore the function of tumor antigen-specific effector T cells.
Complex network of negative regulation in the tumor microenvironment
While engagement of inhibitory receptors expressed on T cells represents one category of negative regulation of anti-tumor immune responses, recent work has established that the tumor microenvironment is more complex and contains a network of suppressive mechanisms that may act in concert to prevent immune-mediated destruction and ultimately allow tumor outgrowth. We have observed in melanoma metastases that PDL1 is usually coexpressed with the tryptophan-catabolizing enzyme indoleamine-2,3-dioxygenase (IDO) and also with Foxp3+ Tregs (unpublished data). These tumors are also deficient in expression of B7-1 and B7-2, arguing for an anergy-promoting environment as well. Additional support for T-cell anergy has come from evidence that T-cell hyporesponsiveness in the tumor microenvironment can in some cases be restricted to anti-tumor T cells while sparing viral antigen-specific T cells in the same tumor site (209). Together, observations such as these suggest the possibility that interference with multiple negative regulatory influences simultaneously may ultimately be necessary for optimal therapeutic benefit. Preclinical studies of combination therapy approaches are beginning to shed light on the most synergistic permutations. Using homeostatic proliferation in lymphopenic recipients as a strategy to reverse T-cell anergy (210) combined with depletion of Tregs using anti-CD25 mAb, we recently have observed powerful rejection of B16 melanoma tumors in vivo (211). Uncoupling PD-L1/PD-1 interactions using PD-1−/− T cells in combination with homeostatic proliferation also appears to be synergistic (unpublished observations). Continued evaluation of logical combinations in preclinical models will provide necessary preparation for when combination treatments become possible with clinical-grade reagents in patients.
Concluding remarks
There are multiple levels at which manipulation of costimulatory and coinhibitory receptors may improve the ability of tumor antigen-specific T cells to control tumor growth in vivo. Such manipulations may potentially improve both the priming phase and the effector phase of the anti-tumor immune response, but the need for augmentation of each of these phases in individual cases may vary. When considering translation to the clinic, it is critical to keep in mind that some individual patients may have already generated a spontaneous anti-tumor T-cell response as detected using functional assays in the blood, and a subset of those may have evidence of migration of activated T cells into tumor sites. However, local negative regulatory pathways may dominate, thus allowing tumor escape from immune destruction. In those instances, vaccination and other manipulations to augment the priming phase may be less critical than uncoupling critical negative regulatory pathways in the tumor site (e.g. PD-L1-PD-1 interactions, T cell anergy, etc). However, other individual patients may have developed systemic peripheral tolerance to dominant tumor antigens, and in those instances, interventions to recruit T cells against subdominant antigens through vaccination, increased B7 costimulation, CTLA-4 blockade, etc. may be the most crucial. On the other hand, some patients may have tumors that completely lack the ability to recruit activated T cells into the tumor microenvironment, and as such even superior induction of circulating functional tumor antigen-specific T cells may be totally ineffective because of a local barrier to T-cell homing. In those instances, promoting improved T-cell migration (e.g. via local application of LIGHT) may represent the highest priority intervention. Therefore, maximally effective clinical/translational development of novel immunotherapeutics will require the development and application of assay systems to appraise the details of the anti-tumor immune response both in the blood and in tumor sites, both to determine the frequency of each type of mechanistic block in the anti-tumor T-cell response, and also to determine the most critical pathways to manipulate towards improved clinical outcome. Such detailed analyses will ultimately identify biomarkers that should facilitate the selection of patients likely to benefit from specific immunotherapeutic interventions.
In addition to inter-patient heterogeneity, a second variable when considering clinical translation is the potential differences in dominant tumor escape mechanisms in different subtypes of cancer. For example, PD-L1 has been observed to be widely expressed in metastatic melanomas, whereas B7-H4/B7.x has not been detected in melanoma but frequently observed in ovarian cancers. In addition, a high percentage of infiltrating γδTCR-expressing T cells with apparent immune suppressive properties has been observed in breast cancer tumors but not melanomas. Thus, one can envision the application of selected immunotherapeutic interventions dictated by the biologic characteristics of the cancer type being targeted.
A final important point is to emphasize the apparent complexity and multiple layers of barriers to the anti-tumor immune response that seem to coexist in patients. For example, metastatic melanomas frequently express PD-L1, but also are B7-poor and contain IDO-expressing APCs and CD4+FoxP3+ Tregs. As such, manipulating one pathway may not be sufficient to restore maximal anti-tumor efficacy, raising the likelihood that combination immunotherapies may be required. Once could imagine providing local expression of B7-1 in the tumor microenvironment to prevent induction of T-cell anergy, but also having to eliminate Tregs, block IDO activity, and/or interfere with PD-L1/PD-1 interactions in a combinatorial fashion in order for tumor regression to occur in the largest fraction of patients. Carefully designed preclinical models and continued analysis of individual cancer patients should enable generation of a prioritized list of combination immunotherapies, including those that modulate costimulatory and coinhibitory receptors, to develop for clinical trial testing. Such studies may demand an unprecedented level of collaboration between academic scientists, multiple pharmaceutical companies, government funding institutes, and regulatory agencies. Inasmuch as several immunotherapy combinations already are supported by sound preclinical data, it is envisioned that clinical translation of these approaches will gain sufficient momentum in the near future.
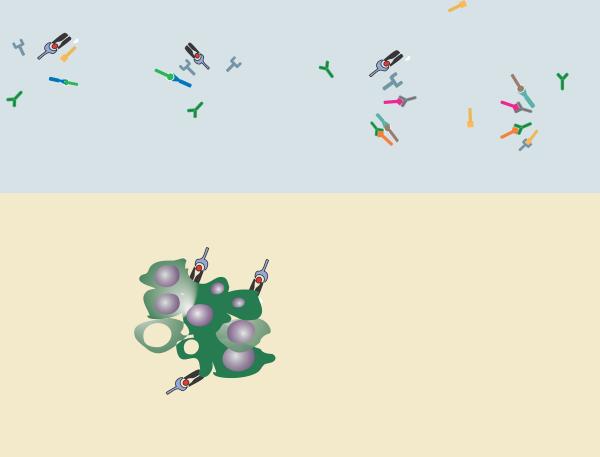
Fig. 1. Schematic of positive costimulatory pathways that could regulate specific stages in an anti-tumor immune response.
Depicted are representations of the priming phase in secondary lymphoid organs, and the effector phase within the tumor microenvironment. Most costimulatory signals can be envisioned to improve aspects of the immune response in both compartments, to improve productive cross-priming by APCs and to help maintain the desired functional properties of effector cell subsets. Specific receptor/ligand interactions are defined in the lower right section.
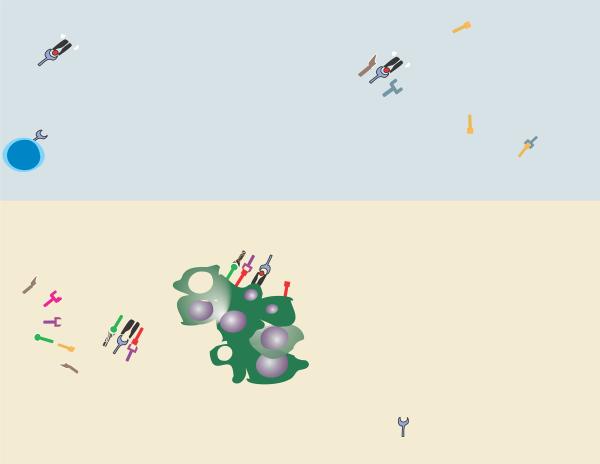
Fig. 2. Schematic of coinhibitory pathways that can be active within tumor-draining lymph nodes and the tumor microenvironment.
Depicted are representations of the priming phase in secondary lymphoid organs, and the effector phase within the tumor microenvironment, along with key coinhibitory pathways that could dampen anti-tumor T-cell responses at each level. Several inhibitory signals, such as PD-L1/PD-1 interactions, can regulate both the effectiveness of tumor antigen cross-priming and the function of effector cells at tumor sites. Not all pathways are necessarily relevant for each subtype of cancer and do not necessarily coexist in all individual tumors. Specific receptor/ligand interactions are defined in the lower right section.
Acknowledgments
Grant support: Work described in this review was supported in part by NIH grants: R01 CA127475, R01 CA118153, R01 CA90575, and P01 CA97296 (T.F.G.), and K23 CA133196 (J.K.). G.D. is a Fellow of the Leukemia and Lymphoma Society
References
- 1.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68(8):2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 2.Staveley-O'Carroll K, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 4.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5(6):677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 5.Radoja S, Saio M, Frey AB. CD8+ tumor-infiltrating lymphocytes are primed for Fas-mediated activation-induced cell death but are not apoptotic in situ. J Immunol. 2001;166(10):6074–6083. doi: 10.4049/jimmunol.166.10.6074. [DOI] [PubMed] [Google Scholar]
- 6.Radoja S, Saio M, Schaer D, Koneru M, Vukmanovic S, Frey AB. CD8(+) tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol. 2001;167(9):5042–5051. doi: 10.4049/jimmunol.167.9.5042. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman GJ, Freedman AS, Segil JM, Lee G, Whitman JF, Nadler LM. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989;143(8):2714–2722. [PubMed] [Google Scholar]
- 10.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damle NK, Linsley PS, Ledbetter JA. Direct helper T cell-induced B cell differentiation involves interaction between T cell antigen CD28 and B cell activation antigen B7. Eur J Immunol. 1991;21(5):1277–1282. doi: 10.1002/eji.1830210527. [DOI] [PubMed] [Google Scholar]
- 13.Gimmi CD, et al. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 16.Azuma M, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 17.Green JM, et al. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1(6):501–508. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 18.Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 19.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 20.Boise LH, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3(1):87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 21.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 22.Song J, Lei FT, Xiong X, Haque R. Intracellular signals of T cell costimulation. Cell Mol Immunol. 2008;5(4):239–247. doi: 10.1038/cmi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 24.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109(6):719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 25.Zha Y, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7(11):1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Zha Y, Gajewski TF. Molecular regulation of T-cell anergy. EMBO Rep. 2008;9(1):50–55. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang F, Gu H. Negative regulation of lymphocyte development and function by the Cbl family of proteins. Immunol Rev. 2008;224:229–238. doi: 10.1111/j.1600-065X.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 28.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 30.Baskar S, Ostrand-Rosenberg S, Nabavi N, Nadler LM, Freeman GJ, Glimcher LH. Constitutive expression of B7 restores immunogenicity of tumor cells expressing truncated major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1993;90(12):5687–5690. doi: 10.1073/pnas.90.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, McGowan P, Hellstrom I, Hellstrom KE, Chen L. Costimulation of tumor-reactive CD4+ and CD8+ T lymphocytes by B7, a natural ligand for CD28, can be used to treat established mouse melanoma. J Immunol. 1994;153(1):421–428. [PubMed] [Google Scholar]
- 32.Chen L, et al. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179(2):523–532. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gajewski TF. B7-1 but not B7-2 efficiently costimulates CD8+ T lymphocytes in the P815 tumor system in vitro. J Immunol. 1996;156(2):465–472. [PubMed] [Google Scholar]
- 34.Fallarino F, Ashikari A, Boon T, Gajewski TF. Antigen-specific regression of established tumors induced by active immunization with irradiated IL-12- but not B7-1-transfected tumor cells. Int Immunol. 1997;9(9):1259–1269. doi: 10.1093/intimm/9.9.1259. [DOI] [PubMed] [Google Scholar]
- 35.Gajewski TF, Fallarino F, Uyttenhove C, Boon T. Tumor rejection requires a CTLA4 ligand provided by the host or expressed on the tumor: superiority of B7-1 over B7-2 for active tumor immunization. J Immunol. 1996;156(8):2909–2917. [PubMed] [Google Scholar]
- 36.Huang AY, Bruce AT, Pardoll DM, Levitsky HI. Does B7-1 expression confer antigen-presenting cell capacity to tumors in vivo? J Exp Med. 1996;183(3):769–776. doi: 10.1084/jem.183.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonia SJ, et al. Phase I trial of a B7-1 (CD80) gene modified autologous tumor cell vaccine in combination with systemic interleukin-2 in patients with metastatic renal cell carcinoma. J Urol. 2002;167(5):1995–2000. [PubMed] [Google Scholar]
- 38.Fishman M, et al. Phase II trial of B7-1 (CD-86) transduced, cultured autologous tumor cell vaccine plus subcutaneous interleukin-2 for treatment of stage IV renal cell carcinoma. J Immunother. 2008;31(1):72–80. doi: 10.1097/CJI.0b013e31815ba792. [DOI] [PubMed] [Google Scholar]
- 39.Raez LE, et al. Allogeneic vaccination with a B7.1 HLA-A gene-modified adenocarcinoma cell line in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22(14):2800–2807. doi: 10.1200/JCO.2004.10.197. [DOI] [PubMed] [Google Scholar]
- 40.Horig H, et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother. 2000;49(9):504–514. doi: 10.1007/s002620000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Mehren M, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6(6):2219–2228. [PubMed] [Google Scholar]
- 42.Kaufman HL, et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res. 2008;14(15):4843–4849. doi: 10.1158/1078-0432.CCR-08-0276. [DOI] [PubMed] [Google Scholar]
- 43.Douillard JY, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 44.Garnett CT, et al. TRICOM vector based cancer vaccines. Curr Pharm Des. 2006;12(3):351–361. doi: 10.2174/138161206775201929. [DOI] [PubMed] [Google Scholar]
- 45.Marshall JL, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23(4):720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 46.Kaufman HL, et al. Local delivery of vaccinia virus expressing multiple costimulatory molecules for the treatment of established tumors. Hum Gene Ther. 2006;17(2):239–244. doi: 10.1089/hum.2006.17.239. [DOI] [PubMed] [Google Scholar]
- 47.Gulley JL, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14(10):3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiPaola RS, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1–5. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaufman HL, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60–70. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufman HL, et al. Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Invest. 2005;115(7):1903–1912. doi: 10.1172/JCI24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13(18 Pt 1):5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 52.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989;86(6):1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pollok KE, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150(3):771–781. [PubMed] [Google Scholar]
- 54.Schwarz H, Valbracht J, Tuckwell J, von Kempis J, Lotz M. ILA, the human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood. 1995;85(4):1043–1052. [PubMed] [Google Scholar]
- 55.Goodwin RG, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23(10):2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 56.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10(6):481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 57.Saoulli K, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187(11):1849–1862. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gramaglia I, Cooper D, Miner KT, Kwon BS, Croft M. Co-stimulation of antigen-specific CD4 T cells by 4-1BB ligand. Eur J Immunol. 2000;30(2):392–402. doi: 10.1002/1521-4141(200002)30:2<392::AID-IMMU392>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 59.Shuford WW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186(1):47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169(9):4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 61.Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176(5):2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 62.Melero I, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3(6):682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 63.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 64.Lynch DH. The promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol Rev. 2008;222:277–286. doi: 10.1111/j.1600-065X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 65.Lee SW, Croft M. 4-1BB as a Therapeutic Target for Human Disease. In: Grewal IS, editor. Therapeutic Targets of the TNF Superfamily. Landes Bioscience; Bothell, Washington, USA: 2009. pp. 120–129. [DOI] [PubMed] [Google Scholar]
- 66.Miller RE, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169(4):1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 67.Uno T, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12(6):693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 68.Xu DP, Sauter BV, Huang TG, Meseck M, Woo SL, Chen SH. The systemic administration of Ig-4-1BB ligand in combination with IL-12 gene transfer eradicates hepatic colon carcinoma. Gene Ther. 2005;12(20):1526–1533. doi: 10.1038/sj.gt.3302556. [DOI] [PubMed] [Google Scholar]
- 69.McMillin DW, Hewes B, Gangadharan B, Archer DR, Mittler RS, Spencer HT. Complete regression of large solid tumors using engineered drug-resistant hematopoietic cells and anti-CD137 immunotherapy. Hum Gene Ther. 2006;17(8):798–806. doi: 10.1089/hum.2006.17.798. [DOI] [PubMed] [Google Scholar]
- 70.Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res. 2006;26(5A):3445–3453. [PubMed] [Google Scholar]
- 71.Sun Y, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002;168(3):1457–1465. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 72.Vinay DS, Cha K, Kwon BS. Dual immunoregulatory pathways of 4-1BB signaling. J Mol Med. 2006;84(9):726–736. doi: 10.1007/s00109-006-0072-2. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, et al. Role of 4-1BB in allograft rejection mediated by CD8+ T cells. Am J Transplant. 2003;3(5):543–551. doi: 10.1034/j.1600-6143.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 74.So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19(3–4):253–262. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sznol MHF, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA) J Clin Oncol. 2008 May 20;26(suppl) abstract 3007. [Google Scholar]
- 76.Paterson DJ, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 77.Stuber E, Neurath M, Calderhead D, Fell HP, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2(5):507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 78.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159(8):3838–3848. [PubMed] [Google Scholar]
- 79.Murata K, et al. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191(2):365–374. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang HC, Klein JR. Multiple levels of activation of murine CD8(+) intraepithelial lymphocytes defined by OX40 (CD134) expression: effects on cell-mediated cytotoxicity, IFN-gamma, and IL-10 regulation. J Immunol. 2001;167(12):6717–6723. doi: 10.4049/jimmunol.167.12.6717. [DOI] [PubMed] [Google Scholar]
- 81.Imura A, et al. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183(5):2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15(3):445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 83.Walker LS, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190(8):1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14(3–4):265–273. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 85.Vu MD, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179(3):1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 87.Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6(4):528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 88.Morris NP, et al. Development and characterization of recombinant human Fc:OX40L fusion protein linked via a coiled-coil trimerization domain. Mol Immunol. 2007;44(12):3112–121. doi: 10.1016/j.molimm.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harrop JA, et al. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol. 1998;161(4):1786–1794. [PubMed] [Google Scholar]
- 90.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195(12):1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwon BS, et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272(22):14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 92.Shaikh RB, et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001;167(11):6330–6337. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 93.Wang J, et al. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest. 2001;108(12):1771–1780. doi: 10.1172/JCI13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee Y, et al. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity. 2006;25(3):499–509. doi: 10.1016/j.immuni.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 95.Tamada K, et al. Cutting edge: selective impairment of CD8+ T cell function in mice lacking the TNF superfamily member LIGHT. J Immunol. 2002;168(10):4832–5. doi: 10.4049/jimmunol.168.10.4832. [DOI] [PubMed] [Google Scholar]
- 96.Tamada K, et al. Blockade of LIGHT/LTbeta and CD40 signaling induces allospecific T cell anergy, preventing graft-versus-host disease. J Clin Invest. 2002;109(4):549–557. doi: 10.1172/JCI13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tamada K, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6(3):283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 98.Yu P, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5(2):141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 99.Fan Z, et al. NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors. Blood. 2006;107(4):1342–1351. doi: 10.1182/blood-2005-08-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu P, et al. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol. 2007;179(3):1960–1968. doi: 10.4049/jimmunol.179.3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daud AI, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McHugh RS, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16(2):311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 103.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29(6):934–46. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 104.Liu X, et al. B7H costimulates clonal expansion of, and cognate destruction of tumor cells by, CD8(+) T lymphocytes in vivo. J Exp Med. 2001;194(9):1339–1348. doi: 10.1084/jem.194.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wallin JJ, Liang L, Bakardjiev A, Sha WC. Enhancement of CD8+ T cell responses by ICOS/B7h costimulation. J Immunol. 2001;167(1):132–139. doi: 10.4049/jimmunol.167.1.132. [DOI] [PubMed] [Google Scholar]
- 106.Ara G, et al. Potent activity of soluble B7RP-1-Fc in therapy of murine tumors in syngeneic hosts. Int J Cancer. 2003;103(4):501–507. doi: 10.1002/ijc.10831. [DOI] [PubMed] [Google Scholar]
- 107.Couderc B, et al. Enhancement of antitumor immunity by expression of CD70 (CD27 ligand) or CD154 (CD40 ligand) costimulatory molecules in tumor cells. Cancer Gene Ther. 1998;5(3):163–175. [PubMed] [Google Scholar]
- 108.Nieland JD, Graus YF, Dortmans YE, Kremers BL, Kruisbeek AM. CD40 and CD70 co-stimulate a potent in vivo antitumor T cell response. J Immunother. 1998;21(3):225–236. doi: 10.1097/00002371-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 109.Lorenz MG, Kantor JA, Schlom J, Hodge JW. Anti-tumor immunity elicited by a recombinant vaccinia virus expressing CD70 (CD27L) Hum Gene Ther. 1999;10(7):1095–1103. doi: 10.1089/10430349950018094. [DOI] [PubMed] [Google Scholar]
- 110.Cohen AD, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66(9):4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ko K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202(7):885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou P, L'Italien L, Hodges D, Schebye XM. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J Immunol. 2007;179(11):7365–7375. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
- 113.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 114.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 115.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 116.Mackey MF, et al. Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997;57(13):2569–2574. [PubMed] [Google Scholar]
- 117.Kikuchi T, Crystal RG. Anti-tumor immunity induced by in vivo adenovirus vector-mediated expression of CD40 ligand in tumor cells. Hum Gene Ther. 1999;10(8):1375–1387. doi: 10.1089/10430349950018049. [DOI] [PubMed] [Google Scholar]
- 118.Vesosky B, Hurwitz AA. Modulation of costimulation to enhance tumor immunity. Cancer Immunol Immunother. 2003;52(11):663–669. doi: 10.1007/s00262-003-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Geldart T, Illidge T. Anti-CD 40 monoclonal antibody. Leuk Lymphoma. 2005;46(8):1105–13. doi: 10.1080/10428190500085255. [DOI] [PubMed] [Google Scholar]
- 120.Sotomayor EM, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5(7):780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 121.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5(5):548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 122.Vonderheide RH, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 123.Vonderheide RH, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19(13):3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 124.Dessureault S, et al. A phase-I trial using a universal GM-CSF-producing and CD40L-expressing bystander cell line (GM.CD40L) in the formulation of autologous tumor cell-based vaccines for cancer patients with stage IV disease. Ann Surg Oncol. 2007;14(2):869–884. doi: 10.1245/s10434-006-9196-4. [DOI] [PubMed] [Google Scholar]
- 125.Brunet JF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328(6127):267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 126.Dariavach P, Mattei MG, Golstein P, Lefranc MP. Human Ig superfamily CTLA-4 gene: chromosomal localization and identity of protein sequence between murine and human CTLA-4 cytoplasmic domains. Eur J Immunol. 1988;18(12):1901–1905. doi: 10.1002/eji.1830181206. [DOI] [PubMed] [Google Scholar]
- 127.Iida T, et al. Regulation of cell surface expression of CTLA-4 by secretion of CTLA-4-containing lysosomes upon activation of CD4+ T cells. J Immunol. 2000;165(9):5062–5068. doi: 10.4049/jimmunol.165.9.5062. [DOI] [PubMed] [Google Scholar]
- 128.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 130.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Linsley PS, et al. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176(6):1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kearney ER, et al. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155(3):1032–1036. [PubMed] [Google Scholar]
- 133.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 134.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 135.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7(6):885–895. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 136.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 137.Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126(2):375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 138.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 139.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 140.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 141.Kwon ED, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94(15):8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang YF, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57(18):4036–4041. [PubMed] [Google Scholar]
- 143.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95(17):10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11(4):483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 145.Sotomayor EM, Borrello I, Tubb E, Allison JP, Levitsky HI. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A. 1999;96(20):11476–11481. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Elsas A, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194(4):481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sutmuller RP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maker AV, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29(4):455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sanderson K, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23(4):741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 153.Weber JS, et al. The efficacy and safety of ipilimumab (MDX-010) in patients with unresectable stage III or stage IV malignant melanoma. Journal of Clinical Oncology. 2007;25:8523. [Google Scholar]
- 154.Hamid O, et al. Dose effect of ipilimumab in patients with advanced melanoma: results from a phase II, randomized, dose-ranging study. Journal of Clinical Oncology. 2008;26(489s) [Google Scholar]
- 155.Ribas A, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23(35):8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 156.Ribas A, et al. Phase 1 trial of monthly doses of the human anti-CTLA4 monoclonal antibody CP--675,206 in patients with advanced malignancies. Journal of Clinical Oncology. 2005;23(716s) [Google Scholar]
- 157.Ribas A, et al. Results of a phase II clinical trial of 2 doses and schedules of CP-675,206, an anti-CTLA4 monoclonal antibody, in patients (pts) with advanced melanoma. Journal of Clinical Oncology. 2007;25(118s) [Google Scholar]
- 158.Ribas A, et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. Journal of Clinical Oncology. 2008;26(486s) [Google Scholar]
- 159.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J. 1992;11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–72. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 161.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 162.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 164.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 165.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 166.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169(7):3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 168.Muhlbauer M, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45(4):520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 169.Strome SE, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63(19):6501–6505. [PubMed] [Google Scholar]
- 170.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 171.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13(18 Pt 1):5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 172.Thompson RH, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101(49):17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hamanishi J, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blank C, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 175.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 177.Blank C, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119(2):317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 178.Hirano F, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–1096. [PubMed] [Google Scholar]
- 179.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 180.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 181.Brahmer JR. Safety and activity of MDX-1106 (ONO-4538), an anti-PD-1 monoclonal antibody, in patients with selected refractory or relapsed malignancies. Proceedings of the American Society of Clinical Oncology; 2008 May 30–Jun 3; Chicago, IL. 2008. [Google Scholar]
- 182.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 183.Tseng SY, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193(7):839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197(9):1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shin T, et al. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198(1):31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shin T, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201(10):1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18(6):863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 188.Sica GL, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 189.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100(18):10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Suh WK, et al. Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol Cell Biol. 2006;26(17):6403–6411. doi: 10.1128/MCB.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Watanabe N, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 192.Choi IH, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171(9):4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 193.Simon I, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66(3):1570–1575. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 194.Krambeck AE, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kryczek I, et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177(1):40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 197.Kryczek I, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67(18):8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 198.Triebel F, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171(5):1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Baixeras E, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176(2):327–337. doi: 10.1084/jem.176.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32(8):2255–2263. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 201.Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169(10):5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 202.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161(8):4058–4065. [PubMed] [Google Scholar]
- 203.Cappello P, et al. LAG-3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER-2/neu transgenic BALB/c mice. Cancer Res. 2003;63(10):2518–2525. [PubMed] [Google Scholar]
- 204.Casati C, et al. Soluble human LAG-3 molecule amplifies the in vitro generation of type 1 tumor-specific immunity. Cancer Res. 2006;66(8):4450–4460. doi: 10.1158/0008-5472.CAN-05-2728. [DOI] [PubMed] [Google Scholar]
- 205.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174(2):688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 206.Grosso JF, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117(11):3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 208.Gandhi MK, et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108(7):2280–2289. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 209.Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55(10):1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006;177(7):4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 211.Kline J, et al. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14(10):3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]