Abstract
Clinical observations reveal that an augmented pace of T-cell recovery after chemotherapy correlates with improved tumor-free survival, suggesting the add-back of T cells after chemotherapy may improve outcomes. To evaluate adoptive immunotherapy treatment for B-lineage non-Hodgkin lymphoma (NHL), we expanded T cells from client-owned canines diagnosed with NHL on artificial antigen presenting cells (aAPC) in the presence of human interleukin (IL)-2 and IL-21. Graded doses of autologous T cells were infused after CHOP chemotherapy and persisted for 49 days, homed to tumor, and significantly improved survival. Serum thymidine kinase changes predicted T-cell engraftment, while anti-tumor effects correlated with neutrophil-to-lymphocyte ratios and granzyme B expression in manufactured T cells. Therefore, chemotherapy can be used to modulate infused T-cell responses to enhance anti-tumor effects. The companion canine model has translational implications for human immunotherapy which can be readily exploited since clinical-grade canine and human T cells are propagated using identical approaches.
Chemotherapy employed for cytoreductive effects can be harnessed to modulate tumors and their microenvironment to present neo-antigens1,2,3,4. However, the cellular immune response to tumor-(TAA) is compromised after chemotherapy due to iatrogenic lymphodepletion. This prompted us to test the hypothesis that add-back of autologous polyclonal T cells after chemotherapy can result in improved immune-mediated anti-tumor responses. This is supported by observations that improved recovery of T-cell numbers after lymphodepleting chemotherapy is predictive of tumor-free survival in patients with colorectal cancer, non-small cell lung carcinoma, cervical tumor, and non-Hodgkin lymphoma (NHL)5,6,7,8,9,10. To generate clinically-sufficient numbers of T cells for adoptive transfer, we and others have developed culturing platforms based on artificial antigen presenting cells (aAPC)11,12,13,14,15. To assess whether infusions of non-specific T cells propagated ex vivo on aAPC improve survival after standard-of-care chemotherapy, we developed the companion canine as an out-bred large animal cancer model for immunotherapy of spontaneously-occurring NHL.
Cancer in client-owned dogs models human malignancies due to their genetic similarity, large size, spontaneous occurrence of a broad diversity of tumor types, and similar treatment modalities16,17. The etiology of spontaneous canine and human cancers is analogous as both arise from genetic abnormalities or predisposition and common environmental exposures. NHL is the most common canine malignancy accounting for up to 24% of all reported neoplasms18,19. Similar to humans, the majority of canine NHL (60–80%) arises from malignant B cells. The most common presentation is a generalized lymphadenopathy corresponding to stage IV to V disease, with stage V describing tumor in blood, bone marrow, and other organ systems. The current standard-of-care treatment for canine B-lineage NHL is the combination chemotherapy regimen of cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP), which induces a temporary remission in approximately 85% of canines, but is rarely curable as the two-year survival rate is less than 20%20. Although other chemotherapy regimens have been compared to CHOP, none have significantly improved the overall survival of canines with NHL20.
In the present study, we report that clinically-sufficient numbers of T cells can be expanded from the peripheral blood (PB) of all 8 treated out-bred canines with spontaneous NHL, using K562 cells genetically modified to function as aAPC. Non-specific autologous ex vivo-propagated T cells were noted to persist after infusion, traffic to secondary lymphoid tissue, and improve the survival of canines receiving CHOP for spontaneously-occurring B-lineage NHL, compared to control dogs matched for disease stage and remission status which received only CHOP. The therapeutic potential of T-cell therapy is dependent on peristence, homing, and effector functions. Biomarkers were developed to report on some of these variables. T-cell engraftment correlated with thymidine kinase (TK) serum levels while decreased neutrophil-to-lymphocyte ratios (NLR) correlated with an improved anti-tumor effect. T-cell effector function was augmented after ex vivo propagation as supported by changes in mRNA profiles and an improvement in canine survival that correlated with up-regulated T-cell expression of granzyme B. In summary, T-cell therapy in out-bred companion canines with cancer is justified not only to improve their survival, but as a model that informs on human immunotherapy of NHL.
Results
Immunophenotype of PB-derived T cells
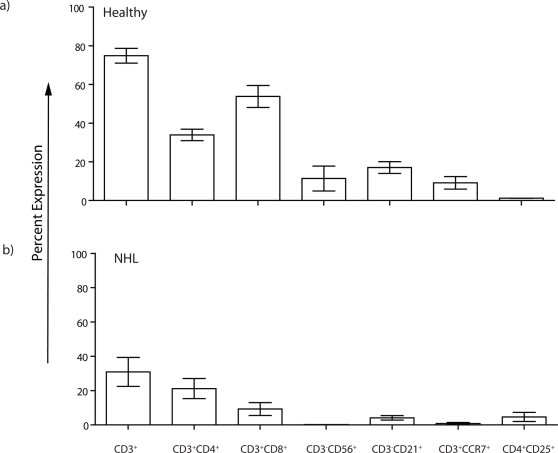
Flow cytometry, analyzed in a lymphocyte gate, was used to reveal protein expression on T cells derived from PB obtained from healthy subjects and canines diagnosed with NHL before their treatment with CHOP (Figure (Fig) 1). In healthy dogs the average CD3+ population was 74 ± 4% (mean ± s.e.m.) with CD3+CD4+ T cells (33 ± 3%) making up a reduced percentage of the overall T-cell population compared to CD3+CD8+ T cells (54 ± 6%). Among the CD3+CD4+ population, 1.2 ± 0.03% co-expressed CD25 which is consistent with a low number of circulating numbers of regulatory T cells (Tregs). Natural killer (NK) cells, described as CD3negCD56+, comprised an average of 11 ± 6%, while CD3negCD21+ B cells were present at 17 ± 3%. In comparison (Fig 1), the percentage of CD3+ T cells in the PB obtained from canines with NHL (30 ± 8%) was lower (p = 0.003). Similarly, the NK-cell (0.2 ± 0.05%) and B-cell populations (4.1 ± 1.3%) were also decreased (p = 0.13 and p = 0.008, respectively). We noted that PB from canines with NHL had an increased percentage of CD3+CD4+ T cells (21 ± 6%) and a decreased percentage CD3+CD8+ T cells (9 ± 4%) which resulted in an “inverted ratio” compared to healthy donors. The difference in percentage of CD3+CD8+ T cells between healthy subjects and canines with NHL was statistically significant (p = 0.0006). A slight rise in the CD4+CD25+ population (4 ± 3%, p = 0.18), presumably reflects an increased number of Tregs, in canines with NHL, compared to healthy subjects and is consistent with a state of immunosuppression. As a marker for both the potential of T cells to migrate to lymph node (LN) and memory phenotype21, we noted that the CD3+CCR7+ T cells in PB from healthy subjects was 9 ± 3%, but was reduced to 0.8 ± 0.6% in PB of canines with NHL (p = 0.04).
Figure 1. Composition of circulating canine T cells.
The expression of T-cell subsets in PB from (a) healthy subjects (n = 4) and (b) canines with NHL (n = 4) prior to CHOP treatment. Percentage expression is based on cells within a lymphocyte gate described by a Forward vs. Side Scatter flow cytometry plot. Mean ± s.e.m. are shown.
Canine T cells can be non-specifically and efficiently propagated on aAPC when co-cultured with IL-21
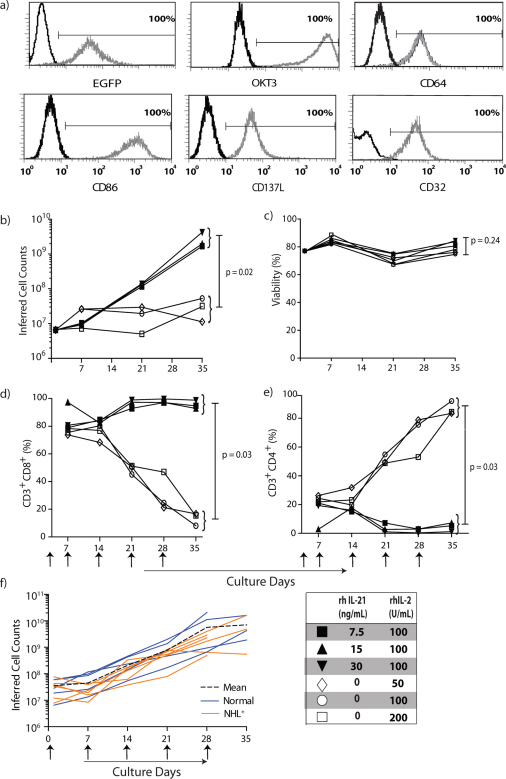
Canine T cells from the PB of healthy subjects were numerically expanded on γ-irradiated aAPC CLN4, pre-loaded via CD64 with OKT3 to cross-link canine CD3, and human recombinant (rh)IL-2 and rhIL-21 (Fig 2A). The homology between human and canine IL-2, IL-15, and IL-21 is shown in Supplementary Table S1. T cells propagated in the presence of graded doses of rhIL-2 had limited replicative potential, for while the T cells remained viable over a 35-day culturing period, they demonstrated only a 6.5 ± 2.4 fold expansion (n = 2). Therefore, we determined whether supplemental signaling through the common γ-cytokine receptor could augment canine T-cell proliferation. The addition of graded doses of rhIL-21 in addition to rhIL-2 maintained T-cell viability (Fig 2C) and resulted in 399 ± 124 fold expansion over 35 days (313 ± 75 fold expansion after 28 days; n = 2, p = 0.03) which was greater than the numeric expansion achieved with only rhIL-2 (Fig 2B). We observed that the largest numbers of T cells were produced at the highest concentration of rhIL-21 (Fig 2B). These data demonstrate that a combination of exogenous rhIL-21 and rhIL-2 in coordination with cross-linking CD3 via OKT3 are required to sustain proliferation of canine T cells on CLN4 aAPC.
Figure 2. rhIL-21 preferentially propagates canine CD8+ T cells on OKT3-loaded aAPC.
T cells isolated from PBMC of healthy dogs were numerically expanded on γ-irradiated OKT3-loaded aAPC at a T-cell : aAPC ratio of 2:1. T cells were re-stimulated with aAPC every 7 days. Recombinant human IL-21 and/or rhIL-2 were added to the co-culture of T cells on aAPC as shown. (a) Parental K562 cells were genetically modified to co-express human CD64 and the human T-cell co-stimulatory molecules CD86, CD137L, and membrane-bound IL-15 (co-expressed with EGFP) and cloned (CLN4). OKT3 was loaded via introduced CD64 on CLN4 and detected with a Fab-specific antibody. CLN4 is shown as gray-open histograms and K562 parental cells as black-open histograms. (b) Numeric expansion of T cells on γ-irradiated OKT3-loaded aAPC (added every 7 days) in the presence of rhIL-2, or both rhIL-2 and rhIL-21. The average cell counts from two donors are shown. (c) The mean viability of T cells during co-culture on aAPC and cytokine(s) (n = 2). Percentage expression (n = 2) of (d) CD3+CD8+ and (e) CD3+CD4+ T cells upon co-culture with aAPC and cytokines(s). (f) Numeric expansion of T cells measured over culture time on aAPC and rhIL-2 and 21 recorded as the mean inferred cell count (black dashed line) based on 5 healthy subjects (blue solid line) and 5 non-trial canines with NHL (orange solid line). Upward arrows indicate timing for the addition of γ-irradiated OKT3-loaded aAPC.
rhIL-21 selectively propagates canine CD8+ T cells
The addition of rhIL-21 selectively propagates human CD8+ T cells22 which is desirable as the cytolytic effector function of infused CD8+ T cells can control tumor growth23. We therefore investigated the influence of rhIL-21 in combination with rhIL-2 to selectively propagate CD8+ versus CD4+ canine T cells. We observed a decrease in the expression of CD3+CD8+ T cells from 76 ± 1% on day 7 to 14 ± 3% on day 35 when T cells were cultured on CLN4 with rhIL-2, whereas the expression of CD3+CD8+ T cells cultured with both rhIL-2 and rhIL-21 increased from 73 ± 2% on day 7 to 95 ± 2% on day 35 (Fig 2D). These data are consistent with the ability of rhIL-21 to preferentially support outgrowth of canine CD8+ T cells which has practical implications for following the fate of infused T cells, as canines with NHL have inverted ratio of CD4:CD8 (Fig 1) and after CHOP preferentially recover CD4+ T cells.
Propagation of T cells from canines with B-lineage NHL
Late stage presentation of NHL and treatment with CHOP leads to T-cell lymphopenia characterized by decreased numbers of CD3+ T cells and low numbers of CD8+ T cells, similar to observations in humans after CHOP (Fig 1)24. The culture conditions established to selectively expand CD8+ T cells from healthy dogs were applied to numerically expand T cells from canines with NHL. After 28 days of co-culture on CLN4 and rhIL-21/2, T cells underwent an average 124 ± 44 fold expansion (n = 6) which was less than achieved using T cells from healthy subjects (221 ± 39 fold expansion, p = 0.04, n = 6) and is likely indicative of an initial partial defect in T cells from canines with NHL to be fully activated for proliferation (Fig 2F). Even so, the aAPC-based expansion methodology was sufficient to achieve the T-cell numbers necessary for adoptive immunotherapy. These data demonstrate that the platform technology of every 7-day additions of γ-irradiated aAPC in combination with rhIL-2 and rhIL-21 can sustain the proliferation of T cells and provides the foundation for infusing ex vivo-propagated autologous T cells in canines diagnosed with advanced-stage NHL.
Characterization of propagated T cells used for adoptive transfer
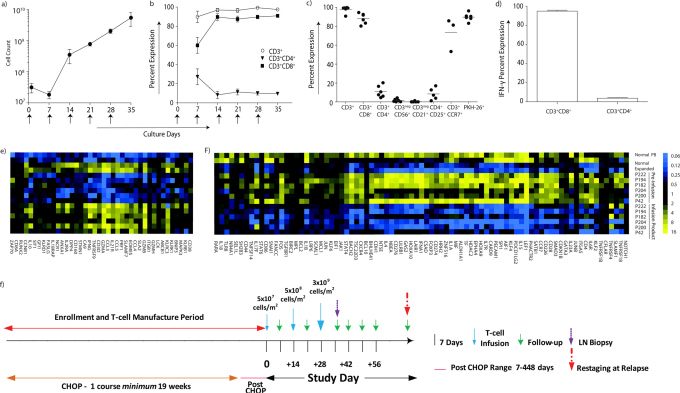
PB-(Supplementary Fig S1 and S2) with NHL recursively stimulated on γ-irradiated CLN4 in the presence of rhIL-2 and rhIL-21 for up to 35 days of culture underwent a mean 109 ± 15 fold expansion (Fig 3A). Seven days after the first aAPC-mediated stimulation the cultures contained T cells that were: 89 ± 6% CD3+, 60 ± 8% CD3+CD8+, and 27 ± 8% CD3+CD4+. By day 14, cultures were predominantly CD3+CD8+ (90 ± 4%) and the mean CD3+CD4+ population had decreased to 8 ± 4% (Fig 3B). T-cell infusion products analyzed for 6 (of the 8) treated dogs at the time of their cryopreservation were on average (Fig 3C): 98 ± 1% CD3+; 88 ± 2% CD3+CD8+; 11 ± 3% CD3+CD4+; 0.2 ± 0.2% CD3negCD21+ (B cells); 73 ± 10% CD3+CCR7+ (central memory); and 90 ± 2% PKH-26+ (fluorescence label to track persistence). To evaluate a T-cell effector function (n = 3) we assessed the IFN-γ production upon cross-linking CD3 with OKT3 loaded on CLN4 (Fig 3D). All the propagated CD3+CD8+ T cells, in contrast to the few numerically expanded CD3+CD4+ T cells, generated IFN-γ (99.1 ± 1% versus 3.7 ± 0.6%). It was noted that of the propagated CD4+ T cells, 8 ± 3% co-expressed CD25 and their reduced production of IFN-γ is consistent with a regulatory function. The selective propagation of CD8+ T cells that could be activated to secrete IFN-γ was exploited for adoptive immunotherapy.
Figure 3. Characterization of T-cell infusion products.
(a) The mean inferred cell count for 6 (of the 8) canines infused with T cells that were propagated over 28–35 days on γ-irradiated OKT3-loaded aAPC (CLN4) in presence of rhIL-2/IL-21. Arrows represent days aAPC were added. (b) Percent expression of CD4+ and CD8+ T-cell subsets during propagation on γ-irradiated aAPC in the presence of rhIL-2/IL-21. Arrows represent days aAPC were added. (c) Immunophenotype of the T-cell infusion products (n = 6) at the time of cryopreservation. The horizontal lines describe mean percentage expression. (d) T-cell infusion products were tested for IFN-γ production after stimulation with OKT3-loaded aAPC in the presence of rhIL-2/IL-21 (n = 3). Analysis of multiplexed digital gene profiling of PB-derived canine T cells before and after propagation from healthy subjects (n = 2) and canines with NHL (n = 6) identified mRNA species which were either significantly (p<0.001) (e) up-regulated (> 2 fold change) or (f) down-regulated (< 2 fold change) in both healthy subjects and in at least 5 of 6 canines with NHL. (g) Study design: Enrollment occurred pre-, post, or during treatment with CHOP. Enrolled canines received one course of CHOP, typically administered over 19 weeks, and received one to three infusions of T cells 14 days apart using an intra-patient dose-escalation scheme.
T-cell gene expression
T cells from canines with NHL exhibited a decreased in vitro proliferative potential on aAPC consistent with T-cell dysfunction. Therefore, we investigated whether the propagation process could produce T cells that were genetically similar to T cells from healthy donors. This was undertaken by multiplex digital profiling using the nCounter analysis system to quantify a panel of selected genes (Supplementary Table S2). The detected mRNA species undergoing a significant change (p<0.001, fold-change >2) were identified in T cells from healthy subjects and patients with NHL by comparing pre-expansion with post-expansion samples and then filtering for uniformity. After propagation for 28 days, 40 mRNA species were up-regulated in at least 5/6 canines with NHL as well as in T cells from healthy subjects (Fig 3E). Similarly, we noted a set of 83 mRNA species that decreased (p<0.001, fold-change <2) after expansion in at least 5/6 canines with NHL and healthy subjects (Fig 3F). Gene products associated with T-cell cytoxicity (granzymes, IFN-γ, perforin), proliferation (EOMES, DPP4, TNFRSF9, LCK, ZAP70), and trafficking to LN and inflammation (CCL3, CCL4, CCL5 receptors) were up-regulated after propagation. Similarly, mRNA species coding for both IL-2 and IL-21 receptors were increased reflecting the use of rhIL-2 and rhIL-21 during the propagation process. mRNA species coding for FOXP3 were significantly decreased in the infusion product, corresponding to the decreased number of Treg produced during expansion. In aggregate, the synchronous direct quantification of multiple mRNA species revealed a pattern of gene expression that was similar whether the T cells were propagated from healthy dogs or canines with NHL.
Adoptive transfer of propagated T cells
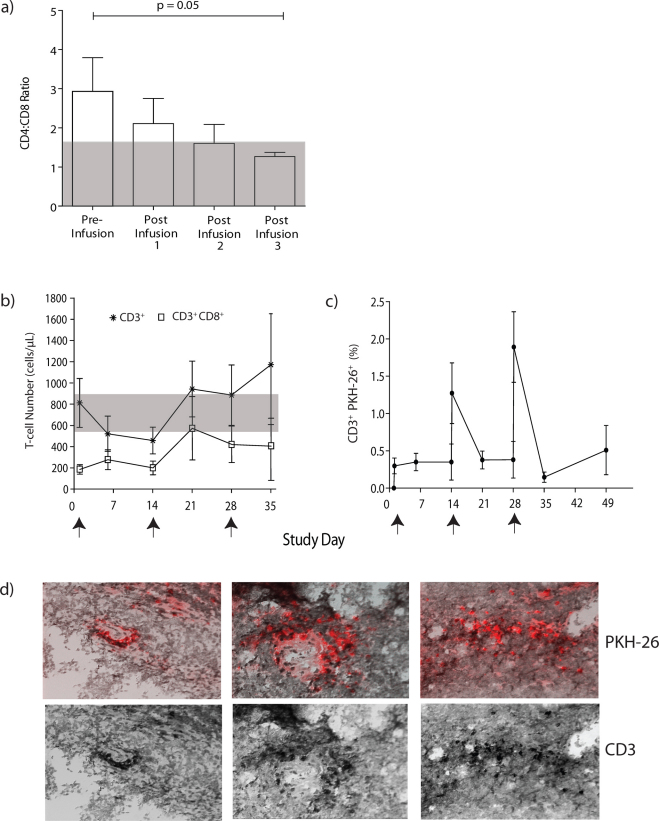
The ratio of CD4:CD8 T cells in PB was serially assessed as a measure of persistence of infused predominately CD8+ T cells (Fig 4A). The CD4:CD8 ratio in healthy subjects was 1.6:1 ± 0.2 (n = 4) while the mean pre-infusion ratio in canines with NHL was 3:1. After each infusion (predominately containing CD8+ T cells), the average ratio decreased to 1:1.2 after the third dose (n = 6), which was significantly reduced compared to pre-infusion measurements (p = 0.05). We also observed that the percentage of CD3+CD8+ T cells in PB increased after each T-cell infusion. The mean number of CD3+CD4+ and CD3+CD8+ T cells pre-infusion was 585 ± 223 cells/µL and 188 ± 44 cells/µL, respectively. By study day 35, the mean CD3+CD4+ and CD3+CD8+ T-cell counts in PB were 305 ± 166 cells/µL and 469 ± 142 cells/µL, respectively. Similarly, the mean absolute lymphocyte count (ALC) increased after the T-cell infusions (Supplementary Table S3) which reflected an increase (from 64% to 79%) in CD3+ T cells above pre-infusion levels over the same 35 day time frame (Fig 4B). To directly examine the persistence of adoptively transferred T cells, the infusion products were pre-labeled with PKH-26, a red fluorescent dye. PKH-26+CD3+ T cells incrementally increased in the PB after each of the three graded T-cell infusions and were detectable for up to 49 days (Fig 4C). Toxicities attributed to T cells were confined within a 72-hour period after the second T-cell infusion with the majority of the seven recorded adverse events being Grade I or II (Table 2). One canine with a Grade III gastrointestinal adverse event required hospitalization for dehydration (24 hours). In aggregate, these data support that multiple doses of ex vivo propagated T cells can be safely administered to canines with NHL and persist after infusion.
Figure 4. Tracking infused T cells.
(a) Ratio of CD4:CD8 T cells in PB after CHOP, but before adoptive transfer of T cells and compared with measurements taken three hours after each T-cell infusion. The grey shaded area represents the mean CD4:CD8 ratio (1.6:1) in healthy subjects (n = 4). (b) Mean T-cell counts in PB from 6 canines before and after adoptive transfer of T cells. The grey shaded area represents the range for CD8+ T cells in healthy subjects (n = 4, 581 to 958 cells/µL). Arrows represent days T cells were infused. (c) Mean expression of CD3+ T cells pre-stained with red fluorescent dye, PKH-26, in the PB of 6 canines. Arrows represent days T cells were infused. (d) Evaluation of fluorescence and staining of CD3 from a LN biopsy. Frozen tissues were viewed with fluorescent microscopy to detect (top) PKH-26+ (red) T cells and (bottom) co-stained with anti-CD3 (black) to validate T-cell presence.
Table 2. Number of canines with adverse events at least possibly attributed to T-cell infusion.
| Symptom | Dose1 | Dose 2 | Dose 3 | |||
|---|---|---|---|---|---|---|
| Grade I, II | Grade III, IV | Grade I, II | Grade III, IV | Grade I, II | Grade III, IV | |
| Lethargy | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 0 | 1 | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 2 | 1 | 0 | 0 |
| Vomiting | 0 | 0 | 2 | 1 | 0 | 0 |
Grading based on Veterinary Co-operative Oncology Group Scale (version 2009).
Adoptively transferred T cells migrate to tumor
Ten days after the last infusion, enlarged LNs were sampled to determine if the administered T cells migrated to sites of tumor. Under fluorescent microscopy, PKH-26+ T cells could be detected throughout a LN and this was concordant with the expression of CD3 (Fig 4D). Thus, we conclude that the infused T cells can traffick to, and persist in, secondary lymphoid tissue containing tumor.
Adoptive transfer of T cells after CHOP improves survival
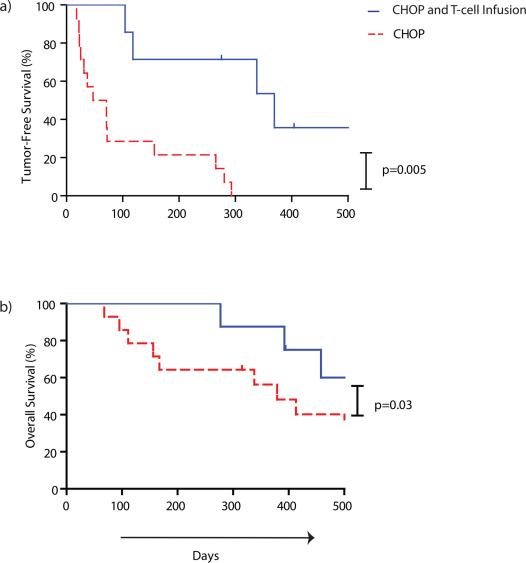
To evaluate the ability of ex vivo-propagated T cells to impact canine survival, we compared the 8 dogs that received CHOP and autologous T cells with a cohort of stage-matched canines with NHL that received only CHOP. Both cohorts were followed for 500 days post initial diagnosis of NHL or obtaining CR for the analysis of tumor-free survival. As expected, 7 of 8 dogs that received T-cell infusions achieved a prior complete remission (CR) from CHOP. However, we observed that the infusion of T cells after CHOP resulted in marked improvements in overall and tumor-free survival and that this was evident when only 8 dogs had been infused (Fig 5). The historical controls which were matched for age, size, disease stage, and achieving CR after CHOP with the canines that received T cells after CHOP. This control population had a median overall survival (n = 12) of 167 days (range from 68 to 413 days) upon initial diagnosis of NHL and a median tumor-free survival (n = 12) of 71 days (range from 23 to 293 days) following the CR achieved after receiving CHOP. Significantly, for the 8 canines receiving CHOP combined with T-cell infusions the median overall survival was improved to 392 days (range 277 to 458 days) and the tumor-free survival improved to 338 days (range 104 to 369 days). The hazard ratio and p-value for the log rank test of overall survival were 4.3 and 0.03, respectively. T-cell infusions significantly increased the period of tumor-free survival when used in conjunction with CHOP therapy (p = 0.005) which implies a clinical benefit to dogs with NHL that received immunotherapy.
Figure 5. Adoptive transfer of ex vivo propagated T cells after CHOP improves survival of canines with NHL.
Differences between 12 historical stage-, age-, and size-matched controls treated with CHOP (red dashed line) and 7 (curve A) or 8 (curve B) research participants treated with CHOP and T cells (blue solid line) over 500 days in evaluating (a) tumor-free survival measured after achieving CR (p = 0.005) and (b) overall survival measured upon diagnosis of NHL diagnosis (p = 0.03). One dog (P42) had progressive disease at the time of T-cell infusions and was not evaluated in the tumor-free survival analysis, but was included in the overall survival analysis. This patient had stable disease for 112 days after study day 14.
Biomarkers
The therapeutic potential of the infused T cells is dependent in part on their persistence and their effector function. TK is elevated during DNA synthesis25 and we tested whether TK could be used to monitor proliferation and survival of infused T cells. To determine whether TK was secreted by proliferating T cells, T cells were co-cultured for 48 hours with γ-irradiated OKT3-loaded CLN4 and cytokines which resulted in elevated TK levels (28.6 ± 2.5 U/L) in the conditioned supernatant compared to γ-irradiated T cells (p = 0.03) and T cells co-cultured with cytokines only (p = 0.04) (Supplementary Figure S3). Therefore, using TK as a biomarker for proliferation, we observed that serum TK levels (Fig 6A) were elevated for 4 weeks after the T-cell infusions and appeared to track with the presence of adoptively transferred T cells (study day 14 average: 24.8 ± 8.3 U/L and study day 28 average: 49.2 ± 18.5 U/L, n = 5). Serum from healthy canines (n = 2) revealed that the mean TK concentration was 5.8 ± 1.8 U/L. These studies indicate that TK may be a biomarker for the presence of infused T cells which presumably propagate after adoptive transfer.
Figure 6. Biomarkers that inform on T-cell engraftment and canine survival.
(a) Serum TK concentration in canines (n = 5) plotted with the number of CD3+CD8+ T cells in PB. Arrows indicate the infusion of T cells. (b) NLR on study day 35 plotted as a function of tumor-free survival. Horizontal arrow indicates NLR in PB of healthy canines. (c) Expression of granzyme B in propagated T cells, assessed at time of cryopreservation, plotted as a function of tumor-free survival.
To investigate the impact of T-cell persistence on canine survival, we measured the NLR, recognizing that the absolute neutrophil count (ANC) is one measurement of hematopoietic recovery after CHOP chemotherapy and thus can serve as a marker for T cells arising from bone marrow reserves versus adoptively transferred. We noted that the NLR decreased (p = 0.04) in patients which experienced a prolonged remission (Fig 6B). The pre-infusion NLR for patients sustaining remission was 6:1 ± 1.2 and it decreased significantly to 2.2:1 ± .5 by study day 35 (p = 0.03). The pre-infusion NLR in canines with an abbreviated remission (including the patient P42 with stable disease) was 6.7:1 ± 3.2 and increased to 35.8:1 ± 30.4 at study day 35. Excluding P42, the NLR at study day 35 was 5.7:1 ± 2. Thus, a decreased NLR ratio may be predictive of improved canine survival. We measured granzyme B as a marker for T-cell effector function and observed that its expression in the infusion product correlated with improved tumor-free survival (Fig 6C). In patients with decreased (≤ 118 days) tumor-free survival (or stable disease), only 28 ± 7% of the propagated T cells expressed granzyme B, compared to 99 ± 0.5% of T cells infused in canines who sustained a remission for longer than 119 days. The correlation between the tumor-free survival and increased percentage of propagated T cells expressing granzyme B was statistically significant (p = 0.0018). These data demonstrate that inspection of the T-cell product may be used to stratify patients at risk for progression of NHL. In summary, both a decreased NLR and an increased expression of granzyme B were associated with improved outcome for canine NHL patients treated with CHOP and T cells.
Discussion
Autologous CD8+ T cells expressing T-cell receptors with undefined specificities (non-specific) were numerically expanded on γ-irradiated OKT3-loaded aAPC in presence of human cytokines and infused into canines with advanced NHL to achieve improved anti-tumor effects after CHOP chemotherapy. The adoptively transferred T cells were able to persist for at least 49 days in the PB, based on the measurement of a fluorescent tag, and traffic to sites of disease. Digital profiling showed that the genetic signature of propagated mostly CD8+ T cells was similar between healthy dogs and canines with NHL. Elevated serum TK levels correlated with persistence and propagation of adoptively transferred T cells. A decreased NLR and increased T-cell expression of granzyme B were predictive of positive outcomes for canines with NHL. These data provide a foundation for further improving the potency of T-cell infusions through the enforced expression of a chimeric antigen receptor to redirect specificity for a lineage-specific antigen on canine malignant B cells.
Cancer in companion canines is a comparative model for human malignancies that has advantages compared to murine studies evaluating adoptive T-cell therapies. Previous immunotherapies in out-bred canines included mAbs, vaccines, and hematopoietic stem-cell transplantation26,27. The large companion dog population and widespread prevalence of canine cancers can power pre-clinical human trials to determine feasibility, efficacy, and safety in order to expand our knowledge of applied cellular therapy28.
The approach to propagating canine T cells mirrors our current methodology to numerically expanding human T cells as both employ recursive additions of the same aAPC, rhIL-2 and rhIL-21. Currently, for our human trial to treat advanced CD19+ lymphoid malignancies (clinicaltrials.gov identifier: NCT00968760), we propagate clinical-grade genetically modified human T cells on these same aAPC (CLN4) in the presence of similar concentrations of soluble rhIL-2 and rhIL-21. The use of aAPC for the canine trial resulted in the robust and preferential outgrowth of CD8+ T cells. Indeed, rhIL-21 in conjunction with rhIL-2 has been shown to maintain the T-cell expression of CD28 and enhance survival, proliferation, antigen affinity, and production of cytokines after antigen stimulation8,22,29,30,31,32,33,34. Of significance to our study, CD8+ T cells treated with rhIL-21 demonstrated increased transcription of genes related to LN homing and cytolytic effector function35.
The approaches to improve propagation of T cells in vitro can be combined with manipulation of the recipient to improve T-cell therapy. Add-back of T cells in the lymphopenic recipient augments adoptive immunotherapy by decreasing undesired regulatory or suppressor cells, elevating cytokines that promote engraftment and modulating the tumor microenvironment. Indeed, chemotherapy that induces lymphopenia may increase the expression of neo-antigens by residual tumor3,36,37,38. Chemotherapy has been previously used as an immune-modulating agent to generate mAbs specific for TAA39 and one explanation for the clinical benefit of the add-back of the propagated T cells in our canine study is that treatment with CHOP resulted in the presentation of TAAs that could be recognized by the infused polyclonal population of T cells2,4.
Augmented recovery of T-cell numbers after chemotherapy has been previously noted to correlate with improved rates of malignant relapse and decreased mortality23. Indeed, longitudinal measurements of ALC can predict improved survival in patients with solid tumors and hematological malignancies7,9,40. Adoptive immunotherapy trials are underway in human patients that may inform on the ability of T cells that have been non-specifically propagated ex vivo to provide anti-tumor responses. It was observed that the number of CD3+CD8+ T cells tended to remain elevated in those canine patients that achieved a CR which is also supportive of a T-cell mediated anti-tumor effect. Recovery of T-cell subsets may also impact the anti-tumor responses. An increased CD4:CD8 ratio has been shown in humans to be an indicator of decreased response to chemotherapy and poor prognosis41,42. We note that in our canine trial, the adoptive transfer of CD8+ T cells was able to normalize the inverted CD4:CD8 ratio assessed pre-infusion. Measuring the recovery of T-cell counts in PB raises the question whether T cells can home to sites of malignant disease as the presence of tumor-infiltrating human CD8+ T cells correlates with improved tumor control and prognosis10,43,44,45. Digital profiling revealed that the propagated canine T cells up-regulated mRNA species coding for desired chemokines receptors for CCL3, CCL4 and CCL5, which is consistent with the ability to home to LNs. This was supported by observation that infused CD8+ T cells demonstrated a capacity to traffick to tumor as they were detected inside LNs containing tumor 10 days post infusion.
In addition to measuring that ability of T cells to home to sites of malignant disease, we investigated biomarkers that inform on T-cell persistence and their cytolytic potential and thus could impact the therapeutic potential of adoptive immunotherapy. Serum TK is a prognostic factor in several human cancers, including NHL and canine malignancies25,46,47 and transient increases in serum TK levels occur after chemotherapy, surgery, inflammation, and infection48. We demonstrate that TK levels also correlated with in vitro propagation and in vivo persistence of infused T cells. An increased NLR has been shown to be a negative prognostic factor in human cancers, including NHL49,50,51 and we report that a decreased NLR correlated with improved canine tumor control. In addition to T-cell numbers, we examined the potential effector function of the infused product and demonstrated that an elevated T-cell expression of granzyme B correlated with improved control of NHL.
In summary, we demonstrate that the companion canine is an informative comparative oncology model regarding clinical benefits for adoptive transfer of propagated non-specific T cells after CHOP. The augmented tumor-free survival after canine T-cell therapy is reminiscent of the improved survival observed in humans that received CD20-specific therapeutic mAbs when combined with CHOP52,54. Our data suggest that chemotherapy can be used as an immune-modulating agent and that add-back of canine or human T cells after chemotherapy may be an approach to improving tumor-free survival, which has implications for NHL, as well as other tumor types.
Methods
Eligibility for clinical trial. Client-owned canines treated at Texas A&M University (TAMU) College of Veterinary Medicine participated with owner's written consent. A diagnosis of stage IV or V B-cell lineage NHL encompassed the inclusion criterion, while the exclusion criterion included: canines too ill to proceed, allergies to bovine or murine products, and insufficient T-cell ex vivo expansion. Ten canines were enrolled and 8 infused with autologous ex vivo-propagated T cells. Before adoptive immunotherapy, one patient died unrelated to NHL or chemotherapy and the other was lost. Enrolled patients were monitored for up to 500 days post NHL diagnosis and actively monitored after the first T-cell infusion.
Study Design. Upon enrollment (2009–2010), fine needle aspirates (FNA) of LNs verified B-lineage NHL (Supplementary Fig S1). Trial participants are described in Table 1, Supplementary Figs S1 and S2, and Supplementary Table S3. All participants (and historical controls with NHL) received 19-weeks of CHOP (chemotherapy in Supplementary Table S4) prior to T-cell infusions. PB-derived T cells were numerically expanded over 24 to 35 days (mean ± s.e.m. = 30 ± 2 days). PB samples were taken at the time of enrollment, before and 3 hours after each T-cell infusion, and continued throughout the active monitoring period. Patients were enrolled before, during, or after CHOP. Employing an intra-patient dose escalation scheme (Fig 3), 1 to 3 T-cell doses (up to 5×107/m2, 5×108/m2, and 3×109/m2) were infused based on canine body surface area (BSA)55,56. T cells were first administered at a median of 14 days (range 7 – 448 days) after CHOP completion (Table 1). P42 relapsed prior to T-cell therapy and stable disease was achieved seven days after the second T-cell infusion, while all other participants were in remission after CHOP and at the time of T-cell infusions.
Table 1. Patient characteristics that received T cells after CHOP.
| UPN | Breed | Sex | Age (yrs) | BSA (m2) | InitialStaging | Chemotherapy | Staging at the time of 1st T-cell infusion | No. T-cell Infusions | No. days T cells cultured before cryopreservation |
|---|---|---|---|---|---|---|---|---|---|
| P42 | Rottweiler | F | 5 | 1.2 | IV | CHOP, P,M,T | PD | 3 | 35 |
| P182 | Labrador Retriever | F | 6 | 1.1 | IV | CHOP | CR | 3 | 35 |
| P194 | Border Collie | F | 6 | 0.9 | IV | CHOP | CR | 3 | 28 |
| P200 | Rottweiler | M | 4 | 1.3 | V | CHOP | CR | 3 | 33 |
| P204 | Labrador Retriever | F | 11 | 0.9 | IV | CHOP | CR | 3 | 24 |
| P222 | Cocker Spaniel | F | 5 | 0.5 | IV | CHOP | CR | 3 | 27 |
| P249 | Labrador Retriever | F | 9 | 1.08 | IV | CHOP | CR | 2 | 35 |
| P263 | Sheltie | M | 6 | 0.66 | IV | CHOP | CR | 1 | 35 |
T = Toceranib; M = MOPP; P = Prednisone; CR = Complete Remission; PD = Progressive Disease
The average T-cell dose for the infusions administered to 8 dogs was 6.45×108±1.65×108 cells/m2 (mean ± s.e.m.) (range: 5×107 to 2.5×109 cells/m2).
Patient P42 relapsed prior to initiation of adoptive immunotherapy and received mechlorethamine, vincristine, procarbazine, and prednisone (MOPP), L-asparaginase, toceranib, and prednisone, concurrent with the T-cell infusions. This dog achieved stable disease after T-cell therapy.
Control dogs. PB was obtained from healthy subjects seen at TAMU during 2010. Size- and age- matched canines with stage IV or V biopsy-proven B-lineage NHL treated at TAMU through the length of the T-cell trial and treated only with CHOP, were used as matched controls for survival curves (n = 12).
Isolation of mononuclear cells from PB. Canine-derived PB mononuclear cells (PBMC), diluted 1:10 with EDTA/PBS (Miltenyi Biotec, Auburn, CA) were isolated by density-gradient centrifugation over Ficoll-Paque-Plus (GE Healthcare BioSciences, Piscataway, NJ) and cryopreserved in freeze media (FM) [10% heat-inactivated Fetal Bovine Serum (FBS, HyClone, Logan, UT), and 10% DMSO (Sigma, St. Louis, MO) in HyQ RPMI 1640 (HyClone)].
aAPC. The human tumor cell line K562 (homogenously expressing endogenous CD32) was transduced with lentiviruses to co-express human CD19, CD64, CD86, CD137L, and membrane bound human IL-15 (co-expressed with EGFP), and cloned (Clone 4, CLN4) by limiting dilution15,57. CLN4 was fingerprinted at MDACC using short tandem repeat PCR and proven to be derived from K562. CLN4 was γ-irradiated (100 Gy) prior to loading with anti-human CD3 (OKT3, Muronmonab, Orthoclone, Raritan, NJ), cryopreserved in FM and was used for propagating T cells.
Flow cytometry. Fluorochrome-conjugated monoclonal antibodies (mAbs) were used at a 1∶25 dilution in flow buffer (FB) [2% FBS and 0.1% sodium azide (Sigma) in PBS (Sigma)]: mouse anti-dog CD3 (AbD Serotec, Raleigh, NC, clone: CA17.2A12, catalog number: MCA1774F), rat anti-dog CD4 (AbD Serotec, YKIX302.9, MCA1038A647), rat anti-dog CD8 (AbD Serotec, YCATE55.9, MCA1039PE), and mouse anti-dog CD21 (AbD Serotec, CA2.1D6, MCA1781PE). The following mouse anti-human antibodies that cross-react with canine homologs were used: CD25 (Dako, Carpinteria, CA, ACT-1, F0801), CD56 (Dako, MOC-1, R7127), CCR7 (BD Pharmingen, San Diego, CA, 3D12, 552176) and CD32 (BD Pharmingen,18.26, 559769). Cells were stained for 30 minutes at 4°C prior to washing and analysis. Granzyme B staining was undertaken on T cells fixed for 20 minutes in BD Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA). After washing in BD perm wash buffer, cells were incubated with mouse anti-human granzyme B (BD Pharmingen, GB11, 560211) and isotype control (BD Pharmingen, mouse anti-Rat IgG2a, 558067) for 30 minutes. To measure IFN-γ expression, cells were incubated with golgi plug at a 1:1000 dilution in culture media (CM) [2 mmol/L Glutamax-1 (Life Technologies-Invitrogen, Carlsbad, CA) and 10% heat-inactivated defined FBS in HyQ RPMI 1640] for 4 hours at 37°C before washing and fixation (as described above), and then stained with mouse anti-bovine IFN-γ mAb (AbD Serotec, CC302, MCA1783PE) that cross-reacts with canine IFN-γ. Flow cytometry confirmed expression of introduced T-cell co-stimulatory molecules and the loading of OKT3 (using 1:100 dilution in FB of F(ab')2 fragment of Goat anti-Mouse IgG fragment F(ab')2 Specific, Jackson ImmunoResearch Laboratories, West Grove, PA, 115-116-072) on CLN4. Data were acquired on a FACS Calibur (BD Bioscience) using Cell Quest Version 2.0 (BD Biosciences) and analyzed with FCS Express Version 3.0 (De Novo Software, Thornhill, Ontario, Canada).
Numeric expansion of canine T cells on γ-irradiated aAPC. PBMC and T cells were cultured at 38.5°C, 5%CO2, and 96% relative humidity in CM. T cells were cultured at a 2:1 (Tcell : aAPC) ratio and re-stimulated every 7–10 days with aAPC. Recombinant human IL-21 (eBiosciences, San Diego, CA) was added (30 ng/mL) three-times-per-week for the first seven days. Recombinant human IL-21 and rhIL-2 (Invitrogen) at 100 U/mL, was added three-times-per-week, for subsequent weekly stimulations beginning with the re-addition of aAPC. Viable T cells were enumerated every 7 days by Trypan blue exclusion using the Auto T4 Cell Counter Cellometer (Nexcelom Bioscience, Lawrence, MA).
Fluorescent-labeling of propagated T cells. T cells were labeled with the red fluorescent dye, PKH-26, using the Cell Linker Kit for General Cell Membrane Labeling (Sigma) based upon manufacturer's instructions. Cells were re-suspended in Diluent C containing 4 µL/mL of PKH-26 for 5 minutes, before adding an equal volume of FBS. To remove unbound dye, cells were washed three times prior to use.
Direct digital detection of mRNA molecules. We generated a custom panel (Supplementary Table S3) to quantify 346 canine genes in a single multiplexed non-enzymatic reaction using the nCounter Prep Station and Digital Analyzer (NanoString Technologies, Model NCT-SYST-120, Seattle, WA)58. T cells obtained directly from PBMC were purified to homogeneity using mouse anti-canine CD3 antibody and a BD FACS Aria II high speed sorter (BD Biosciences) prior to analysis. Expanded T cells (99% CD3+ and ≤ 1% CD32+) were analyzed after 28 days of co-culture and 7 days from the last addition of aAPC. Propagated T cells were cryopreserved, thawed at 37°C and rested with rhIL-2 and rhIL-21 in CM for 2 hours before flash freezing in liquid nitrogen. Sample mRNA was isolated using the AllPrep DNA/RNA Mini kit according to manufacture's instructions (Qiagen, Valencia, CA). For the digital detection, two sequence-specific gene probes, mRNA target sequence-specific capture probe, and a mRNA target sequence-specific fluorescent-labeled color-coded probe were produced for each gene. 30,000 cell equivalents of each sample were hybridized with the probe as previously described58. Data analysis and statistical descriptions are described in the supplementary methods.
Infusion of propagated T cells. Propagated and cryopreserved autologous T-cell products were shipped on dry ice to TAMU for infusion. T cells were thawed at 37°C and intravenously infused within 10 minutes at a rate not exceeding 2 mL per 3 minutes. Adverse reactions were determined by the 2009 Veterinary Co-Operative Oncology Group (VCOG) scale. Day 0 was defined as the first T-cell infusion administered.
Complete blood counts (CBC). These were analyzed at TAMU using Abbott Cell Dyn 3700 (Abbott Park, IL) to obtain ANC, ALC, and platelet counts.
Immunohistochemistry. Popliteal LNs were biopsied and sections snap-frozen and/or formalin-fixed. Frozen sections were evaluated for the presence of CD3 and CD79a using an alkaline phosphatase detection system using an autostainer (Dako), which dispensed 300 µL of reagent per slide. Slides were either acetone or formalin-fixed for 5 minutes prior to staining. Universal Block (KPL Labs, Gaithersburg, MD) was applied to block endogenous phosphatase. Slides were incubated for 60 minutes with the primary antibodies diluted 1:200 in Da Vinci Green Diluent (Biocare Medical, Concord, CA): rabbit anti-human CD3 (Dako, A0452) and mouse anti-dog CD79a (Dako, M7051, HM57). Secondary antibodies, biotinylated goat anti-mouse (KPL Labs, 71-00-29) or goat anti-rabbit (KPL Labs, 71-00-30), were incubated for 60 minutes before streptavidin phosphatase (KPL Labs, 71-00-45). Vulcan fast red (Biocare Medical) was used to detect the antibody binding sites. Tissues were counterstained with hematoxylin (Biocare Medical).
TK measurements
106 T cells and CLN4 were either γ-irradiated at 100 Gy or used as non-irradiated cells. Conditioned supernatants were collected from non-irradiated T cells co-cultured for 48 hours on OKT3-loaded CLN4 with rhIL-2/rhIL-21 in CM as well as separately from γ-irradiated T cells and CLN4. TK concentration was measured using the Liaison TK kit (310960D, DiaSorin, Stillwater, MN) in supernatants and serum. Samples were processed according to manufacture's protocols on the Liaison analyzer (DiaSorin).
Statistical methods
Results for percentage and fold expansion are shown as mean ± standard error (s.e.m.). Further analysis used the Student's t-test with p-values less than 0.05 considered significant. For survival curves, Kaplan-Meier and Log Rank analyses were completed against historical controls with p-values less than 0.05 considered statistically significant. GraphPad (GraphPad Software, San Diego, CA) Prism version 5.0 for Windows was used for all statistical calculations.
Study was approved by the IACUC and CRRC committees at TAMU and by the ACUF committee at MDACC.
Author Contributions
Authors contributions C.M.O.C. designed the trial and performed the experiments, analyzed all data, designed panel for nCounter Assay system (digital multiplexed gene expression) and wrote the manuscript. S.S. gathered historical control and clinical data. C.A.H. performed experiments. H.H. assisted with method development and regulatory affairs. M.J. reviewed tumor histology. S.L.P. assisted with statistical analysis. S.M. assisted with digital profiling. W.M. authored the statistical program for analyzing profiling data. R.E.D. assisted with analysis and generation of gene expression profiles as well as edited manuscript. S.C. assisted with trial. H.W. recruited trial participants and cared for the patients. D.A.L. and R.C. edited manuscript. L.J.N.C. conceptualized the idea and edited the manuscript.
Supplementary Material
Dataset 1
Acknowledgments
Research Support: NIH Training Grant (T32CA009598); Cancer Center Core Grant (CA16672); RO1 (CA124782, CA120956, CA141303); R33 (CA116127); Department of Defense (PR064229); S10 (1S10RR026916); Alex Lemonade Stand Foundation; American Kennel Club Canine Health Foundation; Burroughs Wellcome Fund; Cancer Prevention Research Institute of Texas; CLL Global Research Foundation; Gillson Longenbaugh Foundation; Harry T. Mangurian, Jr., Fund for Leukemia Immunotherapy, Institute of Personalized Cancer Therapy; Leukemia and Lymphoma Society; Lymphoma Research Foundation; Miller Foundation; Mr. and Mrs. Joe H. Scales; National Foundation for Cancer Research; Pediatric Cancer Research Foundation; William Lawrence and Blanche Hughes Children's Foundation. We thank the flow cytometry core at MDACC for their assistance and Terrie Gornet in Laboratory Medicine at MDACC for her assistance measuring TK levels. CLN4 aAPC was kindly provided by Dr. Carl June (University of Pennsylvania).
References
- Rubinfeld B. et al. Identification and immuntherapeutic targeting of antigens induced by chemotherapy. Nat Biotechnol 24, 205–209 (2006). [DOI] [PubMed] [Google Scholar]
- Lee Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs K. S., Segal N. H. & Allison J. P. Targeting immunosupportive cancer therapies: accentuate the positive, eliminate the negative. Cancer Cell 12, 192–199 (2007). [DOI] [PubMed] [Google Scholar]
- Shurin G. V., Tourkova I. L., Kaneno R. & Shurin M. R. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol 183, 137–144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès F. et al. Immune filtration in human tumors: a prognostic factor that should not be ignored. Oncogene 29, 1093–1102 (2010). [DOI] [PubMed] [Google Scholar]
- Lenschow D. J., Walunas T. L. & Bluestone J. A. CD28/B7 system of T cell co-stimulation. Annu Rev Immunol 14, 233–258 (1996). [DOI] [PubMed] [Google Scholar]
- Siddiqui M. et al. Absolute lymphocyte count predicts overall survival in follicular lymphomas. British Journal of Haematology 134, 596–601 (2006). [DOI] [PubMed] [Google Scholar]
- Li Y., Bleakley M. & Yee C. IL-21 Influences the frequency, phenotype, and affinity of the antigen specific CD8 T cell response. J Immunol 175, 2261–2269 (2005). [DOI] [PubMed] [Google Scholar]
- Porrata L. F. et al. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant 14, 807–816 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353, 2654–2666 (2005). [DOI] [PubMed] [Google Scholar]
- Dupont J., Lantouche J. B., Ma C. & Sadelain M. Artificial antigen-presenting cells transduced with telomerase efficiently expand epitope-specific, human leukocyte antigen-restricted cytotoxic T cells. Cancer Res 65, 5417–5427 (2005). [DOI] [PubMed] [Google Scholar]
- Maus M. V. et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol 20, 143–148 (2002). [DOI] [PubMed] [Google Scholar]
- Suhoski M. M. et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther 15, 981–988 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numbenjapon T. et al. Antigen-independent and antigen-dependent methods to numerically expand CD19-specific CD8+ T cells. Exp Hematol 35, 1083–1090 (2007). [DOI] [PubMed] [Google Scholar]
- Singh H. et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res 68, 2961–2971 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoloni M. C. & Khanna C. Translation of New Cancer Treatments from Pet Dogs to Humans. Nature Reviews Cancer 8, 147–156 (2008). [DOI] [PubMed] [Google Scholar]
- Paoloni M. C. & Khanna C. Comparative Oncology Today. Vet Clin North Am Small Anim Pract 37, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H. E. Animal Neoplasms: a systemic review. in Neoplasms: Comparative pathology in animals, plants, and man (ed. Kaiser H. E., ed. ) (Williams & Wilkins, Baltimore, 1981). [Google Scholar]
- Moulton J. E. & Harvey J. W. (eds.). Tumors of Domestic Animals, (University of California Press, Berkeley, Cailf, 1990). [Google Scholar]
- Valerius K. D., Ogilvie G. K., Mallinckrodt C. H. & Getzy D. M. Doxorubicin alone or in combination with asparaginase, followed by cyclophosphamide, vincristine, and prednisone for treatment of multicentric lymphoma in dogs: 121 cases (1987–1995). J Am Vet Med Assoc 210, 512–516 (1997). [PubMed] [Google Scholar]
- Machado L. et al. Expression and function of T cell homing molecules in Hodgkin's lymphoma. Cancer Immunol Immunother 58, 85–94 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher C. et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood 114, 5375–5384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl U. et al. Immune recovery in children undergoing allogeneic stem cell transplantation: absolute CD8+ CD3+ count reconstitution is associated with survival. Bone Marrow Transplant 39, 269–278 (2007). [DOI] [PubMed] [Google Scholar]
- Kurokawa T., Hase M., Tokuman N. & Yoshida T. Immune reconstitution of B-cell lymphoma patients receiving CHOP-based chemotherapy containing rituximab. Hematol Oncol 29, 5–9 (2011). [DOI] [PubMed] [Google Scholar]
- Von Euler H. P. et al. Monitoring therapy in canine malignant lymphoma and leukemia with serum thymidine kinase 1 activity--evaluation of a new, fully automated non-radiometric assay. Int J Oncol 34, 505–510 (2009). [PubMed] [Google Scholar]
- Junghanss C. et al. Adoptive immunotherapy against kidney targets in dog-leukocyte antigen-identical mixed hematopoietic canine chimeras. Transplantation 75, 268–274 (2003). [DOI] [PubMed] [Google Scholar]
- Milner R. J., Salute M., Crawford C., Abbot J. R. & Farese J. The immune response to disialoganglioside GD3 vaccination in normal dogs: a melanoma surface antigen vaccine. Vet Immunol Immunopathol 114, 273–284 (2006). [DOI] [PubMed] [Google Scholar]
- Peruzzi D. et al. A vaccine targeting telomerase enhances survival of dogs affected by B-cell lymphoma. Mol Ther 18, 1559–1567 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquet J. M., Kyparissoudis K. & Pellicci D. G. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 178, 2827–2834 (2007). [DOI] [PubMed] [Google Scholar]
- Hinrichs C. S., Spolski R. & Paulos C. M. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for Adoptive Immunotherapy. Blood 111, 5326–5333 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaka A. S. et al. Genetic Modification of T cells with IL-21 Enhances Antigen Presentation and Generation of Central Memory Tumor-Specific Cytotoxic T-lymphocytes. J Immmunother 32, 726–736 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D. S., Wurster A. L. & Grusby M. J. Biology of IL-21 and the IL-21 Receptor. Immunol Rev 202, 84–92 (2004). [DOI] [PubMed] [Google Scholar]
- Pouw N. et al. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol Immunother 59, 921–931 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H. et al. Reprogramming CD19-Specific T Cells with IL-21 Signaling Can Improve Adoptive Immunotherapy of B-Lineage Malignancies. Cancer Res 71, 3516–3527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs C. S., Gattinoni L. & Restifo N. P. Programming CD8+ T cells for effective immunotherapy. Curr Opin Immunol 18, 363–370 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Powell D. J., Rosenberg S. A. & Restifo N. P. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 6, 383–393 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongu M., Harashima N., Yamada T., Harada T. & Harada m. Immunogenic chemotherapy with cyclophosphamide and doxorubicin against established murine carcinoma. Cancer Immunol Immunother 59, 769–777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneno R. et al. Chemotherapeutic agents in low noncytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol 34, 97–106 (2011). [DOI] [PubMed] [Google Scholar]
- Aquino A. et al. Drug-induced increase of carcinoembryonic antigen expression in cancer cells. Pharmacol Res 49, 383–396 (2004). [DOI] [PubMed] [Google Scholar]
- De Angulo G., Yuen C., Palla S. L., Anderson P. M. & Zweidler-McKay P. A. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: implications for risk stratification and future studies. Cancer 112, 407–415 (2008). [DOI] [PubMed] [Google Scholar]
- Biller B. J., Guthm A., Burton J. H. & Dow S. W. Decreased ratio of CD8+ T cells to regulatory T cells associated with decreased survival in dogs with osteosarcoma. J Vet Intern Med 24, 1118–1123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyuchnikov E. et al. Post-transplant immune reconstitution after unrelated allogeneic stem cell transplant in patients with acute myeloid leukemia. Leuk Lymphoma 51, 1450–1463 (2010). [DOI] [PubMed] [Google Scholar]
- Galon J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006). [DOI] [PubMed] [Google Scholar]
- Piersma S. J. et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res 67, 354–361 (2007). [DOI] [PubMed] [Google Scholar]
- Al-Shibli K. I. et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 14, 5220–5227 (2008). [DOI] [PubMed] [Google Scholar]
- Hallek M., Wanders L., Strohmeyer S. & Emmerich B. Thymidine kinase: a tumor marker with prognostic value for non-Hodgkin's lymphoma and a broad range of potential clinical applications. Ann Hematol 65, 1–5 (1992). [DOI] [PubMed] [Google Scholar]
- Holdenrieder S. et al. Clinical relevance of thymidine kinase for the diagnosis, therapy monitoring and prognosis of non-operable lung cancer. Anticancer Res 30, 1855–1862 (2010). [PubMed] [Google Scholar]
- Li Z. et al. Transient increase in serum thymidine kinase 1 within one week after surgery of patients with carcinoma. Anticancer Res 30, 1295–1299 (2010). [PubMed] [Google Scholar]
- Garcea G. et al. Preoperative Neutrophil-to-Lymphocyte Ratio (NLR) is Associated with Reduced Disease-free Survival Following Curative Resection of Pancreatic Adenocarcinoma. World J Surg 35, 868–872 (2011). [DOI] [PubMed] [Google Scholar]
- Kao S. et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 16, 5805–5813 (2010). [DOI] [PubMed] [Google Scholar]
- Porrata L. F. et al. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol 85, 896–899 (2010). [DOI] [PubMed] [Google Scholar]
- Tobinai K. et al. Randomized phase II study of concurrent and sequential combinations of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy in untreated indolent B-cell non-Hodgkin lymphoma: 7 year follow-up results. Cancer Science 101, 2579–2585 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose J. M. et al. Long-term update of a phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive non-Hodgkin's lymphoma. Leukemia and Lymphoma 46, 1569–1573 (2005). [DOI] [PubMed] [Google Scholar]
- Voulgarelis Giannouli Anagnostou V. S. D. & Tzioufas A. G. Combined therapy with rituximab plus cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) for Sjogren's syndrome-associated B-cell aggressive non-Hodgkin's lymphomas. Rheumatology 43, 1050–1053 (2004). [DOI] [PubMed] [Google Scholar]
- Gerald M. C. & O'Bannon F. V. (eds.). Nursing pharmacology and therapeutics, (Prentice Hall, Englewood Cliffs, 1988). [Google Scholar]
- Hand M. S., Thatcher C. D., Rimillard R. L. & Roudebush P. (eds.). Small Animal Clinical Nutrition, (Walsworth, Marceline, MO, 2000). [Google Scholar]
- Manuri P. V. et al. piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies. Hum Gene Ther 21, 427–437 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss G. K. et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26, 317–325 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset 1