Abstract
Low-density lipoprotein receptor-related protein 2 (LRP2) is a multifunctional cell surface receptor conserved from nematodes to humans. In mammals, it acts as regulator of sonic hedgehog and bone morphogenetic protein pathways in patterning of the embryonic forebrain and as a clearance receptor in the adult kidney. Little is known about activities of this LRP in other phyla. Here, we extend the functional elucidation of LRP2 to zebrafish as model organism of receptor (dys)function. We demonstrate that expression of Lrp2 in embryonic and larval fish recapitulates the patterns seen in mammalian brain and kidney. Furthermore, we studied the consequence of receptor deficiencies in lrp2 and in lrp2b, a homologue unique to fish, using ENU mutagenesis or morpholino knockdown. While receptor-deficient zebrafish suffer from overt renal resorption deficiency, their brain development proceeds normally, suggesting evolutionary conservation of receptor functions in pronephric duct clearance but not in patterning of the teleost forebrain.
Keywords: megalin, forebrain development, LDL receptor-related proteins, pronephros
INTRODUCTION
Low-density lipoprotein (LDL) receptor-related protein 2 (LRP2) is a member of the LDL receptor gene family, a group of multifunctional cell surface receptors that perform many cellular functions including endocytosis, cell migration, antigen presentation, and signal transduction (Herz et al., 2000). LRP2 (also known as megalin) represents the archetypical receptor of the gene family as it is conserved throughout evolution from nematodes to insects to vertebrates (Christensen and Birn, 2002).
Gene targeting experiments in the mouse have elucidated distinct functions for LRP2 in the embryonic and in the adult mammalian organism. In the early mouse embryo, LRP2 is expressed on the apical surface of the neural tube and lack of the receptor in Lrp2−/− mice causes disturbances in forebrain formation and holoprosencephaly (Willnow et al., 1996). The underlying developmental defect has been linked to an impaired dorso-ventral patterning of the forebrain caused by altered expression of sonic hedgehog (Shh) and bone morphogenetic protein (Bmp) 4 (Spoelgen et al., 2005). Although the exact mechanism of LRP2 action in neural tube patterning still awaits elucidation, these observations indicate a crucial role for the receptor in regulation of key morphogen pathways in the developing CNS.
In adult mice, expression of LRP2 is restricted to absorptive tissues including epithelia in the kidney, urogenital tract, and brain ventricles (Zheng et al., 1994). In the proximal tubules of the kidney, LRP2 acts as clearance pathway for filtered plasma proteins that transport lipophilic vitamins and steroid hormones. Loss of the receptor in LRP2-deficient adult mice results in urinary excretion of vitamin-carrier complexes and in multiple vitamin deficiencies (Christensen et al., 1999; Nykjaer et al., 1999). Remarkably, disturbed brain formation and renal resorption deficits are phenotypes shared by humans suffering from Donnai-Barrow syndrome, an autosomal recessive disorder caused by mutations in LRP2 (Kantarci et al., 2007). These findings demonstrate that embryonic and adult functions of LRP2 are conserved in mammalian species.
Genetic defects in LRP2 also have been identified in the orthologue in Caenorhabditis elegans, designated Ce-LRP1. Recessive mutations in ce-Lrp1 produce a complex phenotype, involving arrested larval growth and the inability to shed the old cuticle during molting (Yochem et al., 1999). The larval growth arrest phenotype in ce-Lrp1-deficient larvae resembles features observed in dauer, a specialized diapause stage of larvae induced by starvation (Grigorenko et al., 2004). Because ce-Lrp1-deficient phenotypes are reproduced in wild type larvae by cholesterol depletion, a role for Ce-LRP1 in cellular uptake of carrier-bound sterols has been proposed – a function that resembles the clearance activity of the receptor in the mammalian kidney (Matyash et al., 2004; Entchev and Kurzchalia, 2005).
In the present study, we have extended the characterization of LRP2 to zebrafish as a model organism of receptor (dys)function. We have elucidated the expression domains of the receptor in the embryonic and in the larval fish that recapitulate spatial but not temporal aspects of patterns seen in mammals. In addition, we have studied the consequence of receptor deficiencies for lrp2 and for lrp2b, a receptor homologue unique to fish, using ENU mutagenesis and morpholino knockdown approaches, respectively. Our studies uncovered evolutionary conservation of functions for this receptor pathway in pronephric duct clearance, but not in patterning of the developing central nervous system (CNS), between mammals and teleosts.
RESULTS
Zebrafish lrp2 recapitulates spatial but not temporal aspects of mammalian receptor expression
Using in situ hybridization (ISH), we characterized the expression pattern of lrp2 in zebrafish embryos. At 20 hpf, expression of the receptor becomes apparent in the developing pronephros (Fig. 1A). Prominent pronephric expression persists throughout all embryonic stages (Fig. 1B–D, F–H). Starting at 24 hpf, lrp2 expression appears in the otic vesicles (asterisks in Fig. 1B–D, F–H). At 36 hpf, expression is also visible in the developing CNS with robust signals in the diencephalon (arrows in Fig. 1D–H) and in the rostral dorsal telencephalon (arrowheads in Fig. 1D and E). Cells expressing Lrp2 in the diencephalon were identified as radial glia lining the surface of the ventricles as demonstrated by co-immunostaining of the receptor with the marker brain lipid-binding protein (Blbp) (Fig. 2). At 72 hpf, signals for lrp2 are also detected in the retinal pigment epithelium of the eye (Fig. 1G).
Figure 1. lrp2 expression in early zebrafish development.
Whole mount in situ hybridization (ISH) for lrp2 in zebrafish embryos at the indicated time points. The asterisks mark expression in the otic vesicle. Expression in the developing CNS is highlighted by arrows. The arrowheads indicate lrp2 expression domains in the telencephalon at 36 hpf in lateral (D) and frontal (E) head aspects. Panels D’ and E’ depict ISH using the sense probe as negative control to the respective images shown in D and E. pn, pronephros.
Figure 2. Expression of Lrp2 in the ventricular system of the brain at 48 hpf.
(A–C) Immunohistology on coronal forebrain sections for Lrp2 (A) and brain lipid-binding protein (Blbp; B) demonstrates co-localization of both proteins in radial glia cells lining the ventricular system (merge; C). (D) Whole mount in situ hybridization for lrp2 indicating the plane of section through the diencephalon shown in panels A through C.
Genetic disruption of lrp2 in bugeye and 5cben zebrafish lines
Recently, two zebrafish lines bugeye (lrp2mw1) and 5cben (lrp2p5bnc) were identified in independent ENU screens for adult ocular defects (see method section for details) (Veth et al., 2011). Positional cloning efforts uncovered nonsense mutations in lrp2 as the underlying cause of the adult onset large eye phenotype in both lines (Fig. 3A–C). Mutations in lrp2 completely disrupt receptor expression as shown by immunohistology of embryonic brain ventricles and eyes (Fig. 3D) and pronephros (Fig. 3E). Overall, Lrp2 deficiency does not impact growth, viability, or reproduction of affected fish (data not shown). Eye phenotypes including enlarged eyes with myopia and elevated intraocular pressure in Lrp2-deficient zebrafish have been described elsewhere (Veth et al., 2011). Here, we focused on the consequences of receptor dysfunction for structural and functional integrity of the kidney and the forebrain, essential functions for LRP2 in the mammalian organism.
Figure 3. Loss of Lrp2 expression in bugeye mutants.
(A) Structure of Lrp2 indicating amino acid changes in bugeye and 5cben lines. (B, C) Appearance of adult wild type and bugeye zebrafish in lateral (B) and dorsal (C) views highlighting the large eye phenotype in mutants. (D) Immunohistological detection of Lrp2 (green) in retinal pigment epithelium (arrow) and ventricular system (arrowhead) in wild type but not in bugeye embryos at 48 hpf using polyclonal antisera directed against multiple epitopes in the extracellular domain of the receptor polypeptide. (E) Loss of Lrp2 expression (red) in immunohistological sections of the pronephros in 4 dpf old bugeye larvae. White lines indicate the position of the pronephric ducts. Complete loss of Lrp2 was also documented in 5cben (data not shown).
Loss of Lrp2 disrupts clearance pathways in the pronephros
Prominent expression of lrp2 is seen in the zebrafish pronephros throughout ontogeny (Fig. 1). To evaluate the consequences of receptor deficiency for the fish kidney, we performed detailed analyses of developmental and functional aspects of this organ in lrp2 mutant lines.
Initially, we tested expression of lrp2, disabled 2 (dab2), a cytosolic adaptor required for Lrp2 function (Morris et al., 2002), as well as of wilms tumor 1a (wt1a) in the pronephros of wild type and mutant embryos from 20 to 72 hpf (Suppl. figure 1). Wt1a marks cells in the bilateral nephric primordium (20–24 hpf) that later form the central pronephric glomeruli (48–72 hpf) (Serluca and Fishman, 2001). lrp2 and dab2, on the other hand, are co-expressed in the anterior part of the pronephric duct at 20–24 hpf. The expression domain becomes more restricted to the proximal portion of the pronephric duct as the tubular system of the pronephros condenses (48–72 hpf). Signals for lrp2 transcripts were significantly reduced (but not absent) in bugeye embryos suggesting that loss of receptor activity negatively impacts receptor gene transcription (Suppl. figure 1). Otherwise, no discernable differences were seen in dab2 and wt1a expression domains in mutant compared with wild type embryos (Suppl. figure 1).
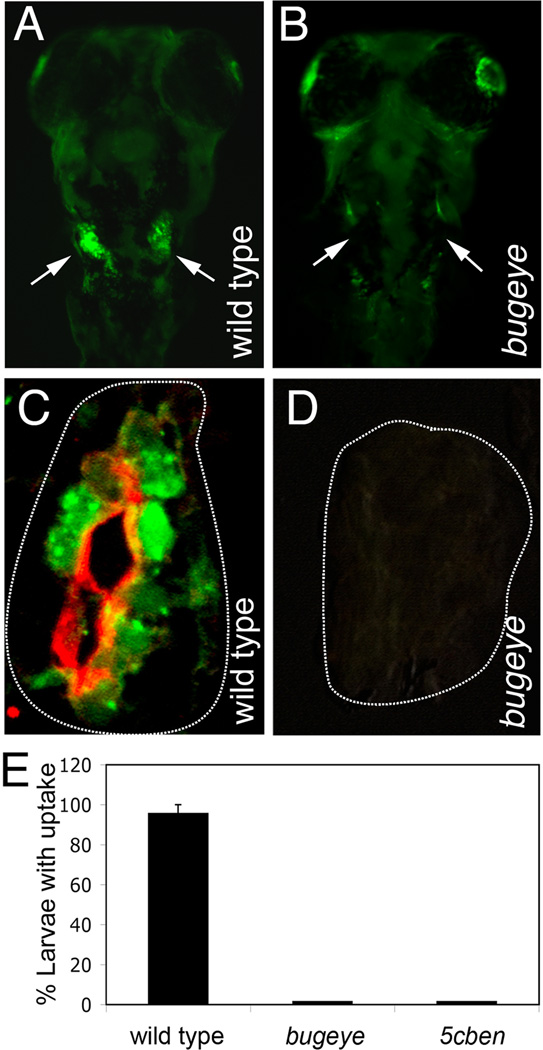
In zebrafish, the vascularization of glomeruli by ingrowing endothelial cells and the onset of blood filtration are completed by 48 hpf (Drummond et al., 1998). In the functional kidney, renal clearance of filtered metabolites from the glomerular effluent proceeds in pronephric duct regions that express Lrp2, suggesting a role for the receptor in tubular reabsorptive processes. Tubular clearance pathways in the zebrafish pronephros can be visualized by injection of fluid-phase tracers such as FITC-labeled dextran into the cardinal vein and by subsequent reabsorption of the dye in the pronephric duct (Drummond et al., 1998). Loss of Lrp2 in bugeye larvae resulted in complete absence of pronephric uptake of FITC-dextran as documented by fluorescence microscopy on whole mounts (Fig. 4B) and on transverse sections through the pronephros (Fig. 4D). Lack of tubular reabsorptive capacity was also confirmed in 5cben using dye uptake experiments (Fig. 4E). Electronmicroscopical analysis failed to detect any appreciable numbers of endosomes or dense apical tubules (the recycling membrane compartment) in epithelial cells of the pronephric duct in mutants whereas these components of the endocytic apparatus were readily visible in wild types (Fig. 5). These findings indicated a complete breakdown of the endocytic machinery in tubule cells lacking Lrp2.
Figure 4. Lrp2 deficiency disrupts clearance pathways in the pronephric duct.
(A, B) Whole mount fluorescence microscopy detects accumulation of fluid phase marker FITC-dextran (green) in the wild type but not in the Lrp2-deficient pronephros (arrows in A and B) upon injection into the common cardinal vein. (C, D) Immunohistological detection of Lrp2 (red) and FITC-dextran (green) in the pronephros of the indicated genotypes. White lines highlight the position of the pronephric ducts. (E) Wild type as well as bugeye and 5cben larvae at 4 dpf were injected with FITC-dextran and the number of larvae with tubular accumulation of tracers evaluated by fluorescence microscopy. Data are given as % ± SEM of all larvae injected. n= 53 (wild type), 15 (bugeye), or 23 (5cben).
Figure 5. Loss of endocytic apparatus in Lrp2-deficient pronephros.
Electronmicroscopical analysis documents almost complete loss of the endocytic apparatus including endosomes (E) and dense apical tubules (arrows) in the 5cben pronephros compared with the wild type control at 4 dpf. BB, brush border of tubular epithelium.
Lack of lrp2 does not impact forebrain patterning in zebrafish
In mammals, LRP2 is a key regulator that acts upstream of the morphogens SHH, FGF8, and BMP4 in patterning of the rostral neural tube (Spoelgen et al., 2005). To explore whether lrp2 has a similar function in control of brain formation in zebrafish, we performed detailed histo-anatomical characterization of early forebrain development in bugeye. Surprisingly, no obvious alterations in head and forebrain anatomy were evident in whole mounts or in histological sections from mutant compared with control embryos between 24 and 72 hpf (Fig. 6). Also, ISH for forebrain markers in zebrafish including one-eyed pinhead (oep), pax6b, emx3, foxa1, shha, shhb, nkx2.1b, netrin, or axial failed to reveal any discernable differences between 20 and 96 hpf comparing wild type and mutant embryos (Fig. 7). Abnormalities in formation of the optic chiasm in zebrafish are indicative of faulty midline symmetry as shown in mutants lacking shha (Schauerte et al., 1998) or fgf8 (Shanmugalingam et al., 2000). In contrast, formation of the optic chiasm was normal in bugeye embryos as shown by immunohistology for marker Zn5 (Suppl. figure 2). Finally, lack of receptor expression did not affect the appearance of radial glia in the ventricular system (Suppl. figure 3) or the proliferative capacity of progenitor cell populations in the developing CNS in zebrafish (Suppl. figure 4), an obvious consequence of receptor deficiency in Lrp2−/− mice (Spoelgen et al., 2005).
Figure 6. Appearance of head and brain structures in bugeye embryos.
Lateral head aspects (A–F) and coronal diencephalic sections stained with toluidine blue are shown. No obvious malformations in head or brain anatomy are seen at the indicated time points comparing wild type and bugeye embryos.
Figure 7. Analysis of forebrain development in bugeye embryos.
Whole mount in situ hybridization of wild type and bugeye embryos at 20, 30, 48 and 96 hpf using the indicated markers of forebrain development. Lateral or dorsal head aspects are shown.
Zebrafish lrp2b does not compensate for loss of lrp2
The occurrence of an event of whole genome duplication at the root of the teleost lineage explains the presence of additional gene copies in teleosts not seen in other vertebrate species (Jaillon et al., 2004). Apparently, this situation also applies to lrp2 because a second gene in the zebrafish genome encodes a homologous protein (GenBank XR_084338.1). Expression of this gene is supported by three ESTs from different libraries (EH509550, EH535651, AL918472), and by a similar sequence in Tetraodon nigroviridis (GenPept 47219712). We termed this protein Lrp2b.
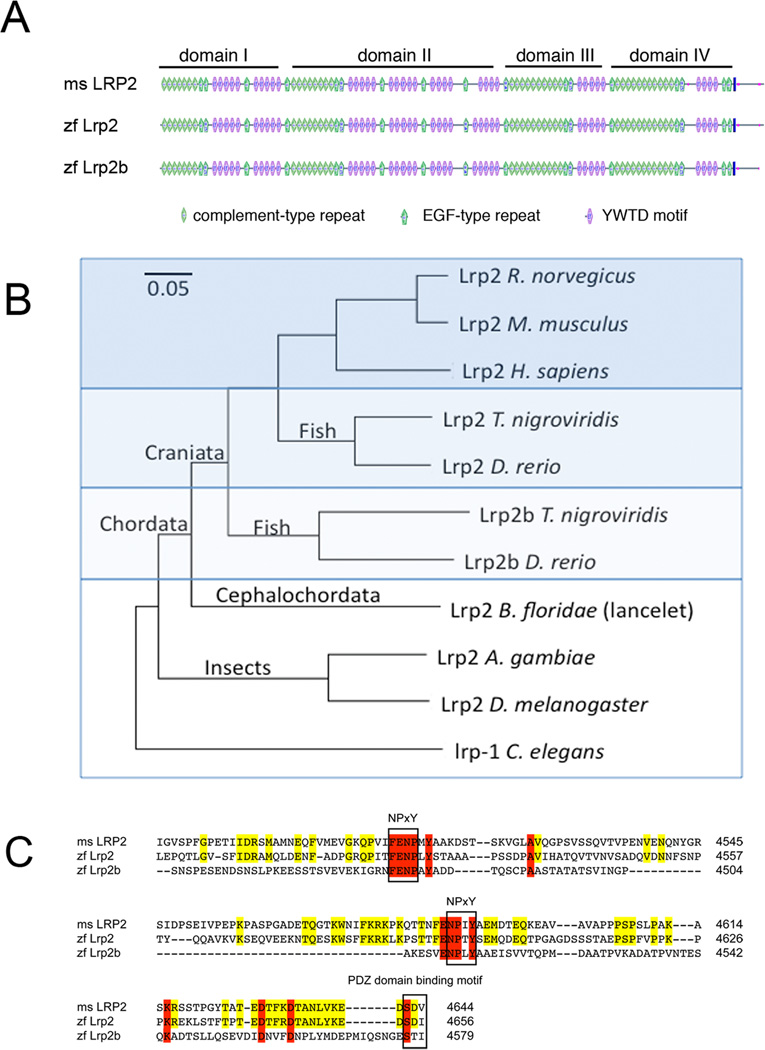
Analysis of the predicted domain structure of Lrp2b revealed an identical arrangement of complement-type and epidermal-growth factor-type repeats as well as YWTD elements compared with mouse and zebrafish Lrp2 (Fig. 8A). At the amino acid level, zebrafish Lrp2 is more closely related to mouse LRP2 than to its homologue Lrp2b (Fig. 8B). This assumption was supported by comparing the amino acid sequence of the cytoplasmic tail in the three receptor variants (Fig. 8C). The cytoplasmic domains of LRPs carry protein-protein interaction motifs that are important for receptor function and that are strictly conserved in individual receptor variants across species. Thus, mouse LRP2 and zebrafish Lrp2 share a higher degree of sequence identity in the cytoplasmic tail sequence than zebrafish Lrp2 and Lrp2b (Fig. 8C). However, two essential NPxY motifs that interact with cytosolic adaptors (Gotthardt et al., 2000) as well as a carboxyl terminal PDZ binding domain motif are conserved in Lrp2b (Fig. 8C).
Figure 8. Comparative analysis of lrp2 and lrp2b.
(A) Structural organization of mouse LRP2 as well as zebrafish Lrp2 and Lrp2b. The vertical solid line indicates the membrane anchor in the receptor polypeptides. (B) Phylogenetic tree of the indicated receptors based on the amino acid sequences from the following GenPept records (unless indicated): Lrp2 R. norvegicus (13562118), Lrp2 M. musculus (124487372), Lrp2 H. sapiens (126012573), Lrp2 T. nigroviridis (47210425), Lrp2 D. rerio (303304950), Lrp2b T. nigroviridis (47219712), Lrp2b D. rerio (GenBank XR_084338.1), Lrp2 B. floridae (260807227), Lrp2 A. gambiae (158300186), Lrp2 D. melanogaster (281360654), and lrp-1 C. elegans (212645014). (C) Amino acid sequence in the cytoplasmic receptor domains indicating identity between mouse (ms) LRP2 and zf Lrp2 (yellow) or between all three receptor variants (red). The NPxY protein-protein interaction sites and the PDZ domain-binding motif common to all three receptor species are highlighted.
We used quantitative RT-PCR on whole zebrafish embryos to investigate the expression of lrp2b. As shown in Fig. 9A, low levels of lrp2b transcript can be detected at 24, 48, or 72 hpf compared with lrp2. Also, there is no increase in expression of lrp2b in bugeye compared to wild type. This finding indicates there is no compensatory up-regulation of this alternative receptor gene in lrp2 mutants (Fig. 9B).
Figure 9. Expression of lrp2 and lrp2b in the zebrafish embryo.
(A) Quantitative RT-PCR of lrp2 and lrp2b transcripts in wild type embryos at the indicated time points. Data are given relative to lrp2 levels in wild types at 24 hpf (set to 1). (B) Comparative RT-PCR of lrp2b transcripts in wild types and bugeye mutants at the indicated time points. Data are given relative to lrp2b levels in wild types at 24 hpf (set to 1).
To test the potential contribution of lrp2b to fish development, we used ATG morpholino (MO) knockdown approaches to block Lrp2b expression in bugeye. As control for knockdown efficiency, we co-injected transcripts encoding mCherry with a modified ATG region that was targeted by the lrp2b MO as well. Two different lrp2b MOs were used in this study and both efficiently blocked expression of mCherry (Suppl. figure 5A–C). However, application of the two MO failed to elicit any obvious morphological alterations in bugeye at 24 hpf (Suppl. figure 5D–F). Apparent undisturbed embryonic development was also seen (data not shown) when using a third MO that targeted a splice site in intron 2 of lrp2 (Suppl. figure 6), suggesting that double deficiency for lrp2 and lrp2b does not elicit major developmental phenotypes. Finally, MO knockdown of lrp2b in wild type zebrafish did not impair pronephric uptake of FITC-dextran (14 out of 15 lrp2b morphants tested positive) compared to control fish (15 out of 16 positive). This observation indicated that tubular clearance is an activity unique to Lrp2.
DISCUSSION
We have characterized in detail the consequences of Lrp2 deficiency in zebrafish, and we have compared our findings to phenotypes observed in receptor-deficient mouse models and in humans. These studies have revealed the surprising fact that functions for the receptor in renal tubular clearance pathways but not in embryonic brain development are conserved between fish and mammals.
The proximal tubules in the kidney represent the major site of LRP2 expression in the mammalian organism. In this tissue, the receptor acts as low affinity but high capacity scavenger for retrieval of numerous plasma proteins filtered through the glomerulus. In particular, LRP2 mediates reabsorption of carrier proteins such as vitamin D binding protein (DBP) and retinol-binding protein (RBP), transporters for vitamin D metabolites and retinoids, respectively, preventing uncontrolled loss of these essential metabolites and regulating systemic vitamin homeostasis (reviewed in (Christensen and Birn, 2001). Consequently, lack of receptor activity in knockout mice and in patients with Donnai-Barrow syndrome results in proteinuria and vitamin deficiencies (Leheste et al., 1999; Kantarci et al., 2007). We have previously reported the impact of splice morpholino-mediated knockdown of lrp2 in zebrafish larvae (Anzenberger et al., 2006). However, expression of the receptor from maternal transcripts precluded analyses of receptor defects in early embryonic stages, including patterning of pronephros and forebrain. The availability of a zebrafish lrp2 mutant has now enabled us to address these issues.
Loss of lrp2 expression does not impact the formation of pronephric structures (Suppl. figure 1). However, it results in the complete absence of fluid phase uptake (Fig. 4) and in a dramatic breakdown of the endocytic architecture (Fig. 5) in the lrp2 mutant pronephros. These findings argue that the receptor plays a similarly important role as renal retrieval pathway in zebrafish as it does in mammals. The nature of the ligands internalized by Lrp2 in the pronephros is unclear at present. However, vitamin carriers such as DBP and RBP are expressed in zebrafish as well (Abe et al., 1975; Tingaud-Sequeira et al., 2006) suggesting a conserved function for this receptor pathway in vitamin handling in pro- and metanephros.
LRP2 has also been shown to have pivotal roles during mammalian forebrain development (Willnow et al., 1996; Spoelgen et al., 2005). Expression of lrp2 in zebrafish embryos faithfully recapitulates the expression pattern seen in rodents, with the main expression domains in the developing forebrain, optic stalk, and otic vesicle. However, there is a remarkable difference inasmuch as expression in fish starts rather late with respect to forebrain patterning. In mice, prominent expression of Lrp2 in the neuroepithelium is seen around embryonic day (E) 9.0 when the forebrain undergoes a complex process of dorso-ventral specification governed by inductive signals provided by SHH from the ventral and by BMP4 from the dorsal neural tube (Spoelgen et al., 2005). Lack of LRP2 in mice results in an aberrant increase in expression and activity of BMP4 in the dorsal and loss of SHH signals in the ventral neuroepithelium. Thus, the receptor is believed to modulate morphogen pathways by acting as activator of SHH and/or inhibitor of BMP4 signals (Spoelgen et al., 2005). This hypothesis is supported by the ability of LRP2 to act as cell surface receptor for BMP4 and SHH (McCarthy et al., 2002; Spoelgen et al., 2005).
In contrast to the mouse, the Shh pathway seems to play a lesser role in providing inductive signals in the floor-plate in zebrafish (Schauerte et al., 1998). Rather, Nodal-related signals are more important in specifying the medial floor-plate, processes that are initiated already during early gastrulation (5 hpf) (Hatta et al., 1991; Schier et al., 1997). Given the onset of Lrp2 expression in the fish forebrain around 36 hpf, the receptor is unlikely to have a decisive function in ventral neural tube specification in this species. This assumption is supported by normal forebrain development observed in zebrafish lacking Lrp2 and the related receptor Lrp2b. Interestingly, robust expression of zebrafish Lrp2 in radial glia cells in the brain ventricles in the larval (Fig. 2) and in the adult brain (data not shown) parallels the pattern for LRP2 in fetal and adult mammalian CNS. In the adult mouse, the receptor is expressed on the apical surface of the ependyma, the epithelial lining of the ventricular system. Recent findings demonstrate that in mouse ependymal cells, LRP2 promotes neurogenesis in the adjacent subventricular zone (SVZ), one of the two neurogenic niches in the adult mammalian brain (Gajera et al., 2010). Likely, the ability of LRP2 in the ependyma to down-regulate anti-proliferative signals provided by BMP4 underlie the neurogenic activity of this receptor pathway (Gajera et al., 2010). Adult neurogenesis in the zebrafish CNS is more complex, involving multiple neurogenic sites (Byrd and Brunjes, 2001; Zupanc et al., 2005). Still, prominent neurogenic activity is seen along the entire telencephalic ventricular zone where Lrp2 is expressed (Adolf et al., 2006). Conceptually, a function for the receptor in adult neurogenesis may also be relevant for zebrafish.
In conclusion, comparative analyses in zebrafish and mouse models have identified functions for LRP2/Lrp2 as clearance receptor in pro- and metanephros. An activity as multifunctional clearance receptor likely reflects a primordial function of this receptor pathway already present in nematodes. In contrast, distinct activities in control of morphogen pathways in forebrain patterning may be more recent evolutionary additions in higher vertebrates.
METHODS
Fish stocks
Zebrafish lines bugeye (lrp2mw1) and 5cben (lrp2p5bnc) with nonsense mutations in lrp2 have been described previously (Veth et al., 2011). In brief, the bugeye mutant was identified in a forward-genetic screen for adult ocular abnormalities. Recombinant mapping panels to genetically position the mutant indicated that the bugeye phenotype is caused by a single recessive mutation. To map the mutant locus, progeny from single pair backcross matings were used for whole-genome linkage analysis. Co-segregation for markers on chromosome 9 and the mutant phenotype was found. Subsequent analysis of candidate genes in this region revealed a T to A conversion that changes a cysteine to a stop codon at amino acid position 23 (C23X) in lrp2. In an independent genetic screen a second adult-onset large eye mutant was identified. Intercrosses between this mutant (allele p5bnc) and bugeyemw1 were non-complementing and sequencing revealed a separate non-sense mutation in lrp2 (Q413X) in lrp2p5bnc.
For upkeep, all fish were maintained and raised at 28.5°C under standard conditions. Embryos were kept in egg water and staged according to (Kimmel et al., 1995). Since homozygous bugeye and 5cben mutants are viable and fertile, maternal/zygotic (MZ) mutant embryos were obtained by mating of homozygous mutants. Lrp2 null offspring were compared to wild type controls matched for genetic background and age.
Identification of lrp2b
Homology search in the NCBI zebrafish genome database identified entry XR_084338.1 as possible lrp2 gene duplication, termed lrp2b by us. The original entry includes an insertion of six nucleotides at position 8603–8609 resulting in a stop codon. This insertion was removed manually to derive an open reading frame encoding a full length LRP of 4579 amino acids depicted in figure 8. Independently, we also cloned a cDNA sequence encompassing the entire extracellular domain of lrp2b (nucleotide positions 1 to 12323) from mRNA isolated from the zebrafish eye (data not shown).
Morpholino injections
Morpholino antisense oligonucleotides (MO) were purchased from GENE TOOLS, USA. Sequences were chosen to target the ATG region of lrp2b (lrp2bMO1: 5’-CACCACTCATGCACTGACCTGACCTGCACA-3’; lrp2bMO2: 5’-TGCTACACTGGAACTCACCACTCAT-3’) or a splice site in intron 2 (lrp2bMO3: 5’- TGTAGACATACTACAGGTCCTTTGC-3’). All MOs were injected at a final concentration of 100 µM in ddH2O using a MPPI-2 microinjector (Applied Scientific Instrumentation, USA). The effects of lrp2bMO1 and lrp2bMO2 were verified by co-injection of a test RNA consisting of mCherry coding sequence fused to the lrp2b ATG region (−15 to +25).
Phenotypic analysis
Phenotypic analysis of embryonic and adult zebrafish by immunohistology, in situ hybridization, and quantitative RT-PCR was performed according to standard protocols. Characterization of pronephric duct clearance by FITC-dextran uptake has been reported (Drummond et al., 1998).
Supplementary Material
ACKNOWLEDGEMENT
We are indebted to Melanie Liekweg and Petra Schrade for expert technical assistance. Studies were funded in part by the SFB 665 (to TEW and AH) and the NIH R01RR020357 (to RGG).
REFERENCES
- Abe T, Muto Y, Hosoya N. Vitamin A transport in chicken plasma: isolation and characterization of retinol-binding protein (RBP), prealbumin (PA), and RBP--PA complex. J Lipid Res. 1975;16:200–210. [PubMed] [Google Scholar]
- Adolf B, Chapouton P, Lam CS, Topp S, Tannhauser B, Strahle U, Gotz M, Bally-Cuif L. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;295:278–293. doi: 10.1016/j.ydbio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Anzenberger U, Bit-Avragim N, Rohr S, Rudolph F, Dehmel B, Willnow TE, Abdelilah-Seyfried S. Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. J Cell Sci. 2006;119:2127–2137. doi: 10.1242/jcs.02954. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 2001;105:793–801. doi: 10.1016/s0306-4522(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol. 2001;280:F562–F573. doi: 10.1152/ajprenal.2001.280.4.F562. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, Nykjaer A, Blomhoff R, Willnow TE, Moestrup SK. Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol. 1999;10:685–695. doi: 10.1681/ASN.V104685. [DOI] [PubMed] [Google Scholar]
- Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entchev EV, Kurzchalia TV. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin Cell Dev Biol. 2005;16:175–182. doi: 10.1016/j.semcdb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Gajera CR, Emich H, Lioubinski O, Christ A, Beckervordersandforth-Bonk R, Yoshikawa K, Bachmann S, Christensen EI, Gotz M, Kempermann G, Peterson AS, Willnow TE, Hammes A. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123:1922–1930. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- Grigorenko AP, Moliaka YK, Soto MC, Mello CC, Rogaev EI. The Caenorhabditis elegans IMPAS gene, imp-2, is essential for Development and is functionally distinct from related presenilins. Proc Natl Acad Sci U S A. 2004;101:14955–14960. doi: 10.1073/pnas.0406462101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K, Kimmel CB, Ho RK, Walker C. The cyclops mutation blocks specification of the floor plate of the zebrafish central nervous system. Nature. 1991;350:339–341. doi: 10.1038/350339a0. [DOI] [PubMed] [Google Scholar]
- Herz J, Gotthardt M, Willnow TE. Cellular signalling by lipoprotein receptors. Curr Opin Lipidol. 2000;11:161–166. doi: 10.1097/00041433-200004000-00009. [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Brunet F, Petit JL, Stange-Thomann N, Mauceli E, Bouneau L, Fischer C, Ozouf-Costaz C, Bernot A, Nicaud S, Jaffe D, Fisher S, Lutfalla G, Dossat C, Segurens B, Dasilva C, Salanoubat M, Levy M, Boudet N, Castellano S, Anthouard V, Jubin C, Castelli V, Katinka M, Vacherie B, Biemont C, Skalli Z, Cattolico L, Poulain J, De Berardinis V, Cruaud C, Duprat S, Brottier P, Coutanceau JP, Gouzy J, Parra G, Lardier G, Chapple C, McKernan KJ, McEwan P, Bosak S, Kellis M, Volff JN, Guigo R, Zody MC, Mesirov J, Lindblad-Toh K, Birren B, Nusbaum C, Kahn D, Robinson-Rechavi M, Laudet V, Schachter V, Quetier F, Saurin W, Scarpelli C, Wincker P, Lander ES, Weissenbach J, Roest Crollius H. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate protokaryotype. Nature. 2004;431:946–957. doi: 10.1038/nature03025. [DOI] [PubMed] [Google Scholar]
- Jowett T, Lettice L. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet. 1994;10:73–74. doi: 10.1016/0168-9525(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, Chassaing N, Lacombe D, Devriendt K, Teebi A, Loscertales M, Robson C, Liu T, MacLaughlin DT, Noonan KM, Russell MK, Walsh CA, Donahoe PK, Pober BR. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet. 2007;39:957–959. doi: 10.1038/ng2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic Development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol. 1999;155:1361–1370. doi: 10.1016/S0002-9440(10)65238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Entchev EV, Mende F, Wilsch-Brauninger M, Thiele C, Schmidt AW, Knolker HJ, Ward S, Kurzchalia TV. Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol. 2004;2:e280. doi: 10.1371/journal.pbio.0020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy RA, Barth JL, Chintalapudi MR, Knaak C, Argraves WS. Megalin functions as an endocytic sonic hedgehog receptor. J Biol Chem. 2002;277:25660–25667. doi: 10.1074/jbc.M201933200. [DOI] [PubMed] [Google Scholar]
- Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the Dab2 adaptor protein in embryonic Development and kidney transport. Embo J. 2002;21:1555–1564. doi: 10.1093/emboj/21.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Perriere G, Gouy M. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie. 1996;78:364–369. doi: 10.1016/0300-9084(96)84768-7. [DOI] [PubMed] [Google Scholar]
- Schauerte HE, van Eeden FJ, Fricke C, Odenthal J, Strahle U, Haffter P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Serluca FC, Fishman MC. Pre-pattern in the pronephric kidney field of zebrafish. Development. 2001;128:2233–2241. doi: 10.1242/dev.128.12.2233. [DOI] [PubMed] [Google Scholar]
- Shanmugalingam S, Houart C, Picker A, Reifers F, Macdonald R, Barth A, Griffin K, Brand M, Wilson SW. Ace/Fgf8 is required for forebrain commissure formation and patterning of the telencephalon. Development. 2000;127:2549–2561. doi: 10.1242/dev.127.12.2549. [DOI] [PubMed] [Google Scholar]
- Spoelgen R, Hammes A, Anzenberger U, Zechner D, Andersen OM, Jerchow B, Willnow TE. LRP2/megalin is required for patterning of the ventral telencephalon. Development. 2005;132:405–414. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Forgue J, Andre M, Babin PJ. Epidermal transient down-regulation of retinol-binding protein 4 and mirror expression of apolipoprotein Eb and estrogen receptor 2a during zebrafish fin and scale Development. Dev Dyn. 2006;235:3071–3079. doi: 10.1002/dvdy.20921. [DOI] [PubMed] [Google Scholar]
- Veth KN, Willer JR, Collery RF, Gray MP, Willer GB, Wagner DS, Mullins MC, Udvadia AJ, Smith RS, John SWM, Gregg RG, Link BA. Mutations in Zebrafish lrp2 Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma. PLoS Genetics. 2011;7:e1001310. doi: 10.1371/journal.pgen.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain Development in mice lacking gp330/megalin. Proc Natl Acad Sci U S A. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J, Tuck S, Greenwald I, Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126:597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- Zheng G, Bachinsky DR, Stamenkovic I, Strickland DK, Brown D, Andres G, McCluskey RT. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpa 2MR, and the receptor-associated protein (RAP) J Histochem Cytochem. 1994;42:531–542. doi: 10.1177/42.4.7510321. [DOI] [PubMed] [Google Scholar]
- Zupanc GK, Hinsch K, Gage FH. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol. 2005;488:290–319. doi: 10.1002/cne.20571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.