Abstract
Although follicular lymphoma remains incurable, recent advances in first-line therapy have resulted in improved response rates and response duration. Maintenance therapy with rituximab (Rituxan) after induction treatment with rituximab alone or chemotherapy in combination with or without rituximab has resulted in further improvement in progression-free survival in both treatment-naive and previously treated patients. Efficacy results from the large phase 3, randomized Primary Rituximab and Maintenance (PRIMA) trial in the first-line setting have dem onstrated significant improvements in progression-free survival, in the rate of patients achieving complete remission, and in the proportion of patients remaining in complete remission using maintenance rituximab.
The use of maintenance therapy is also under study in additional hematological malignancies, including diffuse large B-cell lymphoma and chronic lymphocytic leukemia. Clinical investigation is ongoing to address the optimal duration of maintenance therapy and the question of whether re-treatment upon disease progression is as beneficial as maintenance for follicular lymphoma.
Keywords: follicular lymphoma, maintenance therapy, rituximab
INTRODUCTION
Non-Hodgkin’s lymphoma (NHL) is the most common adult hematological cancer, with 66,360 new cases expected in the U.S. alone in 2011.1 NHL comprises a heterogeneous group of diseases, the most common of which are follicular lymphomas (FLs), comprising approximately 22% of diagnosed cases, and diffuse large B-cell lymphomas (DLBCLs), comprising approximately 31% of cases.2 Unlike the aggressive lymphomas (DLBCLs), indolent lymphomas, such as FLs, are generally considered incurable.3 However, because of the prolonged natural history of low-grade lymphoma and the slow rate of progression, many patients are initially observed without treatment.4
Treatment goals focus not only on survival, but also on maintaining good quality of life (QOL) with minimal symptoms. The indications for treatment at initial diagnosis often include the presence of B symptoms (fevers, night sweats, and weight loss), bulky disease, compromise of normal organ function, and the presence of cytopenias resulting from marrow involvement. If the primary approach is observation, therapy is usually initiated when significant progressive adenopathy is noted but before organ dysfunction occurs.
For patients with advanced-stage FL who require treatment, the aims are to achieve disease control and to minimize lymphoma-related symptoms. Because of the high likelihood of eventual relapse with current therapy, attaining a prolonged disease-free interval with available regimens is a key goal of therapy. Maintenance therapy with rituximab (Rituxan, Roche/Genentech/Biogen Idec) has been found to significantly improve outcomes in patients with both untreated and relapsed FL.5–12 This article reviews the clinical data for maintenance rituximab in FL and discusses its role in treatment.
TREATMENT
Initial Therapy
Treatment with rituximab, in combination with various chemotherapy regimens, has significantly improved progression-free survival and overall survival in patients with FL compared with chemotherapy alone (Table 1).13–17 The two most commonly used chemoimmunotherapy regimens for the treatment of FL include rituximab in combination with cyclophosphamide, vincristine, and prednisone (R-CVP), and rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).13,16
Table 1.
Clinical Efficacy of Induction Therapy in Follicular Lymphoma (FL): Results From Randomized, Controlled Phase 3 Trials
| Study | No. of Patients* | Regimen | Efficacy |
|---|---|---|---|
| Untreated FL | |||
| Rummel, 200917 | 549 | BR vs. R-CHOP |
|
| Marcus, 200816 | 321 | R-CVP vs. CVP |
|
| Salles, 200814 | 358 | R-CHVP-IFN vs. CHVP-IFN |
|
| Herold, 200715 | 201 | R-MCP vs. MCP |
|
| Hiddemann, 200513 | 428 | R-CHOP vs. CHOP |
|
| Relapsed or Refractory FL | |||
| Van Oers, 200619 | 465 | R-CHOP vs. CHOP |
|
| Forstpointner, 200418 | 93 | R-FCM vs. FCM |
|
Number represents enrolled patients with a diagnosis of FL; total trial population may have been larger.
BR = bendamustine + rituximab; CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisone; CHVP = cyclophosphamide, doxorubicin, etoposide, and prednisone; CVP = cyclophosphamide, vincristine, and prednisone; EFS = event-free survival; FCM = fludarabine, cyclophosphamide, and mitoxantrone; IFN = interferon; MCP = mitoxantrone, chlorambucil, prednisone; mo = months; MR = maintenance rituximab; OBS = observation; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; R = rituximab; TTF = time to treatment failure.
In a phase 3 randomized study of 428 patients with advanced-stage FL, R-CHOP was significantly superior to CHOP for all endpoints, including time to treatment failure (P < 0.001), remission rate (P = 0.011), duration of response (P < 0.001), time to next treatment (P < 0.001), and overall survival (P = 0.016).13 Similarly, R-CVP provided clinical improvements, compared with CVP, in a randomized study of patients with previously untreated, advanced-stage FL.16 The complete response rate, time to progression, disease-free survival, duration of response, time to treatment failure, and time to next treatment were all significantly better in the R-CVP arm of the study. Clinical benefits with R-CHOP and R-CVP were maintained across all patient subgroups, regardless of risk profile or age.13,16
Rituximab continues to be studied in combination with new agents with the goal of improving outcomes and patient safety. The phase 3 Study Group Indolent Lymphomas, Germany (StiL) trial compared rituximab plus bendamustine (Treanda, Cephalon) with R-CHOP as first-line treatment in patients with advanced FL.17 The complete response rate was significantly better for rituximab plus bendamustine (40.1% vs. 30.8%, respectively; P = 0.0323), as were the median progression-free survival rate (54.8 vs. 34.8 months, respectively; hazard ratio [HR] = 0.5765; P = 0.0002) and the median event-free survival rate (54 vs. 31 months, respectively; HR = 0.6014; P = 0.0002).17
Rituximab plus bendamustine was associated with fewer serious adverse events (AEs) compared with R-CHOP (49 vs. 74, respectively). The combination regimen was also associated with significantly lower rates of grade 3 or 4 neutropenia (10.7% vs. 46.5%; P < 0.0001) and with significantly lower rates of grade 3 or 4 leukocytopenia (12.1% vs. 38.2%; P < 0.0001). Granulocyte–colony-stimulating factor (G-CSF) was used more often in patients treated with R-CHOP than in those treated with rituximab plus bendamustine (20% vs. 4% of all cycles, respectively).17
Relapsed or Refractory Follicular Lymphoma
Rituximab, as chemoimmunotherapy, has also improved survival outcomes in patients with relapsed or refractory FL (see Table 1).18,19 In a phase 3 study of 147 patients with relapsed or refractory FL or mantle-cell lymphomas, rituximab plus fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) significantly improved the overall response rate (79% vs. 58%; P = 0.01), complete response rate (33% vs. 13%; P = 0.005), median progression-free survival (16 vs.10 months; P = 0.0381), and overall survival (estimated 2-year survival rates: 73% vs. 53%; P = 0.003) compared with FCM alone.18 R-FCM was also superior to FCM in all subgroups, including patients who had received one previous therapy (overall response rate, 82% vs. 71%, respectively), those who had received two or more prior therapies (overall response rate, 74% vs. 41%), and those with disease that was refractory to prior therapy (overall response rate, 62% vs. 20%). A similar survival benefit was observed when R-FCM was compared with FCM in the subset of patients with recurrent FL (2-year survival rates, 90% vs. 70%, respectively).18
The phase 3 European Organization for Research and Treatment of Cancer (EORTC) 20981 trial followed a double-randomization design. Patients received either R-CHOP or CHOP during the initial induction phase, and responders received maintenance rituximab or observation.19 Results from 465 patients in the induction phase (without prior rituximab) showed that the overall response rate was significantly better for R-CHOP compared with CHOP alone (85.1% vs. 72.3%, respectively; P < 0.001), primarily because of a significant increase in the complete response rate (29.5% vs.15.6%; P < 0.001). The median progression-free survival rate and the 3-year overall survival rate were also improved in patients receiving R-CHOP versus CHOP (see Table 1). Results from the maintenance phase of this study are described in the next section.
Maintenance Therapy
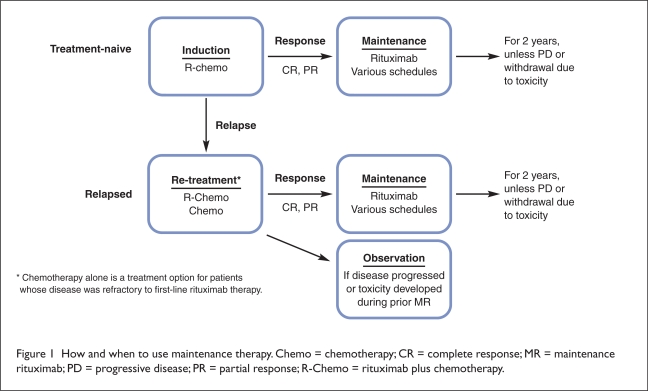
Treatment with maintenance rituximab after a response to induction therapy appears to be an effective approach for extending the duration of disease remission (Figure 1).20 In randomized phase 3 trials in both patients with newly diagnosed FL and in those with relapsed or refractory FL, investigators have reported significantly improved event-free and progression-free survival with maintenance rituximab compared with observation after induction therapy with rituximab combined with various chemotherapy regimens (e.g., CVP, CHOP, and FCM), chemotherapy alone, or rituximab monotherapy (Table 2).5–11
Figure 1.
How and when to use maintenance therapy. Chemo = chemotherapy; CR = complete response; MR = maintenance rituximab; PD = progressive disease; PR = partial response; R-Chemo = rituximab plus chemotherapy.
Table 2.
Clinical Efficacy of Maintenance Rituximab in Follicular Lymphoma
| Study | Disease Setting | No. of Patients | Regimen | Maintenance Schedule | Efficacy |
|---|---|---|---|---|---|
| Salles, 201021 | First-line | 1,217 | R + Chemo + MR vs. R + Chemo + Obs | Single dose every 2 months for 2 years |
|
| Hochster, 20095 | First-line | 311 | CVP + MR vs. CVP + Obs | 4 weekly doses every 6 months for 2 years |
|
| Ardeshna, 201022 | First-line | 720 | R + MR vs. Obs | Single dose every 2 months for 2 years |
|
| Martinelli, 20106 | First-line and recurrent | 202 | R + MR vs. R + Obs | Single dose at 3, 5, 7, and 9 months |
|
| Foá, 201011 | First-line and recurrent | 545 | R + Chemo + MR | Single dose every 2 months for 2 years |
|
| Van Oers, 20108 | Recurrent | 465 | CHOP ± R + MR vs. CHOP ± R + Obs | Single dose every 3 months for 2 years |
|
| Pettengell, 201037 | Recurrent | 280 | R + MR vs. R + Obs | Single dose every 3 months for 2 years |
|
| Forstpointner, 20069 | Recurrent | 125 | R-FCM + MR vs. R-FCM + Obs | 4 weekly doses at 3 months and 9 months after induction |
|
| Hainsworth, 200510 | Recurrent | 90 | R + MR vs. R + Re-treatment | 4 weekly doses every 6 months for 2 years |
|
Chemo = chemotherapy; CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisone; CR = complete response; CRu = complete response unconfirmed; EFS = event-free survival; FCM = fludarabine, cyclophosphamide, and mitoxantrone; HR = hazard ratio; MR = maintenance rituximab; Obs = observation; OS = overall survival; PFS = progression-free survival; PR = partial response; R = rituximab; SD = stable disease.
Various dosing schedules for maintenance rituximab have been investigated in these trials. Dosing schedules have included a single dose administered every 2 or 3 months and once-weekly doses for 4 weeks every 6 months for up to 2 years after initial induction therapy. Improvements in the duration of the response to therapy have been observed across studies regardless of the induction regimen (with or without rituximab initially), the maintenance schedule, or the disease setting (first-line or relapsed). The optimal dose, schedule, and duration of treatment have yet to be established and are under investigation.
Table 2 summarizes the efficacy results from clinical studies of maintenance rituximab by disease setting, induction treatment, and the maintenance schedule employed in each trial. In the phase 3 Eastern Cooperative Oncology Group (ECOG) 1496 study, 311 treatment-naive patients with indolent lymphoma (including 282 patients with FL) received CVP induction and were randomly assigned to maintenance rituximab or observation.5
Maintenance rituximab significantly prolonged median progression-free survival compared with post-treatment observation (51.6 vs. 15.6 months, respectively; P < 0.0001). Maintenance rituximab also demonstrated a trend toward an improved 3-year overall survival rate (91% vs. 86%; P = 0.08). The significant improvement in progression-free survival was maintained in all patient subgroups in terms of tumor burden (low or high), histology (follicular or other), degree of residual disease (minimal or gross), and the Follicular Lymphoma International Prognostic Index (FLIPI; low, intermediate, or high).
The Swiss Group for Clinical Cancer Research (SAKK) 35/98 trial included 138 previously treated patients and 64 untreated patients with FL; all of the previously treated patients were rituximab-naive.6 The patients received single-agent rituximab. If their disease did not progress, they were randomly assigned to no further treatment (observation) or to four additional doses of rituximab given at 2-month intervals.
The long-term follow-up results (median period, 9.5 years) showed that the median event-free survival rate was almost doubled after maintenance rituximab compared with observation—24 months vs. 13 months, respectively (P < 0.001) (see Table 2). Further, 27% of the patients receiving maintenance rituximab were still in remission at 8 years compared with 5% of the observation arm. A subanalysis of initially untreated patients who received induction rituximab showed an even greater benefit after maintenance rituximab, with approximately 45% of these patients still in remission at 8 years.
Results from the phase 3 Primary Rituximab and Maintenance (PRIMA) trial demonstrated a significant benefit in progression-free survival as well as in other clinical outcomes after maintenance rituximab in patients with FL with a high tumor burden (see Table 2).7,21 In this study, patients who responded to induction immunochemotherapy consisting of eight cycles of R-CVP or six cycles of R-CHOP or R-FCM (plus two additional rituximab infusions) were randomly assigned to maintenance rituximab or observation. Maintenance rituximab significantly prolonged progression-free survival in patients who responded to induction with rituximab-based chemotherapy. The risk of disease progression was reduced by 45% (HR = 0.55; P < 0.0001; see Table 2). Three-year progression-free survival rates were 74.9% in the maintenance rituximab arm and 57.6% in the observation arm (P < 0.0001).21
The benefit of maintenance rituximab was observed regardless of the induction regimen (R-CHOP, HR = 0.51; R-CVP, HR = 0.68; and R-FCM, HR = 0.54), the patient’s age (< 60 years, HR = 0.49; > 60 years, HR = 0.67), and the response to induction therapy (complete response or complete response unconfirmed, HR = 0.57; partial response, HR = 0.48).21 Not surprisingly, patients treated with maintenance rituximab also had a significantly longer time before they needed the next treatment (HR = 0.60; P < 0.001).
At the end of maintenance therapy, 72% of the patients in the maintenance rituximab arm attained a complete response compared with 52% of patients in the observation arm (P = 0.0001).21 Further, 52% of responders in the maintenance rituximab arm who had achieved a partial response were able to attain a complete response, in contrast to 30% of responders in the observation arm (P < 0.0001). Additional follow-up in the PRIMA trial will allow the effect of maintenance rituximab on overall survival to be evaluated.
The preliminary results of the phase 3 Watch-and-Wait study, which enrolled 462 patients with asymptomatic, non-bulky, stage 2, 3, or 4 FL, were presented in 2010.22 Patients were randomly assigned to watchful waiting or to treatment with rituximab, which was administered in two different schedules. Patients in both treatment groups received rituximab 375 mg/m2 weekly for 4 weeks, and one group also received maintenance rituximab once every 2 months for 2 years. The study began in September 2004. By September 2007, it was clear that the maintenance rituximab arm was superior to the single-agent rituximab arm (no maintenance rituximab); therefore, the latter treatment arm was discontinued.
At 3 years, 8% of the patients in the watch-and-wait arm had not required further treatment compared with 80% of patients in the rituximab induction arm and 91% of patients who had received induction rituximab, followed by maintenance rituximab.22
Although the study results suggest that progression-free survival is improved with early treatment, the long-term effect of intervention, maintenance therapy, or both, in patients with asymptomatic indolent lymphoma is unknown. Questions regarding the impact on the time to second treatment and overall survival will require longer follow-up.
Preliminary results from follow-up of all three study arms showed that the time to initiation of chemotherapy or radio-therapy was significantly increased by rituximab.22 At 3 years, 49% of patients in the watchful-waiting group had not received treatment compared with 80% in the group receiving rituximab for 4 weeks and 91% in the group receiving rituximab for 4 weeks followed by maintenance rituximab. Progression-free survival was also significantly improved (see Table 2). After 3 years of follow-up, 30% of patients in the watchful-waiting group did not have disease progression, compared with 60% of patients who had received rituximab for 4 weeks and 81% of patients who had received rituximab for 4 weeks and then received maintenance rituximab. No significant differences in overall survival were observed among the three study arms; 96% of the patients were still alive in each group.
In the relapsed setting, long-term follow-up (a median of 6 years after randomization to maintenance) of previously rituximab-naive patients in the EORTC 20981 trial showed that maintenance rituximab significantly prolonged the median progression-free survival, compared with observation (44.4 vs. 15.6 months, respectively; HR = 0.55; P < 0.0001).8 The progression-free survival benefit from maintenance rituximab, compared with observation, was maintained whether or not rituximab was included in the induction regimen—CHOP (3.1 vs. 1.0 years; HR = 0.37; P < 0.001) or R-CHOP (4.4 vs. 1.2 years; HR = 0.69; P = 0.043).
Although not statistically significant, there was a trend toward improved 5-year overall survival in the maintenance rituximab arm compared with the observation arm (74.3% vs. 64.7%, respectively; HR = 0.70; P = 0.07). This benefit was seen with both CHOP (HR = 0.59, P = 0.05) and R-CHOP (HR = 0.80, P = 0.42).
Maintenance Therapy Versus Re-treatment After Relapse
A key clinical question is whether maintenance therapy with rituximab offers comparable or better outcomes than rituximab re-treatment in patients after relapse. A re-treatment approach offers the benefit of fewer doses of rituximab, which might avoid the development of resistance resulting from prolonged exposure in the maintenance setting.
This question was first investigated in the phase 2, randomized Minnie Pearl Cancer Research Network trial, in which 114 patients with relapsed or refractory indolent NHL were initially treated with four once-weekly doses of single-agent rituximab.10 Patients who showed a response to treatment or those with stable disease (N = 90; 62 with FL and 28 with small lymphocytic lymphoma) received either maintenance rituximab (four once-weekly doses repeated at 6-month intervals for up to 2 years) or rituximab re-treatment (four once-weekly doses) at disease progression. In the maintenance rituximab arm, six additional responses were achieved with maintenance therapy, increasing the overall response rate from 39% (after induction) to a best response rate of 52%. The complete response rate also increased from 9% (after induction) to 27%. The median follow-up period for all patients was 41 months.
The median progression-free survival rate was significantly prolonged in the maintenance arm compared with the re-treatment arm (31.3 vs. 7.4 months, respectively; P = 0.007).10 Similarly, in the subset of patients with FL, median progression-free survival was longer in the maintenance arm than in the re-treatment arm (31 vs. 13 months, respectively). Significantly more patients in the maintenance arm (n = 10) had a complete response at the end of the study compared with those in the re-treatment arm (n = 1) (P = 0.03).
There was no significant difference in 3-year overall survival rates between the two groups (72% for maintenance rituximab, 68% for re-treatment). There also was no significant difference between the two groups with regard to rituximab benefit, defined as the time from the date of study entry to the date of the next (non-rituximab) lymphoma treatment (31.3 vs. 27.4 months in the maintenance and observation arms, respectively). The cumulative rituximab dose was approximately 29% lower in the re-treatment group. Both maintenance rituximab and rituximab re-treatment were well tolerated; no treatment-related hospitalizations or patient discontinuations were associated with AEs.
A comparison of maintenance treatment and re-treatment upon relapse is under investigation in the randomized, phase 3 Rituximab Extended Schedule or Re-treatment (RESORT) trial.23 As in the Minnie Pearl study, patients with stage 3 or 4 indolent FL with low tumor burden were initially treated with four once-weekly doses of single-agent rituximab as induction therapy. Patients were re-evaluated 9 weeks after induction treatment. Those with a partial or complete response to induction rituximab were randomly assigned to one of two treatment arms. In Arm 1 (re-treatment rituximab), patients received rituximab once weekly for 4 weeks upon disease progression provided the time to progression was more than 6 months. In Arm 2 (maintenance rituximab), patients received a single dose of rituximab once every 13 weeks until disease progression and in the absence of unacceptable toxicity.
The primary endpoint was the time to rituximab failure. Secondary endpoints included the time to first cytotoxic therapy in patients treated with these regimens, patient response, the duration of response, and health-related QOL. The clinical results of RESORTS have not yet been published.
Safety of Maintenance Therapy
The long-term use of rituximab as maintenance therapy has raised concerns regarding the potential for additional toxicity. Results from the PRIMA study showed that the safety of maintenance rituximab was consistent with the known safety profile of rituximab, with no new or unexpected findings (Table 3).21 The most common AEs in 1,009 patients treated with at least one maintenance dose of rituximab were grade 2 to 4 infections (maintenance rituximab, 39% vs. observation, 24%). Grade 3 to 4 AEs were reported in 24% of patients treated with maintenance rituximab compared with 17% of patients under observation (neutropenia, 4% vs. <1%, respectively, and infections, 4% vs. <1%). A similar rate of infections (4.1%) was reported in the Maintenance Rituximab in Follicular Lymphoma (MAXIMA) study.11
Table 3.
Safety Profile of Maintenance Rituximab
| Study | Key Safety Data |
|---|---|
| Maintenance Rituximab vs. Observation* | |
| Salles, 201021 |
|
| Ghielmini, 200924 |
|
| Forstpointner, 20069 |
|
| van Oer, 20108 |
|
| Hainsworth, 200510 |
|
| Foá, 201011 |
|
| Taverna, 201025 |
|
Specific adverse events (AEs) are grade 3 or grade 4 unless otherwise stated.
In the SAKK 35/98 trial, the rates of grade 3 and 4 neutropenia were comparable for maintenance rituximab (16%) and observation (17%); slightly higher rates of asthenia and other nonhematological toxicities were reported for maintenance rituximab (see Table 3).24 The occurrence of secondary malignancies was not significantly different in patients who received maintenance rituximab and in those undergoing observation (10 and 12 patients with tumors, respectively). Similarly, long-term follow-up in the EORTC 20981 trial showed that the rates of secondary malignancies were similar between maintenance rituximab and observation (5% vs. 8%, respectively) (see Table 3).8
The safety of long-term maintenance rituximab is under investigation in the phase 3 SAKK 35/03 trial, which enrolled 270 patients with treatment-naive FL or relapsed or refractory disease who had received rituximab as induction therapy.25 A total of 167 responders were randomly assigned to receive four doses of maintenance rituximab once every 2 months or maintenance rituximab every 2 months for 5 years. At the time of the analysis, the median duration of maintenance rituximab therapy was 3.3 years. A total of 899 hematological and non-hematological AEs were reported; 26 AEs were grade 3 in severity, and six were grade 4. After randomization, additional cancers developed in five patients.25 The RESORT trial investigators plan to further assess the long-term safety of maintenance rituximab.23
Concern has been expressed about the potential consequences of hypogammaglobulinemia associated with maintenance rituximab. The PRIMA study reported an increased incidence of infectious events, mostly mild to moderate in severity, despite the absence of a significant decrease in serum immunoglobulin levels.21 The safety results after long-term follow-up in the EORTC 20981 study showed that in the observation arm, serum immunoglobulin G (IgG) levels increased from 6.6 g/L at the second randomization to 7.3 g/L at the end of the 2-year observation period.8 In the maintenance arm, IgG levels at the second randomization and at the end of the maintenance period were 6.5 g/L and 6.3 g/L, respectively. No patient was withdrawn from the study because of persistent IgG levels below 3 g/L.8 Trials are continuing to be conducted to investigate hypogammaglobulinemia and its potential consequences.
Quality-of-life data from the PRIMA study indicated no significant differences between maintenance rituximab and observation.21 Similarly, maintenance rituximab was found to be safe in a prospective QOL study in which 122 patients with FL were randomly assigned to receive maintenance rituximab or observation. 26 There were no significant differences in QOL measures between the two groups.
Maintenance Therapy in Aggressive Lymphoma
Current clinical data do not support the use of maintenance rituximab in patients with DLBCL.27,28 In a two-stage randomized trial,27 DLBCL patients 60 years of age and older received CHOP (n = 314) or R-CHOP (n = 318), with a second random assignment to maintenance rituximab or observation in responders (n = 207 and n = 208, respectively). The 2-year failure-free survival rates after the second random assignment were 76% for maintenance rituximab and 61% for observation (P = 0.009). A significant difference in failure-free survival rates at 2 years was noted between maintenance rituximab and observation after CHOP (74% vs. 45%, respectively; P = 0.0004) but not after R-CHOP (79% vs. 77%; P = 0.81). There were no significant differences in overall survival with maintenance rituximab after CHOP (HR = 0.73; P = 0.27) or R-CHOP (HR = 1.28; P = 0.48).27
In another study, 50 untreated patients with high-risk DLBCL who had achieved a complete response after treatment with rituximab and chemotherapy were randomly assigned to maintenance rituximab (n = 21) or observation (n = 29) for up to 2 years.29 Maintenance rituximab significantly increased the disease-free survival rate compared with observation (93.3% vs. 69.4%, respectively; P = 0.046), but overall survival rates did not differ significantly (93.8% vs. 87.7%; P = 0.519).
Ongoing Clinical Investigations of Maintenance Therapy
Clinical studies continue to address important questions concerning the role of maintenance rituximab in patients with FL and seek to identify the optimal dosing schedule in this population. Whether re-treatment has been as beneficial as maintenance therapy will be more evident with the publication of the phase 3 RESORT trial.23
Another area of study is the optimal duration of maintenance therapy with rituximab. The phase 3 MAINTAIN trial, with a planned enrollment of 874 patients with NHL (including FL and mantle-cell lymphoma), will compare a maintenance schedule of one dose every 2 months for 2 years with a schedule of one dose every 2 months for 4 years in responders to rituximab plus bendamustine in the first-line setting.23 The ongoing SAKK 35/03 trial is comparing a single dose of rituximab every 2 months for a total of 8 months with a long-term maintenance schedule of one dose every 2 months for 5 years in previously untreated FL and relapsed or refractory FL.25
Maintenance rituximab is also under investigation in other lymphomas and in chronic lymphocytic leukemia (CLL). The ongoing NHL-13 trial is an open-label, phase 3 study of 600 patients with DLBCL or grade 3b FL who received R-CHOP–like chemotherapy as induction treatment.30 Responders were randomly assigned to maintenance rituximab (one dose every 2 months for 2 years) or observation.
In another study, 256 newly diagnosed or relapsed patients with CLL are being treated with rituximab induction. Responders will be randomly assigned to maintenance rituximab (one dose every 3 months for 2 years) or observation.31
In a study of 200 patients with relapsed CLL, responders to induction therapy (rituximab plus cladribine and cyclophosphamide) will be randomly assigned to maintenance rituximab (one dose every 3 months for 2 years) or observation.32
Current Trends in Maintenance Therapy
Maintenance therapy is used routinely for FL in the practice setting, and patterns of use of maintenance rituximab have been reported in the National LymphoCare Study.33,34 This large prospective, multicenter, observational study (N = 2,734) has collected data on treatment and outcomes in patients with newly diagnosed FL in the U.S. Initial treatment and maintenance or observation decisions were made by the treating physician. Patients without disease progression within 215 days after completing first-line induction were classified as either receiving maintenance rituximab or observation.34
Among 1,046 patients who received rituximab-based induction therapy, 467 (45%) were treated with R-CHOP; 220 (21%) received single-agent rituximab; 195 (19%) received R-CVP; 122 (12%) received rituximab plus fludarabine; and 42 (4%) received other rituximab-based regimens.34 After initial treatment with rituximab monotherapy, 54% of patients received maintenance rituximab and 46% were observed. After initial treatment with rituximab plus chemotherapy, 45% of patients received maintenance rituximab and 55% were observed. Patients receiving maintenance rituximab were more likely to have grade 1 or 2 FL, stage 3 or 4 disease, normal lactate dehydrogenase, involvement of more than four lymph nodes, and two or more extranodal sites.31 There were no differences in maintenance use by age, sex, race, or FLIPI prognostic score.34
Cost Effectiveness
A cost-effectiveness evaluation based on the PRIMA intent-to-treat population showed that first-line maintenance rituximab represented greater value for costs than observational practice.35 In an analysis from the United Kingdom National Healthcare Service, the incremental cost-effectiveness ratio (ICER) was £15,977 ($24,958) per quality-adjusted life-year (QALY) gained—well below the assumed willingness-to-pay threshold of £30,000 ($46,863). The average life expectancy of patients treated with first-line maintenance rituximab was projected to be 1.27 years longer than that in observed patients and to be associated with an additional 1.17 QALYs.35 This is largely because the maintenance rituximab patients spend more time in a progression-free state compared with patients in an observation cohort. Total costs were £14,129 ($22,069) higher for first-line rituximab maintenance compared with observation. However, this was partially offset by the lower costs of second-line rituximabtherapy and by the supportive care incurred during progression. The ICER remained cost-effective in most plausible sensitivity scenarios.35
A U.S.-based cost analysis of maintenance rituximab in the setting of relapsed FL has been performed according to results from EORTC 20981.19,36 Five years after a second induction with R-CHOP, disease-free survival rates were estimated at 47% and 22% for maintenance rituximab and observation, respectively, during the second remission, and overall survival rates were estimated at 73% and 61%. The discounted ICER for the addition of maintenance rituximab was expected to be $19,522 per QALY gained—well below currently accepted thresholds.36
DISCUSSION
When used as part of induction chemotherapy, rituximab is highly active and has consistently been shown to improve outcomes in both untreated and relapsed low-grade lymphoma (Table 4). The results of multiple studies confirming this benefit have led to the near-universal use of rituximab in induction therapy. Unfortunately, despite improvements in progression-free survival and in overall survival, most patients with FL still relapse after initial treatment. Relapses are associated with impaired QOL and are characterized by diminishing durations of response to treatment.
Table 4.
Maintenance Rituximab In Follicular Lymphoma: Key Points
|
Maintenance therapy has the potential to improve the degree of response and to extend remission, thereby improving QOL. Attempts to use chemotherapy as a backbone for maintenance resulted in prolonged disease control, but they were associated with significant toxicity and failed to show a benefit in overall survival. Initial studies using rituximab as part of a maintenance strategy for patients in a first relapse who had achieved a response after treatment were promising, showing a clear benefit in response duration with minimal additional toxicity. However, interpretation of early studies remained difficult because of the absence of rituximab in the induction arms, heterogeneous patient populations, and various dosing schedules.
The large, randomized PRIMA study has confirmed a benefit of rituximab not only in progression-free survival but also in the degree of response in untreated patients after a rituximab-containing induction regimen.7,21 These findings leave little doubt that maintenance rituximab is an effective strategy in responding patients.
Despite these positive results, questions remain. To date, no prospective randomized study has shown a consistent improvement in overall survival with the addition of maintenance rituximab. Although follow-up of the PRIMA trial and other maintenance rituximab studies remains short (see Table 4), results from the EORTC 20891 trial demonstrate that subsequent rituximab use by relapsing patients in both arms make observing a difference in overall survival questionable. The absence of an observed overall survival benefit in multiple reports suggests that re-treatment with rituximab at progression may be as effective as maintenance rituximab. The only published study that compared maintenance therapy with re-treatment showed an improvement in the degree and length of response with maintenance rituximab; however, the duration of benefit was similar between the two arms, and overall survival was not significantly improved with maintenance rituximab.10
Although the optimal duration of rituximab maintenance is unknown, most published studies used a 24-month schedule. In the absence of mature safety data with longer dosing, treatment beyond 24 months is not recommended outside of a clinical study. Although the PRIMA trial used a rituximab regimen of one dose every 2 months, multiple dosing schedules appear to be effective. The choice of a schedule in our clinic is often based on multiple factors, including the patient’s preference and the physician’s experience with a given regimen.
CONCLUSION
For the practicing physician, translating the increasing amount of data from emerging maintenance studies into clinical practice remains challenging. However, it is becoming increasing clear that rituximab dosing after initial therapy results in improved disease control and should be considered in all patients with FL.
Questions regarding the optimal timing, schedule, and length of treatment as well as the effect of maintenance rituximab on overall survival remain unanswered and call for future randomized studies. Until these studies are performed, the decision to recommend maintenance rituximab should include a detailed discussion with the patient about the known potential benefits and risks of extended dosing. The use of maintenance rituximab in my own practice is also influenced by factors such as the presence of pretreatment risk factors and the patient’s response to and tolerance of induction therapy.
Footnotes
Disclosure: Dr. Fowler is a member of the advisory board for Genentech Inc. and receives research funding for ongoing studies from Genentech. Support for third-party writing assistance for this manuscript was provided by Genentech.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures 2011. Available at: www.cancer.org/acs/groups/content/@epidemiology-surveilance/documents/document/acspc-029771.pdf. Accessed August 18, 2011.
- 2.Armitage JO. The changing classification of non-Hodgkin’s lymphomas. CA Cancer J Clin. 1997;47(6):323–325. doi: 10.3322/canjclin.47.6.323. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Gregory SA, Marcus R. Emerging treatments for indolent lymphoma. Clin Adv Hematol Oncol. 2007;5(Suppl 8):1–9. [PubMed] [Google Scholar]
- 4.McLaughlin P. A look in the mirror. J Clin Oncol. 2009;27(8):1158–1159. doi: 10.1200/JCO.2009.19.8655. [DOI] [PubMed] [Google Scholar]
- 5.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 study. J Clin Oncol. 2009;27(10):1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol. 2010;28(29):4480–4484. doi: 10.1200/JCO.2010.28.4786. [DOI] [PubMed] [Google Scholar]
- 7.Salles GA, Seymour JF, Feugier P, et al. Rituximab maintenance for 2 years in patients with untreated high tumor burden follicular lymphoma after response to immunochemotherapy (Abstract 8004) J Clin Oncol. 2010;28(Suppl 15s) [Google Scholar]
- 8.van Oers MH, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: Long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28(17):2853–2858. doi: 10.1200/JCO.2009.26.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide and mitoxantrone (R-FCM) in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG) Blood. 2006;108(13):4003–4008. doi: 10.1182/blood-2006-04-016725. [DOI] [PubMed] [Google Scholar]
- 10.Hainsworth JD, Litchy S, Shaffer DW, et al. Maximizing therapeutic benefit of rituximab: Maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin’s lymphoma: A randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23(6):1088–1095. doi: 10.1200/JCO.2005.12.191. [DOI] [PubMed] [Google Scholar]
- 11.Foá R, Di Rocco A, van Hazel G, et al. Maintenance rituximab every 2 months for 2 years is effective and well tolerated in patients with follicular lymphoma with both standard or rapid infusion: Updated results from the phase IIIb MAXIMA study (Abstract 3945) Blood. 2010;116 [Google Scholar]
- 12.Vidal L, Gafter-Gvili A, Salles G, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: Systematic review and meta-analysis of randomized trials—2010 update (Abstract 1798) Blood. 2010;116 [Google Scholar]
- 13.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 14.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: Results of the GELA-GOELAMS FL2000 study. Blood. 2008;112(13):4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 15.Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: An East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25(15):1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 16.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 17.Rummel MJ, Norbert-Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab is superior in respect of progression-free survival and CR rate when compared to CHOP plus rituximab as first-line treatment of patients with advanced follicular, indolent, and mantle cell lymphomas: Final results of a randomized phase III study of the StiL (Study Group Indolent Lymphomas, Germany) (Abstract 405) Blood. 2009;114(Suppl) [Google Scholar]
- 18.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(10):3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 19.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin’s lymphoma, both in patients with and without rituximab during induction: Results of a prospective randomized phase III intergroup trial. Blood. 2006;108(10):3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 20.van Oers MH. Rituximab maintenance therapy: A step forward in follicular lymphoma. Haematologica. 2007;92(6):826–832. doi: 10.3324/haematol.10894. [DOI] [PubMed] [Google Scholar]
- 21.Salles GA, Catalano J, Pierre Feugier P, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 22.Ardeshna KM, Smith P, Qian W, et al. An intergroup randomised trial of rituximab versus a watch and wait strategy in patients with stage II, III, IV, asymptomatic, non-bulky follicular lymphoma (grades 1, 2, and 3a): A preliminary analysis (Abstract 6) Blood. 2010;116 [Google Scholar]
- 23.National Institutes of Health. Rituximab clinical trials. Available at: www.clinicaltrials.gov/ct2/show/NCT00075946. Accessed July 13, 2010. [Google Scholar]
- 24.Ghielmini ME, Hsu Schmitz S, Martinelli G, et al. Long-term follow-up of patients with follicular lymphoma (FL) receiving single agent rituximab at two different schedules in study SAKK 35/98 (Abstract 8512) J Clin Oncol. 2009;27(Suppl) doi: 10.1200/JCO.2010.28.4786. [DOI] [PubMed] [Google Scholar]
- 25.Taverna CJ, Bassi S, Hitz F, et al. Rituximab maintenance treatment for a maximum of 5 years in follicular lymphoma: Safety analysis of the randomized phase III trial SAKK 35/03 (Abstract 1802) Blood. 2010;116 [Google Scholar]
- 26.Witzens-Harig M, Reiz M, Heiss C, et al. Quality of life during maintenance therapy with the anti-CD20 antibody rituximab in patients with B cell non-Hodgkin’s lymphoma: Results of a prospective randomized controlled trial. Ann Hematol. 2009;88(1):51–57. doi: 10.1007/s00277-008-0560-2. [DOI] [PubMed] [Google Scholar]
- 27.Habermann TM, Weller EA, Morison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network clinical practice guidelines in oncology, non-Hodgkin’s lymphoma. V.3.2011. Available at: www.nccn.org/professionals/physician_gls/pdf/nhl/pdf. Accessed August 23, 2010
- 29.Li YJ, Xia ZJ, Li S, et al. Maintenance rituximab versus observation after R-CHOP or R-EPOCH in patients with untreated poor-prognosis diffuse large B-cell lymphoma and grade III follicular lymphoma (Abstract 8084) J Clin Oncol. 2010;28(Suppl 15s) [Google Scholar]
- 30.National Institutes of Health. A multicentre, randomized phase III study of rituximab as maintenance treatment versus observation in patients with aggressive B-cell lymphoma: NHL-13. Available at: www.clinicaltrials.gov/ct2/show/NCT00400478?term=NHL-13&intr=rituximab&rank=1. Accessed August 23, 2011. [Google Scholar]
- 31.National Institutes of Health. Rituximab versus observation as maintenance therapy in chronic lymphocytic leukemia (chronic lymphocytic leukemia) Available at: www.clinicaltrials.gov/ct2/show/NCT01118234?term=maintenance&cond=CLL&intr=rituximab&rank=3. Accessed August 23, 2011. [Google Scholar]
- 32.National Institutes of Health. A study of maintenance treatment With MabThera (rituximab) in patients with progressive B-cell chronic lymphocytic leukemia. Available at: www.clinicaltrials.gov/ct2/show/NCT00718549?term=maintenance&cond=CLL&intr=rituximab&rank=10. Accessed August 23, 2011. [Google Scholar]
- 33.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: First report of the National LymphoCare Study. J Clin Oncol. 2009;27(8):1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flowers C, Taylor MJ, Hirata J, et al. Use of maintenance rituximab in the United States following R-based induction for follicular lymphoma (FL) (Abstract 8100) J Clin Oncol. 2010;28(Suppl 15s) [Google Scholar]
- 35.Papadakis K, Follows G, Boyer J, et al. Cost-effectiveness analysis of rituximab maintenance in patients with untreated high tumour burden follicular lymphoma after response to immunochemotherapy: A UK National Healthcare Services perspective (Abstract 3833) Blood. 2010;116 [Google Scholar]
- 36.Hayslip JW, Simpson KN. Cost-effectiveness of extended adjuvant rituximab for US patients aged 65–70 years with follicular lymphoma in second remission. Clin Lymphoma Myeloma. 2008;8(3):166–170. doi: 10.3816/CLM.2008.n.020. [DOI] [PubMed] [Google Scholar]
- 37.Pettengell E, Schmitz N, Gisselbrecht C, et al. Randomized study of rituximab in patients with relapsed or resistant follicular lymphoma prior to high-dose therapy as in vivo purging and to maintain remission following high-dose therapy (Abstract 8005) J Clin Oncol. 2010;28(Suppl 15s) [Google Scholar]