Introduction
The U.S. is experiencing a rapidly increasing frequency of drug shortages, which have caused numerous difficulties for clinicians, health care facilities, patients, and federal regulators.1 Drug shortages are caused by many factors, including difficulties in acquiring raw materials, manufacturing problems, regulatory issues, and business decisions, as well as many other disturbances within the supply chain.1,2 They adversely affect patient care by causing substitution of safe and effective therapies with alternative treatments; compromising or delaying medical procedures; or causing medication errors.2 Drug shortages also significantly burden health care provider and health care facility finances and personnel.2 A management strategy that includes clear policies and procedures for information gathering, decision-making, collaboration, and timely communication should be established to effectively handle drug shortages.2
Drug Shortages Are Rapidly Increasing In Frequency
There have been increasingly frequent drug shortages in the U.S. during at least the past decade.3,4 These have been tracked by both the American Society of Health-System Pharmacists (ASHP) and the FDA.3,4 However, these two organizations define the term drug shortage differently. The ASHP defines a drug shortage as “a supply issue that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent,” whereas the FDA focuses only on “products used to prevent or treat a serious or life-threatening disease or medical condition for which there is no other available source with sufficient supply of that product or alternative drug available.”2–5
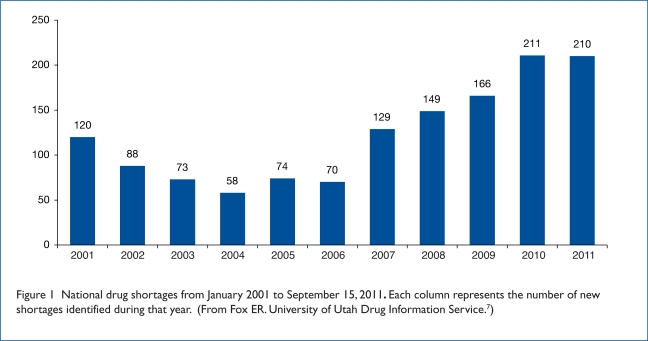
The Drug Information Service at University of Utah Health Care (UUHC), which partners with the ASHP to manage its drug shortage program, tracked a total of 211 drug shortages in 2010—the highest number recorded to date in a single year (Figure 1).3,6,7 By comparison, the ASHP identified 224 drug shortages during the entire six-year period between January 1996 and June 2002.3 An analysis by the Premier Healthcare Alliance in March 2011 also found that more than 240 drugs were in short supply or completely unavailable in 2010, and more than 400 generic medications had been back-ordered for five days or more.8 The number of drug shortages has been rapidly escalating in recent years; ASHP/UUHC reported 70 in 2006, 129 in 2007, 149 in 2008, and 166 in 2009.4,9 For 2011, there have been 210 shortages reported as of September 15. At the time of this writing, 203 drugs were listed on the ASHP/UUHC drug shortage Web site and 73 medically necessary drugs were listed on the FDA Web site.10,11
Figure 1.
National drug shortages from January 2001 to September 15, 2011. Each column represents the number of new shortages identified during that year. (From Fox ER. University of Utah Drug Information Service.7)
Although certain types of drugs are more vulnerable to shortages than others (such as generic cancer drugs), shortages of a variety of drugs have been reported, including heart drugs, pain medications, intravenous (IV) electrolytes, and many others.6 Drugs that have been around for decades are suddenly not available, which can significantly affect patient care.12 These include leucovorin, propofol, morphine, hydromorphone, furosemide, amino acids, and others.6 Shortages can also extend to medical supplies, such as technetium-99m, which was in short supply when the Canadian nuclear reactor that produces it temporarily closed down for repairs in 2007.9
Lack of an Advanced Warning System Is the Main Cause of Problems
Ideally, there would be an early warning system for impending drug product shortages that would provide ample opportunity to prepare for all implications of the shortage.2 Manufacturers are required to give the FDA six months’ advance notice only when they plan to stop producing a single-source, medically necessary drug.2,4 However, even this requirement has been criticized as being “soft,” since “medically necessary” is not statutorily defined; therefore, the manufacturer is free to decide whether or not notification is required.12 Also, the manufacturer isn’t penalized in any way if it fails to provide the required notification.12 Manufacturers are required to notify the FDA of quality problems; however, drug shortages or discontinuations are often caused by a business decision or other factors that don’t require FDA notification.12
Therefore, manufacturers either routinely don’t provide any notice of an impending drug shortage or provide little advance notice and no estimate of the projected duration.12 Without advanced warning of an impending drug supply problem, the FDA can become engaged only when a shortage begins.6 Some drug manufacturers do voluntarily alert the FDA about anticipated shortages.8 If all manufacturers of a product are notified that a drug shortage is impending, they might be able to increase production to avert it.5 Early notification allows the FDA to work with firms to address an impending shortage more quickly; such actions averted 38 shortages during 2010.8 However, even with advance notice of an impending shortage of some agents, the manufacturing process for vaccines or antibiotics is so complex that shortages may occur even if alternative manufacturers are willing to produce the product to make up for the shortfall.2
Distributors have also been inconsistent in providing information to health systems regarding drug shortages.2 Health systems are often unaware of drug shortages until it is no longer possible to purchase a product, causing considerable difficulty in developing an effective management strategy.3,6 Pharmacists need to have reliable and timely information to enable them to successfully manage drug shortages.3
Causes of Drug Shortages
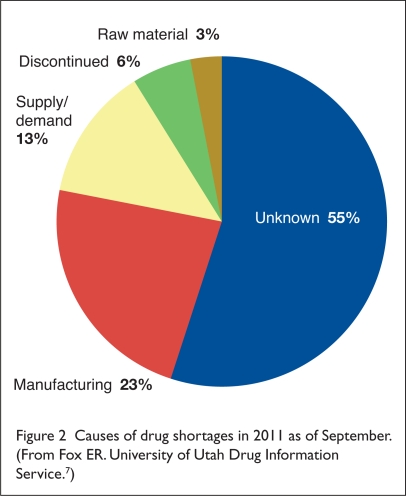
There are a variety of reasons for drug shortages. According to the ASHP/UUHC drug shortage program, as of September 15, 2011, manufacturing problems (23%) and supply/demand issues (13%) were the most common known causes of shortages.13 However, a far greater percentage of drug shortages (55%) were classified as being due to “unknown” causes (Figure 2).13 A discussion of common causes for drug shortages appears in the following section.
Figure 2.
Causes of drug shortages in 2011 as of September. (From Fox ER. University of Utah Drug Information Service.7)
Manufacturing Difficulties
Many factors can contribute to drug-manufacturing difficulties, including antiquated equipment, a shift of a company’s resources from manufacturing to research and development, loss of production and compliance personnel, and many others.2 Changes in a product’s formulation and limited production capabilities can also delay product availability.2,5 The FDA also approves a specific manufacturing line to produce a specific drug at a specific facility.14 Therefore, pharmaceutical manufacturers can’t just set up production of a drug in short supply somewhere else in a facility.14 Manufacturers also often use the same manufacturing equipment for many drug products, so it is difficult to increase production of one product without causing manufacturing shortages and delays for another.5 Consequently, sometimes it is possible to increase drug production only by buying more equipment or contracting with other companies to produce the drug.5 However, this isn’t an easy solution, because there is often a significant lag between a supply shortfall and a production increase at another facility.13 Antitrust laws may also prevent companies from sharing manufacturing information.9
Shortages of Raw Materials
Disruptions in the supply of raw or bulk materials are frequently responsible for drug shortages. These shortages are especially problematic when a primary or sole-source supplier of a raw material delays or discontinues production, affecting multiple manufacturers.2 Even if there are multiple manufacturers of a drug, there may be only one producer of a raw material that is used in producing that drug.2 Therefore, any interruption in the supply of that substance will affect all of the producers of the finished product.5
Drug manufacturers also increasingly import raw materials from other countries, making them reliant on a global supply chain.1 It is estimated that 80% of the raw materials in the pharmaceuticals sold in the U.S. are imported from abroad.2 Suppliers could be in Europe, India, or China, and if one of these foreign companies has a problem, it can lead to disruptions in the American drug supply.1 Problems of availability can arise as a result of armed conflicts, political upheaval, trade disputes, animal diseases, degradation or contamination during transport, climate or other environmental conditions, or a decreased crop yield of plants that are a source of raw materials.2
Raw material shortages can also delay manufacturing increases that are undertaken to compensate for a drug shortage. This can occur because, in addition to a greater quantity of active ingredient, other materials must also be acquired to produce a larger supply of the drug.14 For example, the manufacturer must acquire additional quantities of excipients and packaging.14
Voluntary Recalls
A major drug recall can have a rapid and significant effect on the availability of a product, especially when it is produced by a sole manufacturer.2 Recalls usually affect specific lots and occur because of a lack of confidence in safety or for other reasons, such as technical deficiencies in the drug’s labeling.2 Voluntary recalls are generally temporary and occur because of minor problems in manufacturing that are not governed by FDA regulations.2 A dilemma could arise when it is predicted that a voluntary recall will cause a drug shortage.2
Natural Disasters
Natural disasters can profoundly affect drug product availability.2 Finished drug products or supplies can be affected by fires, hurricanes, tornadoes, and floods.2 Long-term drug shortages can also occur because of damage to manufacturing facilities caused by natural disasters, particularly if the product produced by the site is a sole-source product.2 In 1998, a shortage of several drugs occurred when hurricane George damaged pharmaceutical manufacturing facilities in Puerto Rico.2 Sometimes natural disasters exacerbate drug shortages because they create an unexpected demand for drugs needed to treat disaster victims.2 In 2005, an increase in demand for and a shortage of certain drugs resulted from hurricanes Katrina and Rita.2
Supply and Demand Issues
Occasionally, the demand for a drug can increase beyond expectations or production capacity.2 This can occur in response to the approval of a new indication for an existing product, changes in therapeutic guidelines, the spread of disease, or other unpredictable factors.2 For example, in 2006, a shortage of pediatric flu vaccine occurred when the Centers for Disease Control and Prevention (CDC) changed its guidelines to include children 6 to 59 months of age.2 This change in recommendations put pressure on the suppliers of the only product that the FDA had approved for use in children 6 to 23 months of age.2
Business and Economic Issues
Drug shortages can also occur because of a manufacturer’s business decisions. These may be based on a variety of factors, including insufficient profits, the introduction of generic products, market share, anticipated clinical demand, patent expiration, drug-approval status, regulatory compliance requirements, expense to correct manufacturing problems, or mergers.2 Manufacturers may permanently or temporarily reduce production quantities of certain drugs as they shift production or reallocate resources to another product.2
Mergers often lead to a reduction in product lines or a shift in manufacturing to another facility, causing the delayed availability or discontinuation of a drug.2 The increasing frequency of drug shortages may be partially connected to recent mergers in the pharmaceutical industry.15 Consolidation in the pharmaceutical industry has left only a few manufacturers for many older, less profitable products, meaning that when raw material runs short, equipment breaks down, or the FDA enforces compliance regulations, these problems can quickly give rise to drug shortages.1
Many of the drugs in short supply also tend to be generic medications, which aren’t very profitable, so companies don’t plan for backup capacity.15 Economic pressure on manufacturers can also lead them to maintain lower inventories of low-profit drugs or take them off the market.12 For example, in 2000, a shortage of diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed occurred when one of the manufacturers discontinued these products because of low revenues.2
Regulatory Issues
Some industry representatives blame the drug shortage problem on increased oversight by the FDA, because the agency has made drug safety a higher priority after being criticized for being too lax.1 However, FDA officials dispute that increased government oversight is a major factor in causing drug shortages.1 Instead, FDA officials have said that manufacturing problems are the cause of most shortages.1 Data reported by the ASHP/UUHC for 2010 supports this contention, since manufacturing difficulties accounted for the largest percentage of known reasons for drug shortages (28%), whereas regulatory issues accounted for the fewest (1%).13
The FDA has been paying increased attention to quality control, an especially critical issue for injectable drugs.12 Drug shortages do occur when an FDA enforcement action for non-compliance with current good manufacturing practices (cGMPs) causes the primary or sole manufacturer of a drug to halt or delay production.2 Subcontractors that supply products to pharmaceutical manufacturers can also be subject to cGMP violations.2 Resolution of such issues is often a lengthy process and may require FDA inspections for recertification, issuance of an injunction against the manufacturer, or seizure of products.2
The limited resources of the FDA may also impede the timely inspection and recertification of manufacturing sites after a noncompliance shutdown.16 Some pharmaceutical company officials have also suggested that FDA enforcement actions and drug-shortage issues should be more closely coordinated to avoid interruptions.1
Supply Chain Issues and Health Care System Practices
Once a drug is manufactured, the drug supply chain is composed mostly of wholesalers or distributors, prime vendors, group-purchasing organizations (GPOs), and end-user hospitals and health care systems.2 Business decisions made by these components of the supply chain can also contribute to drug product shortages.2 Most hospitals and health systems obtain most of their drug products through wholesale distributors.2 Distribution methods that are restrictive or that deviate from the usual supply chain can create shortages.2 Market approval requirements or postmarketing surveillance might cause manufacturers to limit drug product availability to only selected suppliers and health systems that comply with manufacturer agreements.2 Health systems may also be required to order a drug directly from a manufacturer or through one specialty distributor; this practice may also restrict supplies.2
Transportation and communication efficiencies have allowed “on-hand” inventories to be reduced throughout the supply chain.2 Consequently, most manufacturers, distribution centers, and health systems now use “just-in-time” inventory management to optimize cash flow and reduce the cost of inventory stock.2 Although this practice is widespread and is considered to be sound, it increases the vulnerability of health care systems to unexpected drug shortages.2 Manufacturers and distributors may also use management strategies to reduce quarter- or year-end inventories or limit shipments according to yearly quotas.2
Poor ordering practices, drug stockpiling in advance of price increases, hoarding in response to rumors of an impending shortage, and delivery delays may also affect drug stock inventories in health care facilities.2 Drug shortages may also occur when too many health care facilities in a geographic area are using the same wholesaler, particularly since some shortages are wholesaler-dependent and may occur only because of delays in supplier contracts.2
Specific Drugs Vulnerable to Shortages
Oncology Medications
In 2010, IV oncology medications topped the list of drug shortages.8 This may be because injectable medications are more vulnerable to cGMP violations issued by the FDA.8 Included among the drugs in short supply were leucovorin/levoleucovorin, bleomycin, cisplatin, carmustine, cytarabine, doxorubicin, etoposide, mechlorethamine, chlormethine, vinblastine, busulfan, and vincristine.15,16 Many of these agents are decades-old; however, they are still mainstays of treatment for certain cancers, such as lymphoma and testicular cancers, which are often curable if the right drugs are available.15 The increasing frequency of cancer drug shortages has prompted a plan by the National Cancer Institute (NCI) to create a reserve of these key medications.15
The shortage of cytarabine was particularly worrisome because there is no substitute for this drug, which is used to treat acute myeloid leukemia (AML).15 Oncologists complained that hospitals had to ration the drug and delay treatment for some patients or give them less effective therapies.15 Cytarabine’s scarcity was caused by a shortage of raw materials for two of three manufacturers and the subsequent discovery of crystals in some shipments.1,15 A third manufacturer could not meet the demand on its own.15 The FDA allowed temporary foreign importation of cytarabine until the crystallization problem was resolved and shipments resumed.15
Injectable Drugs
Many injectable drugs affected by shortages are mainstays of medical care, such as morphine, norepinephrine, and electrolytes.1 Sterile injectables are particularly vulnerable to drug shortages because manufacturing processes are complicated, so they are more prone to production problems.1,4 Illustratively, in 2010, the most common reasons for injectable medication shortages were product quality issues that caused a mandatory or voluntary recall or cessation of production.4 Other reasons for shortages of injectable drugs include product discontinuation, capacity issues, inability to procure raw materials or other components, and a loss of a manufacturing site.4
If quality-control issues arise and a manufacturing plant is shut down until problems can be corrected and compliance with GMPs re-established, extra pressure is placed on other firms that manufacture that product.12 However, because the production of sterile injectables is lengthy and complicated, other manufacturers might not be able, or willing, to increase production.9 In addition, relatively few manufacturers are still making sterile generic drug injectables because of a low return on investment.12 In fact, shortages of pre-loaded epinephrine syringes and propofol occurred when manufacturers discontinued making these products when the FDA requested additional compliance documentation.1
Generic Drugs
Most drug shortages that occur in the U.S. involve generic medications.4,10 These shortages likely occur because manufacturers have little financial incentive to produce off-patent medications.1,9 At most, only a few manufacturers produce a particular generic drug, so shortages are inevitable.9 Shortages of drug classes containing mostly generic drugs, including anesthetics, antibiotics, and cancer treatments, have tripled since 2006.15 Companies may also decide to discontinue production of a trade-name drug once it comes off-patent, and the need to produce an additional product also strains the capacity of generic drug manufacturers.6
Drugs Subject to the FDA’s Unapproved Drugs Initiative
Manufacturers also cite the FDA’s Unapproved Drugs Initiative as the cause of some drug shortages.14 Because of this initiative, the FDA has placed increased regulatory pressure on drugs that were introduced before 1938 and were therefore “grandfathered” into the market before the agency was granted the authority for drug approval.12,14 These drugs therefore lack an approved New Drug Application (NDA) or abbreviated NDA.12,14 The agency encourages unapproved drug manufacturers to file an NDA to seek marketing approval.17 However, the preparation and submission of an NDA is a lengthy, expensive process that might not be profitable for a company and therefore might not be worth pursuing.14 If an NDA is required for these older drugs, the manufacturer may instead choose to discontinue them.14 It has been recommended that an expedited approval pathway, such as an NDA that requires less than the usual amount of data, be established for these products.14
Single-Source Products and Concentrated Market Share
The number of firms producing a specific drug and their respective market share are significant factors affecting shortages.5 Mergers between two companies that produce similar product lines can also typically result in single-source products.2 As the number of manufacturers of a drug decreases, resiliency in the supply chain is also reduced and the supply becomes more vulnerable.2 Many vaccines in the U.S. have become single-source products because of manufacturer discontinuations and consolidations.15 Patented drugs are almost always single-source products.5
Even when a drug is produced by multiple manufacturers, it is not unusual for an individual company to maintain a large market share.5 When a company with a dominant or large market share ceases drug production, it may be difficult for other manufacturers to compensate for the shortfall.5
The Impact of Drug Shortages
Drug shortages have had a profound and widespread impact on the quality of health care in the U.S. and have created significant obstacles. In late 2010, the Institute for Safe Medication Practices (ISMP) conducted a national survey of 1,800 health care practitioners (consisting of approximately two-thirds pharmacists) to assess the impact of drug shortages. The ISMP found that:12
84% of the respondents said they had never received advance warning of a shortage from manufacturers or the FDA.
80% said they faced difficulty obtaining comparable drugs.
78% said there were significant costs to obtaining comparable drugs.
70% said they had been unable to find comparable alternatives.
64% believed that shortages posed a risk of adverse patient outcomes.
respondents reported more than 1,000 adverse events and near-misses attributable to drug shortages.
A discussion of these and other consequences appears in the following section.
Higher Hospital Expenses
One way in which drug product shortages adversely affect hospital or health system finances is by raising the cost of delivering patient care, largely through increased drug acquisition and personnel expenditures.2 After surveying 311 pharmacy experts from 228 hospitals in late 2010, Premier Healthcare Alliance estimated that drug shortages cost hospitals at least $200 million annually because of the need to purchase more expensive therapeutic substitutes.3,8 This cost estimate does not include the indirect costs associated with drug shortages, such as the additional labor required.8 The annual labor costs of drug shortages have been estimated to be an additional $216 million.3
The increased frequency of drug product shortages has also created nontraditional distributors, known as gray-market, open-market, or alternative suppliers.2 When it is clear that a shortage of a particular drug exists or is impending, these distributors buy up the remaining stock and then aggressively market it to hospitals, health systems, home care agencies, and physician practices, which are unable to obtain the drug, at 10 to 1,000 times the usual price.1,2,13 Typically, these distributors have only a small quantity of product, sometimes only enough for one or two patients.2 Many do not offer a return or refund on products.2 There is also no guarantee of proper storage or pedigree, and there is a risk that the product might be counterfeit or compounded.2
Increased Labor Costs
Many shortages can be effectively managed after hospital personnel identify available alternative products or therapeutic equivalents.3 However, additional labor is often needed to make operational adjustments required to accommodate product changes.3 In 2004, a national survey by the ASHP and Johns Hopkins Hospital found that pharmacists were spending a median of 3 hours per week, and non-pharmacists an additional 2.5 hours per week, to manage drug shortages.3 In contrast, a survey of 1,322 ASHP members conducted in 2010 reported that pharmacists and pharmacy technicians spent a median of 9 and 8 hours per week, respectively, managing drug shortages.3 This finding suggests that the time health care facility personnel spent on drug shortages has tripled since 2004.3
Explanations for this increased need for manpower include an increase in the quantity of drugs in short supply, the number of drug shortages that require identification of therapeutic alternatives, and the complexity of health care delivery systems.3 Furthermore, because drug shortage management activities need to be undertaken immediately to avoid an impending crisis, they often take precedence over other important high-impact, high-value activities, such as direct patient care.3 Pharmacists instead need to spend a large amount of time on tasks such as communicating with manufacturers and wholesalers, providing education to facility personnel on alternative agents, developing or modifying policies or clinical guidelines, and updating electronic databases and medication administration systems.3
Safety Risks
Numerous safety risks are associated with drug shortages. A shortage often causes the substitution of one drug for another that might have reduced efficacy or cause side effects.9 Reports of delayed treatments, frantic searches for desperately needed drugs, devastating injuries from mistakes and inadequate therapies, and even possible deaths are also beginning to emerge.1 These and other safety risks are discussed in the following section.
Compromised Clinical Outcomes
Along with being frustrating, drug shortages can also lead to rationing; cancellation of procedures; or the use of alternative treatments that have a worse safety profile, are more expensive, or are more prone to overdoses or medication errors.1,4 Because of the shortages of cancer drugs, oncologists often have to delay or alter carefully timed chemotherapy regimens.1 Sometimes cancer patients need to be “triaged,” and drugs for which there are no substitutes are preserved for those who are considered to have the best prognosis and are more curable.1
For example, cytarabine is highly effective in treating several forms of leukemia and lymphoma, but it must be administered as quickly as possible, especially to patients with AML.1 The availability of cytarabine can therefore literally represent life or death for these patients.1 During the cytarabine shortage, stocks ran low in many hospitals and some facilities ran out completely.1 Many health care facilities therefore had to ration the drug, giving priority to those who they thought most urgently needed it.1
Clinical outcomes can be compromised if a less effective agent is used or a proper therapeutic alternative is unavailable.3 For example, in 2009, severe shortages of leucovorin, used to treat colorectal cancer, prompted the American Society of Clinical Oncology (ASCO) to issue a clinical alert.9 The alert suggested that levofolinate be substituted.9
There is evidence that levofolinate, in combination with irinotecan and fluorouracil, is effective in patients with metastatic colorectal cancer.9 However, it is unclear whether this agent is as effective as leucovorin in treating colorectal cancer; therefore, this strategy is risky.9 In addition, levofolinate is FDA-approved for use only as a rescue drug after high-dose methotrexate in patients with osteosarcoma.9 It is also much more expensive than leucovorin.9
Epinephrine is often used as a replacement for norepinephrine bitartrate injection, a blood pressure medication that is often in short supply.8 However, epinephrine has physiological effects that differ from those of norepinephrine bitartrate and is therefore not always an ideal replacement.8
Medication Errors
Medication errors can occur more frequently as a result of drug shortages. This is because physicians are forced to prescribe alternative agents, product forms, or drug concentrations that they might not be familiar with.3,4 Even if an appropriate substitute exists, the conversion factors for dosage adjustments might not be known for generic drugs.9 Nurses and doctors responding to emergencies may also lose precious time when they need to work with unfamiliar substitutes or have to recalculate dosages, increasing the chances of overdosing or underdosing patients.3,4
The potential for these types of dosage errors is particularly significant, since premeasured, prefilled syringes are often in short supply.1 To compensate, many hospitals are recalibrating electronic medication delivery systems or preparing correct doses ahead of time, especially for the emergency department.1 Emergency personnel are also often forced to administer drugs with an unfamiliar formulation or duration of effect.12 Protocols that have been standardized to reduce medication errors can’t be followed when the drug they apply to isn’t available.12
Despite rigorous attempts to adapt to drug shortages, a long list of errors and near-misses has been reported.1 Earlier this year in Alabama, at least 19 patients were sickened and nine died after receiving an infusion of a solution through their feeding tubes that was apparently contaminated with bacteria when a pharmacy was forced to use an unfamiliar ingredient because of a shortage.1
There have also been reports of medication errors from hospitals that experienced shortages of neuromuscular blockers.12 These reports indicated that multiple patients received an incorrect dose: rocuronium was infused at the rate intended for cisatracurium, and vecuronium was administered according to a protocol for pancuronium.12
The 2010 ASHP survey found that 35% of respondents reported that their facility had experienced a “near-miss” error resulting from a drug shortage in the previous year.3 Of particular concern was the ongoing shortage of syringes prefilled with epinephrine 0.1 mg/mL.12 For most of 2010, this product was available in a 1-mg/mL dilution, in ampules, multidose vials, or prefilled syringes with a 3.5-inch intracardiac needle.12 Epinephrine cannot be prediluted and stored, because it is sensitive to light, heat, and oxygen and is unstable shortly after dilution.12 Therefore, as an alternative, a recommendation was issued for pharmacy managers to make up kits for emergency departments and emergency medical services that contain empty syringes, vials of saline solution, and 1-mg/mL ampules of epinephrine.12 However, the advisory cautioned that this strategy could introduce a high risk of an overdose.12
Death
Drug shortages can affect the ability of health care professionals to keep patients alive, particularly in emergency departments, intensive-care units, and oncology wards.1 In particular, the shortage of cytarabine raised the possibility that drug shortages would not only cause disruptions in care but could also be a death sentence for AML patients.6 Cytarabine’s scarcity became a symbol for a worst-case scenario in which no alternative work-around therapy existed.6 Although the production problems that caused this shortage have since been corrected, uncertainties about a continuous supply of this drug still linger.6
With respect to other cancer drugs, it is unknown whether deaths or complications have occurred because of drug shortages.6 This information isn’t traced by any agency, and many confounding variables exist, such as the patient’s type of cancer, stage of disease, and whether an equally effective therapy can be identified.6 The relationship between cancer therapies and patient outcomes is also not clear-cut, because no cancer drug is known to be 100% effective.6 These factors make the impact of cancer drug shortages likely to remain undefined.6
Drug Shortages Increase Quality Control Risk
Health care facility personnel are often able to formulate solutions to a drug shortage; however, these can often present quality-control risks.1 For example, alternative medications might not contain antimicrobial retardants such as EDTA, sodium metabisulfate, or benzyl alcohol/sodium benzoate.8 Therefore, a strict aseptic technique may need to always be maintained during handling of the substitute medication.2
Purchasing drugs from unfamiliar alternative sources also increases the risk of procuring counterfeit medications.1 The inability to determine a product’s source (which could be outside the U.S.) and to confirm whether it was properly handled through the change in custody are also concerning.2
Compounding pharmacies are sometimes a viable source of drugs that are in short supply.2 However, drug preparations from these pharmacies might not meet applicable state or federal standards (e.g., U.S. Pharmacopeia, chapter 797, or FDA labeling requirements), so caution may be warranted.2 There have also been reports of apparent lapses in quality control by compounding pharmacies that have resulted in serious patient injury or death.2 The sources of raw materials used in compounding pharmacies have also been questioned.2
Drug Shortages Affect Supplies of Alternative Drugs
Another effect of drug shortages is that the original shortage causes a decline in the supply of an alternative agent because of an unexpected increase in demand.4 Recent examples of this phenomenon involved neuromuscular blocking agents or IV furosemide.4,5 When the supply of furosemide became limited, there was subsequently an increased demand for the alternative agent, bumetanide, which also led to a shortage of that medication.4
Drug Substitutions Violate Clinical Trial Protocols
Drug shortages can interfere with clinical trials that use older drugs as controls or in combination therapy with the experimental agent.15 However, If a drug specified by a clinical trial protocol isn’t available, clinicians may need to delay enrollment or deviate from the trial design by substituting an alternative treatment.15 Such substitutions can have a significant impact on the clinical study design and may have repercussions when trial results are analyzed.15 There have been scattered reports of lead investigators for clinical trials needing to modify protocols to allow for substitute treatments.15
Shortages Strain Professional Relationships
Shortages are highly frustrating for everyone involved, including pharmacists, physicians, purchasing agents, nurses, and patients.2 Consequently, drug shortages can compromise relationships with colleagues or patients when frustrations are misdirected.3
The FDA’s Role in Drug Shortages
FDA Limitations in Managing Shortages
A coordinated effort is often required between manufacturers and various components within the FDA to manage drug shortages.5 However, the FDA has only limited authority to assist in managing shortages.2 The Drug Shortage Program is a division of the Center for Drug Evaluation and Research (CDER), one of five centers within FDA.5 The purpose of this program is to ensure that prescription medications, over-the-counter drugs, and generic drugs remain available to the American public.5 The other four centers within the FDA oversee the supply of vaccines, biologic agents, veterinary drugs, medical devices, radiological products, and nutraceuticals.2,5
The FDA’s Drug Shortage Program focuses only on shortages of “medically necessary” drugs, since these products have the greatest impact on public health.2,5 A drug is considered to be medically necessary if it “is used to treat or prevent a serious disease or medical condition, and there is no other available source of that product or alternative drug that is judged by medical staff to be an adequate substitute.”2 The FDA does not investigate drug shortages that are expected to be temporary and self-limiting or that involve only a particular strength or package size.5 The agency does not investigate these types of shortages because of limited resources and the transient nature of temporary shortages, even though they can cause significant problems for health care providers and health care facilities.3,5
When the FDA receives a report of a drug shortage, it first contacts the appropriate manufacturer or distributor for verification.5 When the shortage is confirmed as not being transient or self-limiting, CDER personnel with the appropriate medical or scientific background are notified and consulted to determine whether the drug product is medically necessary.5 Sometimes the opinions of experts outside the FDA are solicited in order to make this determination.5 Both the drug’s approved and off-label uses are considered when experts are making this decision.5 An evaluation of medical necessity essentially relies on a risk–benefit evaluation of the product shortage versus the medical need.2
Even when a drug is considered to be medically necessary, the FDA’s power to resolve a product shortage is limited.5 The FDA cannot require a company to produce a drug, and it has no authority over business decisions made by manufacturers, even if the drugs they produce are medically necessary.2,5 Inconvenience and cost issues regarding drugs that are in short supply are also insufficient reasons to classify a drug as medically necessary and do not fall within the authority of the FDA.2,5
The FDA also has no jurisdiction over shortages that occur because of contractual problems between manufacturers, distributors, or end-users.2,5 According to current law, the FDA also can’t require a manufacturer to provide notification of an anticipated shortage unless it is expected to involve a sole-source, medically necessary drug.5
Actions Within the FDA’s Authority to Resolve Drug Shortages
Once a shortage of a medically necessary drug has been verified, the FDA works with the manufacturer toward resolving the identified problem.5 The FDA doesn’t have the authority to mandate manufacturers to resume or ramp up production to correct drug shortages.12 However, the FDA can provide assistance, even if the shortage is due to business decisions, voluntary recalls, cGMP noncompliance, or other factors.2 In 2010, the FDA was able to avert 24 impending drug shortages through assistance provided to pharmaceutical manufacturers.14
A discussion of some actions that the FDA can take to help resolve drug shortages follows.
Allow Temporary Importation of Foreign Products
In rare cases, the FDA may allow the emergency importation of a product from a foreign manufacturing source until a shortage is resolved.1,2,5 As of June 2011, the FDA had allowed the foreign importation of six drugs.6 Four of these were oncology agents: capecitabine (Xeloda, Genentech/Roche) for metastatic breast cancers and colorectal cancer; a substitute form of leucovorin; thiotepa, a drug used in bone marrow transplants; and ethiodol, a chemoembolization drug used to treat liver metastases.6
In the past, the FDA also allowed the foreign importation of drugs such as propofol and naloxone HCl injection to ease drug shortages.5
Assist in Resolving Manufacturing Issues
There are several ways in which the FDA can help resolve manufacturing difficulties. If a new manufacturing site or supplier needs to be established to resolve production problems, the FDA can expedite a data review to approve such changes.5 The FDA can also expedite the review of new or generic drug applications to approve medications that are alternatives to those that are experiencing manufacturing difficulties.5 This occurred when the FDA expedited the approval of a generic injectable penicillin in response to a shortage of penicillin G sodium for injection.5
Drug shortages can also be caused by cGMP enforcement actions pursued by the FDA to protect the public from potentially unsafe drug products.2 These actions are first evaluated by CDER personnel to determine whether they will create a shortage of a medically necessary drug.2 If an enforcement action involves a medically necessary product, the FDA will help the manufacturer return to compliance or will consider qualifying additional manufacturing sites.2
Sometimes a product is in compliance with FDA cGMP guidelines, but it may be voluntarily recalled by a manufacturer because it deviates from company specifications.5 In such a scenario, an ad hoc committee of FDA scientists can conduct a hazard evaluation to identify any health risks that might be associated with the product.5 If no significant risks are identified, additional protocols for end-product testing may be established in order to monitor the manufacturing process, allowing drug production to continue uninterrupted.5
Help Find Other Suppliers
In response to the shortage of a medically necessary drug, the FDA will assist in establishing a substitute or alternative source for the product.5 Alternative drug products are identified by the CDER review division that is responsible for a particular drug class.5 The FDA may encourage other firms to initiate or increase production of the drug to meet patient needs.5 It can also request that a manufacturer not suspend production until an alternative source is available.2 The FDA also encourages manufacturers of unapproved drugs to resume production when there is a shortage.17
The FDA has been fairly successful in influencing manufacturers to increase production.6 Because of such efforts, Hospira agreed to return to manufacturing potassium phosphates for injection concentrate, an unapproved drug, to address a critical shortage.17 Some companies have even produced drugs at a loss, because they recognized a critical need for them.16
Establish an Allocation Program for Drugs in Short Supply
The FDA may help a drug manufacturer establish an allocation program for remaining product inventory until a shortage is resolved.5 Manufacturers may opt to distribute a product from one central location to control how much product is released, place limitations on quantities sold, or promote the use of alternative products to conserve remaining supplies.5 In some instances, an allocation program may be set up to ensure that a drug is available only on an emergency basis for life-threatening conditions for which no alternative therapy exists.5 In the past, the FDA has helped set up allocation programs for repository corticotropin injection (H.P. Acthar Gel, Questcor), caspofungin acetate (Cancidas, Merck) and betamethasone sodium phosphate–betamethasone acetate (Celestone Soluspan, Schering).5
Alert Alternative Drug Manufacturers About Impending Shortages
The FDA asks manufacturers to provide advance notice of an impending shortage so that the agency can warn manufacturers of related or alternative products about an upcoming increase in demand.5 The companies can then respond by increasing production to avert possible shortages stemming from this heightened demand.5
Proposed Legislation
On February 7, 2011, Amy Klobuchar (D-Minn.) and Robert Casey (D-Pa.) introduced legislation regarding drug shortages to the Senate.3,4,8 The bill, the Preserving Access to Life-Saving Medications Act (Table 1), was introduced at the behest of groups such as ASHP, ASCO, and the American Society of Anesthesiologists (ASA).3,8,9
Table 1.
Pending Federal Legislation on Drug Shortages
| Feature | House Bill 2245 | Senate Bill 296 |
|---|---|---|
| Short title | Preserving Access to Life-Saving Medications Act of 2011 | Preserving Access to Life-Saving Medications Act |
| Target of legislation | Prescription drug and biological products | Prescription drug products, except those that were originally derived from human tissue and have been replaced by a recombinant product |
| Terms with definitions | Average historical demand, discontinuance, drug shortage, and interruption | Drug shortage and shortage |
| Required notifications by manufacturers to HHS Secretary | For planned discontinuance of manufacturing, ≥6 months in advance; for unplanned discontinuance or interruption, ≥6 months in advance or as soon as practicable after manufacturer becomes aware of situation; if manufacturer certifies to HHS Secretary that good cause exists for shorter notification period, <6 months | For discontinuance, planned interruption, or planned adjustment of manufacturing, ≥6 months in advance; for unplanned interruption or adjustment, as soon as practicable after manufacturer becomes aware of situation; HHS Secretary may adjust required time frame |
| Penalties on manufacturers for failing to submit required notification to HHS Secretary | Civil money penalty of $10,000/day, up to $1.8 million per incident, if failure was intentional; specifics to be stated in regulations by HHS Secretary | Civil monetary penalties to be stated in regulations by HHS Secretary |
| Required notifications by HHS Secretary to public | Information on actual drug shortages and manufacturing discontinuances and interruptions; also, when possible, estimate of expected duration of shortage, discontinuance, or interruption | Information on actual drug shortages and manufacturing adjustments related to supply of raw materials, production capabilities, business decisions perhaps affecting manufacture of a drug, change in production output, and other adjustments as determined appropriate by HHS Secretary |
| Notifications by HHS Secretary to manufacturers | When criteria for identifying a drug as vulnerable to shortage have been met | When criteria for identifying a drug as vulnerable to shortage have been met |
| Criteria for identifying drugs as vulnerable to shortage | Number of manufacturers of drug, sources of raw material or active pharmaceutical ingredients, characteristics of supply chain (e.g., production complexities), and availability of therapeutic alternatives | Number of manufacturers of drug, sources of raw material or active pharmaceutical ingredients, characteristics of supply chain (e.g., production complexities), and availability of therapeutic alternatives |
| Collaboration | HHS Secretary, manufacturers of drug identified as vulnerable to shortage, and other stakeholders (e.g., distributors, health care providers) will establish and improve plans for “continuity of supply”; such plans cannot prohibit manufacturer from allocating distribution of its products to manage actual or potential drug shortage | HHS Secretary and manufacturers of drug identified as vulnerable to shortage will establish and improve plans for “continuity of operations” for medically necessary drugs |
| Manufacturing-site re-inspections | –––– | If need for reinspection is due to failure to comply with federal Food, Drug, and Cosmetic Act, then re-inspection will occur ≤90 days after company certifies it has corrected problem |
| Limitations | HHS Secretary may not require manufacturer to make a drug in the event of discontinuance or interruption or to delay or alter discontinuance or interruption | –––– |
| Effective date | 1 year after enactment | When enacted |
| Reports to Congress by HHS Secretary | By 1 year after enactment and every 5 years thereafter on actions taken during previous year to address drug shortages through all parts of supply chain | Annually on actions taken during previous year to address drug shortages through all parts of supply chain |
| Study by GAO | By 1 year after enactment, report on study of FDA’s identification and response to drug shortages, possible causes of such drug shortages, communication between industry, FDA, distributors, and end users, and legislation’s effects on ability of FDA to identify and ameliorate drug shortages; report will also include identification of additional measures that need to be taken to prevent or address drug shortages | –––– |
GAO = Government Accountability Office; HHS = Health and Human Services.
From Thompson C. Am J Health Syst Pharm 2011;68:1379–1381. Available at: www.ashp.org/DocLibrary/News/August-1-table.aspx.17
Among other changes, the Senate bill would require the FDA to revise the definition of a shortage of a “medically necessary” drug to account for drug-use factors.3,18 The legislation redefines a drug shortage as “a period of time when the total supply of all versions of a drug available at the user level will not meet the current demand for the drug at the user level.”18 Further, this legislation requires prescription drug manufacturers to provide the FDA with early notice of any “manufacturing discontinuance, interruption, or other adjustment,” that would “likely” result in a shortage of a product.8,15,18 The legislation also gives the FDA the authority to establish a schedule of civil penalties to be applied to manufacturers that have not provided the required notification.8
The bill further addresses FDA responsibilities with respect to drug shortages.18 It calls for the FDA to develop evidence-based criteria to identify drugs that are vulnerable to a shortage.3,18 Contingency plans for production would then be required for drugs that are identified as vulnerable.3,18 The legislation also mandates the FDA to make it a priority to inspect facilities that manufacture, propagate, compound, or process a drug involved in a drug shortage or that have recently corrected a production problem.18 In addition, the bill requires the FDA to distribute, “to the maximum extent practicable,” information on drug shortages to health care provider and patient organizations.18
On June 21, 2011, similar legislation (the Preserving Access to Life-Saving Medications Act of 2011) was introduced in the House of Representatives by Diana DeGette (D-Col.) and Thomas J. Rooney (R-Fla.).17 The House and Senate bills are similar; the major difference is that the House bill includes biological products and defines civil monetary penalties, whereas the Senate bill doesn’t do either (see Table 1).17 However, neither the House nor Senate legislation covers “unapproved drugs.”17
Prospects for both bills are uncertain. As of June 2011, the Senate bill had only one original cosponsor and 10 additional cosponsors—all Democrats.17 The House bill had only one original cosponsor and no additional cosponsors.17 Neither bill mentioned a date for a committee hearing, which would indicate that the bill had attracted the interest of other legislators.17 In addition, Republican control of the House of Representatives does not augur well for the passage of the bill, since the Republican Party generally supports PhRMA (which is expected to oppose the bill) and is often opposed to what it perceives to be government intervention.8 Some members of the health care community have referred to the legislation as a good first step, although others have expressed doubt that it will help.8
Recommendations Made at the Drug Shortages Summit
On November 5, 2010, the ASHP, ISMP, ASA, and ASCO, hosted a summit on drug shortages in Bethesda, Maryland.3,12,14,15,18 In attendance were about 55 representatives of pharmaceutical manufacturers, wholesalers, group-purchasing organizations, health care providers, trade groups, and the federal government.14 Notably, the representatives from the manufacturers that had experienced the most drug shortages in 2010—APP Pharmaceuticals LLC, Bedford Labs, Hospira Inc., and Teva Pharmaceuticals—were present.8 Representatives from PhRMA and the Generic Drug Manufacturers association also attended the summit.14
This summit produced 20 recommendations in four key areas that affect drug shortages: regulatory and legislative, raw material sourcing and manufacturing, business and marketing, and distribution.3 The recommendations included improved communication between the FDA and the manufacturer as well as increased transparency regarding manufacturing and inventory problems.3 Recommendations to relax drug importation laws (with appropriate safeguards) or to use tax breaks to promote the upgrading of manufacturing lines or the increased manufacturing of drugs were also made.9,15 In addition, it was suggested that the U.S. government create national stockpiles of critical drugs, as is done with pandemic flu vaccines or to counteract bioterrorism.16 It was also recommended that measures that would create other problems be avoided.14
Summit participants agreed to form work groups and continue to collaborate to explore, further prioritize, and develop detailed action plans to carry out the summit’s recommendations.14 Another summit meeting is expected to be held soon.6
Strategies for Managing Drug Shortages
Drug shortages present an ongoing challenge for health care providers and health facility personnel. The management of drug shortages in hospitals and health systems is particularly complex because these facilities routinely treat patients with acute or emergent conditions, deliver a significant number of medically necessary or single-source products, and use high-cost new drug technologies.2 In response to drug shortages, health systems must act rapidly to identify and obtain the drug, or the alternative product, to avoid disruptions in patient care and to provide uninterrupted, therapeutically equivalent, safe drug therapy, preferably at comparable costs.2,3 A drug shortage can also affect established procedures regarding drug procurement, therapeutic decision-making, and other institutional practices.9
Although it is often not possible to predict when a drug shortage will occur, strategies for dealing with shortages should be defined in advance.2 In 2001, the ASHP began providing guidelines for managing drug shortages that, ideally, have the potential to minimize such challenges.2–4 Some highlights of the recommendations issued by the ASHP are summarized in the following section.2
Validate Drug Shortages
When a back order or other notice is received by a health system’s drug-purchasing agent, there is often lead time before actual stock depletion.2 Therefore, the person responsible for procuring drug supplies needs to be aware of fluctuations in the health system’s supply chain that might indicate a potential shortage; for example, a specific strength is difficult to obtain, only a partial order is filled, or most manufacturers have no stock.2 Drug-purchasing agents who are not pharmacists need to work with the pharmacy staff.2
The suspected drug shortage should be verified with the distributor or manufacturer, and, whenever possible, the cause should be determined.2 This information will help to determine when the drug shortage will have an impact and how long it will last, since these factors vary according to the cause of the shortage and where the problem occurred in the supply chain.2 For example, a lack of raw materials may affect all manufacturers that produce the drug, whereas manufacturing problems will likely affect only the supply from a single manufacturer.2 A projected date for resumed product availability should be requested from manufacturers or distributors in order to guide management strategies and to determine the ability to endure the shortage.2
Assess Inventory of Drugs in Short Supply
Once a shortage is confirmed, the inventory on hand needs to be counted and an estimate of the time period it will cover should be made.2 This count should include all available inventory of the drug within the health care facility, including pharmacy stock, inpatient units, ambulatory care clinics, automated medication storage and distribution devices, floor stock, resuscitation carts, and prepared trays.2 Based on available quantities and historical usage, the pharmacy should estimate how long a shortage can be endured.2 Usage data can be obtained from purchasing agent records, distributors, the pharmacy department, billing, and/or automated medication storage and dispensing devices.2 After conservation measures, both current and reduced use rates should be considered in calculations of how long the available inventory of the drug and possible alternative products will last.2 Inventory data and the estimate of the shortage’s duration will allow a threat analysis to be conducted to determine the potential consequences of the shortage to the health care facility.2
Establish Contact with Other Sites or Health Systems
Whenever possible, collaborative arrangements within the health care system or with other regional institutions should be established.2 Large health systems can often survive drug shortages by shifting drug inventory among its sites.18 Information regarding possible alternative therapies should also be shared among facilities.2
Identify Alternative Drugs or Therapeutic Equivalents
An important task in managing drug shortages is to identify alternative therapies that can substitute for the unavailable drug product.2 Decisions regarding alternative agents should be made in collaboration with medical, nursing, and pharmacy representatives and should be approved by the appropriate medical committees.2 Once a decision is made, the therapeutic alternative should also be inventoried to ensure an adequate supply.2 Pharmacists may also need to establish or alter procedures for bar coding; look-alike, sound-alike medications; distribution paths; contract compliance; effects on automation; and final product preparation.2 The health care facility should also be prepared in case a shortage of the best alternative agent subsequently develops.2
Prioritize Patients to Receive Drugs in Short Supply
Criteria for patient prioritization during a drug shortage should be developed by a multidisciplinary team involving pharmacy, medical, and nursing staff.2,3 All patients whose treatment depends on the unavailable drug product and alternative therapies should be identified.2,3 Judicious use of the product in short supply helps to ensure that treatment will be available to patients who cannot be treated with an alternative agent.3,4 Drug prescribing and utilization trend data may also be useful in developing criteria for prioritization.2 Once criteria are established to determine which patients will receive the drug in short supply, good communication is essential to ensure that adequate supplies will be available to complete entire courses of therapy.2
National organizations, such as the CDC or professional medical societies, may be sources of guidance.2 Health facility risk managers or the ethics staff may also be consulted for help with patient prioritization when drug supplies are severely limited.15 For example, during the influenza vaccine shortage of 2004–2005, many health care facilities did not have a sufficient supply to follow recommended CDC guidelines, so they had to develop guidelines to further prioritize which patient groups would receive the vaccine.2
Follow Good Inventory-Management Practices
Inventory management can be a challenge during a drug shortage. Health care facilities should resist the temptation to stockpile drugs in anticipation of a shortage, because this can cause additional problems.2 Hoarding drugs in advance of a feared shortage can deplete existing inventory and worsen the shortage by diverting any supplies away from other facilities and patients in need.2,9,18 Stockpiling drugs can also be expensive and can cause a facility to be left with excess inventory, which is costly and might not be absorbed if the shortage is averted or isn’t as severe as had been anticipated.2
A shortage may fail to materialize when problems that are a legitimate potential threat arise but never reach end-users.2 This could happen when the supply chain contains enough inventory to provide an adequate supply to meet the usual demand, allowing the problem that might have caused a shortage to be resolved in the meantime.2 In addition, during shortages, manufacturers and distributors often allocate a product according to usage history, so a sudden stockpiling order generally has no effect on increasing this drug allocation.2
Modify Clinical Guidelines and Other Policies
Establishing an organizational approach to decision-making is an essential step in managing drug shortages.2 Committee structures and responsibilities should be predetermined for every phase of the drug shortage management process. Because many drug products have limited therapeutic alternatives, the effect on patient care and costs needs to be evaluated.2 A threat analysis that evaluates all factors relevant to the shortage (e.g., duration, current inventory, medical necessity, and alternative sources or therapies) is helpful in determining the potential effect of the shortage on patient care and costs.2 The impact of a drug shortage on a health care facility is often dependent on the scope, level of services, and the population that the facility serves.2
Drug shortages can also have a significant impact on safe medication practices, as well as processes for medication distribution and administration, within a health care facility.2 There is a particular need for clear guidelines when a critical drug can be obtained only from an alternative distributor or a compounding pharmacy or when it is completely unavailable.2 Patients who feel that they have received improper care or have experienced an unanticipated adverse event as a result of delays, prioritization, alternative therapy, nontraditional drug product sources, or drug unavailability may pursue litigation.2 Risk managers and legal representatives should therefore be notified immediately if all acceptable options for obtaining a drug or therapeutic alternative have been exhausted.2
The economic impact of obtaining a drug through gray-market distributors or purchasing more expensive therapeutic equivalents also needs to be estimated.15,16 Once this has been determined, the financial impact must be communicated through budgetary channels to request and justify contingency funds.2 Any additional expenses that are caused by a drug product shortage (e.g., overtime, extra delivery charges, or costs for in-house compounding) must also be documented to support budget variances and future budget proposals.2
Develop Timely Communication Systems and Strategies
Effective communication strategies are necessary to manage a drug shortage within a health care facility and should be developed.2 Some health care systems may designate a specific department to manage communications; however, others may pursue a more collaborative strategy that involves multiple departments and committees.2 The pharmacy staff should assist prescribers and the nursing staff by thoroughly informing them about the drug product shortage and its projected duration, alternative therapies, temporary therapeutic guidelines, and implementation plans.2 This information should be communicated to the clinical staff in order to ensure patient safety and prevent medication errors that could be caused by differences between drug dosages, time to onset, durations, and other factors.2
The Joint Commission requires that drug prescribers who might be affected by a shortage be directly notified.15 Plans for communication to reach medical, nursing, and pharmacy staff members who work during other shifts also need to be implemented.2
Patients or family members should also be counseled when a drug product shortage is expected to delay or compromise care, especially when patients have been stabilized on a drug and the available alternatives might not be as effective.2 Patients should be informed about the available therapies so that they can participate in the decision-making process.4
Sources of Information
The ASHP Drug Shortage Resource Center Web site (www.ashp.org/shortages) is a major source of information about drug shortages.10 Updates on shortages of medically necessary drugs are available on the FDA’s Web site.2,3,11 Information about blood and vaccine shortages can be found on the Web site for the FDA’s Division of Biological Evaluation and Research (CBER) (www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Shortages/default.htm).2,19 Manufacturers, distributors, and the CDC can also be contacted to determine the cause of a shortage and its expected duration.2 Data from a 2010 ASHP member survey rating the quality of these information sources are presented in Table 2 (see page 756).3
Table 2.
Respondents’ Ratings of Existing Information Sources on Drug Shortages
| Rated Characteristic* | ASHP Web Site | FDA Web Site | Wholesaler Web Site or Communication | GPO Web Site or Communication | Direct Communication With Manufacturer |
|---|---|---|---|---|---|
| Timeliness | 3.93 | 3.40 | 3.38 | 3.42 | 3.02 |
| Reason for, duration of shortage provided | 3.80 | 3.23 | 2.66 | 3.23 | 2.78 |
| Suggested alternatives provided | 3.97 | 2.74 | 1.81 | 3.04 | 1.92 |
ASHP = American Society of Health-System Pharmacists; GPO = group-purchasing organization.
Mean rating, based on a 5-point Likert scale, where 1 = very poor, 2 = poor, 3 = neutral, 4 = good, and 5 = very good.
From Kaakeh R, et al. Am J Health Syst Pharm 2011;68:1811–1819.3
The FDA encourages the lay and professional public to report drug shortages to the ASHP or FDA drug shortage program Web site.2 E-mails can also be sent to the FDA’s CDER division (drugshortages@fda.hhs.gov).2 Vaccine or blood product shortages can be reported by e-mail to the FDA’s CBER division (CBERshortage@fda.hhs.gov).2
Establishing contact with national professional and patient organizations or the media can also raise awareness of a drug shortage.2 Drawing attention to a drug shortage in this way may increase awareness of its impact, encourage production by alternative manufacturers, initiate collaborative efforts to develop alternative therapies or guidelines, or spur ad hoc training about the use of substitute treatments.2
Conclusion
The increasing frequency of drug shortages creates complex challenges for health care providers and facilities.2 Drug shortages have a profound impact on patient safety, clinical outcomes, quality control, health care facility management, and other important factors. Although it is impossible to predict or prepare for every drug shortage, careful planning can prevent the consequent problems from turning into a crisis.2 Establishing clear procedures and guidelines for managing drug shortages is essential.2 Proper information-gathering, extensive collaboration, and timely communication strategies are critical elements of an effective drug shortage management plan.2
References
- 1.Stein R. Shortages of key drugs endanger patients. The Washington Post. 2011 May 1; Available at: www.washingtonpost.com/national/shortages-of-key-drugs-endanger-patients/2011/04/26/AF1aJJVF_story.html?hpid=z5. Accessed September 30, 2011. [Google Scholar]
- 2.Fox E, Birt A, James K, et al. ASHP guidelines on managing drug product shortages in hospitals and health systems. Am J Health Syst Pharm. 2009;66:1399–1406. doi: 10.2146/ajhp090026. [DOI] [PubMed] [Google Scholar]
- 3.Kaakeh R, Sweet B, Reilly C, et al. Impact of drug shortages on U.S. health systems. Am J Health Syst Pharm. 2011;68:1811–1819. doi: 10.2146/ajhp110210. [DOI] [PubMed] [Google Scholar]
- 4.Johnson TJ. Drug shortages: An increasing problem for patients and clinicians. S D Med. 2011;64(1):14–15. [PubMed] [Google Scholar]
- 5.Jensen V, Kimzey L, Goldberger MJ. FDA’s role in responding to drug shortages. Am J Health Syst Pharm. 2002;59:1423–1425. doi: 10.1093/ajhp/59.15.1423. [DOI] [PubMed] [Google Scholar]
- 6.Jenks S. Efforts underway to curb drug shortages. J Natl Cancer Inst. 2011;103(12):914–915. doi: 10.1093/jnci/djr234. [DOI] [PubMed] [Google Scholar]
- 7.Fox ER. Drug Status Update. Available at: www.fda.gov/downloads/Drugs/NewsEvents/UCM274565.pdf.
- 8.Barlas S. Severe drug shortages impose heavy costs on hospital pharmacies: Senate bill might help ... or not. P&T. 2011;36(5):242–302. [PMC free article] [PubMed] [Google Scholar]
- 9.Shortages of cancer drugs in the USA. Lancet. 2011;12:313. doi: 10.1016/S1470-2045(11)70087-0. [DOI] [PubMed] [Google Scholar]
- 10.Drug Shortages: Current Drugs ASHP Drug Shortage Resource Center. Available at: www.ashp.org/DrugShortages/Current. Accessed October 3, 2011.
- 11.Current Shortages FDA Drug Shortage Program. Available at: www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm050792.htm. Accessed October 3, 2011.
- 12.McKenna M. Hospital pharmacists scrambling amid vast drug shortages: Emergency physicians between rock and hard place. Ann Emerg Med. 2011;57(2):13A–15A. doi: 10.1016/j.annemergmed.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 13.American Society of Health-System Pharmacists Drug Shortages Summit Report. Available at: www.ashp.org/drugshortages/summitreport. Accessed October 1, 2011.
- 14.Thompson C. Stakeholders in supply chain discuss shortages. Am J Health Syst Pharm. 2011;68:9–10. doi: 10.2146/news100088. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser J. Shortages of cancer drugs put patients, trials at risk. Science. 2011;332:523. doi: 10.1126/science.332.6029.523. [DOI] [PubMed] [Google Scholar]
- 16.Cancer drug shortages persist. P&T. 2011;36(7):397. [Google Scholar]
- 17.Thompson C. House legislation proposes early warning system for drug shortages. Am J Health Syst Pharm. 2011;68:1379–1381. doi: 10.2146/news110052. [DOI] [PubMed] [Google Scholar]
- 18.Thompson C. Senator proposes drug shortage law. Am J Health Syst Pharm. 2011;68:461. doi: 10.2146/news110013. [DOI] [PubMed] [Google Scholar]
- 19.Biologic Product Shortages FDA Center for Biologic Research and Evaluation. Available at: www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/Shortages/default.htm. Accessed October 3, 2011.