Abstract
Dendritic cells (DCs) and natural killer (NK) cells have central roles in antiviral immunity by shaping the quality of the adaptive immune response to viruses and by mediating direct antiviral activity. HIV-1 infection is characterized by a severe dysregulation of the antiviral immune response that starts during early infection. This Review describes recent insights into how HIV-1 infection affects DC and NK cell function, and the roles of these innate immune cells in HIV-1 pathogenesis. The importance of understanding DC and NK cell crosstalk during HIV infection for the developement of effective antiviral strategies is also discussed.
Introduction
Despite some recent progress in HIV vaccine strategies, HIV infection remains a world-wide problem. Growing evidence indicates that the immune system is dysregulated during HIV infection, resulting in compromised efficacy of potential immune therapies for HIV. Humoral immune responses occur late in HIV-1 infection, with neutralizing antibodies appearing 3 months or more after initial infection 1. T cell responses are elicited around 1–2 weeks after infection, but are largely ineffective owing to the early emergence of antigen escape variants of HIV 1. Recent reports have highlighted the dual role of innate immunity in both early viral control and in contributing to disease pathology. Dendritic cells (DCs) and natural killer (NK) cells are crucial mediators of innate immunity and promote the development of adaptive immune responses. DCs are crucial for activating and conditioning virus-specific T cells, a process that is largely influenced by the preceding innate immune response. NK cells impede early spread of viruses by producing cytokines and directly killing infected cells. HIV vaccine strategies that use DCs, either through in vitro manipulation of DCs isolated from patients or in vivo targeting of DC subsets, are currently being investigated and the success of these approaches will depend upon a proper understanding of how DC biology is affected by HIV-1 infection. NK cells may be crucial for early control of HIV infection and can have important roles in editing the function of DCs, thereby affecting the ability of DCs to prime antiviral effector T cells. This Review focuses on the roles of these two innate cell types during HIV-1 infection.
DCs bridge innate and adaptive immunity
Human DCs are rare potent antigen-presenting cells that can be generally divided into myeloid CD11c+ ‘conventional’ DCs (cDCs) or plasmacytoid DCs (pDCs) 2 (Table I). Both subsets specialize in detecting viruses and initiating innate and adaptive immune responses that lead to viral elimination or control. DCs express several receptors for recognizing viruses 3, including pattern recognition receptors (PRRs) such as the Toll-like receptors (TLRs) and C-type lectins. DCs detect viruses in peripheral tissue sites and, following activation and viral uptake, migrate to draining lymph nodes, where they trigger adaptive immune responses and promote NK cell activation (Table I) 4. Activated cDCs produce cytokines such as interleukin-12 (IL-12), IL-15 and IL-18. IL-12 is critical for cDCs to induce T helper 1 (TH1) cell responses, which subsequently promote potent cytotoxic T lymphocyte (CTL) responses that are necessary for clearing virus-infected cells 5. Both IL-12 and IL-15 produced by cDCs can activate NK cells (Table I) 4. pDCs produce more type I interferons (IFNs) in response to HIV than any other cell in the body, and stimulate cDCs in a bystander fashion as well as directly activating NK cells 6. In this section, we describe recent observations that have been made concerning DC biology and function during HIV-1 infection. Specifically, we focus on how DCs bind and recognize HIV virions, and how DCs are in turn modulated by the virus, ultimately leading to their dysregulation in vivo. The implications of these events underlie the need to develop vaccine strategies that enhance the ability of DCs to prime potent T cell responses that can subvert the potential inhibitory effects of HIV on the immune system.
Table 1.
DC subsets and their role in HIV infection
| Subset | Conventional DC (cDC) (HLA-DR+, CD11c+) | Plasmacytoid DC (pDC) (HLA-DR+, CD123+) | References | ||||
|---|---|---|---|---|---|---|---|
| Langerhans Cells | Dermal DCs | Blood DCs | 2, 7, 9, 10, 15, 27 | ||||
| CD103− | CD103+ | BDCA-3− | BDCA-3+ | ||||
| Location | Epidermis, gut lumen | Dermis | Dermis | Blood, Secondary Lymphoid organs | Blood | Blood Secondary Lymphoid Organs Peripheral tissue (skin, lung, etc.) |
|
| C-type lectin expression | Langerin | DC-SIGN, DEC-205 | Langerin, DEC-205 | DEC-205, DCIR | CLEC9A | BDCA-2, BDCA-4 | |
| Role in HIV infection | Internalize HIV into degradative Birbeck granules | Bind to HIV and and transmit virus to T cells in draining LNs | ?? | ?? | ?? |
|
9, 10, 43, 45, 53 |
| TLR expression | TLR2,3,5* | TLR2,3,4,5* | TLR2,3,4,5* | TLR2,3,4,7,8 | TLR2,3,8 | TLR7,9 | 2, 15, 27, and below |
| Cytokine production | IL-12, IL-15, IL-23, IL-6, TNFα, IL-1β | IFNα, IFNβ, IL-6, TNFα | 2, 4, 5, 6 | ||||
| Function | Priming of antigen-specific CD4+, CD8+ T cells, B cells; NK cell activation via IL-12 |
Induction of Tregs; Induction of plasma cells NK cell activation via type I IFN |
2, 4, 5, 6 | ||||
| Pathology during HIV infection |
|
|
25, 26, 28, 31, 32, 33, 34, 35, 36, 37 | ||||
confounding reports regarding TLR distribution on Langerhans cells and dermal DCs
→ Van der Aar et al. 2007. J. Immunol. 178: 1986
Peiser et al. 2008. J. Leukoc. Bio. 83: 1118
Angel et al. 2007. Int. Immunol. 19: 1271
Flacher et al. 2006. J. Immunol. 177: 7859
Binding and recognition of HIV by DCs
HIV entry receptors
Binding and internalization of HIV by DCs is mediated through the various HIV entry receptors (CD4, CC-chemokine receptor 5 (CCR5) and CXC-chemokine receptor 4 (CXCR4)). Other chemokine receptors, including CCR3, CCR8, CCR9 and CXCR6 7–8, have been suggested to participate in HIV-1 entry. In addition, cDCs express C-type lectins that bind to HIV, including DC-specific ICAM3-grabbing non-integrin (DC-SIGN), langerin and C-type lectin domain family 4 member A (CLEC4A, also known as DCIR) 7, 9 (Table I). Expression of these receptors varies based on cDC subtype, localization, and activation state. Although pDCs express C-type lectins, such as blood DC antigen 2 (BDCA2) and BDCA4, binding of HIV to pDCs is generally mediated by interaction of HIV’s gp120 envelope protein with CD4 on pDCs (Table 1) 10
Depending on the receptors utilized for HIV binding, virus–cDC interactions can affect virus fate. HIV binding to DC-SIGN through its gp120 envelope protein leads to internalization of the virus into early endosomal compartments in DCs, where HIV is not degraded. This may enable DCs to deliver intact HIV virus to draining lymph nodes, where DC–T cell interactions could promote infection of T cells through viral synapses and/or cell–cell fusion. The contribution of this mode of transmission has been called into question as the transfer of HIV-1 to T cells can occur independently of DC-SIGN 11. However, another report highlights how interaction of HIV with DC-SIGN may be important for transmission of HIV. Clusterin in semen is differentially glycosylated than clusterin in blood and specifically blocks the HIV–DC-SIGN interaction, thereby preventing HIV transmission to CD4+ T cells. The differential efficacy of clusterin in semen and clusterin in blood to block HIV–DC-SIGN interactions may explain why exposure to HIV sexually is less likely to result in infection than blood-borne exposure 12. CLEC4A was also recently described to bind to HIV virions and, similar to DC-SIGN, promotes transmission of infectious virus to CD4+ T cells 13.
The C-type lectin langerin, which can bind and internalize HIV, is selectively expressed by epidermal Langerhans cells and migratory Langerhans cells are believed to enhance sexually transmitted HIV infection by promoting spread of the virus to T cells in draining lymph nodes. In contrast to DC-SIGN-mediated internalization, HIV internalized through langerin is trafficked to Birbeck granules, where the virus is rapidly degraded 14; this suggests that Langerhans cells also function as a barrier to HIV infection. However, high viral concentrations enable langerin-independent internalization of HIV by Langerhans cells, resulting in the transport of intact HIV to T cells by migratory Langerhans cells. In accordance with this, others have shown that in vitro-derived Langerhans cells can transmit infectious HIV to T cells 15. The differential fate of HIV depending on the particular cDC receptors used to bind the virus emphasizes how different DC subsets can contribute to or hinder HIV infection.
Innate recognition of HIV by DCs
Whereas binding and internalization of HIV is mediated by a variety of DC-expressed surface receptors, activation of DCs by HIV is mainly thought to involve intracellular members of the TLR family. Human pDCs express TLR7 and TLR916 and recognition of single-stranded RNA from HIV-1 by TLR7 leads to pDC production of type I IFN and other inflammatory cytokines. The initial gp120–CD4 interaction promotes endocytosis of HIV-1 by pDCs and the subsequent activation of TLR7 by viral RNA 17. Genomic RNA from HIV-1 contains several immunostimulatory GU-rich sequences, which activate the TLR7 pathway 18, and owing to their constitutive expression of interferon-regulatory factor 7 (IRF7), pDCs rapidly secrete high levels of IFNα following the detection of HIV by this route 19.
However, HIV does not induce maturation of cDCs by activating TLRs17. This is surprising considering that blood cDCs express TLR7 and TLR8, both of which can be activated by HIV-derived single-stranded RNA. One possible explanation is low viral replication within cDCs; only 1–3% of DCs in culture become infected in vitro and circulating blood DCs isolated from patients with HIV do not appear to be infected with the virus 20–21. A recent study using pseudotyped virus that lacked envelope protein showed that when this strain of HIV-1 carries the vpx gene, infection of DCs promotes their maturation and production of type I IFN, and facilitates anti-viral T cell immunity. This response is mediated by interaction of newly synthesized HIV-1 capsid with cellular cyclophilin A (CYPA), and activation of the type I IFN inducing transcription factor IRF3 through an unknown cytoplasmic sensor22. Thus, it is possible that there are too few virions within HIV-1-exposed cDCs to trigger TLR signaling, or that vpx is essential for this interaction. However, although viral replication in pDCs is also low, pDCs rapidly respond to HIV-1 through TLR7. Therefore another explanation is that HIV interaction with C-type lectins may abrogate subsequent TLR responsiveness in cDCs. HIV, similar to Mycobacterium spp., has been implicated in inhibiting TLR stimulation of cDCs through its interactions with DC-SIGN23 and this is discussed in more detail below.
Can TLR signalling promote HIV replication in DCs?
De novo replication of integrated HIV-1 in immature cDCs can be initiated by TLR8- and DC-SIGN-mediated signal-transduction events. HIV is targeted to TLR8-containing endosomal compartments by DC-SIGN, initiating transcription of integrated HIV-1 DNA through TLR8 activation and subsequent nuclear factor-kB (NFkB) signalling 24. The binding of gp120 to DC-SIGN leads to RAF1-mediated phosphorylation of the p65 subunit of NF-kB, thereby permitting transcription elongation of nascent HIV-1 transcripts and productive transcription within infected DCs 24. Thus both TLR8 and DC-SIGN ligation are required for induction of signal-transduction pathways that promote synthesis of complete viral transcripts from proviral integrated DNA. Coinfection with Candida albicans or Mycobaterium tuberculosis also triggers RAF1-dependent phosphorylation, suggesting that coinfection could enhance the productive transcription of HIV 24. Although this in vitro work argues for a role of TLR8 in promoting HIV replication in cDCs, it is unclear whether this is a physiological mechanism for productive infection in cDCs as replication within cDCs is very low. However, it raises the issue of whether the use of TLR8 agonists as immunotherapeutic adjuvants might increase productive replication within cDCs containing integrated HIV-1.
Autophagy and HIV recognition
Autophagy leads to the delivery of cytosolic components (such as signalling molecules and damaged organelles) to lysosomal compartments, which contain PRRs 25. Following HIV-1 infection, fusion between endosomes and autophagosomes is inhibited, thereby preventing autophagy-mediated viral degradation26. This inhibition occurs through activation of mammalian target of rapamycin (mTOR), a serine-threonine kinase that is a negative regulator of autophagy. In accordance with inhibition of autophagy, HIV-1 replication in DCs leads to decreased cathepsin activity in these cells, possibly due to a blockade of lysosomal fusion that is necessary for cathepsin acitivition. This results in enhanced survival of HIV-1 in phagosomes and decreased presentation of viral antigens 27–29.
In contrast to the aforementioned study, which showed that HIV can activate TLR8, HIV-mediated inhibition of autophagy was shown by others to inhibit DC responsiveness to the classical TLR4 and TLR8 agonists, LPS and imiquimod 26. One explanation for this discrepancy may be that activation of TLR8 by HIV precedes autophagy inhibition. Alternatively, selective blockade of autophagosomes by HIV may depend on the particular HIV receptor used for viral entry. Clearly additional studies are necessary to understand how HIV-1 can modulate cDC responsiveness to TLR agonists. It will also be important to determine how HIV affects different blood cDC subtypes such as the newly described BDCA3+ blood DC subset 30.
Dysregulation of DC responses in HIV-1 infection
Decreased DC frequency
HIV-1 infection leads to a progressive decrease in blood DC numbers (both cDCs and pDCs), which correlates with increasing plasma virus load and disease progression 31. A decrease in DC frequency occurs early during acute HIV infection and is sustained over later stages of infection. Patients with chronic HIV infection also have fewer blood DCs compared with uninfected controls 31.
Direct infection of DCs has been suggested to be responsible for decreased DC frequency during HIV infection 31. Several studies have evaluated whether DCs from the blood of chronic HIV-1 patients are infected with HIV, with some showing evidence of infection but others suggesting that no infection occurs21, 32. Given the low level of HIV-infected DCs, it is unlikely that direct infection can adequately explain the decreased DC frequency in HIV patient blood.
Instead, the decline in blood DC frequency during viral infection may be due to indirect mechanisms. Aberrant IFNα production during HIV infection may impede the differentiation of cDCs from monocytes 33 or other precursors. Also, DCs from patients with HIV show increased apoptosis compared with DCs from uninfected individuals 34.
A third possibility is that decreased DC frequencies in the blood may be due to the redistribution of DCs to secondary lymphoid organs. Indeed, several groups have reported an increase of pDCs in the lymph nodes 35–36 and spleens 37 of patients with HIV. The overall functional capacity of DCs found in secondary lymphoid organs of patients with HIV remains to be adequately evaluated.
Finke et al. reported that cDC numbers returned to normal levels following antiretroviral therapy (ART) 38. A recent study showed no effect of primary HIV-1 infection on cDC levels and, although pDC and CD4+ T cell numbers were reduced, they were restored following ART39. Differential restoration of cDC and pDC numbers may stem from multiple components of the immune system going awry beyond repair in the chronic phase. Altogether, these data warrant a closer scrutiny of the functional status of DC subsets during the immune recovery associated with ART.
Altered cDC function
Although there is overall consensus that blood DCs decline during HIV infection, it is still unclear whether DCs from patients with HIV are functionally impaired. As indicated previously, HIV interaction with DC-SIGN might negatively affect TLR-induced activation of cDCs. Accordingly, HIV-1 infection interferes with the maturation of blood cDCs and monocyte-derived DCs in response to in vitro activation stimuli 28, 40.
Other studies have reported that cDCs are not functionally defective following HIV exposure 20–21, showing that ex vivo isolated cDCs from patients with acute HIV infection may even be hyperresponsive to TLR stimulation 20 and produce increased levels of IL-12, IL-6, TNF and CCL3 (MIP1β) compared with cDCs from uninfected controls. By contrast, compared to pDCs isolated from uninfected controls, pDCs isolated from patients with acute HIV infection can show decreased IFNα production during the early stages infection, but increased IFNα production at later stages of infection 20.
These discordant results may result from the limitations of analyzing isolated DCs ex vivo. Modulation of DC function in vivo may not necessarily be a result of direct HIV exposure, but instead be due to factors that are produced by the host in response to infection. Moreover, depending on the stage of HIV infection, DCs may be affected by the microenvironment. For example, during chronic HIV infection, monocytes have been shown to upregulate programmed cell death 1 (PD1) and produce IL-10, possibly in response to the high levels of gut-derived microbial products that are present in the plasma of these patients 41. Circulating LPS and other pathogen-derived factors have been implicated in promoting the chronic immune activation that is observed during HIV infection either through direct activation of DCs, or indirectly, through stimulation of cells such as macrophages 42. Such stimuli may lead to partial maturation of cDCs in vivo, making these cells tolerant to subsequent stimuli ex vivo. Accordingly, several reports have indicated that cDCs from chronically infected patients are less efficient at stimulating T cell activation than cells from uninfected individuals 31, 40.
Understanding exactly how cDCs are functionally impaired during HIV infection is crucial for effective HIV vaccine design. This is especially true if functional impairment of DCs results from bystander mechanisms during HIV infection as these mechanisms are likely to impede most DC-targeting approaches, including the reintroduction of ex vivo manipulated DCs into patients.
Alterered pDC function
As with cDC function, it is unclear whether pDC function is impaired during HIV infection. Several groups have argued that type I IFN production by blood pDCs is attenuated during both acute and chronic HIV infection 43–44, whereas others have indicated that circulating pDCs in HIV viremic patients show a normal type I IFN response45. In the former case, it is possible that circulating pDCs were activated in vivo by HIV prior to isolation and were therefore hyporesponsive to a secondary stimulus delivered ex vivo. Consistent with this, activation markers are upregulated by blood pDCs during acute HIV infection, suggesting that these pDCs have responded to virus in vivo. Furthermore, IFN-producing pDCs accumulate in draining lymph nodes of HIV patients 35–36, although these pDCs are prone to apoptosis 34, 36. Nonetheless, evidence is emerging that pDCs have a crucial role in the immune dysregulation that leads to the development of AIDS.
pDCs that are exposed to HIV upregulate the enzyme indoleamine 2,3-dioxygenase (IDO), which metabolizes tryptophan to kynurenine, and this upregulation is dependent on the gp120–CD4 interaction. Through expression of IDO, pDCs promote the differentiation of naive T cells into T regulatory (TReg) cells, which suppress effector T cell activation 46. It remains to be elucidated if increased frequencies of TReg cells prevent effective HIV-specific adaptive immune responses and exacerbate HIV-assiociated pathology, or whether TReg cells increase as a result of chronic immune activation in an effort to limit associated immunopathology. By promoting TReg cell responses, pDCs may be responsible for the decrease in TH17 cells that occurs during HIV infection 47 and is thought to lead to the loss of gut integrity and microbial translocation in patients with HIV 42.
Response to HIV-1 in humans: an overkill?
Uncontrolled and persistent inflammation contributes to the pathology associated with HIV infection. IFNα-producing pDCs can contribute to the T cell loss that is characteristic of patients with AIDS by upregulating the expression of the pro-apoptotic molecules TNF-related apoptosis-inducing ligand (TRAIL), DR5, FAS and FASL on CD4+ T cells, 48,49. This may lead to apoptosis of uninfected CD4+ T cells and promote generalized CD4+ T cell loss. Interestingly, HIV-infected women are almost two times more likely to develop AIDS than HIV-infected men with similar viral loads, and this may be because activated pDCs from HIV-infected women produce more type I IFN than pDCs from HIV-infected men 50.
Studies of non-human primate models of SIV infection support the idea that pDCs have pathological roles. Comparing SIV infection in African green monkeys (Cercopithecus aethiops), sooty mangabeys (Cercocebus atys) and macaques (Macaca sp.), it was shown that SIV infection in macaques progresses to AIDS at a rate that is similar to progression to AIDS in HIV-1-infected humans 51. African green monkeys and sooty mangabeys, both of which are natural hosts for SIV, do not develop AIDS despite showing high levels of virus replication 52. Attenuated type I IFN responses are believed to contribute to the lack of SIV-induced immune pathology in sooty mangabeys 53. Gene profiling has shown that in both non-pathogenic (African green monkeys and sooty mangabeys) and pathogenic (macaques) models of SIV infection there is a comparable IFN-inducible response to acute SIV infection, but only in pathogenic models is this IFN response maintained during chronic infection 54–55.
Additional data from non-human primate models suggests that pDCs can facilitate HIV infection. In the vaginal mucosa of macaques, an initial founder population of T cells becomes SIV-infected but the recruitment of additional target T cells is required for viral spread. It was shown that an early influx of activated pDCs occurs at the initial site of SIV infection and these cells subsequently recruit T cells by secreting chemokines such as CCL5 (also known as RANTES) 56.
As already mentioned above, HIV-exposed pDCs can promote the development of TReg cells, and these may prevent the induction of effector T cells by inhibiting cDC activation. Thus pDCs may promote the wide-spread dysregulation of DC function that occurs during HIV infection (Figure 1). Furthermore, since DCs can promote NK cell activation, DC dysfunction may contribute to dysregulated NK cell activity during HIV infection. This is discussed in more detail below.
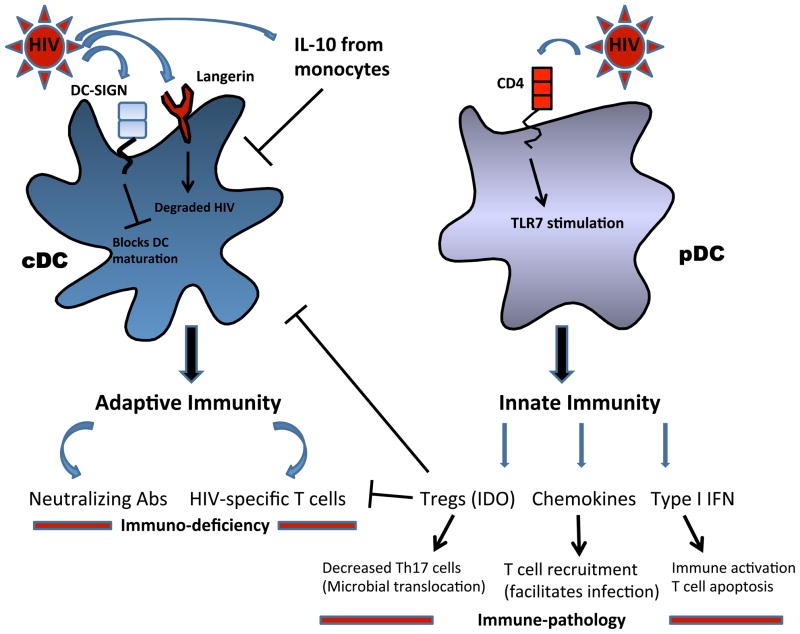
Figure 1. cDCs and pDCs during HIV infection.
Conventional dendritic cells (cDCs) are responsible for initiating antiviral adaptive immunity. Evidence indicates that cDC function is impaired during HIV-1 infection either through direct viral interaction (DC-SIGN interaction, autophagy blockade) or through indirect mechanisms, such as interleukin-10 (IL-10) produced during infection. This cDC impairment could contribute to a lack of effective antiviral adaptive immunity. In contrast, plasmacytoid (pDCs) are mediators of innate immunity. pDCs activated by HIV-1 produce type I interferon (IFN), which in addition to inhibiting viral replication, may also contribute to bystander CD4+ T cell death. Furthermore, evidence shows that pDCs produce T cell-attracting chemokines, which may facilitate viral spread by providing a source of new T cells for HIV to infect. Finally, HIV-exposed pDCs prime Tregs which could impair cDC function and block effector T cell activity, further blunting adaptive immunity. Thus, pDCs can promote deleterious immunopathology during HIV-1 infection.
NK cells in HIV-1 infection
NK cells and their receptors
NK cells promote anti-viral and anti-tumour immunity57 by producing pro-inflammatory cytokines and by lysing infected or transformed cells. In addition, NK cells interact with T cells and DCs to shape the magnitude and quality of adaptive immune responses 58–59. To date, no specific NK cell receptors that directly recognize HIV-1-infected cells have been identified, and the NK cell response to HIV-1 infected cells appears to be regulated by the balance of inhibitory and activating signals delivered to NK cells by these infected cells. Below, we discuss the mechanisms by which NK cell receptors determine NK cell function in response to viral infections with particular focus on HIV-1.
NK cell responses are induced against cells that lack expression of MHC class I molecules for NK cell inhibitory receptors 60 (‘missing self’ recognition) or by cells that overexpress ligands for NK cell activating receptors. In addition to their function in controlling NK cell effector functions, cognate interactions between inhibitory receptors and their ligands during NK cell development confers both self-tolerance and functionality to NK cells, a process termed ‘licensing’ (Box 1).
Box 1. NK cell licensing.
Natural Killer (NK) cell inhibitory receptors for self MHC class I molecules have important roles in controlling the NK cell response to potential target cells, and more recently, have been shown to ensure NK cell tolerance towards self. Studies in humans and mice have shown that during development NK cells undergo an education process, in which MHC class I-specific inhibitory receptors are involved in the calibration of NK cell effector functions97, 99. Engagement of these receptors with MHC class I molecules provides a positive signal to NK cells that leads to licensing of fully competent mature peripheral NK cells that can sense and lyse autologous cells that have downregulated MHC class I molecules97. NK cells that fail to undergo this education process through lack of engagement of MHC-specific inhibitory receptors are unlicensed and have reduced (but not absent) functional activity compared with licensed NK cells97–99. Thus two major types of self-tolerant NK cells exist with regard to MHC class I: NK cells that are licensed and are self-tolerant because they express inhibitory receptors for self, and unlicensed NK cells are also self-tolerant as they are not functionally competent. NK cell licensing does not seem to be an ‘on–off’ switch but more a dynamic and quantitative process125–126. The strength of interaction between inhibitory MHC class I-specific receptors with cognate MHC class I molecules appears to be proportional to the strength of functional responsiveness. NK cells licensed on stronger inhibitory receptor–MHC interactions respond with increased strength and frequency than NK cells licensed on weaker inhibitory signals125. Indeed, a hierarchy of responses to MHC class I-negative cells by NK cells has been observed that is proportional to the number of different killer inhibitory receptors (KIRs) for self-HLA. Certain KIR and HLA alleles are also associated with more responsive NK cells100, 127, suggesting that KIR–HLA combinations can differentially impact NK cell effector capacities.
The most frequently studied inhibitory receptors include the highly polymorphic killer immunoglobulin-like receptors (KIRs), which are specific for classical MHC class I molecules, and the non-polymorphic CD94–NKG2A receptor that recognizes the non-classical MHC molecule HLA-E. NK cell activating receptors include NKG2D, activating KIRs, the natural cytotoxicity receptors (NCRs) and CD16. A comprehensive list of inhibitory and activating receptors expressed on NK cells is beyond the scope of this Review and is summarized elsewhere61.
The major population (approximately 90%) of NK cells in the peripheral circulation are CD56low, constitutively express a large amount of cytolytic granules and have the capacity to spontaneously lyse NK-sensitive targets in the absence of prior sensitization. CD56low NK cells express abundant levels of KIRs, C-type lectins and natural cytotoxicity receptors (NCRs), and are thought to be functionally and phenotypically mature cells. The remaining 10% of circulating NK cells are CD56hi and are often referred to as the ‘immunoregulatory’ NK cell subset as they are poorly cytotoxic, but following stimulation, secrete large amounts of pro-inflammatory cytokines. CD56hi NK cells express high levels of C-type lectins and NCRs, but express little or no KIRs. Although originally thought to be distinct cell lineages, it is now believed that these two NK cell subsets represent different stages of NK cell maturation with CD56hi NK cells being less mature than CD56low NK cells62–63. In addition, a third population of CD56− NK cells has been described that accumulates during chronic viral infections 64–65,66. These CD56− NK cells express a similar receptor profile to CD56low NK cells, but are poorly cytotoxic and do not secrete cytokines. However, cord blood CD3−CD56− NK cells appear to be functionally competent67, suggesting differences in the differentiation status of these phenotypically similar NK cell subsets.
NK cells and the antiviral response
Several mechanisms have been proposed for NK cell-mediated recognition of HIV-1 infected cells (Figure 2). NK cells may be able to detect HIV-infected cells directly through receptor-mediated interactions that as yet have not been identified, or indirectly, following antibody-mediated cross-linking of CD16 (an Fc receptor for IgG). NK cell responses to HIV-1-derived peptides have also been observed, although whether these responses are mediated through CD16 or other NK cell receptors remains to be elucidated 68–69. Thus identification of receptors that mediate NK cell recognition of HIV-1 remains an important research topic.
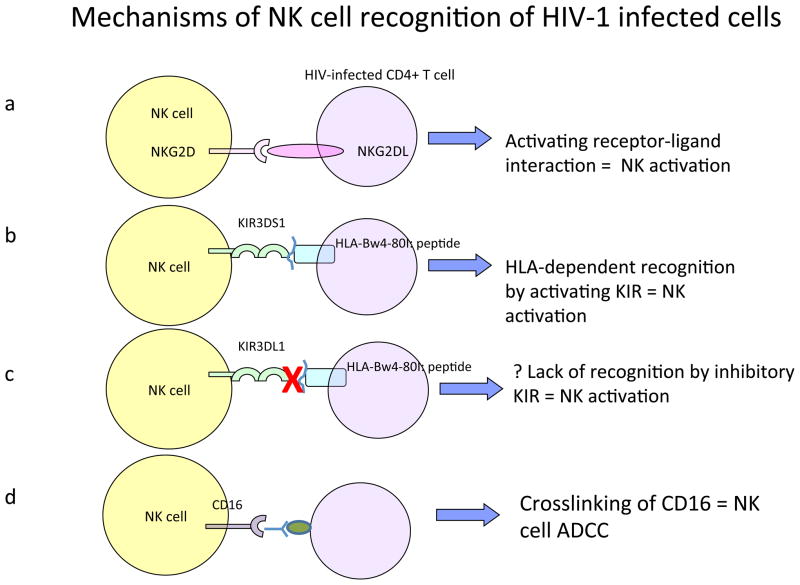
Figure 2. NK cell-mediated recognition of HIV-1-infected cells.
To date, it is not fully understood how natural killer (NK) cells recognize HIV-1-infected cells, and different mechanisms have been proposed. The expression of ligands for activating NK cells receptors on infected cells, such as NKG2D-ligands, results in the direct activation of NKG2D+ NK cells and target cell lysis (a). Changes in the epitopes presented by HLA class I molecules might allow for the engagement of activating killer inhibitory receptors (KIR) receptors, and resulting NK cell activation (b). Similarly, changes in HLA class I presented epitopes on HIV-1-infected cells can result in the disruption of the binding of inhibitory KIRs, leading to NK cell activation (c). Finally, antibodies binding to HIV-1-infected cells can crosslink CD16 and activate CD16+ NK cells (d).
A number of studies have suggested that HIV-1 uses specific strategies to evade NK cells. The HIV-expressed negative regulatory factor (NEF) is known to selectively downregulate expression of HLA-A and HLA-B, but not HLA-C or HLA-E, in infected cells. This allows HIV to evade CTL responses, which are directed against HLA-A and HLA-B, but preventing killing of HIV-infected cells by NK cells, which express inhibitory receptors for HLA-C and/or HLA-E 70–72.. Furthermore, NEF can impair NK cell activity by mediate downregulating ligands for NKG2D on infected cells, specifically MICA, ULBP1 and ULBP2 73.
Altered NK cell phenotype and function during HIV-1 infection
HIV-1 infection is associated with a functional impairment of NK cells that is evident early after infection and continues during disease progression. There is an inverse correlation between NK cell-mediated suppression of HIV-1 replication and viraemia, which may be attributed to several possible mechanisms 74. First, peripheral NK cells in HIV-1-infected individuals have decreased intracellular stores of perforin and granzyme A, and this may account for the decreased cytotoxic capacity of NK cells 75. Persistent HIV-1 viraemia has also been shown to result in aberrant expression of several inhibitory and activating NK cell surface receptors. Lower expression of NCRs is associated with decreased in vitro cytotoxicity of NK cells from HIV-infected individuals 76, which may affect NK cell-mediated clearance of virus-infected cells in vivo. However, in other studies NCR-mediated NK cell activation has been suggested to contribute to pathology by promoting the loss of uninfected CD4+ T cells during HIV infection77. Therefore, the role of NCRs in controlling or contributing to HIV-1 pathology is currently not understood and further investigation is required. Second, a significant proportion (15–25%) of NK cells in the peripheral circulation of HIV-1 infected individuals express some (for example, HLA-DR, CD69) but not other (for example, CD25, NKp44) markers of activated NK cells 78. This suggests that there is an incomplete activation of NK cells in these individuals, which may be due to chronic stimulation resulting in NK cell exhaustion and anergy. Additionally, although overall NK cell numbers are unchanged during HIV infection, infected individuals have fewer CD3−CD56+ NK cells and show expansion of the functionally anergic CD3−CD56− NK cell subset 64–65.
HIV-1 infection has also been associated with an expansion of NK cell populations expressing the activating NKG2C receptor and decreased expression of the inhibitory NKG2A receptor (predominantly on CD56low NK cells) 79. This may alter the balance of NK cells responses towards activation. NKG2C is functional in HIV+ individuals 80; however it is not known how NKG2C functions in HIV infection in the context of diminished NCR-mediated responses. An increase in circulating NKG2C+ NK cells in healthy individuals correlates with cytomegalovirus (CMV) seropositive status 79 and it is possible that the expansion of NKG2C+ NK cell populations during HIV-1 infection may be attributable to reactivation of CMV in HIV-1 infected individuals. Indeed, perturbation of the NK cell compartment during HIV-1 infection may have serious consequences for protection from opportunistic infections.
Finally, NK cells that express CD4 and CXCR4 can be infected with HIV-1 in vitro, resulting in altered function81. This suggests that CD4+ NK cells may be a reservoir for HIV-1 in vivo and further investigation is required to explore this possibility.
KIRs and HLA molecules in HIV infection
Early studies showed that the control of HIV replication is associated with the expression of certain HLA-Bw4 alleles 82, including HLA-B*57 and HLA-B*27, which serve as ligands for the NK cell inhibitory receptor KIR3DL1 and as putative ligands for the NK cell activating receptor KIR3DS1 (Figure 2).
KIR3DS1 and HLA-Bw4 80I
Martin et al 83 first demonstrated the impact of KIRs and HLA molecules on the outcome of HIV infection. The first study to demonstrate a link between expression of KIR and HLA molecules and the outcome of HIV infection, showed that individuals expressing KIR3DS1 in conjunction with HLA-Bw4 80I (HLA-Bw4 alleles that have an isoleucine at position 80) have a slower progression to AIDS than individuals that express either one or none of these molecules. These findings were supported by studies demonstrating that KIR3DS1+ NK cells preferentially expand during primary HIV-1 infection in HLA-Bw4 80I+ subjects 85 and efficiently suppress HIV-1 replication in HLA-Bw4 80I+ target cells84. Furthermore, KIR3DS1 expression has been associated with enhanced NK cell function during primary HIV-1 infection86, and a higher proportion of individuals homozygous for KIR3DS1 was observed in one cohort of HIV-1-exposed but persistently uninfected individuals 87.
However, studies aimed at demonstrating direct interaction of KIR3DS1 and HLA-Bw4 80I have been unconvincing 88–89. Two hypotheses are proposed in the literature. First, KIR3DS1 may recognize HLA-Bw4 80I in the presence of HLA-presented viral peptides or stress-induced self peptides 90. Indeed HIV-1 infection has been shown to uniquely alter the presentation of host-encoded peptides by MHC class I molecules 91. Second, recognition of HLA-Bw4 80I by KIR3DS1 might require an additional cellular protein that is expressed during HIV-1 infection. This hypothesis is supported by evidence from a model of murine cytomegalovirus infection (MCMV), where the activating receptor Ly49P was shown to interact with H2-Dk and provide protection from MCMV only in the presence of a third, as-yet undefined protein 92–93.
KIR3DL1 and HLA-Bw4 80I
Certain alleles of the inhibitory receptor KIR3DL1 have been shown to provide protection against HIV-1 disease progression 94. KIR3DL1 is highly polymorphic, and different alleles result in different levels of KIR3DL1 protein expression on NK cells 95–96. A genetic study on a large cohort of HIV-1 infected individuals revealed that individuals co-expressing high levels of KIR3DL1 and HLA-Bw4 80I had slower HIV-1 disease progression than individuals co-expressing low levels of KIR3DL1 and HLA Bw4-80I94. Furthermore, KIR3DL1+ NK cell populations are expanded in HIV+ individuals in the presence of HLA-Bw4-80I, highlighting the importance of this receptor–ligand interaction in HIV-1 infection85.
These data showing a protective effect for an inhibitory KIR in HIV infection seem to contradict previous reports, which suggested NK cell activation is protective during HIV infection, but could be explained by the interactions of KIR with HLA during NK cell development. As described in Box 1, developing NK cells undergo a licensing process, where engagement of inhibitory KIRs by HLA molecules arms NK cells with functional capacity 97–99. The strength of interaction between inhibitory KIRs and HLA molecules at this licensing step proportionately strengthens later functional capacity. Indeed, studies have shown that KIR3DL1+ NK cells from individuals homozygous for HLA-Bw4 respond more potently to MHC class I-negative cells than individuals expressing only one copy of HLA-Bw4 100. KIR3DL1hi NK cells licensed on strong inhibitory signals would therefore respond more strongly to NEF-mediated downregulation of HLA-Bw4 than KIR3DL1low NK cells. Interestingly, protection against HIV-1 disease progression was also observed in individuals with the KIR3DL1*004, an allotype of KIR3DL1 this is not expressed on the cell surface, but detected intracellularly 94. The mechanism behind this protection is unknown but suggests a role for intracellular KIR–HLA interactions in modulating antiviral immunity.
NK cell licensing might also explain the protective effect of a single nucleotide polymorphism (SNP) that was recently identified in a genome-wide association study (GWAS) 101. In this study, a dimorphism 35kb upstream of the HLA-C locus that is associated with higher levels of HLA-C mRNA and surface expression 101–102 was shown to be associated with better control of HIV-1 viraemia. It is possible that during NK cell development, the interaction of NK cells expressing inhibitory KIR2D receptors with cells expressing high levels of HLA-C (the ligand for KIR2D receptors) may promote the functional capacity of these NK cells.
In summary, recent genetic and functional studies have shown a protective effect for certain KIRs and HLA molecules during HIV-1 disease progression. The mechanisms underlying protection are thought to involve increased NK cell activity against HIV-1-infected cells; however, no data currently exists regarding the role of NK cells during HIV-1 infection in vivo. A better understanding of the functions of NK cells during HIV-1 infection will be required in order to harness these important innate effector cells in vaccine-induced responses, particularly in the context of the recent description of NK cell-mediated recall responses in mice 103–104
DC and NK cell crosstalk in HIV
In addition to their own antiviral functions, NK cells can regulate antiviral immunity by modulating DC function58, 105–110. Crosstalk of NK cells and DCs results in activation of both cell types, with DCs upregulating NK cell effector functions and NK cells inducing further maturation of DCs (Figure 3). Fernandez et al. 58 initially showed that DCs can promote NK cell activity against tumours in vivo and several subsequent studies have explored DC-mediated ‘priming’ of NK cells4, 111. Both cytokine production and cell–cell interactions have been found to be involved in this process. Cytokines produced by cDCs, such as IL-12 and IL-18, can promote NK cell production of IFNγ in vitro4, 106, 108, 111, and pDC production of type I IFNs as well as cell-cell cont act is required to promote NK cell proliferation and cytotoxicity58, 105, 107–108 (Figure 3).
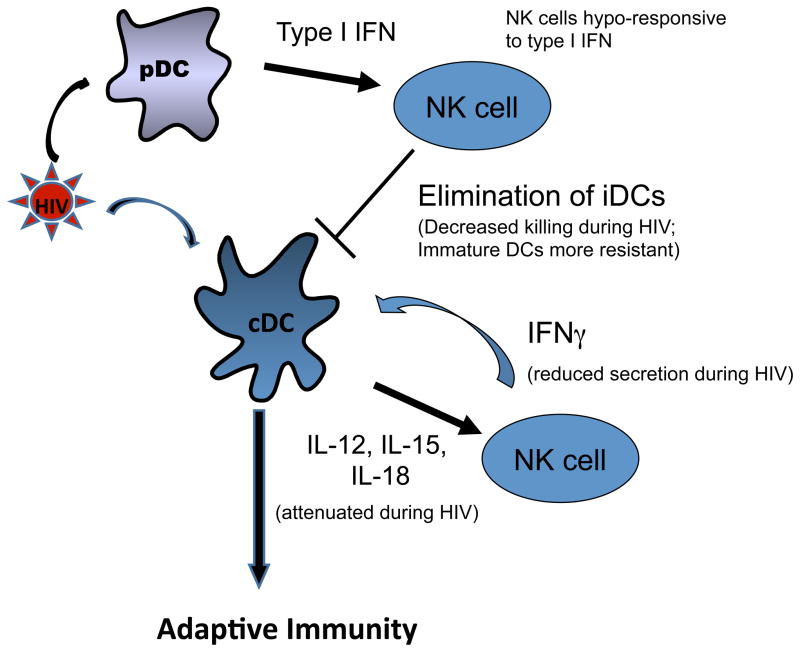
Figure 3. DC–NK cell crosstalk.
Dendritic cells (DCs) are activated by HIV-1 and secrete pro-inflammatory cytokines, including interleukin-12 (IL-12), IL-15 and type I interferon (IFN) that stimulate natural killer (NK) cells. Activated NK cells secrete IFNγ that promotes DC maturation and skewing towards T helper 1 (TH1)-type immune responses. Furthermore, NK cells can eliminate incompletely matured DCs, and promote the induction of adaptive T cell immunity through this editing process. HIV-1-induced functional impairment of NK cells, as well as of DCs, interferes with this crosstalk.
Activated NK cells can boost ongoing adaptive responses by producing IFN-γ, which promotes TH1 cell polarization105, 109. Reciprocally, NK cell-mediated activation of DCs has been shown to promote the differentiation of DCs that are more capable of inducing efficient CTL responses110. Furthermore, NK cell-mediated lysis of virally infected cells can provide a source of apoptotic bodies for uptake by DCs; this promotes DC maturation and their presentation of viral antigen to T cells.
NK cells can also kill immature DCs in a process referred to as ‘DC editing’111. In vitro studies have demonstrated that a low ratio of NK cells to immature DCs promotes DC maturation, whereas a higher NK cell to iDC ratio can result in NK-mediated killing of DCs110. This editing process is dependent on NK cell expression of NKp30 111, and seems to be mediated by NK cell subsets that express CD94–NKG2A but lack inhibitory KIRs 112, although other receptors and mechanisms may be involved.
Recent evidence suggests that the crosstalk of NK cells and DCs is disrupted during HIV-1 infection. cDCs from HIV-1-infected individuals show impaired secretion of IL-12, IL-15 and IL-18, resulting in decreased NK cell activation. Additionally, activation of NK cells by pDCs is impaired during HIV infection since NK cells are less responsive to type I IFNs 113. Furthermore, the anergic CD56− NK cells that accumulate during progressive HIV-1 infection do not produce IFNγ and TNFα following stimulation with MHC-devoid target cells, decreasing their ability to promote DC activation 64–65. NK cell-mediated DC editing is also severely compromised during progressive HIV-1 infection, and NK cells from individuals with chronic HIV-1 infection show a decreased ability to kill immature DCs 114. The defect appears to be largely due to an increase in the proportion of CD56− NK cells with impaired NKp30 function 115.
The precise mechanisms by which HIV-1 impairs NK cell and DC crosstalk remain to be fully elucidated. Effects of HIV-expressed NEF and transactivator of transcription (TAT) on DCs and NK cells have been described116–117. HIV-1-infected DCs become resistant to NK cell-mediated lysis and this is associated with the upregulation of cell death inhibitors by infected DCs. These inhibitors are upregulated by the high-mobility group box 1 protein (HMGB1) and protect HIV-1-infected DCs from TRAIL-dependent apoptosis118. Furthermore, crosstalk with NK cells was found to promote viral replication in HIV-1-infected DCs, but this could be prevented by blocking HMGB1 activity119. In addition, increased production of IL-10 during HIV-1 infection can protect immature DCs from NK cell-mediated lysis, resulting in accumulation of partially mature, poorly immunogenic DCs in the lymph nodes of infected individuals 120.
Overall, the dysregulated crosstalk that occurs between NK cells and DCs during progressive HIV-1 infection appears to be a consequence of an impairment of both DC and NK cell functions (Figure 3). Given the critical role of DCs in determining the quality of the adaptive immune response to infections, this compromised editing of DC function by NK cells during HIV-1 infection might have significant consequences for the antiviral T and B cell response. In order to develop interventions aimed at enhancing immunity to HIV-1, further research is required to better understand the molecular mechanisms involved in NK cell–DC crosstalk and how this crosstalk is disrupted during infection with HIV-1
Harnessing DC and NK cells for vaccination
Although HIV-1 infection is considered a chronic infection, immune dysregulation can occur early in infection and affect subsequent disease progression. Given the complexity of HIV infection, it remains unclear how the innate immune system can be harnessed to induce effective protective immunity mediated by T cells and B cells. Understanding how DCs and NK cells are affected during HIV infection may provide new targets for vaccine design or even therapeutic modulation of disease. Administration of inactivated HIV with myeloid DCs enhances immune control of HIV 121 suggesting that functionally intact APCs will be required to limit viral replication. Adjuvants and vaccine vectors that target cDCs and pDCs simultaneously could promote adaptive immunity and limit TReg cell induction in order to control virus entry at mucosal sites, as well as systemically122,123. Improving upon the approach used by a recent vaccine trial in Thailand (which used an HIV envelope immunogen and a canarypox–HIV vector) by using DC-targeted adjuvants and superior vectors in a “prime-boost strategy” could be one such approach.
Given the speed with which HIV gains entry into cells, innate defences need to be rapidly mobilized. DCs could be targeted to activate specific NK cells and promote their cytolytic functions. Epidemiological data has indicated that certain NK receptors, such as KIR3DS1, are important for controlling HIV disease83 and in mouse models, NK cells can develop into protective virus-specific memory cells103, 124. DC-based vaccine strategies that elicit HIV-specific NK cell responses and that maintain memory may be a crucial factor in ensuring the success of future vaccines. Finally, pDCs are emerging as a population with important roles in contributing to HIV-induced pathology. As such, vaccine strategies that aim to promote cDC and NK cell responses during HIV infection would have to be balanced in order to prevent any deleterious consequences of immune activation.
GLOSSARY TERMS
- viral synapses
Polarized synaptic contact points for cell to cell transmission of virus, such as between DCs and CD4+ T cells
- clusterin
Ubiquitously expressed glycoprotein which is an extracellular chaperone and is widely expressed in different types of cancer
- Birbeck granules
Membrane-bound rod- or tennis racket-shaped structures with a central linear density, found in the cytoplasm of Langerhans cells. Their formation is induced by langerin, an endocytic C-type lectin receptor that is specific to Langerhans cells
- Autophagy
An evolutionarily conserved process in which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation, through fusion to secondary lysosomes
- Perforin
A component of cytolytic granules that participates in the permeabilization of plasma membranes, allowing granzymes and other cytotoxic components to enter target cells
- Granzyme A
A member of a family of serine proteinases that are found, primarily, in the cytoplasmic granules of cytotoxic T lymphocytes and natural killer cells. Granzymes enter target cells through perforin pores, then cleave and activate intracellular caspases and lead to target-cell apoptosis
Biographies
Davor Frleta, Ph.D.
Dr. Frleta received his Ph.D. from Dartmouth College (2004). He was a post-doctoral fellow under Jacques Banchereau and Karolina Palucka (Baylor Research Institute, Dallas, TX) working on dendritic cell (DC)-based cancer vaccines. He currently works with Nina Bhardwaj at NYU Langone Medical Center, studying human DCs during HIV infection.
Lena Fadda, Ph.D.
Dr. Fadda received her PhD from Imperial College studying the impact HLA class I presented peptides on NK cell recognition. She is a Ragon Fellow and currently works with Marcus Altfeld at the Ragon Institute of MGH, MIT and Harvard studying the mechanisms by which NK cells recognize HIV-1-infected cells.
Marcus Altfeld, MD/PhD
Dr. Altfeld received his MD and PhD in Medicine from the University of Cologne, and studies the T cell response to HIV-1 as a postdoctoral fellow at the Partners AIDS Research Center, MGH. He is currently an Associate Professor at HMS and directs the Innate Immunity Program at the Ragon Institute of MGH, MIT and Harvard.
Nina Bhardwaj, MD/PhD
Dr. Bhardwaj received her MD and PhD in Medicine from the New York University. She trained in Medicine at the Brigham and Womens Hospital and in Rheumatology at the Hospital for Special Surgery. Following a post-doctoral fellowship at Rockefeller University, she was an Associate Professor there. She is now Professor of Medicine, Pathology and Dermatology at the NYU Langone Medical Center and Director, Tumor Vaccine Program of the NYU Cancer Institute.
References
- 1.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–62. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 3.Rescigno M, Borrow P. The host-pathogen interaction: new themes from dendritic cell biology. Cell. 2001;106:267–70. doi: 10.1016/s0092-8674(01)00454-8. [DOI] [PubMed] [Google Scholar]
- 4.Ferlazzo G, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606–11. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani C, et al. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur J Immunol. 2005;35:2452–8. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 7.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–68. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ignatius R, et al. The immunodeficiency virus coreceptor, Bonzo/STRL33/TYMSTR, is expressed by macaque and human skin- and blood-derived dendritic cells. AIDS Res Hum Retroviruses. 2000;16:1055–9. doi: 10.1089/08892220050075318. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B, Ashlock BM, Foster H, Fujimura SH, Levy JA. HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology. 2005;343:256–66. doi: 10.1016/j.virol.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 11.Boggiano C, Manel N, Littman DR. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J Virol. 2007;81:2519–23. doi: 10.1128/JVI.01661-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabatte J, et al. Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J Virol. 2007;81:13723–34. doi: 10.1128/JVI.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112:1299–307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Witte L, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–71. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 15.Fahrbach KM, et al. Activated CD34-derived Langerhans cells mediate transinfection with human immunodeficiency virus. J Virol. 2007;81:6858–68. doi: 10.1128/JVI.02472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beignon AS, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–75. doi: 10.1172/JCI26032. This study shows how HIV stimulates plasmacytoid dendritic cells (pDCs) for type I interferon (IFN) production through Toll-like receptor 7 (TLR7) signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier A, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–91. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda K, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 20.Sabado RL, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010 doi: 10.1182/blood-2010-03-273763. This study examines changes that occur in conventional (cDC) and pDC frequency and function over the course of early HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smed-Sorensen A, et al. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J Virol. 2005;79:8861–9. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manel N, et al. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–7. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gringhuis SI, et al. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–26. doi: 10.1038/ni.1858. This work indicates how productive HIV infection in cDCs is induced by TLR8 signalling that initiates viral RNA transcription and DC-SIGN signalling which promotes transcript elongation. [DOI] [PubMed] [Google Scholar]
- 25.Deretic V. Multiple regulatory and effector roles of autophagy in immunity. Curr Opin Immunol. 2009;21:53–62. doi: 10.1016/j.coi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanchet FP, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–69. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granelli-Piperno A, Shimeliovich I, Pack M, Trumpfheller C, Steinman RM. HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J Immunol. 2006;176:991–8. doi: 10.4049/jimmunol.176.2.991. [DOI] [PubMed] [Google Scholar]
- 28.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci U S A. 2004;101:7669–74. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harman AN, et al. HIV-1-infected dendritic cells show 2 phases of gene expression changes, with lysosomal enzyme activity decreased during the second phase. Blood. 2009;114:85–94. doi: 10.1182/blood-2008-12-194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulin LF, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–71. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 32.Cameron PU, et al. Preferential infection of dendritic cells during human immunodeficiency virus type 1 infection of blood leukocytes. J Virol. 2007;81:2297–306. doi: 10.1128/JVI.01795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodama A, et al. Impairment of in vitro generation of monocyte-derived human dendritic cells by inactivated human immunodeficiency virus-1: Involvement of type I interferon produced from plasmacytoid dendritc cells. Hum Immunol. 2010;71:541–50. doi: 10.1016/j.humimm.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Meera S, Madhuri T, Manisha G, Ramesh P. Irreversible loss of pDCs by apoptosis during early HIV infection may be a critical determinant of immune dysfunction. Viral Immunol. 2010;23:241–9. doi: 10.1089/vim.2009.0112. [DOI] [PubMed] [Google Scholar]
- 35.Dillon SM, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehmann C, et al. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS One. 2010;5:e11110. doi: 10.1371/journal.pone.0011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nascimbeni M, et al. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-alpha expression. Blood. 2009;113:6112–9. doi: 10.1182/blood-2008-07-170803. [DOI] [PubMed] [Google Scholar]
- 38.Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–52. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 39.Killian MS, Fujimura SH, Hecht FM, Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS. 2006;20:1247–52. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- 40.Martinson JA, et al. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Said EA, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4(+) T cell activation during HIV infection. Nature Medicine. 2010;16:452–U136. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 43.Kamga I, et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–10. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 44.Sachdeva N, Asthana V, Brewer TH, Garcia D, Asthana D. Impaired restoration of plasmacytoid dendritic cells in HIV-1-infected patients with poor CD4 T cell reconstitution is associated with decrease in capacity to produce IFN-alpha but not proinflammatory cytokines. J Immunol. 2008;181:2887–97. doi: 10.4049/jimmunol.181.4.2887. [DOI] [PubMed] [Google Scholar]
- 45.Lehmann C, et al. Increased interferon alpha expression in circulating plasmacytoid dendritic cells of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2008;48:522–30. doi: 10.1097/QAI.0b013e31817f97cf. [DOI] [PubMed] [Google Scholar]
- 46.Manches O, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–9. doi: 10.1172/JCI34823. This paper shows how HIV-stimulated pDCs induce regulatory T (TReg) cell differentiation through upregulation of indoleamine 2,3-dioxygenase (IDO) on pDCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Favre D, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herbeuval JP, et al. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2005;102:13974–9. doi: 10.1073/pnas.0505251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbeuval JP, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006;103:7000–5. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meier A, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMichael AJ. HIV vaccines. Annu Rev Immunol. 2006;24:227–55. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- 52.Manches O, Bhardwaj N. Resolution of immune activation defines nonpathogenic SIV infection. J Clin Invest. 2009;119:3512–5. doi: 10.1172/JCI41509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mandl JN, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–87. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 54.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–72. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacquelin B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–55. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Q, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez NC, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 59.Mailliard RB, et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–73. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 60.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 61.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romagnani C, et al. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–55. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 63.Yu J, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115:274–81. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mavilio D, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A. 2005;102:2886–91. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alter G, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–9. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez VD, et al. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol. 2009;183:6612–8. doi: 10.4049/jimmunol.0901437. [DOI] [PubMed] [Google Scholar]
- 67.Lu X, et al. CD16+ CD56- NK cells in the peripheral blood of cord blood transplant recipients: a unique subset of NK cells possibly associated with graft-versus-leukemia effect. Eur J Haematol. 2008;81:18–25. doi: 10.1111/j.1600-0609.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 68.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82:5450–9. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tiemessen CT, et al. Cutting Edge: Unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J Immunol. 2009;182:5914–8. doi: 10.4049/jimmunol.0900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 71.Cohen GB, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–71. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 72.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–94. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 73.Cerboni C, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88:242–50. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 74.Kottilil S, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187:1038–45. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 75.Portales P, et al. Interferon-alpha restores HIV-induced alteration of natural killer cell perforin expression in vivo. AIDS. 2003;17:495–504. doi: 10.1097/00002030-200303070-00004. [DOI] [PubMed] [Google Scholar]
- 76.De Maria A, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–8. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 77.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Natl Acad Sci U S A. 2005;102:10981–6. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fogli M, et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol. 2004;34:2313–21. doi: 10.1002/eji.200425251. [DOI] [PubMed] [Google Scholar]
- 79.Mela CM, et al. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: lack of reversion with highly active antiretroviral therapy. AIDS. 2005;19:1761–9. doi: 10.1097/01.aids.0000183632.12418.33. [DOI] [PubMed] [Google Scholar]
- 80.Guma M, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–71. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 81.Bernstein HB, et al. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. Virology. 2009;387:59–66. doi: 10.1016/j.virol.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flores-Villanueva PO, et al. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc Natl Acad Sci U S A. 2001;98:5140–5. doi: 10.1073/pnas.071548198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin MP, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. This study first demonstrated the protective effect of KIR–HLA combinations in HIV-1 disease progression and showed that individuals expressing KIR3DS1 in conjunction with its putative ligand (HLA-Bw4 80I) have a slower progression to AIDS compared with individuals lacking either or both receptor–ligand pairs. [DOI] [PubMed] [Google Scholar]
- 84.Alter G, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–36. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alter G, et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83:6798–805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Long BR, et al. Conferral of enhanced natural killer cell function by KIR3DS1 in early human immunodeficiency virus type 1 infection. J Virol. 2008;82:4785–92. doi: 10.1128/JVI.02449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boulet S, et al. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS. 2008;22:595–9. doi: 10.1097/QAD.0b013e3282f56b23. [DOI] [PubMed] [Google Scholar]
- 88.Gillespie GM, et al. Lack of KIR3DS1 binding to MHC class I Bw4 tetramers in complex with CD8+ T cell epitopes. AIDS Res Hum Retroviruses. 2007;23:451–5. doi: 10.1089/aid.2006.0165. [DOI] [PubMed] [Google Scholar]
- 89.Carr WH, et al. Cutting Edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J Immunol. 2007;178:647–51. doi: 10.4049/jimmunol.178.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Altfeld M, Goulder P. ‘Unleashed’ natural killers hinder HIV. Nat Genet. 2007;39:708–10. doi: 10.1038/ng0607-708. [DOI] [PubMed] [Google Scholar]
- 91.Hickman HD, et al. Cutting edge: class I presentation of host peptides following HIV infection. J Immunol. 2003;171:22–6. doi: 10.4049/jimmunol.171.1.22. [DOI] [PubMed] [Google Scholar]
- 92.Lee SH, et al. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet. 2001;28:42–5. doi: 10.1038/ng0501-42. [DOI] [PubMed] [Google Scholar]
- 93.Kielczewska A, et al. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J Exp Med. 2009;206:515–23. doi: 10.1084/jem.20080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin MP, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–40. doi: 10.1038/ng2035. This large genetic study shows that individuals encoding for a KIR3DL1hi and HLA-Bw4 80I+ phenotype had slower HIV-1 disease progression than individuals encoding for a KIR3DL1low and HLA-Bw4 80I+ phenotype, indicating that KIR–HLA interactions can enhance the protective effect of HLA-Bw4 alleles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yawata M, et al. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006;203:633–45. doi: 10.1084/jem.20051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomas R, et al. Novel KIR3DL1 alleles and their expression levels on NK cells: convergent evolution of KIR3DL1 phenotype variation? J Immunol. 2008;180:6743–50. doi: 10.4049/jimmunol.180.10.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 98.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 100.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105:3053–8. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fellay J, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–7. doi: 10.1126/science.1143767. This genome-wide association study showed that a protective single-nucleotide polymorphism associated with higher HLA-C expression correlates with better control of HIV-1 viraemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas R, et al. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat Genet. 2009;41:1290–4. doi: 10.1038/ng.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–16. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 105.Kikuchi T, et al. Dendritic cells pulsed with live and dead Legionella pneumophila elicit distinct immune responses. J Immunol. 2004;172:1727–34. doi: 10.4049/jimmunol.172.3.1727. [DOI] [PubMed] [Google Scholar]
- 106.Gerosa F, et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gerosa F, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–34. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 108.Borg C, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–75. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 109.Martin-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 110.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferlazzo G, et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Della Chiesa M, et al. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657–66. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]
- 113.Reitano KN, et al. Defective plasmacytoid dendritic cell-NK cell cross-talk in HIV infection. AIDS Res Hum Retroviruses. 2009;25:1029–37. doi: 10.1089/aid.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tasca S, et al. Escape of monocyte-derived dendritic cells of HIV-1 infected individuals from natural killer cell-mediated lysis. AIDS. 2003;17:2291–8. doi: 10.1097/00002030-200311070-00003. [DOI] [PubMed] [Google Scholar]
- 115.Mavilio D, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203:2339–50. doi: 10.1084/jem.20060894. This paper shows that natural killer (NK) cell-mediated DC editing is severely impaired in progressive HIV-1 infection, and appears to be due to an increased accumulation of CD56− NK cells with impaired NKp30 function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quaranta MG, et al. HIV-1 Nef impairs the dynamic of DC/NK crosstalk: different outcome of CD56(dim) and CD56(bright) NK cell subsets. FASEB J. 2007;21:2323–34. doi: 10.1096/fj.06-7883com. [DOI] [PubMed] [Google Scholar]
- 117.Poggi A, et al. NK cell activation by dendritic cells is dependent on LFA-1-mediated induction of calcium-calmodulin kinase II: inhibition by HIV-1 Tat C-terminal domain. J Immunol. 2002;168:95–101. doi: 10.4049/jimmunol.168.1.95. [DOI] [PubMed] [Google Scholar]
- 118.Melki MT, Saidi H, Dufour A, Olivo-Marin JC, Gougeon ML. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLoS Pathog. 2010;6:e1000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saidi H, Melki MT, Gougeon ML. HMGB1-dependent triggering of HIV-1 replication and persistence in dendritic cells as a consequence of NK-DC cross-talk. PLoS One. 2008;3:e3601. doi: 10.1371/journal.pone.0003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alter G, et al. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010;120:1905–13. doi: 10.1172/JCI40913. This work highlights that interleukin-10 (IL-10) secreted in response to HIV-1 renders immature DCs resistant to NK cell-mediated killing resulting in the accumulation of poorly immunogenic DCs in the lymph nodes of infected patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–65. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 122.Hansen SG, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wyand MS, et al. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73:8356–63. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–41. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 126.Yu J, et al. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–89. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 127.Yawata M, et al. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008;112:2369–80. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]