Abstract
Using the data on all live births (~400,000) and criteria pollutants from the Chicago Metropolitan Statistical Area (MSA) between 2000 and 2004, this paper empirically demonstrates how mismatches in the spatiotemporal scales of health and air pollution data can result in inconsistency and uncertainty in the linkages between air pollution and birth outcomes. This paper suggests that the risks of low birth weight associated with air pollution exposure changes significantly as the distance interval (around the monitoring stations) used for exposure estimation changes. For example, when the analysis was restricted within 3 miles distance of the monitoring stations the odds of LBW (births < 2500g) increased by a factor of 1.045 (±0.0285 95% CI) with a unit increase in the average daily exposure to PM10 (in μg/m3) during the gestation period; the value dropped to 1.028 when the analysis was restricted within 6 miles distance of air pollution monitoring stations. The effect of PM10 exposure on LBW became null when controlled for confounders. But PM2.5 exposure showed a significant association with low birth weight when controlled for confounders. These results must be interpreted with caution, because the distance to monitoring station does not influence the risks of adverse birth outcomes, but uncertainty in exposure increases with the increase in distance from the monitoring stations, especially for coarse particles such as PM10 that settle with gravity within short distance and time interval. The results of this paper have important implications for the research design of environmental epidemiological studies, and the way air pollution (and potentially other environmental) and health data are collocated to compute exposure. The paper also calls for time-space resolved estimate of air pollution to minimize uncertainty in exposure estimation.
Keywords: Exposure uncertainty, spatiotemporal misalignment, health risks
INTRODUCTION
We have made great strides in developing an understanding of the mechanism through which air pollution retards respiratory and cardiovascular health (Basu and Samet, 2002; Peters et al., 2001; Pope et al., 1999) and fetus growth (Jedrychowski et al., 2004). But we have often failed to precisely quantify the burden of disease and health risks associated with air pollution for two important reasons. First, most epidemiological studies rely on secondary (observational) data and trace exposure retrospectively at the location and time that might have been responsible for the health response(s) in question. The direct exposure estimates at the spatiotemporal scales of health data are rarely available (with the exception of personal exposure in cohort-based prospective studies) and there are subtle mismatches in the spatial-temporal resolutions, scales and intervals at which health and air pollution data are made available and aggregated. Air pollution is monitored at sparsely distributed sites and data from these sites are generally aggregated at coarse spatiotemporal scales to match the spatiotemporal scales of health data: for example, researchers average the values from all monitoring stations within a county and assign it to all mothers within that county on the same day (Bell et al., 2007; Parker et al., 2005). However, recent literature suggests that there are subtle spatiotemporal variations in air pollution concentrations in urban areas: for example, on January 1, 2000, the concentration of airborne particulates ≤ 2.5μm in aerodynamic diameter (PM2.5) ranged from 22.7μg/m3 to 34.407μg/m3 within Cook County, IL (EPA, 2008) and the value of PM2.5 ranged from 1.6 μg/m3 to 49.1μg/m3 on February 29, 2008 across six sites in Cleveland MSA, OH (Kumar et al., 2011). Therefore, aggregation of air pollution at coarser spatiotemporal scale results in generalization and exposure misclassification. This, in turn, is likely to result in uncertainty in the health risk estimated using such exposure data. Second, the uncertainty can also result from sampling bias (as a consequence of limited number of observations (or data points) at the chosen spatiotemporal intervals and scales of health data), and the lack of control for confounding factors.
Utilizing air pollution data on the criteria pollutants and ~ 400k live births in the Chicago MSA from 2000 to 2004, this paper evaluates how the mismatch in the spatial-temporal resolution of air pollution and health data result in differential risks of adverse birth outcomes associated with air pollution (Fig. 1). The findings of this research are likely to have important implications for future research design to study the linkages between air pollution and health, and the ways environment and health data are collocated. The remainder of this paper presents methodology, results, and a discussion of our findings within the relevant literature.
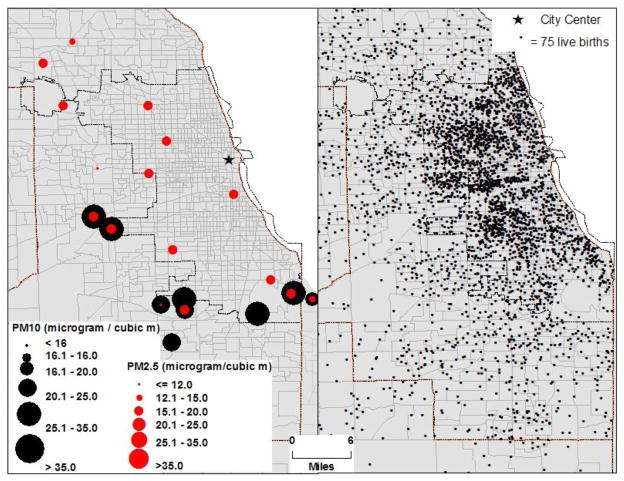
Fig 1.
concentration of PM10 and PM2.5 monitoring stations and live births in Chicago MSA, 2000-04
MATERIALS AND METHODS
Study Area
Metropolitan Chicago is ideally suited to evaluate health effects of air pollution, because it is a highly diverse city in terms of the spatial distribution of air pollution (Fig. 1), and socio-economic and demographic characteristics. According to the 2000 US Census, about 20% of the population in the study area was white, 39% Hispanic, and 20% African American; the average PM2.5 exposure during the complete gestational period ranged from as low as 10 μg/m3 to as high as 31μg/m3 (Fig. 1). Likewise, exposure to other criteria pollutants varied significantly (Supplemental Fig. 1a through 1f and Table 1).
Birth data
Birth data for mothers residing in Chicago Metropolitant Statistical Area, whose entire pregnancy occurred during the study period (January 1, 2000 to December 31, 2004), were extracted from the Illinois Department of Public Health annual birth certificate records for all live births occurring to residents of Cook, DuPage, Kane, Lake, McHenry, and Will counties. A total of 398,120 live births were included in the analysis (Fig. 1). Each record included a clinical estimate of gestational age, birth weight, date of birth, gender, street address, census tract (Chicago residents only), zip code, city and county of residence at the time of birth, prenatal care (measured by Kessner’s Adequacy of Prenatal Care Index), maternal age, maternal race/ethnicity, marital status, maternal education and country of origin, maternal alcohol and tobacco use during pregnancy, parity, time interval between pregnancies, maternal weight gain, delivery method, maternal medical risk factors, and congenital anomalies in the newborn. We excluded records with plural births (3.9%), gestational periods less than 37 weeks (pre-term delivery (PTD)) and greater than 44 weeks (11.0%), birth weight less than 500 grams (0.3%), or impossible clinical estimates of gestational age and weight combinations (1.0%) (Parker et al., 2005). Since PTD is a well-known pathophysiological process which results in low birth weight due to less time for fetal growth (Salam, 2008), it was necessary to exclude PTDs from the final analysis to separate the influence of air pollution exposure on low birth weight from those resulting from PTD. To demonstrate this point, the preliminary analysis compared the risk factors of LBW with and without PTDs.
Chicago resident births with unknown or out-of-range census tract information (n = 1,794) were geocoded by address and zip code using ESRI ArcInfo 9.3 and geocoding services administered by the City of Chicago Department of Information Technology’s GIS Division. Almost all non-Chicago resident births were geocoded by address, city, and zip code using ESRI ArcInfo 9.3 and the geocoding service StreetMap USA packaged with ArcInfo 9.3 (TeleAtlas North America, Inc./Geographic Data Technology, Inc., ESRI, Series Issue 2005). The final study population was composed of 398,120 newborns in the Chicago MSA (Fig. 1), excluding 9% of new births that were not geocoded or were outside the census tract range of the study area.
Census and other data
The Census data were available for a variety of socio-economic and demographic characteristics by census tract and other census units. In the final analysis, however, we included only the percentage of households (in the census tract) receiving public assistance (U.S. Census, 2000). Not only did this variable observe a significant association with many other socio-economic and demographic variables, including vehicles per households, real estate taxes, income, % owner-occupied housing units, % white population, and median number of rooms in the household, but also showed the strongest (among the rest of census variables) association with the risks of low birth weight. The residential locations of the study subjects derived by the geocoding process were aggregated by spatial-join to the corresponding census tract, allowing us to collocate the Census data with the birth data.
Pollution data
We utilized the clinical estimate of gestational age directly listed on the birth certificate to estimate the average air pollution exposure for each newborn by trimester (1–13 weeks, 14–26 weeks, and 27 weeks to birth) and total pregnancy. Criteria pollutants were monitored at spatially dispersed sites k =1,…,K (Supplemental Fig. 2). Daily estimates of these data were acquired from the EPA (EPA, 2008). Each monitoring site was a point location on the two-dimensional geographic space, represented by a pair of coordinates. The geocoded birth outcome data were point locations i = 1,…,N represented by pair of coordinates of the centroid of the census tract of residence (Supplemental Fig. 1a through 1f and 2), because exact location of residence was not available due to confidentiality issue. It is important to note that there were several monitoring stations in some Census Tracts and none within three or six mile distance from the centroid of many Census Tracts (Supplemental Fig. 3). The number of data points used in calculating exposure and the sample size within each of the five selected intervals vary significantly (Table 1 and Supplemental Table 2). Under the distance decay hypothesis, we assume that the exposure of mothers, who resided in the census tracts which had two or more monitoring stations within the specified distance thresholds, is likely to be more robust and reliable than the exposure of mothers computed using values from a distant monitoring station(s).
Table 1.
Descriptive statistics of exposure to criteria pollution within different distance interval to monitoring sites. Mean (standard deviation) and the sample size i.e. # of observations.
| Criteria Pollutant | 3 miles | 6 miles | 9 miles | 12 miles | County | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Sample Size | Mean (SD) | Sample Size | Mean (SD) | Sample Size | Mean (SD) | Sample Size | Mean (SD) | Sample Size (N) | |
| PM2.5 (μg/m3) | 18.0 (4.7) | 43,078 | 17.9 (4.7) | 84,055 | 18.0 (4.6) | 114,332 | 18.0 (4.6) | 114,332 | 18.1 (4.5) | 228,254 |
| PM10 (μg/m3) | 26.5 (4.8) | 10,250 | 26.6 (4.8) | 18,711 | 27.1 (5.1) | 24,540 | 27.1 (5.1) | 24,540 | 27.5 (5.1) | 64,701 |
| NO2 (ppb) | 24.9 (4.2) | 18,200 | 25.2 (4.2) | 37,864 | 25.0 (4.4) | 54,587 | 25.0 (4.4) | 54,587 | 25.1 (4.1) | 134,910 |
| CO (ppb) | 854.0 (311.9) | 13,191 | 822.4 (299.7) | 27,892 | 822.3 (303.0) | 39,347 | 822.3 (303.0) | 39,347 | 815.1 (281.3) | 96,932 |
| O3 (ppb) | 22.8 (5.5) | 35,907 | 22.9 (5.7) | 67,840 | 22.8 (5.6) | 94,017 | 22.8 (5.6) | 94,017 | 22.6 (4.9) | 218,151 |
| SO2 (ppb) | 4.7 (1.3) | 15,696 | 4.7 (1.3) | 34,165 | 4.6 (1.3) | 50,315 | 4.6 (1.3) | 50,315 | 4.6 (1.3) | 129,172 |
The location of air pollution, monitored at sparsely distributed sites, does not overlap (or correspond) with the locations of mothers’ residence (Fig. 1 and Supplemental Fig. 3). Therefore, indirect method was used to interpolate exposure at mothers’ residence during the gestation period. Interpolation method involves an important decision about time-space intervals (or areal unit of aggregation) for searching for sample data. This paper demonstrates how the selection of different distance intervals can result in differential risks of adverse birth outcomes associated with air pollution.
Exposure was computed using different distance intervals (3, 6, 9, and 12 miles) between mother’s residence and air pollution monitoring stations. In order to facilitate the comparison of the results of this study with the other epidemiological studies, exposure was also computed by counties by averaging the values available at all monitoring stations within the county. The time interval for exposure computation remained constant, i.e. different trimesters and the entire gestation. The procedure to compute exposure is detailed below.
Let Aktc denote criteria pollutants c=1,…,C monitored on days t={1,…,T} at spatially dispersed sites k={1,…,K} and Aij(t-l)c, exposure of mothers i = {1,…,n} who gave births on tth days, to cth criteria pollutants, and lived in census tracts j ={1,…,J} during the gestational length (in days) l={1,…,L}. Then their average daily exposure (Aij(t-l)c) to a criterion pollutant in the census tract of their residence was calculated as
| (1) |
Where l = days before the birth date (t): ∀jk = 1 if the distance between jth census tract centroid and kth monitoring site is ≤ h, 0 otherwise. Since the extent of uncertainty in exposure is likely to increase with the increase in distance from the monitoring site, h must be chosen with caution, because setting a low value of h will result in many cases without exposure estimate, and setting h to a high value will increase uncertainty in exposure (Table 1; and Supplemental Table 1 and 2 with details for each trimester).
Linear and logistic regressions were used to model birth weight and odds of LWB, respectively, with the cluster options in STATA (2010). The cluster option specifies that the standard errors allow for intra-group correlation. That is, the observations are independent across groups (clusters) but not necessarily within groups. This option was required because the matrix X′ij consisted of variables at two different geographic scales: individual and census tract. Census tract variables were likely to be the same for all mothers within the same tract, and specifying clusters allowed for intra-tract correlation within the Census variables. Linear regression was also employed to model birth weight (as a continuous variable) with respect to the same covariates and the criteria pollutants.
RESULTS
Descriptive statistics – birth outcomes
Descriptive statistics of exposure and birth outcomes are presented in Table 1 and Table 2, respectively; detailed results of the summary statistics of exposure for each trimester and entire pregnancy are presented in Supplemental Table 1, and the estimate of birth weight with respect to individual covariates, such as age, smoking, race/ethnicity and education, are presented in Supplemental Table 3). The average birth weight (in Chicago and its surrounding counties) was 3344.8±1.7g (95% CI); the value for the city of Chicago was 113g less than that for its surrounding counties. The results are in agreement with the previous literature: birth weight and odds of LBW vary significantly by marital status, smoking, alcohol consumption during pregnancy, congenital anomaly, race/ethnicity, maternal age at birth, educational level, and pregnancy interval (Table 2; Supplemental Table 3) (American College of Obstetricians and Gynecologists, 2000; Bell et al., 2007; Berghella, 2007; U.S. Department of Health and Human Services, 2004; Woodruff et al., 2003). The incidence of low birth weight (i.e. < 2500g) in the study area was 5.8%. Most known risks factors, including smoking, race, medical risk factor, Kessner index, and mother’s education, emerged as significant predictors for LBW (Table 3).
Table 2.
Odds of LBW with respect to different socio-economic and demographic covariates.
| Variables | LBW (all live births) | Crude Odds Ratio ± 95% CI | ||
|---|---|---|---|---|
| # of Births (%) | Incidence (%) | All live births | Live births (with => 37 weeks old) | |
| Total | 398120 (100.0) | 23103 (5.8) | NA | |
| Infant Sex | ||||
| Male | 203784 (51.2) | 10911 (5.35) | 0.85±0.022* | 0.66±0.029* |
| Female (ref) | 194336 (48.8) | 12192 (6.27) | 1 | |
| Year of Birth | ||||
| 2000 (ref) | 25059 (6.3) | 1833 (7.31) | 1 | 1 |
| 2001 | 99235 (24.9) | 5588 (5.63) | 0.76±0.041* | 0.87±0.081* |
| 2002 | 94142 (23.7) | 5281 (5.61) | 0.75±0.041* | 0.87±0.082* |
| 2003 | 92050 (23.1) | 5262 (5.72) | 0.77±0.042* | 0.89±0.083 |
| 2004 | 87634 (22.0) | 5139 (5.86) | 0.79±0.044* | 0.85±0.081* |
| Season of Birth | ||||
| Fall (ref) | 108281 (27.2) | 6362 (5.88) | 1 | 1 |
| Winter | 97331 (24.5) | 5344 (5.66) | 0.96±0.036 | 0.95±0.059 |
| Spring | 94456 (23.7) | 5600 (5.71) | 0.97±0.036 | 0.98±0.059 |
| Summer | 98052 (24.6) | 5797 (5.96) | 1.01±0.037 | 1.05±0.062 |
| Material Race/Ethnicity | ||||
| Non-Hispanic White (ref) | 160133 (42.70) | 6467 (4.04) | 1 | 1 |
| Non-Hispanic Black | 74808 (19.95) | 9163 (12.25) | 3.03±0.100* | 3.67±0.203* |
| Hispanic | 119215 (31.79) | 6039 (5.07) | 1.25±0.045* | 1.43±0.087* |
| Non-Hispanic Other | 20861 (5.56) | 1434 (6.87) | 1.70±0.100* | 2.51±0.222* |
| Maternal Age at Birth (years) | ||||
| 18 or younger | 27676 (7.0) | 2591 (9.36) | 1 | 1 |
| 19–25 | 120821 (30.4) | 7627 (6.31) | 0.65±0.030* | 0.68±0.049* |
| 26–34 | 187853 (47.2) | 9229 (4.91) | 0.50±0.023* | 0.45±0.033* |
| 35 or older | 61770 (15.5) | 3656 (5.92) | 0.61±0.032* | 0.54±0.046* |
| Maternal Weight Gain | ||||
| Less than 25 lbs. gained (ref) | 111361 (28.0) | 10900 (9.40) | 1 | 1 |
| 25–35 lbs. gained | 160170 (40.2) | 8304 (5.18) | 0.53±0.016* | 0.60±0.029* |
| More than 35 lbs. gained | 121985 (30.6) | 3899 (3.20) | 0.32±0.012* | 0.39±0.023* |
| Maternal Education | ||||
| Less than high school (ref) | 96248 (24.2) | 6753 (7.02) | 1 | 1 |
| High school graduate | 102780 (25.8) | 6813 (6.63) | 0.94±0.033* | 0.91±0.050* |
| Some college | 77039 (19.6) | 4623 (6.00) | 0.85±0.033* | 0.74±0.047* |
| College graduate or more | 122053 (30.7) | 4914 (4.03) | 0.56±0.021* | 0.45±0.028* |
| Maternal Country of Birth | ||||
| United States (ref) | 265474 (66.7) | 16915 (6.37) | 1 | 1 |
| Immigrants | 132646 (33.3) | 6188 (4.67) | 0.72±0.021* | 0.79±0.038* |
| Marital Status | ||||
| Married (ref) | 254340 (63.9) | 11014 (4.33) | 1 | 1 |
| Unmarried | 143780 (36.1) | 12089 (8.41) | 2.03±0.054* | 2.29±0.100* |
| Parity | ||||
| First birth (ref) | 138943 (34.9) | 9094 (6.55) | 1 | 1 |
| One or more children | 259177 (65.1) | 14009 (5.41) | 0.82±0.022* | 0.72±0.032* |
| Pregnancy Interval | ||||
| First birth | 138943 (34.9) | 9094 (6.55) | 1 | 1 |
| Less than 1 year | 7334 (1.8) | 900 (12.27) | 2.00±0.146* | 1.26±0.177* |
| 1 year or more | 251843 (63.3) | 13109 (5.21) | 0.78±0.022* | 0.71±0.031* |
| Prenatal Care (Modified Kessner Index) | ||||
| Adequate (ref) | 297466 (74.7) | 15040 (5.06) | 1 | 1 |
| Intermediate | 72402 (18.2) | 5084 (7.02) | 1.42±0.047* | 1.46±0.077* |
| Inadequate | 28252 (7.1) | 2979 (10.54) | 2.21±0.092* | 2.04±0.142* |
| Maternal Smoking During Pregnancy | ||||
| No (ref) | 375001 (94.2) | 20157 (5.38) | 1 | 1 |
| Yes | 23119 (5.8) | 2946 (12.74) | 2.57±0.106* | 3.15±0.194* |
| Maternal Alcohol Consumption During Pregnancy | ||||
| No (ref) | 396301 (99.5) | 22846 (5.76) | 1 | 1 |
| Yes | 1819 (0.5) | 257 (14.13) | 2.69±0.358* | 2.85±0.590* |
| Delivery Method | ||||
| Vaginal (ref) | 307275 (77.2) | 15551 (5.06) | 1 | 1 |
| Primary C-Section | 53698 (13.5) | 5350 (9.96) | 2.08±0.068* | 1.44±0.083* |
| Repeat C-Section | 37147 (9.3) | 2202 (5.93) | 1.18±0.054* | 0.91±0.073 |
| Maternal Medical | ||||
| None (ref) | 287689 (72.3) | 13230 (4.60) | 1 | 1 |
| At least one | 110431 (27.7) | 9873 (8.94) | 2.04±0.055* | 1.52±0.069* |
| Congenital Anomalies | ||||
| None (ref) | 393034 (98.7) | 22456 (5.71) | 1 | 1 |
| At least one | 5086 (1.3) | 647 (12.72) | 2.41±0.201* | 2.15±0.307* |
Significant at 5%; ref = reference category
Table 3.
Low birth weight and the selected covariates.
| Covariates | Odds Ratio | Regression Coefficient | ||
|---|---|---|---|---|
| Low Birth Weight | Birth Weight (g) | |||
| All Births | > 37 weeks | All Births | > 37 weeks | |
| Medical risk category | 1.945*** | 1.435*** | −65.19*** | −5.839*** |
| (±−0.06) | (±−0.08) | (±−4.25) | (±−3.74) | |
| Race category (1=non Hispanic black | 1.860*** | 1.893*** | −156.0*** | −122.4*** |
| (±−0.08) | (±−0.12) | (±−6.35) | (±−5.68) | |
| Smoking (0=no, 1=yes) | 1.740*** | 2.136*** | −168.9*** | −152.6*** |
| (±−0.08) | (±−0.14) | (±−7.92) | (±−7.08) | |
| Kessner Index (0=adequate, 1=inadequate) | 1.287*** | 1.185*** | −55.33*** | −36.11*** |
| (±−0.04) | (±−0.06) | (±−4.27) | (±−3.76) | |
| Marital Status | 1.250*** | 1.275*** | −49.97*** | −40.85*** |
| (±−0.04) | (±−0.08) | (±−4.96) | (±−4.61) | |
| Age Groups (1–4; coded in ascending order) | 1.051*** | 1.025 | 31.71*** | 40.50*** |
| (±−0.02) | (±−0.04) | (±−2.86) | (±−2.45) | |
| Mother’s Education (coded in ascending order) | 0.953*** | 0.915*** | 11.21*** | 10.59*** |
| (±−0.02) | (±−0.02) | (±−2.12) | (±−1.96) | |
| Alcohol Consumption (0=no, 1=yes) | 1.230*** | 1.265** | −44.72*** | −26.43* |
| (±−0.18) | (±−0.25) | (±−30.91) | (±−26.95) | |
| ln(% households receiving public assistance in the census tract of mother’s residence) | 1.058*** | 1.077*** | −13.79*** | −12.72*** |
| (±−0.02) | (±−0.04) | (±−2.90) | (±−2.69) | |
| City code (0=Chicago, 1=Outside Chicago) | 1.078*** | 1.103*** | −16.27*** | −12.23*** |
| (±−0.04) | (±−0.06) | (±−5.94) | (±−5.55) | |
| Constant | 0.0295*** | 0.0141*** | 3342*** | 3352*** |
| (±0.00) | (±0.00) | (±−9.72) | (±−8.72) | |
| Observations | 381,905 | 352,667 | 381,905 | 352,667 |
| R-squared | NA | NA | 0.0615 | 0.0565 |
p<0.01,
p<0.05,
p<0.1 (95% confidence interval in parentheses); NA = not applicable
Distance interval used for computing exposure and sampling bias
It is important to note the sample size varied significantly with respect to distance interval chosen for computing exposure. For example, there were only 10,250 mothers who lived within three miles of PM10 monitoring stations. Given this constraint it was not possible to compute exposure for those who lived beyond 3 miles distance of monitoring station(s) (Supplemental Table 1). Therefore, the analysis within 3 miles distance interval included only 10,250 mothers. The sample size increased to 64,701 mothers when PM10 exposure was computed by county. Likewise the sample size varied for other criteria pollutants for different distance intervals and by county level exposure estimation (Table 1; Supplemental Table 1). Because some counties did not have any monitoring stations, exposure of mothers lived in these counties could not be computed. Therefore, even in the county level analysis we were unable to include all live births. The sampling bias (due to lack of air pollution data outside the chosen distance intervals) have important implications for exposure estimation and interpretation of the results of this paper for several reasons. First, the uncertainty in exposure is likely to increase as the distance from the monitoring sites increases. Second, sample size and its composition in terms of socio-economic and demographic characteristics may vary with respect to different distance intervals from the monitoring stations. However, the exploratory analysis did not suggest any systematic change in birth weight, incidence of low birth weight respect to change in distance interval to monitoring stations (Supplemental Table 4).
From the visual inspection of Supplemental Fig. 1a through 1f two important findings emerge. First, most monitoring stations that record criteria pollutants are concentrated in and around the City of Chicago, and some of the counties in the study area (i.e. Chicago Metropolitan Statistical Area) do not have a single monitoring station. Moreover, all monitoring stations do not record data on all criteria pollutants (Supplemental Fig. 2). Second, most criteria pollutants show a spatial gradient. For example, PM2.5 ranges from 13μg/m3 to 18μg/m3. The concentration of PM2.5 in the City of Chicago is relatively high with the exception of a few pockets of low concentration in the western parts of the city. PM10 ranges from 16μg/m3 to 40μg/m3. Because most PM10 monitoring stations are at the peripheral parts of the city, it is difficult to generalize spatial distribution of PM10 for the entire city. NO2 concentration in the City and near the airport was relatively high (~42ppb). Central parts of the City also show high concentration of O3 (~31ppb). Since all criteria pollutants are not monitored at the same location (Fig. 1; Supplemental Fig. 2), it was not plausible to model the combined (or synergistic) effects of all criteria pollutants on birth outcomes. Therefore, the effect of each criteria pollutant was modeled separately with and without the confounding factors.
Air pollution exposure – descriptive statistics
The average exposure to criteria pollutants was calculated for all three trimesters and the entire gestation separately for each mother. The average daily exposure during the entire gestation was within the National Ambient Air Quality Standards (EPA, 2010). For example, the average daily concentrations of PM2.5 and PM10 for all mothers who resided within 3 miles of monitoring stations were 18.0μg/m3 and 26.5μg/m3, respectively (Table 1; results of exposure by trimester and total pregnancy are in Supplemental Table 1).
Air pollution exposure and birth outcomes
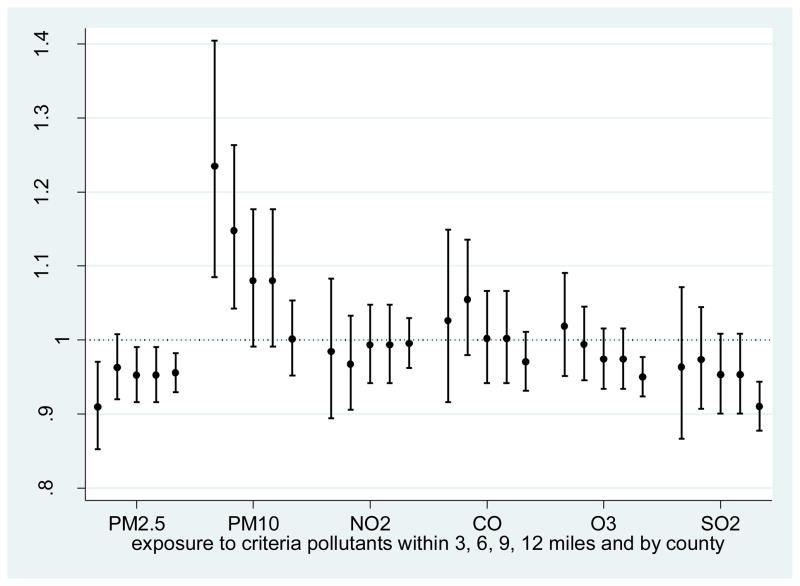
Birth weight as continuous variable and discrete variable (>=2,500 = 1, 0 otherwise) was modeled with respect to air pollution exposure (during all trimesters and for the entire gestational period) with and without the confounding factors. The results for the entire gestation period are presented in Table 4, and detailed results by each trimester are presented in Supplemental Tables 5a and 5b. The analysis was performed separately for different distance intervals and by county. From this analysis, two important findings emerge. First, the association between birth weight and exposure to different criteria pollutants changes significantly as the distance threshold changes (Table 4; Fig. 2). For example in the analysis that did not control for confounders, a unit increase in the daily average PM10 exposure during the entire gestation was associated with 5.3g decline in birth weight when analysis was restricted within 3 miles distance of PM10 monitoring stations; regression coefficients were significant for all three trimesters. When analysis was restricted within 6 miles of PM10 monitoring stations, the effect of PM10 exposure on birth weight dropped significantly, and its effect became statistically insignificant when analysis was extended to 9 miles around the monitoring stations. The effect of PM10 became positively significant when analysis was conducted by county: a unit (i.e. 1μg/m3) increase in the average daily PM10 was significantly associated with 0.76g increase in birth weight, which is counter-intuitive, i.e. higher exposure to PM10 and higher birth weight and vice-versa, and contradictory to fetus growth retardation assumption. Likewise in the that analysis that did not control for confounders, the average daily increase in SO2 and NO2 exposure (when distance to the monitoring station was within 6 miles of mothers’ residence) was associated with the highest decline in birth weight, and the values of the regression coefficients dropped as the distance interval increased further (Fig. 2). While PM10 was associated with an increased risk for LBW in the model with the air pollutant alone, PM2.5 showed the reverse trend, i.e. a decrease of risk for LBW with increasing exposure.
Table 4.
Air pollution exposure and birth outcomes (LBW and Odds of LBW) within different distance intervals.
| Criteria pollutant | Without the control for confounding factors | With confounding factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 miles | 6 miles | 9 miles | 12 miles | County | 3 miles | 6 miles | 9 miles | 12 miles | County | |
| Birth weight (g) and air pollution exposure (95% confidence intervals in parenthesis) | ||||||||||
| PM2.5(μg/m3) | 1.136* (0.158 – 2.114) | 0.549 (−0.160 – 1.257) | 0.811* (0.198 – 1.423) | 1.077* (0.526 – 1.629) | 1.223* (0.779 – 1.666) | −0.507 (−1.575 – 0.561) | −1.107* (−1.883 – − 0.331) | −1.127* (−1.879 – − 0.375) | −0.777* (−1.407 – − 0.147) | −1.122* (−1.657 – − 0.586) |
| PM10 (μg/m3) | −5.318* (−7.315 – − 3.320) | −2.434* (−3.875 – − 0.993) | −0.755 (−1.944 – 0.433) | −2.013* (−3.030 – − 0.997) | 0.763* (0.026 – 1.500) | −1.425 (−4.192 – 1.342) | −0.651 (−2.482 – 1.180) | −0.337 (−1.853 – 1.179) | −0.450 (−1.685 – 0.785) | 0.058 (−0.884 – 1.001) |
| NO2 (ppb) | 0.073 (−1.607 – 1.753) | −1.781* (−2.962 – − 0.600) | −1.466* (−2.403 – − 0.529) | −1.026* (−1.858 – − 0.193) | −0.010 (−0.666 – 0.645) | −2.047 (−4.165 – 0.071) | −2.096* (−3.527 – − 0.665) | −1.965* (−3.004 – − 0.927) | −1.814* (−2.758 – − 0.870) | −1.038* (−1.796 – − 0.280) |
| CO (ppb) | −0.003 (−0.030 – 0.024) | 0.026* (0.007 – 0.045) | 0.036* (0.020 – 0.052) | 0.049* (0.035 – 0.063) | 0.028* (0.018 – 0.039) | 0.034 (−0.002 – 0.070) | 0.024 (−0.004 – 0.052) | 0.015 (−0.007 – 0.036) | 0.020 (−0.001 – 0.040) | 0.006 (−0.009 – 0.020) |
| O3 (ppb) | 0.132 (−0.789 – 1.054) | −0.465 (−1.118 – 0.187) | 0.483 (−0.082 – 1.048) | 0.871* (0.367 – 1.375) | 1.410* (0.975 – 1.844) | −0.582 (−1.781 – 0.617) | −0.950* (−1.806 – − 0.094) | −0.812* (−1.582 – − 0.041) | −1.036* (−1.679 – − 0.392) | −1.048* (−1.594 – − 0.503) |
| SO2 (ppb) | 5.257 (−0.537 – 11.050) | 7.886* (3.947 – 11.825) | 7.340* (4.050 – 10.630) | 9.118* (6.252 – 11.983) | 14.217* (12.143 – 16.292) | −1.858 (−6.739 – 3.024) | 1.263 (−2.465 – 4.991) | −1.325 (−4.762 – 2.113) | −0.359 (−3.408 – 2.690) | 0.685 (−1.633 – 3.004) |
| Odds of LBW and air pollution exposure (95% confidence intervals in parenthesis) | ||||||||||
| PM2.5(μg/m3) | 0.9800* (0.966 – 0.994) | 0.9924 (0.987 – 0.997) | 0.9901* (0.986 – 0.994) | 0.9921* (0.988 – 0.996) | 0.9911* (0.988 – 0.994) | 0.9874 (0.974 – 1.001) | 1.0012 (0.991 – 1.011) | 0.9995 (0.991 – 1.009) | 1.0011 (0.993 – 1.009) | 1.0034 (0.997 – 1.010) |
| PM10 (μg/m3) | 1.0450* (1.017 – 1.074) | 1.0284* (1.018 – 1.039) | 1.0148 (1.006 – 1.023) | 1.0142* (1.007 – 1.021) | 0.9983 (0.993 – 1.003) | 1.0263 (0.986 – 1.068) | 1.0178 (0.991 – 1.045) | 1.0116 (0.990 – 1.033) | 1.0043 (0.987 – 1.022) | 1.0034 (0.992 – 1.015) |
| NO2 (ppb) | 0.9961 (0.974 – 1.019) | 0.9914 (0.984 – 0.999) | 0.9982 (0.992 – 1.004) | 1.0002 (0.995 – 1.006) | 0.9990 (0.995 – 1.003) | 1.0045 (0.981 – 1.028) | 0.9892 (0.972 – 1.007) | 0.9958 (0.984 – 1.008) | 1.0010 (0.990 – 1.012) | 1.0050 (0.996 – 1.014) |
| CO (ppb) | 1.0001 (1.000 – 1.000) | 1.0002 (1.000 – 1.000) | 1.0000 (1.000 – 1.000) | 0.9999 (1.000 – 1.000) | 0.9999 (1.000 – 1.000) | 1.0000 (1.000 – 1.000) | 1.0002 (1.000 – 1.000) | 1.0001 (1.000 – 1.000) | 1.0000 (1.000 – 1.000) | 1.0001 (1.000 – 1.000) |
| O3 (ppb) | 1.0032 (0.991 – 1.016) | 0.9988 (0.994 – 1.003) | 0.9945 (0.991 – 0.998) | 0.9902* (0.987 – 0.994) | 0.9885* (0.986 – 0.991) | 1.0061 (0.993 – 1.019) | 1.0010 (0.992 – 1.010) | 1.0011 (0.993 – 1.009) | 1.0006 (0.994 – 1.008) | 1.0037 (0.997 – 1.010) |
| SO2 (ppb) | 0.9723 (0.897 – 1.054) | 0.9801 (0.954 – 1.007) | 0.9638 (0.943 – 0.985) | 0.9515* (0.933 – 0.970) | 0.9295* (0.916 – 0.943) | 1.0350 (0.979 – 1.094) | 1.0226 (0.967 – 1.082) | 1.0208 (0.974 – 1.070) | 1.0134 (0.976 – 1.052) | 1.0091 (0.982 – 1.037) |
NOTE: Cases with 37 or more weeks of gestation were included in the analysis. Control for confounding factors include all covariates included in Table 3.
p<0.05
Fig. 2.
Odds of low birth weight (< 2500g) with one standard deviation increase in the daily average exposure to criteria pollutants.
NOTE: The first four lines of each criteria pollutant include mothers within 3, 6, 9 and 12 miles distance to monitoring stations, respectively, and the fifth line is for county level aggregated analysis.
Second, when adjusted for confounders (including age, gender, smoking, medical risks, education, race, city, and neighborhood contexts), only PM2.5, NO2, and O3 emerged as significant predictors of birth weight. Unlike PM10 alone (without the confounding factors), a unit (i.e. 1μg/m3) increase in the average daily PM2.5 exposure (when analysis was restricted to mothers resided within 9 miles of the PM2.5 monitoring stations) during the first trimester showed the highest decline on the birth weight. The average daily NO2 exposure, however, was associated with the most significant decline in birth weight when analysis was restricted within 3 miles distance of the monitoring stations: its impact on birth weight gradually declined with the increase in distance from the monitoring stations (Supplemental Table 4). In the county level analysis, the effect of NO2 exposure was lowest, though still statistically significant. A unit increase in the average daily O3 exposure during the third trimester was associated with the highest decline in the birth weight when analysis was restricted within 3 miles of the monitoring stations (Supplemental Table 5b).
The risks of LBW associated with exposure to criteria pollutants were examined separately and with the selected confounding factors. In the model with the air pollution exposure (alone), PM10, NO2, and CO showed a significant association with the birth outcomes. A unit (i.e. 1μg/m3) increase in the average daily PM10 exposure during the entire pregnancy, and for all three individual trimesters, was associated with a significant increase in the risks of being LWB. When analysis was restricted to mothers who resided within 3 miles of the monitoring stations, the risk of LBW increased to 4.5% with a unit increase in the average daily exposure to PM10. But when controlled for confounding factors, the PM10 exposure did not show any significant risks of LBW.
Distance to monitoring stations and sampling bias
As discussed earlier the sample size increased with the increase in distance to monitoring stations. Thus, we evaluate whether increase in the sample size influences characteristics of the sample. We examined the selected socio-demographic characteristics of the samples and incidence of low birth weight within the selected distance intervals: 3, 6, 9 and 12 miles, and County. The incidence of low birth weight was not significantly different with 3 and 6 miles distance interval; likewise other socio-demographic characteristics (such as race/ethnicity, education and income values) did not show significant differences within 3 and 6 miles distance intervals. But income level was significantly higher within 12 miles as compared to the income for the sample within 3 miles (Supplemental Table 4). But the incidence of low birth weight was insignificant for the samples across all distance intervals.
DISCUSSION
While the experimental research provides insight into the causal mechanism of the adverse effects of air pollution, it has been increasingly difficult to quantify precise health risks and burden of disease associated with air pollution, especially through the observational and retrospective studies. Mismatch in the spatiotemporal scales of air pollution and health data that results in uncertainty in exposure computation is one of the major reasons behind uncertainty and inconsistency in the linkages between air pollution and health outcomes. As evident from Fig. 1 and Supplemental Fig. 2, air pollution data are monitored at sparsely distributed sites and there are significant spatial and temporal gradients in the distribution of air pollution. Aggregating data from these sparsely distributed sites alone at a coarser spatial resolution, such as county, may not adequately represent the population exposure.
Our analysis suggests that the risks of low birth weight associated with air pollution exposure changes significantly as the distance interval (around the monitoring stations) used for exposure estimation changes. For example, when the analysis was restricted within 3 miles distance of the monitoring station the sample size was 10,250 mothers and the risk of LBW (without the control for confounding factors) increases 5% with a unit increase in the average daily exposure to PM10 during the entire gestation. The number of cases increased to 18,711 when analysis was restricted to 6 miles distance around the monitoring stations, and the risk of LBW for PM10 exposure dropped to 2.8%. According to the positivist ideology, the true health risks associated with air pollution exposure must be consistent for the same population, irrespective of the spatial-temporal resolutions of exposure data. Therefore, the above conclusion should be interpreted with caution, because it does not suggest that the distance to monitoring station influences health risks as demonstrated in the result section. But it does communicate three important messages. First, uncertainty in exposure, estimated using the data from distantly located monitoring stations, is likely to increase. Second, imposing distance constraints results in sampling biases, because exposure computation is not possible for the population residing outside the chosen distance threshold, and a sub-set of population is included in the analysis. Third, population composition and its characteristic may change with respect to distance from the monitoring station. To verify the role of the second and third, birth weight and covariates were examined with respect to different distance intervals to monitoring stations. The sample size increased with the increase in distance to monitoring stations, but there were little differences in the birth weight and incidence of low birth weight and most socio-economic and demographic characteristics within different distance intervals with the exception of income and race/ethnicity. Therefore, the changing risks of adverse birth outcomes analyzed within different distance intervals from the air pollution monitoring stations can largely be attributed to change in uncertainty in exposure estimation, especially for the coarse particles, such as PM10 because the coarse particles settle with gravity more quickly and within shorter distance and time intervals as compared to fine particulate (PM2.5) and gaseous pollutants
With regard to traditionally used (and well-identified) socio-economic and demographic covariates, the general trends of our findings are consistent with previous studies: the selected covariates, including mother’s age, alcohol consumption, smoking, marital status, etc., emerge as significant risk factors for LBW (American College of Obstetricians and Gynecologists, 2000; Bell et al., 2007; Berghella, 2007; U.S. Department of Health and Human Services, 2004; Woodruff et al., 2003). Although the reliability and robustness of the results reported in this paper and in many other studies can be questioned because of uncertainty in the air pollution exposure and a comparison of our results with other studies substantiate the main thesis of this research, i.e. mismatches in the spatial-temporal resolution, scale and interval at which health and air pollution data are aggregated and analyzed, sampling bias, and lack of control for confounding factors, are partly responsible for uncertainty and inconsistency in the linkages between air pollution and birth outcomes (and other related health outcomes). For example, Bell and others also concluded that African-American mothers were more likely to have a LBW baby as compared to white mothers. Their analysis included PTD, which is likely to bias the risk (upward or downward) associated with SES, other covariates, and air pollution exposure. For example, in our analysis the risk of LBW was 3.03 times greater for an African American mother than for a white mother when cases of PTD were included, and this risk increased to 3.67 when PTD was excluded from the analysis (Table 2) (Bell et al., 2007). Since the spatiotemporal scale of air pollution data and methods of aggregation and exposure computation have been the same for other health effect studies, the findings of this research (about how mismatch in the spatiotemporal scales and location of air pollution and birth data) may be extrapolated to other health effects studies.
Several studies have documented the association between birth outcomes and exposure to PM2.5 and PM10 during the gestational period in different parts of the world, including Sao Paulo, Brazil (Gouveia et al., 2004), Sydney, Australia (Mannes et al., 2005), Massachusetts and Connecticut (Bell et al., 2007), and Los Angeles, California, USA (Parker et al., 2005; Wilhelm and Ritz, 2005). Likewise, other pollutants, namely CO, (Bell et al., 2007; Gouveia et al., 2004; Ha et al., 2001; Liu et al., 2003; Maisonet et al., 2001; Mannes et al., 2005; Ritz and Yu, 1999; Wang et al., 1997; Woodruff et al., 2003) SO2 (Bell et al., 2007; Bobak, 2000; Bobak and Leon, 1999; Ha et al., 2001; Rogers et al., 2000; Wang et al., 1997) and NO2 (Bobak, 2000; Bobak and Leon, 1999; Ha et al., 2001; Rogers et al., 2000) have been identified as important predictors of low birth weight. A recent review of the literature on the effects of air pollution on birth outcomes is summarized in Supplemental Table 7. Although a number of studies have been published during the last decade, the results reported in this research are somewhat inconsistent with that reported in three other studies Bell et al. (2007), Ritz et al. (2007), and Parker et al. (2008)).
Although there has been an increasing interest in quantifying the risk of ambient air pollution associated with birth outcomes, and many studies have been conducted during the last decade, our understanding of the effects of air pollution on birth outcomes is still inconclusive, and major gaps exist in the literature (Woodruff et al., 2009). This paper empirically documents how uncertainty in exposure estimation (due to increase in distance from monitoring stations) can lead to inconclusiveness and inconsistency in the association between birth outcomes and air pollution. A vast majority of studies conducted in recent years point to the fact that research design, mismatches in the spatial-temporal resolutions of exposure and birth outcome data, and selection bias (i.e., restricting the cases included in the analysis to certain areas and groups), can greatly influence the association between air pollution and birth outcomes (Parker and Woodruff, 2008; Slama et al., 2008; Woodruff et al., 2008; Woodruff et al., 2009). The ad-hoc approaches to selecting spatial-temporal intervals, such as 3 miles or 30 miles (Ostro et al., 2010), are likely to result in greater uncertainty in exposure computation.
This paper calls for time-space resolved estimates of air pollution data to address uncertainty in the linkages between air pollution and health outcomes. This, in turn, raises an important question “how to compute reliable time-space resolved estimates of air pollution exposure”? There are two potential ways to answer this question. First, identify spatial and temporal intervals within which robust and reliable estimates of exposure can be estimated, and restrict the analysis to these intervals only. This can be achieved by using a spatial-temporal variogram and Kriging (De Iaco et al., 2002; Mendes and Turkman, 2002). Since the number of monitoring stations is small, this approach is unlikely to address the problem of sampling bias, because it will eliminate a substantial proportion of population from the analysis. Second, a hybrid approach that builds on the respective strengths of atmospheric remote sensing (Kumar et al., 2008; van Donkelaar et al., 2010), chemical transport models (CTM), and spatial-temporal dynamic modeling (Kumar, 2010), can be used to compute time-space resolved estimates of air pollution (Supplemental Fig. 4). Satellite remote sensing provides reliable estimates of aerosol optical depth (AODs) at a fine spatial resolution, and CTM that is a data-driven method can produce AODc at fine temporal scale. Integrating these two methods along with spatial-temporal dynamic modeling can produce time-, space- (and potentially by source- if complete emission inventory data are available) resolved estimates of air quality at the finest spatial and temporal resolutions at which health data are available.
Supplementary Material
Highlights.
How uncertainty in exposure affect risk estimates
Mismatch in the spatial scale of health and air pollution data
Hybrid approaches necessary to compute robust exposure
Challenges the finding of previous studies
Acknowledgments
Funding Support: This work was partly funded by NIH/NIEHS - 1 R21 ES014004-01A and NIH/NICHD - 1 R21 HD046571-01A1 grants.
The author would like to thank two anonymous referees for their thoughtful comments and suggestions, and Dr. Kirsti Bocskay, Chicago Department of Public Health, Chicago, for providing birth data, and Kevin Gibbs for geocoding these data.
Footnotes
Disclaimers/Competing Interests Declaration: This is to declare that the author has no competing interests with this research work. Birth data were supplied by the Chicago Department of Public Health. The department specifically disclaims responsibility for any analyses, interpretations, or conclusions reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College of Obstetricians and Gynecologists. Intrauterine Growth Restriction. ACOG Practice Bulletin; 2000. [Google Scholar]
- Basu R, Samet JM. An exposure assessment study of ambient heat exposure in an elderly population in Baltimore, Maryland. Environmental Health Perspectives. 2002;110:1219–1224. doi: 10.1289/ehp.021101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghella V. Prevention of Recurrent Fetal Growth Restriction. Obstet Gynecol. 2007;110:904–912. doi: 10.1097/01.AOG.0000267203.55718.aa. [DOI] [PubMed] [Google Scholar]
- Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108:173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak M, Leon DA. The effect of air pollution on infant mortality appears specific for respiratory causes in the postneonatal period. Epidemiology. 1999;10:666–670. [PubMed] [Google Scholar]
- De Iaco S, Myers DE, Posa D. Space-time variograms and a functional form for total air pollution measurements. Comput Stat Data An. 2002;41:311–328. [Google Scholar]
- EPA. Envirofacts Data Warehouse. Environmental Protection Agency; 2008. [Google Scholar]
- EPA. National Ambient Air Quality Standards (NAAQS) U.S. Government Printing Office of Environmental Protection Agency; Research Triangle Park, North Carolina: 2010. [Google Scholar]
- Gouveia N, Bremner SA, Novaes HM. Association between ambient air pollution and birth weight in Sao Paulo, Brazil. J Epidemiol Community Health. 2004;58:11–17. doi: 10.1136/jech.58.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EH, Hong YC, Lee BE, Woo BH, Schwartz J, Christiani DC. Is air pollution a risk factor for low birth weight in Seoul? Epidemiology. 2001;12:643–648. doi: 10.1097/00001648-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Bendkowska I, Flak E, Penar A, Jacek R, Kaim I, Spengler JD, Camann D, Perera FP. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: An epidemiologic prospective cohort study in Poland. Environmental Health Perspectives. 2004;112:1398–1402. doi: 10.1289/ehp.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. What Can Affect AOD–PM2.5 Association? Environmental Health Perspectives. 2010;118:A2–3. doi: 10.1289/ehp.0901732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Chu A, Foster A. Remote sensing of ambient particles in Delhi and its environs: estimation and validation. International Journal of Remote Sensing. 2008;29:3383–3405. doi: 10.1080/01431160701474545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Chu AD, Foster AD, Peters T, Willis R. Satellite Remote Sensing for Developing Time and Space Resolved Estimates of Ambient Particulate in Cleveland, OH. Aerosol Sci and Technol. 2011;45:1090–1108. doi: 10.1080/02786826.2011.581256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect. 2003;111:1773–1778. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Bush TJ, Correa A, Jaakkola JJ. Relation between ambient air pollution and low birth weight in the Northeastern United States. Environ Health Perspect. 2001;109(Suppl 3):351–356. doi: 10.1289/ehp.01109s3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannes T, Jalaludin B, Morgan G, Lincoln D, Sheppeard V, Corbett S. Impact of ambient air pollution on birth weight in Sydney, Australia. Occup Environ Med. 2005;62:524–530. doi: 10.1136/oem.2004.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes JM, Turkman KF. A simple spatio-temporal procedure for the prediction of air pollution levels. J Chemometr. 2002;16:623–632. [Google Scholar]
- Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, Henderson KD, Bernstein L. Long-Term Exposure to Constituents of Fine Particulate Air Pollution and Mortality: Results from the California Teachers Study. Environmental Health Perspectives. 2010;118:363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, Woodruff TJ. Influences of study design and location on the relationship between particulate matter air pollution and birthweight. Paediatr Perinat Epidemiol. 2008;22:214–227. doi: 10.1111/j.1365-3016.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- Parker JD, Woodruff TJ, Basu R, Schoendorf KC. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115:121–128. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Pope CA, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas M, Gold DR, Dockery DW. Heart rate variability associated with particulate air pollution. American Heart Journal. 1999;138:890–899. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M, Hoggatt KJ, Ghosh JK. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am J Epidemiol. 2007;166:1045–1052. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- Ritz B, Yu F. The effect of ambient carbon monoxide on low birth weight among children born in southern California between 1989 and 1993. Environ Health Perspect. 1999;107:17–25. doi: 10.1289/ehp.9910717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JF, Thompson SJ, Addy CL, McKeown RE, Cowen DJ, Decoufle P. Association of very low birth weight with exposures to environmental sulfur dioxide and total suspended particulates. Am J Epidemiol. 2000;151:602–613. doi: 10.1093/oxfordjournals.aje.a010248. [DOI] [PubMed] [Google Scholar]
- Salam MT. Air pollution and birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2008;116:A106. doi: 10.1289/ehp.10631. author reply A106–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, Glinianaia S, Hoggatt KJ, Kannan S, Hurley F, Kalinka J, Sram R, Brauer M, Wilhelm M, Heinrich J, Ritz B. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116:791–798. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. STATA/SE Version 10.1. 2010. [Google Scholar]
- US Census. StataCorp LP; College Station, Texas 77845: 2000. [Google Scholar]
- U.S. Department of Health and Human Services, 2004. The Health Consequences of Smoking: A Report of the Surgeon General, 2004. Centers for Disease Control and Prevention, Office on Smoking and Health; Atlanta, GA: May, 2004. [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Kahn R, Levy R, Verduzco C, Villeneuve PJ. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: development and application. Environ Health Perspect. 2010;118:847–855. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ding H, Ryan L, Xu X. Association between air pollution and low birth weight: a community-based study. Environ Health Perspect. 1997;105:514–520. doi: 10.1289/ehp.97105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Ritz B. Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect. 2005;113:1212–1221. doi: 10.1289/ehp.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Darrow LA, Parker JD. Air pollution and postneonatal infant mortality in the United States, 1999–2002. Environ Health Perspect. 2008;116:110–115. doi: 10.1289/ehp.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Parker JD, Darrow LA, Slama R, Bell ML, Choi H, Glinianaia S, Hoggatt KJ, Karr CJ, Lobdell DT, Wilhelm M. Methodological issues in studies of air pollution and reproductive health. Environ Res. 2009;109:311–320. doi: 10.1016/j.envres.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Perspect. 2003;111:942–946. doi: 10.1289/ehp.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.