Abstract
Objective
There are several pathways that mediate the aberrant metabolism of glucose and that might induce greater vascular damage in the setting of diabetes. The polyol pathway mediated by aldose reductase (AR) has been postulated to be one such pathway. However, it has been reported that AR reduces toxic lipid aldehydes and, under some circumstances, might be anti-atherogenic.
Methods and Results
Atherosclerosis development was quantified in two lines of transgenic mice expressing human AR (hAR) crossed on the apoE knockout (apoE−/−) background. The transgenes were used to increase the normally low levels of this enzyme in wild type mice. Both generalized hAR overexpression and hAR expression via the Tie 2 promoter increased lesion size in streptozotocin (STZ) diabetic mice. In addition, pharmacologic inhibition of AR reduced lesion size.
Conclusion
Although in some settings AR expression might reduce levels of toxic aldehydes, transgenic expression of this enzyme within the artery wall leads to greater atherosclerosis.
Keywords: Aldose reductase, apoE−/−, atherosclerosis, diabetes, endothelial dysfunction
INTRODUCTION
The reason diabetes mellitus (DM) causes increased cardiovascular disease is unclear. Both type 1 and type 2 DM are associated with multiple metabolic abnormalities including reduced insulin signaling, dyslipidemia, hyperglycemia and aberrant glucose metabolism.1 This latter process has been studied primarily in vitro and is associated with increased production of reactive oxygen species (ROS), DNA strand breaks, and protein glycation.1 Amongst the several pathways thought to do this is the polyol pathway. Aldose reductase (AR) converts glucose into sorbitol, which is then metabolized to fructose and trioses. AR may create excess ROS due to altered NADH/NADPH and produce sugars like fructose that are more likely to create advanced glycation end products (AGEs). AR expression is increased in inflammatory macrophages and AR enzymatic activity increases production of 4-hydroxynonenal (4-HNE).2
Both the in vivo actions of AR and its role in atherosclerosis are controversial. On the LDL receptor knockout (Ldlr−/−) background generalized overexpression of human AR (hAR) increased atherosclerosis, but only in diabetic mice.3 These data suggested that hyperglycemia was needed to provide sufficient substrate for the vasculo-toxic effects of this enzyme. Mice with a knockout of AR have minimal phenotype; their only obvious alteration is a reduction in renal concentration.4 However, on the apoE knockout (apoE−/−) background AR deficiency was reported to promote atherosclerosis and increase vascular content of 4-HNE5, perhaps secondary to reduced oxidation of phospholipids.6
AR expression and activity vary amongst species. Ischemia/reperfusion injury in the rat heart is reduced by AR inhibitors (ARIs).7 But, mice do not respond in a similar manner unless their relatively low endogenous AR expression is supplemented by a transgene expressing hAR.3 Transgenic expression of hAR increases mouse macrophage AR expression to levels found in humans.3 Thus, it is argued that hAR expression in the mouse mimics human biology.
We asked the following questions: Does hAR expression increase atherosclerosis in diabetic apoE−/− mice? If atherosclerosis is increased by AR expression, do ARIs correct this? Does endothelial cell (EC) production of AR alter EC biology and atherosclerosis development? Data showing that the answer to each of these questions is yes is provided.
Methodology
All studies were performed with the approval of the Institutional Animal Care and Use Committee at Columbia University, New York. The animal characterization and study design is listed in the supplement.8, 9
Total cholesterol and triglyceride levels were determined in fasting mice using commercially available kits (Thermo Electron). Glucose levels were determined from samples of tail vein blood using a glucometer (Abbott Diabetes Care Co.). AR enzymatic activity in aorta was measured as previously described.3, 10
Atherosclerosis assays were performed at the indicated times of sacrifice. Details for staining with sudan-IV, oil red O, as well as en face lesion area of the aorta (using ImagePro Plus software, version 4.1.0.0; Media Cybernetics) and atherosclerotic lesion area (using a Zeiss microscope and image analysis system AxioVision 4.5) used published methods11, and are described in the supplement.
Staining and immunofluorescence details for AR, CD31 detection are provided in the supplemental data.
Endothelial vasorelaxation was studied in 14 week diabetic mice and its citrate controls as described previously.12, 13
Oxidized human LDL (Sigma) was prepared and characterized as published in the literature.14, 15 For cell culture studies three different lines of wild-type (WT) and AR+ murine aortic ECs were established from 3 individual mouse aortas as described previously.16 Confluent cells were incubated either with high glucose (25 mM D-glucose, HG) or HG with the ARI zopolrestat (200 μM), or siRNA against hAR (20 nM) or osmotic controls (5.5 mM D-glucose and 19.5 mM D-mannitol) or oxLDL (5 μg/ml) or pre incubated with zopolrestat (200uM) for 1 hr followed by incubation with oxLDL for 4 hrs. (See supplement for details)
Detailed methodology of western blotting and zymography done on tissue samples and the cell lysates are provided in the supplement.
Statistical analysis
All data are reported as mean ± SD unless notified. Data were analyzed by one way ANOVA using commercially available software (Statview, version 5.0.1, Berkeley, CA, USA). Probability values ≤ 0.05 were considered statistically significant.
Results
AR activity and vascular AR expression in mouse aorta
AR activity was measured in WT and apoE−/− mice aorta with and without diabetes and has been reported to increase with diabetes.17 AR activity was greater in 12 weeks diabetic apoE−/− mouse aorta, 10.80 ± 1.80 nmols NADPH/min/mg protein versus 4.30 ± 1.30 nmols NADPH/min/mg protein in controls. However, AR activity in diabetic apoE−/− mice was lower than that in non-diabetic hAR aorta (16.50 ± 3.40 nmols NADPH/min/mg protein), whereas in diabetic hAR mice AR activity was more than 2 fold greater (31 ± 4 nmols NADPH/min/mg protein).
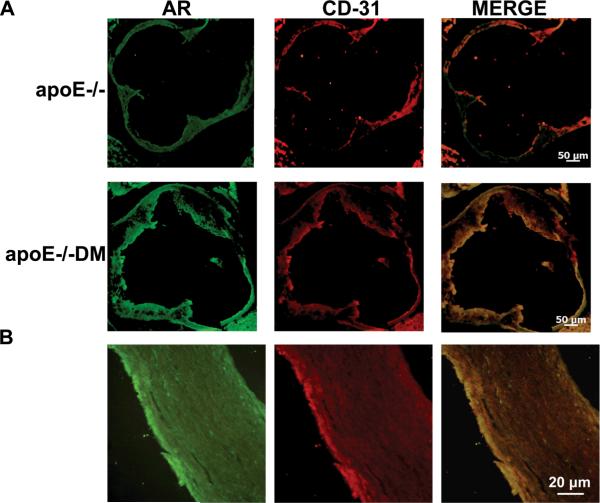
AR expression in the aortic root of non-diabetic and diabetic apoE−/− mice (14 weeks) of age was evident in ECs; it colocalized with CD31 (Figure 1A). Some AR expression was also found in other areas of the artery, probably indicative of some macrophage AR expression.2 Atherosclerotic human carotid artery also revealed colocalization of AR with CD-31 (Figure 1B).
Figure 1.
A) Immunofluorescence sections of aortic root double-stained with anti-AR IgG (green) and anti-CD31 IgG (red) from 14 week-old non-diabetic and diabetic apoE−/− male mice; merged image is also shown. B) Immunofluorescence sections of aorta from atherosclerotic human carotid artery double-stained with anti-AR IgG (green) and anti-CD-31 (red); merged image is also shown.
Effects of generalized hAR expression on atherosclerosis
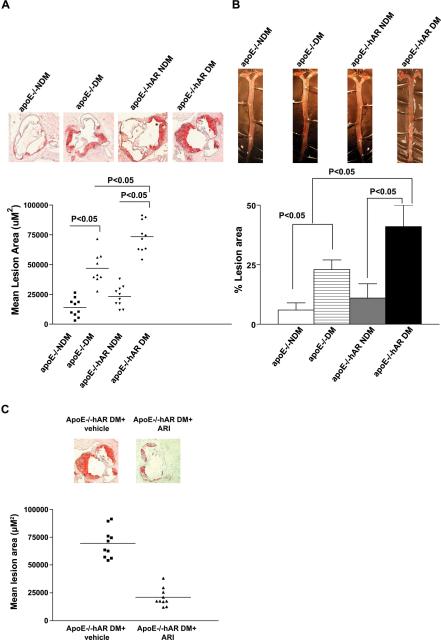
Plasma glucose, triglyceride (TG) and total cholesterol levels were higher in diabetic apoE−/− and apoE−/−hAR+ mice compared to non-diabetic controls (Table 1). Several inflammatory markers increased with diabetes (IL1, IL2, IL6, IL10, IL12, and MCP1), but hAR expression did not alter these markers. At 14 weeks duration of diabetes, mean atherosclerotic lesion area at the aortic sinus was 52,486 ± 3604 μm2 in apoE−/− mice and 79,758.67 ± 6354.37 μm2 in apoE−/−hAR+ mice (P < 0.0001) (Figure 2A). Diabetes, as expected, led to greater lesion areas in ± hAR, a result that correspond with greater plasma cholesterol. Sudan IV stain for aortic en face area also showed greater lesion area in diabetic apoE−/−hAR+ mice compared to diabetic apoE−/− mice (Figure 2B, P<0.05).
Figure 2.
Impact of diabetes and AR expression on atherosclerosis at 14 weeks after diabetes. Shown are representative images of aortic root sections stained with oil red O (A) and sudan IV stained aortic enface (B). Hearts were retrieved from non-diabetic and diabetic apoE−/− (n = 10 and 9, respectively), non-diabetic and diabetic apoE−/−hAR+ (n = 10 per group) mice, diabetic apoE−/−hAR+ mice treated with and without ARI (n=10 and 10 respectively) (C) and mean atherosclerotic lesion area were determined.
Effects of AR inhibition on atherosclerosis
If AR activity was a factor in atherosclerosis development, its inhibition should reduce lesions. When apoE−/−hAR+ were treated with a competitive inhibitor, atherosclerosis decreased >70% in apoE−/−hAR+ diabetic mice, from 63,480 ± 7580 μm2 to 19,011 ± 4824 (Figure 2C).
hAR expression created more inflammatory-appearing lesions
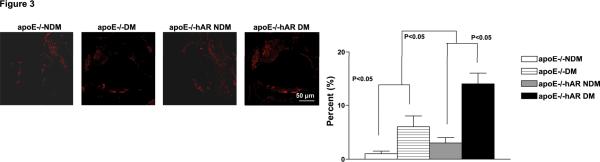
Infiltration of macrophages as detected by CD-68 (Mac-1) staining of the aortic roots was greater in diabetic apoE−/−hAR+ mice than non-diabetic controls and diabetic apoE−/− mice (Figure 3A). Ananlysis of gross composition of the lipid core did not reveal any significant changes of other cell types.
Figure 3.
Immunofluorescence stained on the sections of aorta root (A) from 14 week-old diabetic and non-diabetic apoE−/− and apoE−/−hAR+ male mice with anti-CD-68 IgG. Percent lesion area of macrophage and smooth muscle actin (SMA). Percent infiltration was calculated by measuring the area of macrophages and smooth muscle cells in the lesion to that of total lesion area and represented in terms of percentage.
AR affects endothelial dysfunction and vascular inflammation
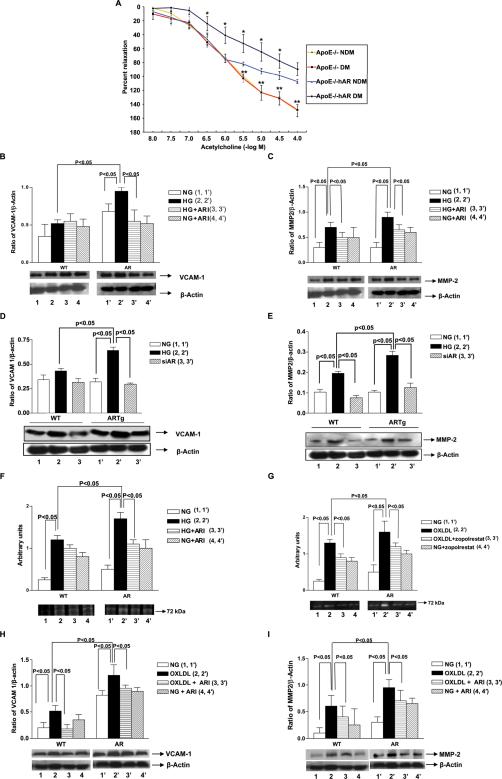
Upon exposure of aortic rings to increasing doses of acetylcholine, there was a significant decrease in the endothelium-dependent relaxation in diabetic apoE−/−hAR+ compared with diabetic apoE−/− mice (Figure 4A). However, there were no significant changes in the diabetic apoE−/− mice compared to its non-diabetic control. Sodium nitroprusside dependent vasorelaxation remained unchanged in all the groups (Data not shown). Although alterations in plasma lipids especially TG are associated with changes in endothelial function, lipid profiles were similar in apoE−/− and apoE−/−hAR+ mice (Table 1a).
Figure 4.
A) Endothelium-dependent vasorelaxation tested in isolated mouse aortic rings from non-diabetic and diabetic apoE−/− (n=4 and 5, respectively), apoE−/−hAR+ (n = 4 and 6, respectively) sacrificed at 14 weeks of age. Relaxation is reported as percent of initial phenylephrine precontraction. Comparisons were conducted among groups for each agonist dose. *P < 0.05, diabetic apoE−/−hAR+ versus diabetic apoE−/− (doses > 10–6 M); **P < 0.05, non-diabetic apoE−/−hAR versus diabetic apoE−/− (doses >10–5.5 M). Murine aortic ECs from the indicated mice were subjected to Western blotting for detection of VCAM-1, MMP-2 antigen and MMP-2 activity. ECs were exposed to high glucose +/− AR inhibitor zopolrestat or siRNA against hAR for 24 hours. Similarly cells were incubated with and without zopolrestat along with oxLDL for 4 hrs. Western blotting was performed to detect VCAM-1 (B, D, H) and MMP-2 (C, E, I) followed by anti–actin IgG. (F, G) Zymography for detection of MMP-2 activity was performed.
Table 1a.
Biochemical parameters between the study groups in apoE−/−hAR and the respective apoE−/− littermates
| Control | STZ | |||
|---|---|---|---|---|
| apoE−/− | apoE−/−hAR−/− | apoE−/− | apoE−/−hAR | |
| N | 8 | 9 | 9 | 10 |
| Fasting glucose (mg/dl) | 144 ± 9 | 148 ± 7 | 321 ± 25* | 319 ± 15# |
| TC (mg/dl) | 472 ± 19 | 462 ± 26 | 981 ± 108* | 1197 ± 56# |
| TG (mg/dl) | 148 ± 16 | 154 ± 32 | 244 ± 20* | 272 ± 6# |
P<0.05 compared to control apoE−/−
P<0.05 compared to control apoE−/−hAR
ECs isolated from hAR transgenic mice had greater VCAM-1 and MMP-2 expression than WT cells treated with HG (Figure 4B, C). Treatment with the ARI zopolrestat decreased VCAM-1 and MMP-2 expression in HG-treated endothelial cells. Similar results were seen in MMP-2 activity (Figure 4D). Alternatively silencing of AR using a siRNA against hAR decreased the VCAM (Figure 4D) and MMP2 (Figure 4E) expression complementing the ARI data. Upon treatment with oxLDL, ECs from AR mice showed increased expression of VCAM-1 and MMP-2 compared to WT control and untreated cells (Figure 4F, G). ARI treatment significantly decreased VCAM-1 and MMP-2 expression in the cells treated with oxLDL (Figure 4H, I). MMP-2 activity was also significantly increased in cells treated with oxLDL compared to untreated cells and the ARI significantly reduced MMP-2 activity in oxLDL treated cells (Figure 4G).
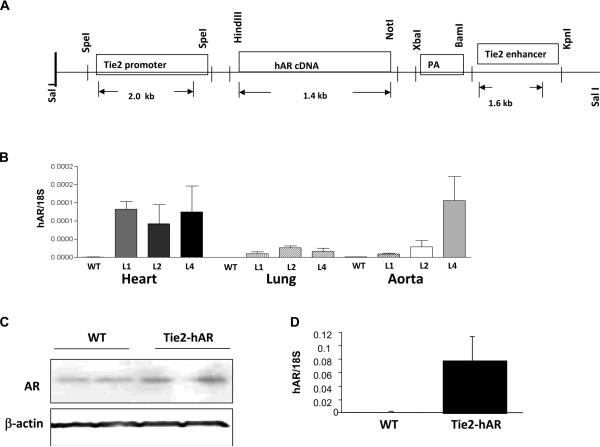
Creation of Tie2-hAR mice
Markedly different expression patterns in heart, lung, and aorta were found in three lines of Tie2- hAR transgenic mice (Figure 5B). The line with the greatest aortic expression was studied further. Protein expression of hAR in aorta tissues is shown in Figure 5C. CD31 antibody positive cells from heart isolated by FACS sorting showed robust hAR gene expression (Figure 5D). No expression was found in peritoneal and bone marrow derived macrophages.
Figure 5.
A) The endothelial specific transgenic construct in which the hAR transgene is located between promoter and enhancer sequences in Tie2 gene. B) Gene expression of hAR was determined by quantitative real-time PCR in different tissues from 3 different lines, 1, 2, 4 (n=3–4 mice/each groups). C) hAR protein expression in aorta from line 4 mice and control mice. D) hAR gene expression in ECs from heart, from line 4 and control mice (n=3/each groups). ECs from hearts were isolated by immunoreactivity to CD31 using FACS sorting.
Atherosclerosis in Tie2-hAR/apoE−/−
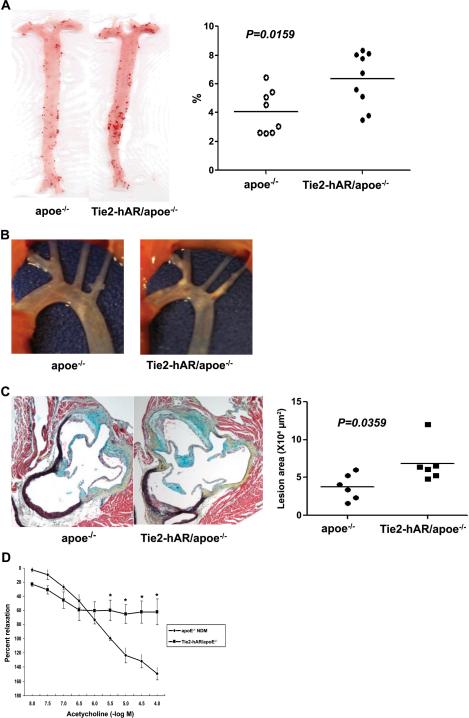
Plasma cholesterol concentrations in diabetic mice were significantly higher than non-diabetic mice but were similar between the apoE−/− and Tie2-hAR/apoE−/− mice (Table 1b). Eight weeks after STZ diabetes Tie2-hAR/apoE−/− animals had significantly larger lesions than diabetic apoE−/− mice; en face area increased from 4.0±0.5% to 6.3±0.6%, p<0.05. (Figure 6A). Direct visualization of the aortic arch is shown in Figure 6B. Lesion area in the proximal aorta increased from 37,740±6,880 to 68,580±10,710 μm2, p<0.05 (Figure 6C). Thus, EC AR expression increased lesions in the apoE−/− background.
Table 1b.
Biochemical parameters between the study groups in Tie2-hAR/apoE−/− and the respective apoE−/− littermates
| Control | STZ | |||
|---|---|---|---|---|
| apoE−/− | Tie2-hAR/apoE−/− | apoE−/− | Tie2-hAR/apoE−/− | |
| N | 9 | 6 | 8 | 9 |
| Fasting glucose (mg/dl) | 113 ± 24 | 109 ± 20 | 419 ± 24 | 439 ± 24 |
| TC (mg/dl) | 309 ± 78 | 391 ± 112 | 1122 ± 183 | 1314 ± 161* |
| TG (mg/dl) | 94.1 ± 22 | 74.5 ± 14 | 114.6 ± 22.4 | 145.6 ±15.6 |
p<0.05 vs control apoE−/−.
Figure 6.
Whole aorta and aortic root lesions of STZ-induced diabetic apoE−/− and Tie2-hAR/ apoE−/− male mice in early atheroscleorosis. Mice were injected with STZ (50mg/kg mouse body weight) for 5 days to induce diabetes and remained on chow diets for 12 weeks until sacrificed for atherosclerosis analysis. A) Oil red O enface staining in whole aorta and the percentage of oil-red O positive area (n=8–9/each groups) p<0.01. B) Images of aortic arch of STZ-induced diabetic apoE−/− and Tie2-hAR/ apoE−/− male mice. C) The lesion areas of aortic roots with MOVAT staining. Individual lesion positive areas in aortic root cross sections from mice were measured. D) Endothelium-dependent vasorelaxation tested in isolated mouse aortic rings from non-diabetic apoE−/−and Tie2-hAR/apoE−/− (n=4 and 3 respectively), sacrificed at 20 weeks of age. Relaxation is reported as percent of initial phenylephrine precontraction. Comparisons were conducted among groups for each agonist dose. *P < 0.05, apoE−/− versus Tie2-hAR/ apoE−/− (doses >10–5.5 M).
hAR expression leads to altered vasodilatation
Tie2-hAR/apoE−/− mice had decreased acetylcholine dependent vaso-relaxation of the thoracic aorta compared to the apoE−/−mice (Figure 6D). Thus, one effect of AR actions is to create endothelial dysfunction.
DISCUSSION
There are a number of pathways leading to aberrant metabolism of glucose that are thought to be pathological. One of these pathways is through the polyol pathway, leading to sorbitol and fructose production. Oxidation of glucose to sorbitol by AR causes oxidative stress because its co-factor NADPH is converted to NADP; this leads to reduced glutathione and increased ROS.18, 19 We had shown in a previous study that STZ induced diabetes accelerated atherosclerosis in STZ-treated hAR-expressing mice. Greater atherosclerosis was associated with diminished cellular antioxidant capacity in aorta.3 In the current study, we show that hAR expression in apoE−/− mice is atherogenic and that expression specifically in ECs also leads to more disease. Moreover, we show that use of a competitive inhibitor of AR that reduces, but does not eliminate AR activity, reduces atherosclerosis.
The mechanisms by which AR mediates/accelerates diabetic complications have been studied. AR has been postulated to produce excess ROS.20 Macrophages from the hAR transgenic mice express higher levels of scavenger receptors and reduced levels of genes that regulate regeneration of glutathione compared to the wild-type cells.3 OxLDL induced upregulation of AR in human macrophages might be pro-inflammatory in foam cells.2 This was suggested as one of the ways by which AR might amplify effects of hyperlipidemia and diabetes leading to atherosclerosis.2
Endothelial dysfunction and upregulation of VCAM-1 are crucial events in atherosclerosis and occur at early stages after lipid infiltration in the vessel. The sites of expression of VCAM-1 are prominent areas of plaque development.21 In this study we show colocalization of AR with CD-31 by immunohistochemistry in both diabetic apoE−/− mice and also in human atherosclerotic carotid artery. Greater expression of AR led to endothelial dysfunction and increased expression of VCAM-1 and MMP-2. STZ-induced diabetic hAR overexpressing mice aorta had decreased acetylcholine mediated endothelial vasorelaxation compared to the WT aorta. EC expression of hAR had the same effect. Interestingly, although there was a significant rise in AR activity in non-diabetic apoE−/− and 14 week diabetic apoE−/− aorta, the activity did not reach the threshold to elicit endothelial dysfunction.
Endothelial function was improved and VCAM-1 and MMP-2 expression reduced by both pharmacological inhibition and targeted silencing of AR in ECs exposed to high glucose and oxLDL. These findings are similar to the improvement in endothelial function observed by blockade of RAGE in atherosclerotic apoE−/− mice.22 Studies in smooth muscle cells incubated with AGE-bovine serum albumin (AGE-BSA) resulted in greater increments of ICAM-1 and monocyte chemoattractant protein-1 (MCP-1), migration and monocyte adhesion in AR transgenic versus wild-type cells.23 These AGE-BSA-mediated increases were suppressed either by ARI (zopolrestat) or AR antisense oligonucleotides.24 Therefore, AR may aggravate AGE linked RAGE activation and this, in turn, could accelerate atherosclerosis.
Pharmacological studies have suggested that AR may inhibit endothelial-dependent relaxation changes in diabetic vessels. It was shown that zopolrestat improves abnormal acetylcholine- and adenosine diphosphate-induced relaxation of aortas from diabetic rabbits.25 Defective endothelium-dependent relaxation to acetylcholine in diabetic rat aortas was corrected by ARI treatment.26 Impaired endothelium-dependent relaxation in galactosemic rats was also prevented by the ARI ponalrestat.26 These findings suggest that increased activity of the AR pathway in hyperglycemia is responsible at least in part for the abnormal endothelium-dependent relaxation in diabetic blood vessels. Consistent with these studies, our data highlight the importance of EC AR actions in vascular response.
In some studies of diabetic mice others have shown changes in lesion morphology. Johansson et al. showed lesional hemorrhage in brachial-cephalic arteries from hyperlipidemic type 1 diabetic mice.27 They attributed the hemorrhage to the hyperlipidemia that accompanies diabetes. In Tie2hAR/apoE−/− background with STZ induced diabetes we also found hemorrhage within lesions, but at the aortic root (Supplement Figure 1), documented by appearance and immunohistochemical staining for TER-199, a red blood cell antigen. Like in the studies by others, hemorrhage correlated with lesion size and was found in the setting of diabetes.
Although the current studies in apoE−/−hAR+ and prior studies in Ldlr−/− mice overexpressing hAR3 show that hAR increases atherosclerosis, contrary findings linking AR with reduced atherosclerosis have been recently reported.5 Srivastava and colleagues showed increased lesions in AR knockout mice in the apoE−/− background. In contrast and consistent with a proatherogenic action of AR in atherosclerosis, we observed increased AR in human atherosclerotic tissue. While we observed reduced lesion size and decreased inflammatory marker levels in ARI zopolrestat treated diabetic apoE−/−hAR+ mice, earlier studies using the ARI lidorestat in human AR expressing Ldlr−/− mice did not find reduced lesion size. But critically, the ARI lidorestat did not increase lesion size.28 The absence of atheroprotection in AR knockout mice in the apoE−/− background warrants investigation of potential compensation from other genes that may have adversely affected the phenotype. Or this suggests that a minimal amount of AR activity is necessary for optimal arterial health, while greater amounts can be toxic.
Studies in cultured cells and in injured arteries are consistent with a role for the AR pathway in vascular stress. Treatment of cultured human umbilical vein ECs with ARIs sorbinil and tolrestat reduced TNF-alpha-stimulated activation of NF-kB, phosphorylation and degradation of IkB-α and the nuclear translocation of NF-kB, in parallel with reduced up-regulation of ICAM-1 and VCAM-1.29 In other studies, ARIs or antisense oligonucleotides suppressed TNF-α- mediated activation of NF-kB.25, 29 AR inhibition also suppressed neointimal production after balloon injury.24, 29 Gleissner et al.2 reported expression of AR in CD68+ cells (monocytes/macrophages) in human atherosclerotic plaque macrophages.2 This group also found that monocyte-derived macrophages isolated from human blood incubation with oxLDL had increased AR gene expression and activity along with increased ROS. Inhibition of AR in these oxLDL-stimulated cells attenuated ROS generation. Thus, both EC and macrophage AR might be important in vascular pathobiology.
Like all animal studies that attempt to reproduce human disease, interpretation of our data has several limitations. We argue that diabetic studies in hAR-expressing mice recapitulate human biology more closely than studies in non-hAR mice. The rationale for this is that AR activity is very low in mice and introduction of the hAR transgene increases AR expression in macrophages3 and hearts1 to levels that are comparable to humans. However, a limitation of our models is that it is unlikely that we have produced human-like levels of AR expression in all cells; some might have higher expression and some lower. A second limitation of our study is the form of diabetes. STZ is an islet cell toxin that leads to insulin deficiency and is therefore a model of type 1, but not type 2 diabetes. Use of the apoE−/− background also has limitations. The lipid levels are higher than most humans, apoE clearly has multiple in vivo functions, and these mice might have other biologic alterations. Nonetheless, this animal is one of the standard models for atherosclerosis research. A potential limitation on the use of ARIs is the specificity of these inhibitors for AR vs aldehyde reductases. Published studies have demonstrated that the carboxylic class of AR inhibitors, such as zopolrestat, is 200 fold more selective for AR over aldehyde reductase.30 Hence, at the concentrations used in this study, the inhibition of aldehyde reductase is unlikely to have played any part in the lesion size reduction in diabetic mice.
There are a number of postulated reasons for increased vascular disease in diabetic patients. Aside from effects of reduced insulin actions and greater fatty acid levels, toxic effects of glucose are implicated. For this reason, insulin treatment and better glucose control appears to be beneficial in type 1 diabetes.31 In type 2 diabetes, the ACCORD trial showed a marked reduction in non-fatal myocardial infarction rate in a better glucose-controlled group of type 2 diabetic patients despite the tendency to greater mortality.32 There are several pathways leading from glucose to altered cellular/tissue function. One of these is via AR. The genetic insufficiency of AR in the mouse might be one reason why mice often do not show greater atherosclerosis with diabetes.33 Humanizing the mouse by transgenic expression of this enzyme may illustrate one route to understanding pathological processes in diabetic humans.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Latoya Woods for helping in preparation of the manuscript. The studies were supported, in part, by grants from NIH (PO1HL60901, RO1 HL61783, and UO1 HL87945), and Juvenile Diabetes Research Foundation. These studies were supported by grants P01-HL54591 and U01-HL087945 and a mentored postdoctoral research award from the American Diabetes Association.
Footnotes
Authors do not have any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Ramasamy R, Goldberg IJ. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ Res. 2010;106:1449–1458. doi: 10.1161/CIRCRESAHA.109.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gleissner CA, Sanders JM, Nadler J, Ley K. Upregulation of aldose reductase during foam cell formation as possible link among diabetes, hyperlipidemia, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1137–1143. doi: 10.1161/ATVBAHA.107.158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho HT, Chung SK, Law JW, Ko BC, Tam SC, Brooks HL, Knepper MA, Chung SS. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Mol Cell Biol. 2000;20:5840–5846. doi: 10.1128/mcb.20.16.5840-5846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava S, Vladykovskaya E, Barski OA, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ Res. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava S, Spite M, Trent JO, West MB, Ahmed Y, Bhatnagar A. Aldose reductase-catalyzed reduction of aldehyde phospholipids. J Biol Chem. 2004;279:53395–53406. doi: 10.1074/jbc.M403416200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramasamy R, Oates PJ, Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes. 1997;46:292–300. doi: 10.2337/diab.46.2.292. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura C, Matsuura Y, Kokai Y, Akera T, Carper D, Morjana N, Lyons C, Flynn TG. Cloning and expression of human aldose reductase. J Biol Chem. 1990;265:9788–9792. [PubMed] [Google Scholar]
- 9.Yamaoka T, Nishimura C, Yamashita K, Itakura M, Yamada T, Fujimoto J, Kokai Y. Acute onset of diabetic pathological changes in transgenic mice with human aldose reductase cDNA. Diabetologia. 1995;38:255–261. doi: 10.1007/BF00400627. [DOI] [PubMed] [Google Scholar]
- 10.Hwang YC, Sato S, Tsai JY, Yan S, Bakr S, Zhang H, Oates PJ, Ramasamy R. Aldose reductase activation is a key component of myocardial response to ischemia. Faseb J. 2002;16:243–245. doi: 10.1096/fj.01-0368fje. [DOI] [PubMed] [Google Scholar]
- 11.Ohashi M, Runge MS, Faraci FM, Heistad DD. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2331–2336. doi: 10.1161/01.ATV.0000238347.77590.c9. [DOI] [PubMed] [Google Scholar]
- 12.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 13.Hein TW, Liao JC, Kuo L. oxLDL specifically impairs endothelium-dependent, NO-mediated dilation of coronary arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H175–183. doi: 10.1152/ajpheart.2000.278.1.H175. [DOI] [PubMed] [Google Scholar]
- 14.Heinecke JW, Kawamura M, Suzuki L, Chait A. Oxidation of low density lipoprotein by thiols: superoxide-dependent and -independent mechanisms. J Lipid Res. 1993;34:2051–2061. [PubMed] [Google Scholar]
- 15.Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, Yan SF. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. Faseb J. 2009;23:1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005;12:138–142. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Hwang YC, Ananthakrishnan R, Oates PJ, Guberski D, Ramasamy R. Polyol pathway and modulation of ischemia-reperfusion injury in Type 2 diabetic BBZ rat hearts. Cardiovasc Diabetol. 2008;7:33. doi: 10.1186/1475-2840-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, Heinecke JW, Bornfeldt KE. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morre DM, Lenaz G, Morre DJ. Surface oxidase and oxidative stress propagation in aging. J Exp Biol. 2000;203:1513–1521. doi: 10.1242/jeb.203.10.1513. [DOI] [PubMed] [Google Scholar]
- 20.Cheng HM, Gonzalez RG. The effect of high glucose and oxidative stress on lens metabolism, aldose reductase, and senile cataractogenesis. Metabolism. 1986;35:10–14. doi: 10.1016/0026-0495(86)90180-0. [DOI] [PubMed] [Google Scholar]
- 21.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 22.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–851. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 23.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dan Q, Wong R, Chung SK, Chung SS, Lam KS. Interaction between the polyol pathway and non-enzymatic glycation on aortic smooth muscle cell migration and monocyte adhesion. Life Sci. 2004;76:445–459. doi: 10.1016/j.lfs.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Tesfamariam B, Palacino JJ, Weisbrod RM, Cohen RA. Aldose reductase inhibition restores endothelial cell function in diabetic rabbit aorta. J Cardiovasc Pharmacol. 1993;21:205–211. doi: 10.1097/00005344-199302000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Cameron NE, Cotter MA. Impaired contraction and relaxation in aorta from streptozotocin-diabetic rats: role of polyol pathway. Diabetologia. 1992;35:1011–1019. doi: 10.1007/BF02221675. [DOI] [PubMed] [Google Scholar]
- 27.Cameron NE, Cotter MA. Contraction and relaxation of aortas from galactosaemic rats and the effects of aldose reductase inhibition. Eur J Pharmacol. 1993;243:47–53. doi: 10.1016/0014-2999(93)90166-f. [DOI] [PubMed] [Google Scholar]
- 28.Noh HL, Hu Y, Park TS, DiCioccio T, Nichols AJ, Okajima K, Homma S, Goldberg IJ. Regulation of plasma fructose and mortality in mice by the aldose reductase inhibitor lidorestat. J Pharmacol Exp Ther. 2009;328:496–503. doi: 10.1124/jpet.108.136283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramana KV, Bhatnagar A, Srivastava SK. Inhibition of aldose reductase attenuates TNF-alpha-induced expression of adhesion molecules in endothelial cells. Faseb J. 2004;18:1209–1218. doi: 10.1096/fj.04-1650com. [DOI] [PubMed] [Google Scholar]
- 30.Mylari BL, Beyer TA, Siegel TW. A highly specific aldose reductase inhibitor, ethyl 1-benzyl-3-hydroxy-2(5H)-oxopyrrole-4-carboxylate, and its congeners. J Med Chem. 1991;34(3):1011–8. doi: 10.1021/jm00107a020. [DOI] [PubMed] [Google Scholar]
- 31.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 33.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, Rabadan-Diehl C, Goldberg IJ. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007;100:1415–1427. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.