Abstract
Individuals who use heroin and illicit opioids are at high risk for infection with human immunodeficiency virus (HIV) and other blood-borne pathogens, as well as incarceration. The purpose of the randomized trial reported here is to compare outcomes between participants who initiated methadone maintenance treatment (MMT) prior to release from incarceration, with those who were referred to treatment at the time of release. Participants who initiated MMT prior to release were significantly more likely to enter treatment postrelease (P < .001) and for participants who did enter treatment, those who received MMT prerelease did so within fewer days (P = .03). They also reported less heroin use (P = .008), other opiate use (P = .09), and injection drug use (P = .06) at 6 months. Initiating MMT in the weeks prior to release from incarceration is a feasible and effective way to improve MMT access postrelease and to decrease relapse to opioid use.
Keywords: HIV prevention, incarceration, medication-assisted treatment, methadone
INTRODUCTION
With nearly 2.3 million people housed in prisons and jails and another 7 to 10 million people arrested and released from correctional facilities each year, the United States has the highest incarceration rate in the world (1, 2). The tremendous surge in the prison and jail population over the past 3 decades is primarily the result of the US government’s “war on drugs.” Law enforcement efforts to control drug use have resulted in a 3-fold increase in drug-related arrests over this period (3–5). More than 200,000 heroin users, most of whom are injection drug users (IDUs), pass through a jail or prison each year (6), while almost 20% of state correctional facility inmates are IDUs (5). Incarcerated populations, especially IDUs, have a disproportionately higher burden of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), hepatitis B (HBV) and C (HCV) viruses, and tuberculosis, as well as sexually transmitted diseases (STDs) and mental illness than the general population (4–7). Fifty-five percent of former prisoners relapse to drug use within 1 month of release from incarceration (8). Return to drug use is typically accompanied by increased criminal activity and recidivism (9, 10), disproportionately high risk of HIV infection (11), and markedly increased risk for death from drug overdose (12). Former inmates who inject drugs are even more likely to return to prison: 57% of IDUs have been incarcerated 5 or more times, compared with only 26% of non-IDUs (13).
Federal, state, and local resources to address heroin and other drug use have focused on the criminal justice system using a custody and control approach, rather than substance use treatment. As a result, individuals at high risk for HIV infection continually pass through incarcerated settings, thus providing public health opportunities for preventive interventions (14–18).
Methadone has been used for over 50 years to treat opiate addiction and has been shown to reduce heroin use and imprisonment rates (19, 20), mortality (21), and HIV infection (22). Previous studies among male inmates have demonstrated that offering methadone prior to release increases rates of entry to community methadone providers and reduces heroin use (23–28). Higher methadone doses are associated with improved reporting to community methadone treatment programs after discharge (29). In addition, successful linkage of correctional-based medication-assisted treatment (MAT) programs to community providers has been shown to decrease mortality, reincarceration, and hepatitis C infection (30).
Methadone maintenance treatment (MMT) prevents withdrawal symptoms and drug cravings, blocks the euphoric effects of other opiates, and prevents relapse to illicit use of opiates (31–33). The primary goal of MMT is to eliminate heroin use by stabilizing dependent persons on long-term treatment programs. MMT is highly effective in reducing heroin use (34–36), mortality, criminality, and recidivism (30), and is far less expensive than incarceration (37). Furthermore, many people undergoing MMT are able to secure employment and pay for their treatment themselves (33).
Just over half of US prison systems offer any methadone and those that do only offer it to very few prisoners under limited circumstances (6, 16). The lack of medication-assisted treatment is often due to the preference for drug-free detoxification, security concerns, logistical barriers due to tight federal regulation of methadone, and philosophical opposition to MAT among staff at multiple levels (16). Even among correctional systems that do provide MAT, linkage to aftercare postrelease remains a challenge and many participants report financial barriers and difficulty negotiating community placement in treatment (38). However, programs that link individuals directly with treatment and provide funding for treatment eliminate many of the problems facing released inmates. To determine whether initiating MMT prior to release from incarceration is an effective strategy for reducing drug use, increasing drug treatment retention, and reducing relapse to injection drug use, we conducted a randomized trial examining methadone initiation prior to release as compared to referral and/or linkage to methadone treatment programs postrelease at the Rhode Island Department of Corrections (RIDOC) (17). We report here 6-month follow-up data.
METHODS
Setting
The Rhode Island Department of Corrections (RIDOC) is located on a single campus and functions as both a jail and a prison for the entire state, with an average census of 4000 inmates and nearly 20,000 admissions per year. The current RIDOC methadone protocol maintains individuals who are arrested and incarcerated while on MMT on their confirmed community dosage for 1 week and then reduces their dose by 2 mg per day. Most people who are released within 30 days can resume treatment at their community-based methadone program immediately. Depending upon how long they have been incarcerated, their dose at release could be very low or zero. RIDOC guidelines stipulate that all inmates on methadone for addiction be detoxified unless the inmate is pregnant or under special circumstances at the discretion of the correctional physician. Therefore, only the male and female intake (or pretrial) facilities are set up to provide methadone administration (2 out of 8 facilities on campus). All methadone at RIDOC is coordinated through the CODAC program, which is the largest, oldest, and only nonprofit provider of methadone treatment services in Rhode Island.
Study Design
The study is a 3-arm randomized controlled trial with 12-month follow-up. The research was developed based upon our experience conducting a Substance Abuse and Mental Health Service Administration (SAMHSA) funded 5-year initiative. The 3 study arms include Arm 1—initiation of methadone prerelease with continued treatment in the inmates’ methadone program of choice and short-term (12 weeks full and 12 weeks half) payment of treatment costs; Arm 2—referral to the participants’ methadone program of choice upon release from incarceration with provision of the same short-term financial assistance; Arm 3—referral to the participants’ methadone program of choice upon release from incarceration without financial assistance. The SAMHSA intervention provided linkage to MMT postrelease and financial support for up to 6 months to offset the costs of MMT for participants in Arms 1 and 2 (39). All 3 arms received HIV risk reduction and overdose prevention counseling and assistance with linkage to the methadone program of their choice at the time of release. The counseling session was conducted prior to release from incarceration during the same session that arrangements were made for the first clinic appointment postrelease. Details involved with arranging the first clinic appointment postrelease included ongoing communication with the clinic, forwarding notice of payment (Arms 1 and 2), assisting with documentation such as ID and social security card, and arranging transportation to the first clinic visit.
Eligible participants were (1) currently incarcerated at the RIDOC with a scheduled release date at least 28 days after enrollment; (2) serving a sentence of less than 2 years at the time of enrollment; (3) heroin dependent with self-reported heroin injection in the month preceding incarceration or enrolled in a methadone treatment program for heroin addiction with a history of injection drug use just prior to incarceration; (4) desire to enter methadone treatment upon release and plan to secure funding for methadone treatment after the temporary payment for methadone runs out; (5) experienced with at least 1 MMT episode prior to incarceration and tolerated methadone in the past; (6) experienced with at least 1 drug-related incarceration; (7) willing to be randomized and to conduct follow-up interviews for up to 24 months; (8) able to provide the name of at least 2 verifiable locator persons; (9) English-or Spanish-speaking; (10) planning to remain in Rhode Island for 24 months; (11) able to give informed consent; (12) age 18 years or older; and (13) not housed in a segregation unit within the prison.
Exclusion criteria were not fulfilling all of the inclusion criteria and the following: (1) currently receiving methadone at the RIDOC; (2) currently undergoing a detoxification protocol from illicit opiates at the RIDOC. This exclusion was in place due to concern that undergoing acute withdrawal from illicit opiates may interfere with the ability of the participant to give voluntary and informed consent and thus increase the potential for coercion.
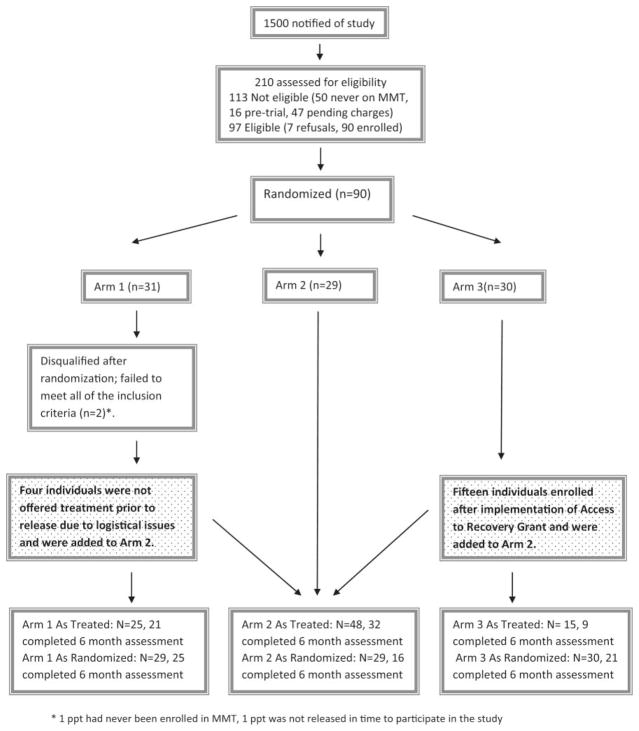
Individuals were recruited through referral from discharge planning staff and self-referral through word of mouth. Additionally, in 3 facilities we out reached to all inmates within 30 days of release. Casting a wide net was necessary because there was no reliable means of identifying opiate users. In this manner we spoke individually with over 1500 inmates during recruitment, and identified 168 who were eligible and interested. The most common reason for ineligibility was no prior MAT experience. Eligible participants were randomized to 1 of the 3 study arms using a computer generated random permutation. Urn randomization procedures were used to stratify individuals based on gender and race. We conducted both intent-to-treat and as-treated analyses.
Figure 1 outlines enrollment and randomization of participants. The figure also outlines arm crossovers during the course of study implementation. Although we performed both intent-to-treat and as-treated analyses, given the significant unintentional crossover between arms, as described below, we have chosen to focus this article on the as-treated analysis, as it gives a more accurate description of the study outcomes.
FIGURE 1.
As-treated analysis.
* 1 ppt had never been enrolled in MMT, 1 ppt was not released in time to participate in the study
All aspects of the study were approved by the Miriam Hospital Institutional Review Board, the RIDOC Medical Research Advisory Group (MRAG) and the federal Office of Human Research Protection. This trial was registered with ClinicTrials.gov (no. NCT00142935).
Data Collection
The Addiction Severity Index (ASI; Version 5) was administered at baseline, 2 weeks postrelease and at 6- and 12-month follow-ups. The ASI includes the following domains: medical status, employment and support, drug use, alcohol use, legal status, family and social status, and psychiatric status. Data regarding methadone treatment access and retention were verified through methadone clinic chart review. Reincarceration data were verified through review of a public access RIDOC database.
Change in Environment During the Study
During implementation of this research, the state of Rhode Island was awarded a federal Access to Recovery (ATR) grant from SAMHSA. This grant provided treatment and recovery support services to individuals in several priority populations, including individuals being released from the RIDOC. The ATR grant provided financial assistance (up to 6 months) for any treatment modality, including MMT. Any individual who was randomized to Arm 3 (referral to treatment postrelease without financial assistance), thus became eligible to participate in the state’s ATR grant program and receive 6 months of treatment for MMT. At the time of ATR’s implementation (July 2008), we had already enrolled 15 Arm 3 participants. The next 15 participants enrolled in Arm 3 were offered ATR financial assistance. Thus 15 participants in Arm 3 received the identical intervention component as participants in Arm 2: linkage to MMT postrelease with up to 6 months of financial assistance, effectively eliminating the intended difference between Arms 2 and 3 after ATR implementation.
Analysis
We completed 6-month assessments with 62 participants. Table 1 outlines the demographic and descriptive analyses for these participants. To examine differences between the groups with respect to outcomes of interest at the 6-month time point, we conducted contingency table analyses, using the Fischer’s exact chi-square test, and means testing using t test Wilcoxon rank-sum tests.
TABLE 1.
Baseline Characteristics of Study Participants Who Completed 6 Months of Follow-Up (n = 62)
| Baseline characteristics | n (%) |
|---|---|
| Mean age (SD) [range] | 40.7 [22–58] |
| Gender | |
| Male | 44 (71.0) |
| Female | 18 (29.0) |
| Ethnicity | |
| Hispanic/Latino | 13 (21.0) |
| Non-Hispanic/Latino | 49 (79.0) |
| Race | |
| White | 45 (72.5) |
| Other | 4 (6.4) |
| Not Reported | 13 (20.9) |
| Usual employment | |
| Full/part time past 3 years | 17 (27.4) |
| Retired/disability | 21 (33.9) |
| Unemployed | 13 (21.0) |
| Incarcerated | 11 (17.7) |
| Highest level of education completed | |
| Less than High School | 26 (41.9) |
| High School equivalent or higher | 36 (58.1) |
| Heroin, median years use [range] | 10 [0–37] |
| Past 30 day drug use prior to incarceration | |
| Heroin | 46 (74.2) |
| Other opiates (not prescribed by a physician) | 19 (30.7) |
| Cocaine/Crack | 38 (61.3) |
| Marijuana | 17 (27.4) |
| ETOH to intoxication | 22 (35.5) |
| Polydrug | 44 (71.0) |
| Ever overdosed | 43 (69.4) |
| Times in methadone maintenance program | |
| 1–2 | 35 (56.4) |
| 3–5 | 20 (32.3) |
| >5 | 6 (9.7) |
| Missing | 1 (1.6) |
| Any injection drug use in the 30 days prior to incarceration | 40 (64.5) |
| Median [range] times injected 30 days prior to incarceration | 50 [0–300] |
| In 30 days prior to incarceration: | |
| Engaged in sexual activity | 50 (80.7) |
| Median [range] number of sexual partners | 1 [0–99] |
| Self-reported health status | |
| HIV positive | 1 (1.6) |
| HBV positive | 15 (24.2) |
| HCV positive | 42 (67.7) |
| Ever prescribed medication for psychiatric/emotional problem | 38 (61.3) |
| Incarceration history | |
| Median months of ever being incarcerated [range] | 75 [2–96] |
Intent-to-Treat
We initially performed an intent-to-treat-analysis and briefly review these findings in the Results section and Table 3. To further examine the impact of the intervention as participants experienced it, we performed as-treated analyses. The 6-month outcomes presented in Table 2 are from the as-treated analyses.
TABLE 3.
Outcomes, Intent-to-Treat: Overdose, Relapse to Drug Use, Incarceration History—6-Month Follow-Up (n = 62)
| Variable | Randomization
|
Test statistic P value |
||
|---|---|---|---|---|
| Arm 1 (n = 25) n (%) |
Arm 2 (n = 16) n (%) |
Arm 3 (n = 219) n (%) |
||
| MMT Tx within 30 days postrelease | 20 (80.0) | 9 (56.3) | 4 (19.1) | <.001 |
| Mean days (SD) to first clinic visit postrelease [range] | 2 (4.7) [0 to 21] |
10.7 (10.0) [0 to 28] |
6.5 (6.0) [0 to 13] |
.01 |
| Drug use, past 30 days | ||||
| Heroin | 6 (24.0) | 8 (50.0) | 11 (52.4) | .09 |
| Other opiates | 0 | 4 (25.0) | 3 (14.3) | .02 |
| Crack/Cocaine | 6 (24.0) | 6 (37.5) | 11 (52.4) | .14 |
| ETOH to intoxication | 3 (12.0) | 5 (31.3) | 0 | .01 |
| Marijuana | 2 (8.0) | 5 (31.3) | 4 (19.1) | .15 |
| Polydrug | 10 (40.0) | 8 (50.0) | 12 (57.1) | .53 |
| Any drug injecting, past 30 days | 3 (12.0) | 6 (37.5) | 8 (38.1) | .09 |
| Currently on MMT | 17 (68.0) | 8 (50.0) | 8 (38.1) | .14 |
| Other treatment in past 6 months (not MMT) | ||||
| Outpatient | 3 (12.0) | 2 (12.5) | 3 (14.3) | 1.0 |
| Residential/Inpatient | 1 (4.2) | 2 (12.5) | 4 (19.1) | .31 |
| Self-help group | 11 (44.0) | 5 (31.3) | 5 (23.8) | .37 |
| Prison/Jail/Other | 2 (8.0) | 0 | 4 (19.1) | .18 |
| Insurance status | ||||
| None | 18 (72.0) | 10 (62.5) | 15 (71.4) | .83 |
| Medicaid/Medicare or equivalent | 7 (28.0) | 6 (37.5) | 6 (28.6) | |
| Other | 0 | 0 | 0 | |
| Nonfatal overdose, past 6 months | 3 (12.0) | 3 (18.8) | 2 (9.5) | .80 |
| Fatal overdose, past 6 months | 0 | 1 (3.1) | 1 (11.1) | NA |
| Arrested in past 6 months | 9 (36.0) | 3 (18.8) | 6 (28.6) | .53 |
| Median months incarcerated, past 6 months [range] | 0 [0–4] | 0 [0–97] | 0 [0–90] | .86 |
TABLE 2.
Outcomes, As-Treated: Overdose, Relapse to Drug Use, Incarceration History—6-Month Follow-Up (n = 62)
| Variable | Randomization
|
Test statistic P value |
||
|---|---|---|---|---|
| Arm 1 (n = 21) n (%) |
Arm 2 (n = 32) n (%) |
Arm 3 (n = 9) n (%) |
||
| MMT Tx within 30 days postrelease | 18 (85.7) | 13 (40.6) | 2 (22.2) | <.001 |
| Mean days (SD) to first clinic visit postrelease [range] | 1.9 (5.0) [−3 to 21] |
9 (9.0) [0 to 28] |
5 (7.1) [0 to 10] |
.03 |
| Drug use, past 30 days | ||||
| Heroin | 3 (14.3) | 18 (56.3) | 4 (44.4) | .008 |
| Other opiates | 0 | 6 (18.8) | 1 (11.1) | .09 |
| Crack/Cocaine | 4 (19.1) | 13 (40.6) | 6 (66.7) | .05 |
| ETOH to intoxication | 3 (14.3) | 5 (15.6) | 0 | .68 |
| Marijuana | 2 (9.5) | 8 (25.0) | 1 (11.1) | .40 |
| Polydrug | 8 (38.1) | 17 (53.1) | 5 (55.6) | .57 |
| Any drug injecting, past 30 days | 2 (9.5) | 12 (37.5) | 3 (33.3) | .06 |
| Currently on MMT | 14 (66.7) | 15 (46.9) | 4 (44.4) | .35 |
| Other treatment in past 6 months (not MMT) | ||||
| Outpatient | 3 (14.3) | 5 (15.6) | 0 | .68 |
| Residential/Inpatient | 1 (5.0) | 5 (15.6) | 1 (11.1) | .56 |
| Self-help group | 9 (42.9) | 9 (28.1) | 3 (33.3) | .58 |
| Prison/Jail/Other | 2 (9.5) | 2 (6.3) | 2 (22.2) | .34 |
| Insurance status | .18 | |||
| None | 16 (76.2) | 19 (59.4) | 8 (88.9) | |
| Medicaid/Medicare or equivalent | 5 (23.8) | 13 (40.6) | 1 (11.1) | |
| Other | 0 | 0 | 0 | |
| Nonfatal overdose, past 6 months | 3 (14.3) | 4 (12.5) | 1 (11.1) | 1.0 |
| Fatal overdose, past 6 months | 0 | 1 (3.1) | 1 (11.1) | NA |
| Arrested in past 6 months | 7 (33.3) | 9 (28.1) | 2 (22.2) | .86 |
| Median months incarcerated, past 6 months [range] | 0 [0–4] | 0 [0–6] | 0 [0–6] | .78 |
Note. Arm 1: original (minus those not offered prerelease MMT). Arm 2: original + Arm 3 ATR + Arm 1 ppts not offered prerelease MMT. Arm 3: original (minus those offered ATR).
As-Treated
As noted in Figure 1, some Arm 1 participants (4 of 25) were not offered MMT prior to release due to reasons beyond the control of the participant. However, all 4 were offered MMT upon release from incarceration with payment assistance. For the purposes of an as-treated analysis, these 4 participants crossed over from Arm 1 to Arm 2. All Arm 2 participants were offered MMT upon release with payment assistance. Of the 30 participants randomized to Arm 3, 15 were offered MMT upon release with payment assistance (through ATR; see results) and therefore crossed over from Arm 3 to Arm 2. As depicted in Figure 1, participants with 6-month follow-up data in the as-treated arms were as follows: Arm 1, n = 21; Arm 2, n = 32; Arm 3, n = 9 (Table 2). All statistical tests were conducted in STATA version 10 (College Station, TX) at the α = .05 level.
RESULTS
Participant Characteristics
Between October 2006 and February 2009, 90 participants were enrolled and randomized to 1 of the 3 study arms. Assessments at 6 months were completed on 62 participants (70% follow-up). The study population was predominantly white males with an average age of 41 years; most had not completed high school, were never married, and had no insurance or publicly funded health insurance upon their release (Table 1). No significant differences were found in participant characteristics among the 3 groups in either the intent-to-treat or the as-treated analyses.
Methadone Initiation During Incarceration
Patients who received methadone while incarcerated were begun on 5 mg the first day and increased by 2 mg daily until reaching their individualized target dose or until they were released. Of the 22 Arm 1 participants who were dosed with methadone before release, the average number of days participants received doses was 15 (range: 1–30 days), with an average dosage prior to release of 33 mg (range: 5–38 mg).
One participant began methadone treatment before release, but was discontinued due to possible adverse reactions with psychiatric medications. This participant did not resume methadone treatment while incarcerated nor again during the course of the study.
Accessing MMT Postrelease and Time Until Entering Treatment
In both analyses (intent-to treat and as-treated; Tables 2 and 3), of those who entered treatment in the community, Arm 1 participants initiated MMT treatment within 30 days postrelease at significantly higher rates than participants in either Arm 2 or 3. In the as-treated analysis, 86% of Arm 1 participants entered treatment within 30 days as compared to 41% of Arm 2 participants and 22% of Arm 3 participants (P value < .001; Table 2). Likewise, in both analyses, Arm 1 participants entered treatment within a shorter period of time. In the as-treated analysis, Arm 1 participants entered treatment within 2 days; Arm 2 and Arm 3 participants entered MMT within 9 and 5 days, respectively (P value = .03; Table 2).
Opiate Overdose Postrelease
Two individuals died within days of release as a result of acute opiate intoxication. Neither received methadone prior to release or had yet attended a methadone clinic postrelease. Eight individuals reported nonfatal overdoses. These occurred equally across the 3 arms. Five of the 8 participants reporting a nonfatal overdose did not enroll in community methadone treatment postrelease or at any point in the following 6 months.
Relapse to Drug Use and Incarceration at 6-Month Follow-Up
There were statistically significant differences in short-term (prior 30-day) drug use for heroin and cocaine use (Table 2) across treatment arms. Only 14% of participants who were offered MMT prerelease relapsed to heroin use at the 6-month follow-up (Arm 1) compared to 56% of participants referred to MMT postrelease with payment assistance (Arm 2) and to 44% of participants with MMT referral only (P = .008). Also at 6 months, statistically significantly fewer participants reported cocaine use in Arm 1 (19%) compared to Arm 2 (41%) and Arm 3 (67%), respectively (P value = .05). Though only approaching statistical significance, fewer Arm 1 participants (10%) reported any injection drug use in the prior 30 days compared to Arm 2 (38%) and Arm 3 (33%) (P value = .06).
Whereas the proportion of participants reporting heroin use was also significantly lower among Arm 1 participants in the intent-to-treat analysis, differences in cocaine use did not reach significance. In both analyses, participants in Arm 1 reported a trend of less overall substance use than participants in the other 2 arms. We did not observe any statistically significant differences between study arms with respect to arrest or history of incarceration at 6-month follow-up.
DISCUSSION
Despite the decades long American “war on drugs,” and the resulting dramatic increase in incarceration of people with opiate addictions, and over 50 years of research supporting the effectiveness of methadone in reducing heroin use and imprisonment (19), there is scarce utilization of methadone for correctional populations (28). The time up to enrollment in community-based MMT, after release from incarceration, is critically important given the high risk for relapse, crime, disease transmission, and overdose in the period immediately after release from incarceration (8–10, 40, 41). A major finding of this study is that offering prerelease MMT initiation and payment assistance postrelease was significantly associated with increased enrollment in postrelease MMT and reduced time to enter community-based MMT. Further, offering prerelease MMT initiation and payment assistance postrelease was significantly associated with reduced heroin use at 6-month follow-up as compared to the other 2 study arms. These findings support evidence from the Key Extended Entry Program at the Rikers Island jail in New York City, where in-jail MMT initiation has been associated with higher retention rates in community-based treatment at 6 months after release (27).
The findings from this study complement those from a recently completed randomized trial conducted by Kinlock et al. in Baltimore, which examined differences in pre- and postrelease initiation of MMT among incarcerated men. Both studies found that methadone initiation prior to release from incarceration significantly increased the likelihood of entering community-based treatment centers and decreased the time to treatment postrelease (23, 25, 26). In the Baltimore trial, participants who received methadone prerelease were more likely to remain in drug treatment programs (23, 24, 28) at 3-, 6-, and 12-month follow-up. In our research findings, although more participants who were offered MMT prerelease were enrolled in MMT at 6 months (68%) compared to Arm 2 (47%) and Arm 3 (44%), this difference did not reach statistical significance. In contrast with the Baltimore study, our study was limited to only inmates with previous MMT experience. Also, this study included men and women, whereas the Baltimore study recruited only men.
Another important difference between the Baltimore and Providence studies is the length of dosing prior to release from incarceration. The Baltimore study initiated MMT dosing 3 months prior to release. On average, our study participants received only 15 days of methadone prior to release, with an average dose of 33 mg at release. Initiating MMT requires a conservative approach with gradual increase of dosing and close monitoring of patients due to the loss of opiate tolerance that typically occurs during incarceration. Initiating and continuing methadone for induction at dosages typically used when enrolling active opioid user’s risks toxicity and even overdose. We reviewed clinic attendance data for all Arm 1 participants who dosed prior to release (n = 22) and despite the short period of dosing, and the relatively low dose prerelease, 21 inmates successfully linked to community based MMT postrelease. This suggests that benefits of inducting MMT prior to release may not require many months of gradual dose escalation, or even the attainment of an opioid-blocking dose.
Because Northeastern states have higher rates of opioid addiction, corrections departments in those states may be the best candidates for implementing prerelease MMT programs. Prerelease MMT initiation may prove challenging for many reasons, including uncertain inmate release dates, facility licensing, dosing schedules, averting medication diversion, arranging follow-up in the community after release, and program costs. It is important to note that MMT is most effective when it is part of a treatment plan that also includes counseling and supportive social services. Cost to the individual is a significant barrier to retention in postrelease MMT. However, when the cost of MMT is compared with the direct and indirect costs of heroin addiction, estimated to be at $21.9 million in the United States (42), it is minimal. Overall, MMT, at approximately $90 per week or $4680 per patient per year, is a cost-effective component of an opiate addiction treatment approach for incarcerated populations as they transition back to the community. Importantly, many people with addiction have underlying mental health problems that are best treated outside the jail/prison environment.
This study is subject to several limitations. Illicit drug use outcomes were self-reported without confirmation through urine testing, so there is the possibility of our data being subjected to social desirability bias. For analysis purposes, half of Arm 3 participants were merged with Arm 2. The “standard of care” essentially changed mid-study, such that inmates eligible for this research were also eligible for receiving substance use treatment through federal ATR funding. Secondly, 4 participants randomized to Arm 1 never received methadone before they were released due to logistical reasons. In addition, overall enrollment was relatively low, 90 participants were randomized (31 to Arm 1, 29 to Arm 2, and 30 to Arm 3). We have reported previously the challenges of participant recruitment within corrections and the barriers to initiating MMT prerelease in the correctional setting (17). It is important to note that we restricted participation to individuals with prior experience with MMT. In hindsight, this criterion was overly conservative and should not be a requirement of future studies given the dramatic benefits seen in our study and others (28) with initiating methadone prior to release. Another limitation is 70% follow-up rate of participants at 6 months. To mitigate this limitation, we performed an analysis of demographic characteristics stratified by follow-up status and did not observe any statistically significant differences. Despite these limitations, we found statistically significant differences in the key outcomes: postrelease treatment initiation, time to treatment initiation, and heroin use at follow-up.
CONCLUSIONS
Our study demonstrates that initiating MMT in the weeks prior to release from incarceration is feasible and an effective way to improve MMT access postrelease. Both initiating methadone prior to release, even with the relatively short duration and low dose of methadone, and ensuring a means of payment for methadone after release were beneficial. These findings have important implications for policy makers, correctional administrators, politicians, and drug treatment programs that serve individuals with opiate dependence and/or addiction, namely that ways to overcome obstacles to initiation of methadone during and after reentry should be explored aggressively.
Acknowledgments
This article was supported by the Lifespan/Tufts/Brown Center for AIDS Research (grant P30AI42853), as well as grants K24DA022112, P30DA013868, R01DA18641, and R01DA027211 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. The authors would like to acknowledge the wonderful staff at the Miriam: Christina Anastacio, Candelaria Barroso, Chandra Cannon, Maria Garcia, Ricky Lugo, Skye Tirado; the staff at the RIDOC, Dr. Michael Poshkus; and most of all, the participants who volunteered to be part of this study.
Contributor Information
Michelle McKenzie, The Miriam Hospital, Providence, Rhode Island, USA; Brown University Medical School, Providence, Rhode Island, USA; and The Center for Prisoner Health and Human Rights, Providence, Rhode Island, USA.
Nickolas Zaller, The Miriam Hospital, Providence, Rhode Island, USA; Brown University Medical School, Providence, Rhode Island, USA; and The Center for Prisoner Health and Human Rights, Providence, Rhode Island, USA.
Samuel L. Dickman, The Miriam Hospital, Providence, Rhode Island, USA; Brown University Medical School, Providence, Rhode Island, USA; and The Center for Prisoner Health and Human Rights, Providence, Rhode Island, USA.
Traci C. Green, Brown University Medical School, Providence, Rhode Island, USA; The Center for Prisoner Health and Human Rights, Providence, Rhode Island, USA; and Rhode Island Hospital, Providence, Rhode Island, USA.
Amisha Parihk, The Miriam Hospital, Providence, Rhode Island, USA; and The Center for Prisoner Health and Human Rights, Providence, Rhode Island, USA.
Peter D. Friedmann, Brown University Medical School, Providence, Rhode Island, USA; The Center for Prisoner Health and Human Rights, Providence, Rhode Island, USA; and Rhode Island Hospital, Providence, Rhode Island, USA.
Josiah D. Rich, The Miriam Hospital, Providence, Rhode Island, USA; Brown University Medical School, Providence, Rhode Island, USA; and The Center for Prisoner Health and Human Rights, Providence, Rhode Island, USA.
References
- 1.West H. Bureau of Justice Statistics: Statistical Tables. Washington, DC: Bureau of Justice; Jun, 2010. [Accessed December 13, 2011]. Prison inmates at midyear 2009: statistical tables. NCJ 230113. Available at: http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=2200. [Google Scholar]
- 2.Walmsley R. International Centre for Prison Studies Web site. 8. London: Kings College of London; [Accessed December 13, 2011]. World Prison Opulation List. Available at: http://www.kcl.ac.uk/depsta/law/research/icps/downloads/world-prison-pop-seventh.pdf. [Google Scholar]
- 3.Drucker E. Drug prohibition and public health: 25 years of evidence. Public Health Rep. 1999;114:14–29. doi: 10.1093/phr/114.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greifinger R. Thirty years since estelle v. gamble. In: Greifinger R, editor. Public Health Behind Bars: From Prisons to Communities. New York: Springer; 2007. pp. 1–12. [Google Scholar]
- 5.Mumola C, Karberg J. Bureau of Justice Statistics Special Report (NCJ 213530) Washington, DC: US Department of Justice; 2006. Drug Use and Dependence, State and Federal Prisoners, 2004. [Google Scholar]
- 6.Rich JD, Boutwell AE, Shield DC, et al. Attitudes and practices regarding the use of methadone in US state and federal prisons. J Urban Health. 2005;82:411–419. doi: 10.1093/jurban/jti072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammett TM. HIV/AIDS and other infectious diseases among correctional inmates: transmission, burden, and an appropriate response. Am J Public Health. 2006;96:974–978. doi: 10.2105/AJPH.2005.066993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurco DN, Hanlon TE, Kinlock TW. Recent research on the relationship between illicit drug use and crime. Behav Sci Law. 1991;9:221–242. [Google Scholar]
- 9.Hanlon TE, Nurco DN, Kinlock TW, Duszynski KR. Trends in criminal activity and drug use over an addiction career. Am J Drug Alcohol Abuse. 1990;16:223–238. doi: 10.3109/00952999009001585. [DOI] [PubMed] [Google Scholar]
- 10.Nurco DN, Stephenson PE, Hanlon TE. After-care/relapse prevention and the self-help movement. Int J Addict. 1990;25:1179–1200. doi: 10.3109/10826089109081041. [DOI] [PubMed] [Google Scholar]
- 11.Inciardi JA, Needle RH. Editors’ introduction: HIV/AIDS interventions for out-of-treatment drug users. J Psychoactive Drugs. 1998;30:225–229. doi: 10.1080/02791072.1998.10399696. [DOI] [PubMed] [Google Scholar]
- 12.Weatherburn D, Lind B. Heroin harm minimization: do we really have to choose between law enforcement and treatment? New South Wales Crime Justice Bull. 1999;46:1–11. [Google Scholar]
- 13.Dolan K, Hall, Wodak A. The provision of methadone within prison settings. In: Ward J, Mattick R, Hall W, editors. Methadone Maintenance Treatment and other Opioid Replacement Therapies. Sydney, Australia: Harwood Academic Publishers; 1998. [Google Scholar]
- 14.Bick J. HIV and viral hepatitis in corrections: a public health opportunity. In: Greifinger R, editor. Public Health Behind Bars: From Prisons to Communities. New York: Springer; 2007. pp. 103–126. [Google Scholar]
- 15.Rich JD, McKenzie M, Shield DC, et al. Linkage with methadone treatment upon release from incarceration: a promising opportunity. J Addict Dis. 2005;24:49–59. doi: 10.1300/J069v24n03_04. [DOI] [PubMed] [Google Scholar]
- 16.Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depend. 2009;105:83–88. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenzie M, Nunn A, Zaller ND, Bazazi AR, Rich JD. Overcoming obstacles to implementing methadone maintenance therapy for prisoners: implications for policy and practice. J Opioid Manag. 2009;5:219–227. doi: 10.5055/jom.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaller ND, Holmes L, Dyl AC, et al. Linkage to treatment and supportive services among HIV-positive ex-offenders in Project Bridge. J Health Care Poor Underserved. 2008;19:522–531. doi: 10.1353/hpu.0.0030. [DOI] [PubMed] [Google Scholar]
- 19.Dole VP, Robinson JW, Orraca J, Towns E, Searcy P, Caine E. Methadone treatment of randomly selected criminal addicts. N Engl J Med. 1969;280:1372–1375. doi: 10.1056/NEJM196906192802502. [DOI] [PubMed] [Google Scholar]
- 20.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence. JAMA. 2000;283:1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 21.Langendam MW, van Brussel GH, Coutinho RA, van Ameijden EJ. The impact of harm-reduction-based methadone treatment on mortality among heroin users. Am J Public Health. 2001;91:774–780. doi: 10.2105/ajph.91.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorrensen J, Copeland A. Drug abuse treatment as an HIV prevention strategy: a review. Drug Alcohol Depend. 2000;59:17–31. doi: 10.1016/s0376-8716(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 23.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37:277–285. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinlock TW, Gordon MS, Schwartz RP, O’Grady KE. A study of methadone maintenance for male prisoners: 3 month post-release outcomes. Crim Justice Behav. 2008;35:34–47. doi: 10.1177/0093854807309111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinlock TW, Gordon MS, Schwartz RP, O’Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: results at 1-month post-release. Drug Alcohol Depend. 2007;91:220–227. doi: 10.1016/j.drugalcdep.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobmaier PP, Kunoe N, Gossop M, Katevoll T, Waal H. Naltrexone implants compared to methadone: outcomes six months after prison release. Eur Addict Res. 2010;16:139–145. doi: 10.1159/000313336. [DOI] [PubMed] [Google Scholar]
- 27.Magura S, Rosenblum A, Lewis C, Joseph H. The effectiveness of in-jail methadone maintenance. J Drug Issues. 1993;23:75–99. [Google Scholar]
- 28.Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addiction. 2008;103:1333–1342. doi: 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris A, Selling D, Luther C, et al. Rate of community methadone treatment reporting at jail reentry following a methadone increased dose quality improvement effort. Subs Abuse. 2012;33:70–75. doi: 10.1080/08897077.2011.620479. [DOI] [PubMed] [Google Scholar]
- 30.Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction. 2005;100:820–828. doi: 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 31.Kreek MJ. Rationale for maintenance pharmacotherapy of opiate dependence. Res Publ Assoc Res Nerv Ment Dis. 1992;70:205–230. [PubMed] [Google Scholar]
- 32.Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann N Y Acad Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 33.Gerra G, Ferri M, Polidori E, Santoro G, Zaimovic A, Sternieri E. Long-term methadone maintenance effectiveness: psychosocial and pharmacological variables. J Subst Abuse Treat. 2003;25:1–8. doi: 10.1016/s0740-5472(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 34.Dolan KA, Shearer J, MacDonald M, Mattick RP, Hall W, Wodak AD. A randomised controlled trial of methadone maintenance treatment versus wait list control in an Australian prison system. Drug Alcohol Depend. 2003;72:59–65. doi: 10.1016/s0376-8716(03)00187-x. [DOI] [PubMed] [Google Scholar]
- 35.Gottheil E, Sterling RC, Weinstein SP. Diminished illicit drug use as a consequence of long-term methadone maintenance. J Addict Dis. 1993;12:45–57. doi: 10.1300/J069v12n04_04. [DOI] [PubMed] [Google Scholar]
- 36.Busch M, Haas S, Weigl M, Wirl C. Long term substitution treatment (maintenance treatment) of opioid dependent persons. GMS Health Technol Assess. 2007;3:Doc04. [PMC free article] [PubMed] [Google Scholar]
- 37.Harwood HJ, Hubbard RL, Collins JJ, Rachel JV. The costs of crime and the benefits of drug abuse treatment: a cost benefit analysis using TOPS data. In: Leukefeld CG, Tims FM, editors. Compulsory Treatment of Drug Abuse: Research and Clinical Practice. NIDA Research Monograph 86. Rockville, MD: US Department of Health and Human Services; 1988. pp. 209–235. [PubMed] [Google Scholar]
- 38.Tomasino V, Swanson AJ, Nolan J, Shuman HI. The Key Extended Entry Program (KEEP): a methadone treatment program for opiate-dependent inmates. Mt Sinai J Med. 2001;68:14–20. [PubMed] [Google Scholar]
- 39.McKenzie M, Macalino G, McClung C, Shield DC, Rich JD. Opiate replacement therapy at time of release from incarceration: Project MOD, a pilot program. J Opioid Manag. 2005;1:147–151. doi: 10.5055/jom.2005.0034. [DOI] [PubMed] [Google Scholar]
- 40.Lipton D. Correctional drug abuse treatment in the United States: an overview. In: Leukefeld C, Tims F, editors. Drug Abuse Treatment in Prisons and Jails. National Institute on Drug Abuse Monograph Series. Washington, DC: US Department of Health and Human Services; 1992. pp. 8–30. [Google Scholar]
- 41.Gore SM, Bird AG, Burns SM, Goldberg DJ, Ross AJ, MacGregor J. Drug injection and HIV prevalence in inmates of Glenochil prison. BMJ. 1995;310:293–296. doi: 10.1136/bmj.310.6975.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depend. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]