Abstract
We recently found that microRNA-34a (miR-34a) is downregulated in human glioma tumors as compared to normal brain, and that miR-34a levels in mutant-p53 gliomas were lower than in wildtype-p53 tumors. We showed that miR-34a expression in glioma and medulloblastoma cells inhibits cell proliferation, G1/S cell cycle progression, cell survival, cell migration and cell invasion, but that miR-34a expression in human astrocytes does not affect cell survival and cell cycle. We uncovered the oncogenes c-Met, Notch-1 and Notch-2 as direct targets of miR-34a that are inhibited by miR-34a transfection. We found that c-Met levels in human glioma specimens inversely correlate with miR-34a levels. We showed that c-Met and Notch partially mediate the inhibitory effects of miR-34a on cell proliferation and cell death. We also found that mir-34a expression inhibits in vivo glioma xenograft growth. We concluded that miR-34a is a potential tumor suppressor in brain tumors that acts by targeting multiple oncogenes. In this extra view, we briefly review and discuss the implications of these findings and present new data on the effects of miR-34a in glioma stem cells. The new data show that miR-34a expression inhibits various malignancy endpoints in glioma stem cells. Importantly, they also show for the first time that miR-34a expression induces glioma stem cell differentiation. Altogether, the data suggest that miR-34a is a tumor suppressor and a potential potent therapeutic agent that acts by targeting multiple oncogenic pathways in brain tumors and by inducing the differentiation of cancer stem cells.
Keywords: miR-34a, glioma, stem cells, c-Met, Notch, differentiation
Introduction
microRNAs are small noncoding regulatory RNA molecules, with profound impact on a wide array of biological processes.1,2 microRNAs modulate protein expression by binding to the 3′ untranslated region (3′UTR) of mRNA and promoting RNA degradation, inhibiting mRNA translation, and also affecting transcription.3 microRNAs are aberrantly expressed in a wide variety of human cancers where they are thought to play important roles by regulating the expression of various oncogenes and tumor suppressors.3–6
microRNA-34a (miR-34a) has been the focus of cancer-associated research in the last few years. It was originally uncovered as a potential tumor suppressor that is downregulated and that induces apoptosis in neuroblastoma cells.7 Shortly afterwards, it received great notoriety when a few studies almost simultaneously reported that it was a transcriptional target of p53.8–11 Since then, a number of studies have addressed the deregulation, functions and mRNA targets of miR-34a in various cancers. It was shown that miR-34a is downregulated in cells or tumors of cancer including colon cancer, leukemia, hepatocellular carcinoma and non-small cell lung cancer.12–15 Functionally, miR-34a was found to affect tumor cell apoptosis, senescence, proliferation and invasion.7,10,12,14,16 miR-34a possesses hundreds of predicted mRNA targets. A few of these have been experimentally verified and include oncogenes such as MYC, CDK6, SIRT1 and c-Met.11,16–18
In a recently published study, our group investigated the expression, functional effects, targets and potential therapeutic use of miR-34a in human brain tumors.19 We showed that miR-34a is downregulated in glioblastoma tumors and that its expression levels inversely correlate with the levels of c-Met in the tumors. We found that miR-34a inhibits glioblastoma cell proliferation, survival, migration and invasion by targeting c-Met and Notch. We also showed that in vivo expression of miR-34a inhibits glioblastoma xenograft growth.19 In this extra view, we briefly review and discuss the implications of these findings and present new data on the effects of miR-34a on glioma stem cell malignancy and differentiation.
Deregulation of miR-34a in Brain Tumors
We recently found that miR-34a expression is lower in human glioblastoma tumors than in normal human brain.19 Within the tumor samples, miR-34a levels were lower in mutant p53 tumors than in wildtype p53 samples but the difference in miR-34a levels was smaller than the one measured between tumors and normal tissue. This suggests that p53 mutations do not completely account for the decrease in miR-34a expression in glioblastoma and that other pathological mechanisms are involved in miR-34a downregulation in these tumors. miR-34a was reportedly located within chromosome 1p36.17 Allelic loss at 1p is seen in 70% to 85% of oligodendrogliomas and 20% to 30% of astrocytomas and most 1p deletions in gliomas involve almost the entire chromosome arm.20 Therefore, miR-34a expression loss might be partly a resultant of its gene deletion. It was also recently shown that miR-34a is inactivated by aberrant CpG methylation in multiple types of cancer, although brain tumors were not investigated.21 Therefore, miR-34a down-regulation in glioblastoma could be a resultant of multiple mechanisms including transcriptional downregulation due to p53 mutations, chromosomal deletion and epigenetic regulation. Understanding the relative contribution of these mechanisms could be of relevance especially for oligendendrogliomas where 1p deletions are very frequent and where the resulting miR-34a loss could contribute to the malignancy of this glioma subtype.
Targets and Functional Effects miR-34a in Brain Tumors
miR-34a has a very large number of predicted targets among which are several oncogenes. We recently assessed the effects of miR-34a on a few oncogenes that were selected based on their known roles in brain tumors. We found that miR-34a expression inhibits c-Met in human glioma and medulloblastoma cells and Notch-1, Notch-2 and CDK6 protein expressions in glioma cells but did not affect PDGFRA expression in any brain tumor cells.19 miR-34a expression also inhibited c-Met reporter activities in glioma and medulloblastoma cells and Notch-1 and Notch-2 3′-untranslated region reporter activities in glioma cells and stem cells, indicating that c-Met, Notch-1 and Notch-2 are direct targets of miR-34a. Notably also, c-Met levels in the human glioma specimens described in the previous section inversely correlated with miR-34a levels measured in the same tumors. This suggests that miR-34a is an important regulator of c-Met expression and that miR-34a downregulation might be partly responsible for c-Met oncogene overexpression as observed in human gliomas.22 Functionally, miR-34a strongly inhibited cell proliferation, cell cycle progression, cell survival and cell invasion in glioma and medulloblastoma cell lines as well as in vivo glioma xenograft growth, but did not affect cell survival and cell cycle status in human astrocytes. Expression of c-Met or Notch-1/Notch-2 transcripts lacking the 3′-untranslated region sequences partially rescued the effects of miR-34a on cell cycle arrest and cell death in glioma cells and stem cells, respectively. Therefore, miR-34a inhibited brain tumor malignancy and growth in part by directly inhibiting the expression of c-Met and Notch, two pathways that play important roles in glioma and medulloblastoma malignancy.23–30 Together with the expression data described in the previous section, these findings suggest that miR-34a is a tumor suppressor in gliomas that acts via inhibition of c-Met, Notch and probably also other oncogenic pathways. The involvement of additional oncogenes in mediating the tumor suppressive effects of miR-34a is supported by the incomplete rescue by c-Met or Notch of miR-34a-mediated tumor suppression. The data also suggest that miR-34a could serve as a new potent experimental therapeutic agent in gliomas. In fact, miR-34a exhibited a very strong anti-tumor effect in both glioma cells and xenografts but not in astrocytes. The potent anti-tumor effects of miR-34a can be explained by the simultaneous inhibition of several oncogenes. The differential effect on tumor cells vs. normal cells could be due to the fact that restoration of miR-34a to cells in which it is downregulated will inhibit malignancy via inhibition of upregulated oncogenes, while transfection into cells in which miR-34a has normal expression levels and in which oncogene expression is already low will not affect cell death. Importantly, for miR34a to be successfully used as a therapeutic agent, it should theoretically also exert inhibitory effects on cancer stem cell malignancy. We therefore assessed the effects of miR-34a on glioma stem cell malignancy and differentiation and present these new findings below.
Results and Discussion
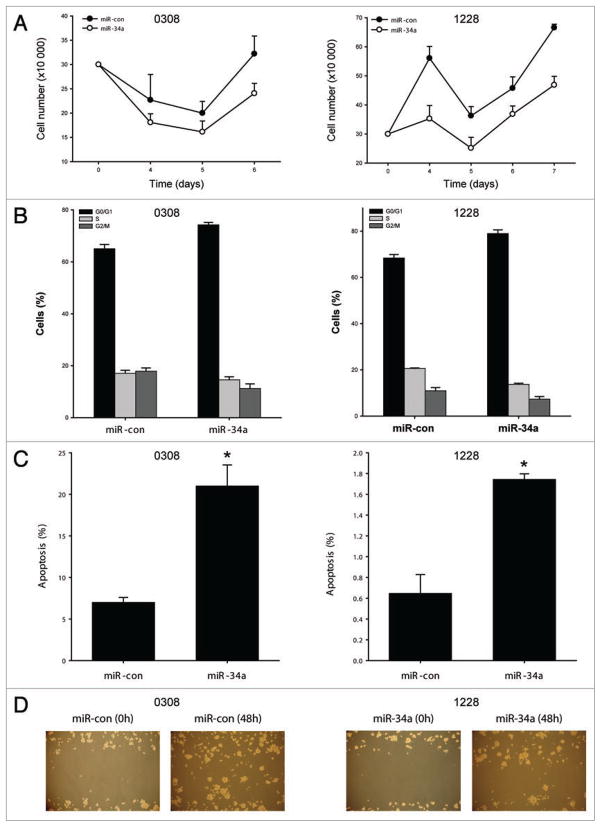
miR-34a transfection into 0308 and 1228 glioma stem cells led to a modest inhibition of cell proliferation (Fig. 1A). miR-34a also induced a modest G1/S cell cycle arrest in both glioma stem cell lines (Fig. 1B). Therefore, unlike in differentiated glioma cells where miR-34a substantially inhibited proliferation, miR-34a only slightly affected glioma stem cell proliferation. miR-34a significantly induced apoptosis in both glioma stem cells that were tested (Fig. 1C). miR-34a also inhibited migration in one glioma stem cell line (Fig. 1D). Therefore, miR-34a inhibits selected malignancy endpoints in glioma stem cells but these effects are generally less pronounced than in differentiated glioma cells as we had shown in our previous study.
Figure 1.
miR-34a inhibits glioma stem cell malignancy. (A and B) Glioma stem cells 0308 and 1228 were transfected with miR-34a or miR-con (30 nM). The cells were harvested 4 days post-transfection and assessed for cell proliferation by cell counting over 5 days, or for cell cycle by propidium iodide flow cytometry 5 days post-transfection. The results show that miR-34a slightly inhibits cell proliferation and induces G1/S cell cycle arrest in both glioma stem cells. (C) The glioma stem cells 0308 and 1228 were transfected with miR-34a or miR-con (30 nM). The cells were assessed for apoptosis 5 days post-transfection using Annexin V flow cytometry. The results show that miR-34a induces apoptosis in both glioma stem cells. (D) 1228 glioma stem cells were seeded on L-poly-polyornithine-coated plates and transfected with miR-34a or miR-con (30 nM). 48 hours post-transfection, the cells were assessed for migration using the scratch/wound assay. Cells that migrated into the scratch were photographed at 40x magnification. The results show that miR-34a inhibits cell migration in 1228 stem cells *p < 0.05.
Importantly, miR-34a significantly induced the differentiation of glioma stem cell lines. Transfection of miR-34a into 0308 and 1228 cells led to decreases in immunostaining of the stem cell markers CD133 and nestin and increases in immunostaining of the astrocyte marker GFAP, and the oligodendrocyte marker claudin-11 in both stem cell lines and the neuronal marker Tuj-1 in 0308 cells (Fig. 2). These data suggest that miR-34a reduces glioma stemness and induces cell differentiation into astrocytes, neurons and oligodendrocytes.
Figure 2.
miR-34a induces glioma stem cell differentiation. 1228 and 0308 glioma stem cells were plated in L-poly-ornithine-coated dishes overnight and then transfected with pre-miR-34a or pre-miR-con for 3 days. The cells were then subjected to immunohistochemistry staining for the stem cell markers CD133 and nestin and the differentiation markers GFAP (astrocytes), Tuj-1 (neurons) and Claudin-11 (oligodendrocytes). Representative photomicrographs show that miR-34a decreases CD133 and nestin immunostatining and increases GFAP, and claudin-11 immunostaining in both stem cell lines and Tuj-1 immunostaining in 0308.
The above data show for the first time that miR-34a regulates glioma stem cell malignancy and differentiation. Interestingly, preliminary data from our lab show that miR-34a levels in glioma stem cells are significantly lower than in differentiated wildtype p53 glioma cell lines. Together, the above data indicate a role for miR-34a in glioma stem cells and suggest that dysregulation of this microRNA might have a role in gliom-agenesis. The data also suggest that if feasible, restoration of miR-34a expression for therapeutic purposes could achieve strong anti-tumor effects not only by inhibiting differentiated glioma cell malignancy but also by targeting glioma stem cells and inducing their differentiation.
Effects of miR-34a on Glioma Stem Cell Malignancy and Differentiation
Methods
The effects of miR-34a on glioblastoma stem cell proliferation, death, migration and differentiation were investigated as described below. The stem cells (0308 and 1228) were a kind gift from Dr. Howard Fine (NIH).31 They were grown in serum-free neurobasal media (Invitrogen), supplemented with human recombinant EGF, bFGF (R&D systems) at 37°C in 5% CO2–95% O2. Pre-miR-34a and control microRNA (miR-con) (Ambion), transfections (30 nM) were performed using Oligofectamine reagent (Invitrogen) according to the instructions of the manufacturer.
To assess the effect of miR-34a on stem cell growth, 30,000 cells/well were transfected with pre-miR-34a or pre-miR-control. The cells were harvested every day, starting from the fourth day after transfection, counted for 5 days with a hemocytometer and growth curves were established. To assess the effects of miR-34a on the cell cycle, the stem cells were transfected with pre-miR-34a or pre-miR-con. Four days after transfection, the cells were fixed in 70% (v/v) ethanol, treated with 20 μg DNase-free RNase and stained with propidium iodide. The cells were then analyzed on a FACscan (Becton-Dickinson, Fullerton) and G0/G1, S and G2/M fractions were determined.
The effects of miR-34a expression on cell apoptosis were assessed by Annexin V-PE flow cytometry as previously described.32 Briefly, 0308 and 1228 glioma stem cells were transfected with pre-miR-34a or pre-miR-con for 5 days. The cells were harvested and stained with Annexin V-PE according to the instructions of the manufacturer. Cell samples were analyzed on a FACsan and apoptotic fractions were determined.
To investigate the effects of miR-34a on glioma stem cells migration, 6-well plates were coated with L-poly-ornithine to induce stem cell attachment to the plates. 1228 glioma stem cells were seeded in the coated wells and scratch wounds were made in the middle of the plates with sterile pipette tips. The glioma stem cells were transfected with pre-miR-34a or pre-miR-con. Forty eight hours later, pictures of migrating cells were taken.
To study the effect of miR-34a on stem cell differentiation, 0308 and 1228 glioma stem cells were plated on L-poly-ornithine-coated dishes and then transfected with pre-miR-34a or pre-miR-con. Three days later, the cells were harvested, plated on slides by cytospin and subjected to immunohistochemistry staining for the stem cell markers CD133 and nestin, and the differentiation markers GFAP (astrocytes), Tuj-1 (neurons) and claudin-11 (oligodendrocytes). Representative fields were photographed at 40x magnification. Of note is that this assay does not allow the identification of cell shape and morphology as all cells are centrifuged immediately before fixation and therefore appear round in shape.
All experiments were performed in triplicates. Numerical data were expressed as mean ± standard deviation. Two group comparisons were analyzed by two-sided Student’s t test. p values were determined and p < 0.05 was considered significant.
Conclusions
miR-34a is a putative tumor suppressor that is downregulated in gliomas via multiple mechanisms. It exerts significant inhibitory effects on brain tumor malignancy and growth by targeting oncogenic molecules and pathways including c-Met and Notch. It also inhibits glioma stem cell malignancy and induces stem cell differentiation. miR-34a is therefore appears to be an important player in brain tumor malignancy and a promising therapeutic agent that might have greater differential effects on tumor cells and tumor stem cells than on the normal brain.
Acknowledgments
Supported by NIH grant RO1 NS045209 (R. Abounader) and a University of Virginia Cancer Center Pilot Project Grant (R. Abounader). We thank Ms. Joanne Lannigan for assistance with flow cytometry.
References
- 1.Ambros V, Lee RC. Identification of microRNAs and other tiny noncoding RNAs by cDNA cloning. Methods Mol Biol. 2004;265:131–58. doi: 10.1385/1-59259-775-0:131. [DOI] [PubMed] [Google Scholar]
- 2.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–26. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldas C, Brenton JD. Sizing up miRNAs as cancer genes. Nat Med. 2005;11:712–4. doi: 10.1038/nm0705-712. [DOI] [PubMed] [Google Scholar]
- 5.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 6.Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, et al. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is downregulated in glioblastoma. Cancer Res. 2008;68:3566–72. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 7.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–22. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 8.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–52. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–43. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–93. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 11.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–4. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA. 2007;104:15472–7. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dijkstra MK, van Lom K, Tielemans D, Elstrodt F, Langerak AW, van ‘t Veer MB, et al. 17p13/TP53 deletion in B-CLL patients is associated with microRNA-34a downregulation. Leukemia. 2009;23:625–7. doi: 10.1038/leu.2008.264. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y, et al. miR-34a inhibits migration and invasion by downregulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Gallardo E, Navarro A, Vinolas N, Marrades RM, Diaz T, Gel B, et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis. 2009;30:1903–9. doi: 10.1093/carcin/bgp219. [DOI] [PubMed] [Google Scholar]
- 16.Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R, et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582:1564–8. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 17.Wei JS, Song YK, Durinck S, Chen QR, Cheuk AT, Tsang P, et al. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27:5204–13. doi: 10.1038/onc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105:13421–6. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–76. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbashina V, Salazar P, Holland EC, Rosenblum MK, Ladanyi M. Allelic losses at 1p36 and 19q13 in gliomas: correlation with histologic classification, definition of a 150-kb minimal deleted region on 1p36, and evaluation of CAMTA1 as a candidate tumor suppressor gene. Clin Cancer Res. 2005;11:1119–28. [PubMed] [Google Scholar]
- 21.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama T, Kataoka H, Kawano Y, Yokogami K, Nakano S, Goya T, et al. Comparative analysis of expression of hepatocyte growth factor and its receptor, c-met, in gliomas, meningiomas and schwannomas. Canc Lett. 1998;124:149–55. doi: 10.1016/s0304-3835(97)00469-2. [DOI] [PubMed] [Google Scholar]
- 23.Abounader R, Ranganathan S, Lal B, Fielding K, Book A, Dietz H, et al. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c-met expression. J Natl Cancer Inst. 1999;91:1548–56. doi: 10.1093/jnci/91.18.1548. [DOI] [PubMed] [Google Scholar]
- 24.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro-oncol. 2005;7:436–51. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Guessous F, DiPierro C, Zhang Y, Mudrick T, Fuller L, et al. Interactions between PTEN and the c-Met pathway in glioblastoma and implications for therapy. Mol Cancer Ther. 2009;8:376–85. doi: 10.1158/1535-7163.MCT-08-0627. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Lal B, Kwon S, Fan X, Saldanha U, Reznik TE, et al. The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res. 2005;65:9355–62. doi: 10.1158/0008-5472.CAN-05-1946. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Guessous F, Johnson EB, Eberhart CG, Li XN, Shu Q, et al. Functional and molecular interactions between the HGF/c-Met pathway and c-Myc in large-cell medulloblastoma. Lab Invest. 2008;88:98–111. doi: 10.1038/labinvest.3700702. [DOI] [PubMed] [Google Scholar]
- 28.Guessous F, Li Y, Abounader R. Signaling pathways in medulloblastoma. J Cell Physiol. 2008;217:577–83. doi: 10.1002/jcp.21542. [DOI] [PubMed] [Google Scholar]
- 29.Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–63. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 30.Purow BW, Sundaresan TK, Burdick MJ, Kefas BA, Comeau LD, Hawkinson MP, et al. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis. 2008;29:918–25. doi: 10.1093/carcin/bgn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Guessous F, Kwon S, Kumar M, Ibidapo O, Fuller L, et al. PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Res. 2008;68:1723–31. doi: 10.1158/0008-5472.CAN-07-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]