Abstract
Background
Exposure to uncontrollable stressors often increases anxiety-like behavior in both humans and rodents. In rat, this effect depends upon stress-induced activity within the dorsal raphé nucleus (DRN). However, the role of serotonin in DRN projection regions is largely unknown. The goals of the current study were to 1) determine if DRN activity during a post-stress anxiety test is involved in anxiety-like behavior, 2) assess the effect of uncontrollable stress on extracellular serotonin in the basolateral amygdala during the anxiety test, and 3) determine the role of the serotonin 2C receptor (5-HT2C) in uncontrollable stress-induced anxiety.
Method
Rats were exposed to tailshocks that were uncontrollable. On the following day anxiety-like behavior was assessed in a JSE test. BLA extracellular serotonin concentrations were assessed during JSE by in vivo microdialysis 24 h after uncontrollable stress, controllable stress or no stress. In separate experiments drugs were administered before the JSE test to inhibit the DRN or to block 5-HT2C receptors.
Results
Exposure to uncontrollable shock reduced later social exploration. Prior uncontrollable stress potentiated serotonin efflux in the BLA during social exploration, but controllable stress did not. Intra-DRN 8-OH-DPAT and systemic and intra-BLA 5-HT2C receptor antagonist SB 242084 prevented the expression of potentiated anxiety in uncontrollably stressed rats. Intra-BLA injection of the 5-HT2C agonist CP 809101 mimicked the effect of stress.
Conclusion
These results suggest that the anxiety-like behavior observed after uncontrollable stress is mediated by exaggerated 5-HT acting at BLA 5-HT2C receptors.
Keywords: rat, learned helplessness, ptsd, serotonin, social exploration, 5-HT2c
INTRODUCTION
The pathobiologies of stress-induced anxiety disorders, such as Acute Stress Disorder (ASD) and Post Traumatic Stress Disorder (PTSD), are poorly understood, yet PTSD has an estimated lifetime prevalence of 7.8% (1) and treatment of PTSD is inadequate (2). ASD and PTSD symptoms include avoidant, anxiety-like behaviors that present just after stressor exposure or develop with time, respectively (3). Stress victims that display ASD symptoms are more likely to develop chronic PTSD (4) and early intervention can reduce the occurrence of chronic PTSD (5). Although much has been reported about the therapeutic mechanisms of PTSD pharmacotherapy, relatively little is known about the biological basis of anxiety expression after trauma.
The sequelae of a stressor depend on both environmental and genetic factors. In terms of environmental factors, controllable stressors tend to have less measurable impact than those that are not (6), and a lack of behavioral control over stress may be critical to the development of PTSD (7, 8). In one well-characterized paradigm, exposure to inescapable tailshock (IS) induces a number of behavioral consequences that do not follow exposure to exactly equal escapable tailshocks (ES). These outcomes include shuttle escape failure, enhanced fear conditioning, and reduced social exploration, among others collectively described as “learned helplessness effects” (for a review see (6)).
Exaggerated anxiety-like behavior is one of the most striking consequences of exposure to IS, relative to ES, as well as to acute trauma in humans. Rats exposed to IS later show greater fear conditioning (9), post-shock freezing (10), neophobia (11), and reductions in social interaction (12, 13) than do rats exposed to equal ES. The development of anxiety-like behaviors after IS depends upon an intense activation of serotonergic neurons in the dorsal raphé nucleus (DRN; for a review see (6)) during stress that is thought to “sensitize” DRN-serotonin (5-HT) neurons to respond to later stressors in exaggerated fashion (14–16). Acute activation of 5-HT neuronal systems is thought to be a critical component of anxiety expression because acute administration of selective serotonin reuptake inhibitors (SSRIs) evokes anxiety-like behaviors in both humans and rodents (17–20) and mimics the effects of IS (21). We have reported that IS, but not ES, reduces exploration of both adult (13) and juvenile (12) conspecifics, a behavior that is commonly thought to “assay” anxiety-like behavior (22). Furthermore, the effect of IS was dependent upon DRN activation at the time of stress (12).
Only a subset of neurons in the caudal DRN respond differentially to IS and ES (23), and precisely this subset projects to the basolateral amygdala (BLA; (24). The BLA is a key structure in the mediation of anxiety. Environmental information about threatening stimuli is relayed to the BLA, a structure with glutamatergic projections to a wide range of limbic structures involved in the mediation of overt fear and anxiety behaviors (25, 26). Importantly, BLA output is modulated by 5-HT (25). 5-HT2C receptor agonists produce anxiogenic effects (18) and lead to activation in BLA projection regions (27), while 5-HT2C receptor antagonists are anxiolytic (28). Recently, Strong et al. (29) demonstrated that a systemic 5-HT2C antagonist blocked, and 5-HT2C agonists mimicked, the effects of IS on later freezing and shuttle escape behavior.
If IS leads to an anxiety-like state that is dependent upon DRN sensitization, then it is possible that 5-HT released in the BLA, which is increased in response to anxiogenic stimuli after IS (30), plays a critical role in the generation of IS-potentiated anxiety-like behavior. The idea here is that after IS, when the rat is presented with a stimulus such as a juvenile conspecific, 5-HT levels in the BLA rise and activate 5-HT2C receptors, which may in turn enhance BLA output and increase anxiety-like behaviors. Utilizing the juvenile social exploration (JSE) test the current set of experiments aimed to determine whether: a) prior IS exaggerates the release of 5-HT in the BLA produced by a juvenile social interaction, b) DRN 5-HT activation is required to produce IS augmented anxiety, c) BLA 5-HT2C receptors are critical to the anxiety-like effect of IS, and d) 5-HT2C agonism is sufficient to mimic IS effects on JSE (See supplemental materials).
MATERIALS and METHODS
Rats
Adult (60–70 days old and weighing 275–350 gm at the time of testing) and juvenile (28–32 days old and weighing 90–100 gm at the time of testing) male Sprague-Dawley (Harlan, Indianapolis, IN) rats were used in all experiments. Rats were housed with free access to food and water in groups of 2 for microinjection experiments, in groups of 4 when drugs were administered i.p., and in single cages for the microdiaylsis experiment. The vivarium maintained a 12 h light/dark cycle; all experimental procedures were conducted within the first 6 hours of the light phase. All procedures were conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the University of Colorado Institutional Animal Care and Use Committee.
Stress Induction Procedures
In experiments involving escapable stress (ES), 100 electric tailshocks were administered as previously described (14) (See Supplemental Materials). In brief, rats were restrained and shock was delivered through the tail on a variable interval 60 s schedule. Turning a wheel inside the restraining box terminated the shock. Shock terminated automatically if no response was made within 30 s. Rats in the yoked-inescapable shock group (IS) received exactly equal shock but were not permitted control—turning the wheel had no consequence. Animals in the homecage control (HC) remained in the vivarium. When only IS was involved, 100, 5-s shocks were delivered on a variable interval 60-s schedule.
Juvenile Social Exploration Tests
JSE testing was conducted as described previously (31). In a separate testing room, each experimental subject was allocated a single plastic tub cage with shaved wood bedding and a wire lid. To begin the test rats were placed into the test cage and after 45 min a 28 (± 2) day-old juvenile was introduced to the cage for 3 min and an observer, blind to treatment, timed exploratory behaviors (sniffing, pinning, and allogrooming) initiated by the adult (31). In order to observe JSE during in vivo microdialysis the observers were not always blind to treatment and scored two tests simultaneously. In all other experiments the observers were naïve to treatment conditions and scored only one rat at a time. Juveniles were used for multiple tests but were never used for the same adult rat. Testing order was counterbalanced for stress and drug treatments.
Surgical Procedures
All surgery was performed under inhaled isoflurane anesthesia (3% in O2). Microdialysis and microinjection guide cannulae were implanted and fixed in place with stainless steel screws and acrylic cement as previously described (14). Exact coordinates and cannulae specifications are listed in Table 1. A stylet was placed in the cannulae and each rat was inoculated with 0.25ml/kg (s.c.) penicillin (Combi-Pen, Agrilabs). At the end of the experiment, brains were collected, sliced at 40 µm, and stained with cresyl violet for cannulae verification. Subjects were only included if tissue damage from the cannulae tips fell within the target nucleus.
Table 1.
Type and location coordinates of mirodialysis and microinjection cannulae.
| Target | Cannula Type | AP | LM | DV |
|---|---|---|---|---|
| BLA* | CMA 12 (Carnegie Medicine, Sweden) | −3.0 | +4.8 | −6.2 |
| DRN | 26g, 15.5mm length (Plastics One, Roanoke, VA) | −8.1 | 0 | −5.1 |
| BLA | 26g, 7.5mm length (Plastics One) | −3.0 | ±4.8 | −6.2 |
| CeA | 26g, 7.5mm length (Plastics One) | −2.0 | ±4.0 | −6.2 |
Coordinates are in mm and were measured from bregma and dura.
Unilateral microdialysis cannulae. AP = anterior-posterior, LM = lateral-medial, DV = dorsal-ventral, BLA = basolateral amygdala, DRN = dorsal raphé nucleus, CeA = central nucleus of the amygdala.
In Vivo Microdialysis and Quantification of 5-HT
Microdialysis probes (CMA 12, MW cut-off 20kD, 2mm) were inserted into the guide and Ringer’s solution (145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl) was perfused through the probes at a flow rate of 0.2 µl/min. After 12 hr, the flow rate increased to 1.5 µl/min. After a 90 min equilibration period, 8 samples were collected at 15 min intervals. Dialysates were immediately placed in an −80°C freezer until analysis. Samples were analyzed with high-pressure liquid chromatography (HPLC) using standard methods (14).
Drugs
The selective 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT, Sigma, St. Louis, MO) was dissolved in 0.9% saline. The selective, brain-penetrant 5-HT2C antagonist 6-Chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride (SB 242084, Tocris, Ellisvile, MO) was dissolved in 0.9% saline.
Experimental Procedures
Overview
Except where noted all experiments were conducted in this fashion: Rats were implanted with cannulae and allowed 7–10 days to recover. JSE baseline tests were given and 24 h later stress was administered. Since the focus of the current experiments was on the expression of stress-induced anxiety, pharmacological manipulations were made before a post-stress JSE test given 24h after stress.
In vivo microdialysis and quantification of BLA 5-HT
Rats were assigned to either escapable (ES) or yoked inescapable (IS) stress or HC treatment (n = 7/group). After stress, microdialysis began as described above. Rats were left alone in the dialysis room with food and water for at least 12 h on a light cycle that matched the vivarium. Sample collection began 24 h after stress. Beginning 8 min into the 5th sample a juvenile was added to the dialysis bowl for 5 min. The next sample was collected 2 min after the juvenile was removed. This procedure was used to isolate in time the acute change in 5-HT that would be elicited by the JSE test and to distinguish long lasting effects of juvenile exposure to the remaining samples. Here we sought to determine whether as a result of a sensitized DRN, rats in the IS group would exhibit greater extracellular 5-HT in the BLA as a result of the social encounter than ES or HC rats.
Inhibition of DRN by 8-OH-DPAT prior to Social Exploration
Rats were assigned to either inescapable shock (IS) or home cage (HC) and 8-OH-DPAT or saline treatments in a 2 × 2 factorial design (n = 10/group). On the next day, rats were exposed to either 100 trials of IS or left in the home cage. On the day after stress rats received microinjections of 2mg/ml 8-OH-DPAT or saline (injection volume: 0.5µl), immediately placed in the JSE test cage and 45 min later a juvenile was added and social investigation was quantified for 3 min. This dose was based on previous efficacy in our laboratory (12). Microinjections were made in a quiet room near the testing area. Rats were gently restrained and a microinjector was inserted that extended 1mm beyond the cannulae tip (33 g). Injections were made at a rate of 1µl/min; injectors remained in place for 1 min to permit diffusion. DRN serotonin neurons are regulated by the somatodendritic 5-HT1A autoreceptor (32) and the 5-HT1A agonist 8-OH-DPAT is a potent inhibitor of 5-HT neuronal activity (33). Therefore, it was predicted that the IS-8-OH-DPAT group would have social investigation behavior comparable to HC controls.
Pharmacological blockade of the 5-HT2C receptor
One set of rats was given a JSE baseline test and received either IS or HC 24 hr later. The next day they received either saline, 0.1 or 0.5mg/kg body weight SB 242084 (SB) i,p. in saline [a 2 (stress) by 3 (drug) factorial design, n = 10/group] followed by placement into the JSE test cage. After 45 min a juvenile was added. Systemic doses of SB were based on pilot studies and the literature (20, 34). A second set of rats, implanted with bilateral cannulae for microinjection in the BLA, was given a baseline JSE test and then 24 h later randomly assigned to either IS or HC. 24 hr after treatment they received either intra-BLA saline, 10µM, 10mM, or 50mM SB (0.5µl injection volume/side in saline), n = 8/group. Microinjection was followed by JSE testing as above. Intra-BLA concentrations of SB were chosen to capture a range of doses comparable to the only other report of microinjection of this drug (35) and a low dose. Additional groups were added as controls. First, to determine if 5-HT2C signaling was involved in the induction of stress-induced anxiety a group of rats received either saline or 50mM SB prior to IS treatment, but no injection before JSE testing. Second, a group was implanted with guide cannulae to the CeA as a site-specificity control and these rats were treated with IS and either saline or 50mM SB before JSE testing.
Statistical Analysis
Rats with pre-stress JSE lower than 60 s were excluded (31). Data were analyzed with analysis of variance (ANOVA) with stress and drug dose treated as between-subjects variables, while microdialysis samples were treated as within-subjects variables. Main effects and interactions were considered significant if p < 0.05 and were explored with Fisher’s Protected Least Significance Difference (PLSD) post hoc tests to control the experiment-wise error to α = 0.05.
RESULTS
Extracellular BLA 5-HT during and following social exploration
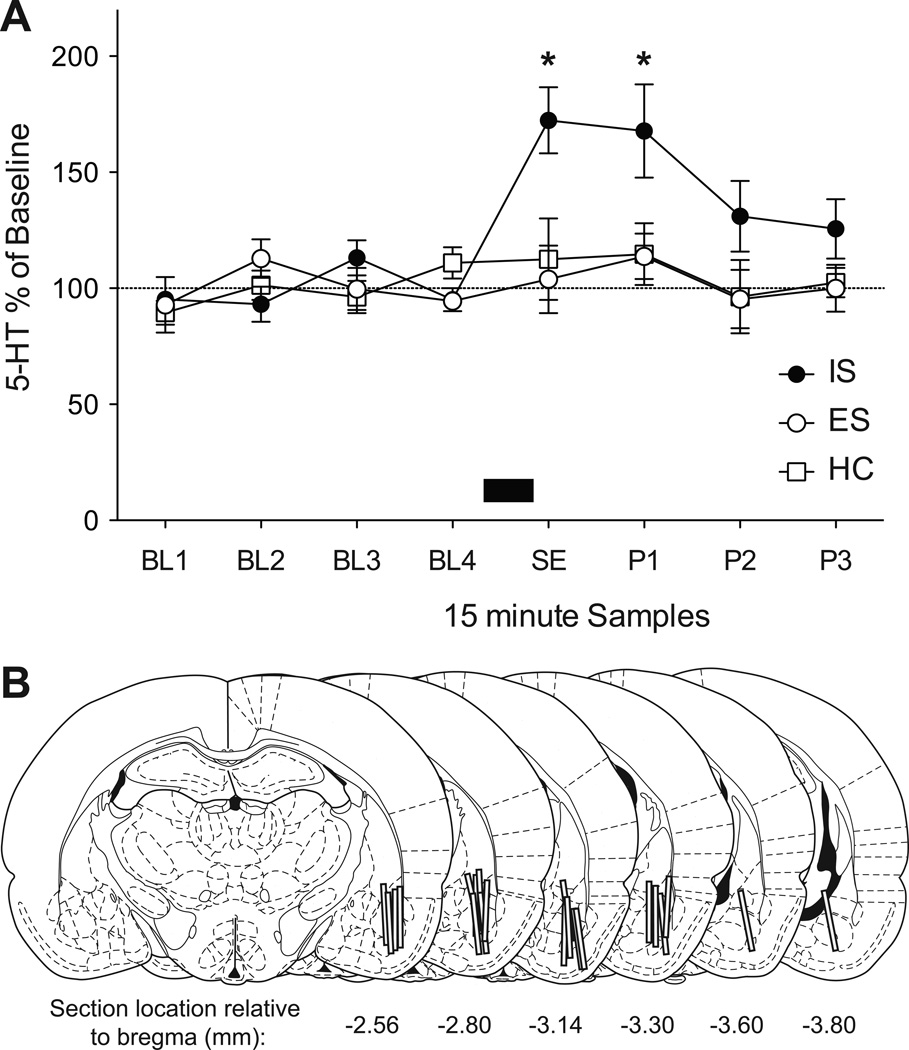
Importantly, all rats in the ES group learned to escape the tailshock, quickly reached the maximum response criterion and maintained escape behavior as previously reported (14). Only rats with dialysis probes at least 50% within the BLA were included (Figure 1); the resulting groups had ns = 6–7. Prior stress did not alter basal 5-HT, means(S.E.M.): ES = 0.272(0.03), IS = 0.267(0.05), HC = 0.274(0.04) pg/20 µl. Extracellular 5-HT was converted to percentage of baseline by dividing each sample by the average of the first 4 samples X 100 (Figure 1). Introducing the juvenile did not raise 5-HT efflux in previously untreated subjects. In contrast, prior IS caused the 5 min juvenile encounter to increase extracellular 5-HT within the BLA while prior ES did not influence the 5-HT response to the juvenile. A 3 (Stress: ES, IS or HC) by 8 (Samples) ANOVA revealed a significant effect of Stress: F(2, 17) = 9.982, p = 0.001, Sample: F(7, 119) = 4.744, p < 0.001, and the Stress by Sample interaction: F(14, 119) = 2.336, p = 0.007. Prior IS caused significantly greater extracellular 5-HT than did either ES or HC treatment during the social exploration sample (SE) and the first post-SE sample (P1; ps < 0.05). Within group comparisons between baseline, SE and post-SE samples revealed a significant elevation in 5-HT from baseline only in the IS group; the SE and all post-SE means were significantly greater than the last baseline sample (BL4; ps < 0.05). ES and HC groups did not differ from each other or their baselines at any time.
Figure 1.
(A) Mean(± S.E.M.) extracellular 5-HT in the BLA before, during and after a 5 m juvenile social exploration (SE). Rats received prior escapable tailshock (ES, n = 7), inescapable tailshock (IS, n = 7), or home cage control (HC, n = 6) treatment. 5-HT is expressed as a percentage of the average of 4 baseline samples (B1–B4). A juvenile conspecific was added to the dialysis bowl 8 m after B4 was collected, remained in the cage for 5 m and was removed 2 m before the SE sample was collected (depicted as a solid black box). Prior IS significantly increased BLA 5-HT during (SE) and after (P1) compared to ES and HC groups, *ps < 0.05. (B) Graphic reconstruction of 2mm dialysis probe placements within the BLA. Illustrations were adapted from the atlas of Paxinos and Watson (51) and are listed in relationship to bregma in mm.
Social exploration was quantified during the 5 min juvenile exposure. The means(SEM): ES = 99.42(5.62), IS = 59.18(8.23), and HC = 87.72(9.51) were comparable to our prior work (12, 36). A one-way ANOVA identified a significant main effect of Stress, F (2, 17) = 7.361, p = 0.005. IS significantly reduced JSE compared to ES and HC treated rats, ps < 0.05, which did not differ from each other.
Inhibition of DRN by 8-OH-DPAT prior to JSE testing
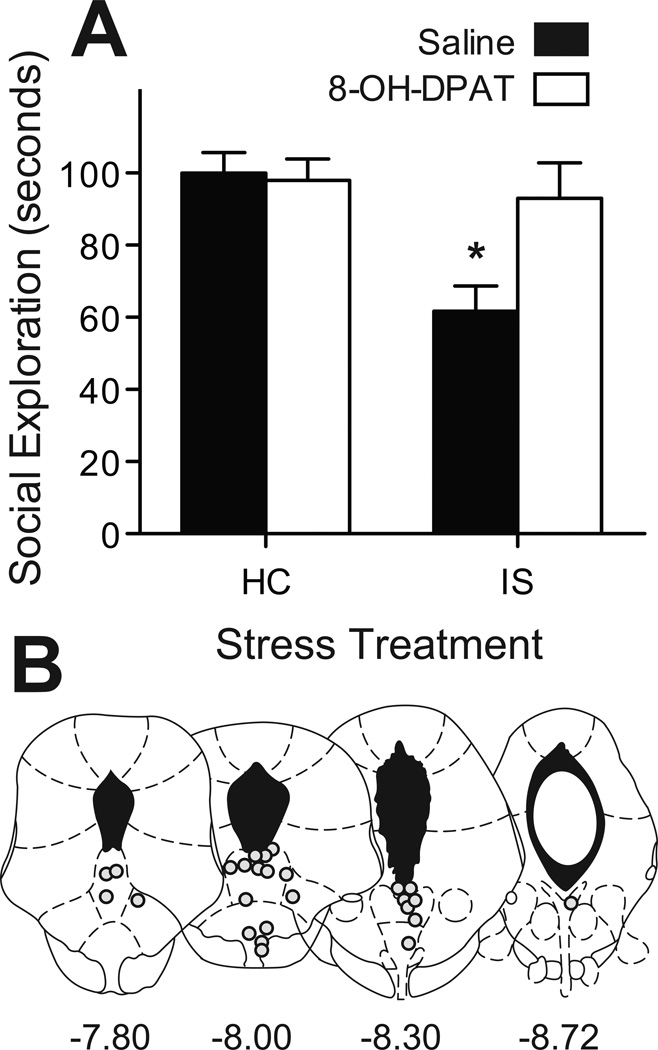
The mean time spent in JSE is depicted in Figure 2. Clearly, intra-DRN 8-OH-DPAT blocked the reduction in exploration produced by prior IS, but did not by itself alter social exploration. Only rats with cannulae placements within the DRN were included in the analysis; the resulting groups had ns = 7–9. A 2 (Stress: IS vs. HC) by 2 (Drug: saline or 8-OH-DPAT) ANOVA revealed a significant main effect of Stress: F (1, 28) = 8.558, p < 0.01 and a significant Stress by Drug interaction: F (1, 28) = 5.073, p < 0.05. Post hoc comparisons revealed a reduction in exploration in the IS-saline group, which was significantly lower than all other groups (ps < 0.05). Importantly, the IS-8-OH-DPAT group did not differ from either HC group.
Figure 2.
(A) Mean(+ S.E.M.) time spent in exploration during a 3 m juvenile social encounter. Prior IS resulted in a significant reduction in exploration compared to a HC control treatment, *p < 0.05. The effect of IS was completely blocked by intra-DRN injection of the 5-HT1A agonist 8-OH-DPAT 45 m before the encounter. (B) Graphic reconstruction of microinjector tip placements within the DRN. Illustrations were adapted from the atlas of Paxinos and Watson (51) and are listed in relationship to bregma in mm.
Systemic 5-HT2C receptor antagonism
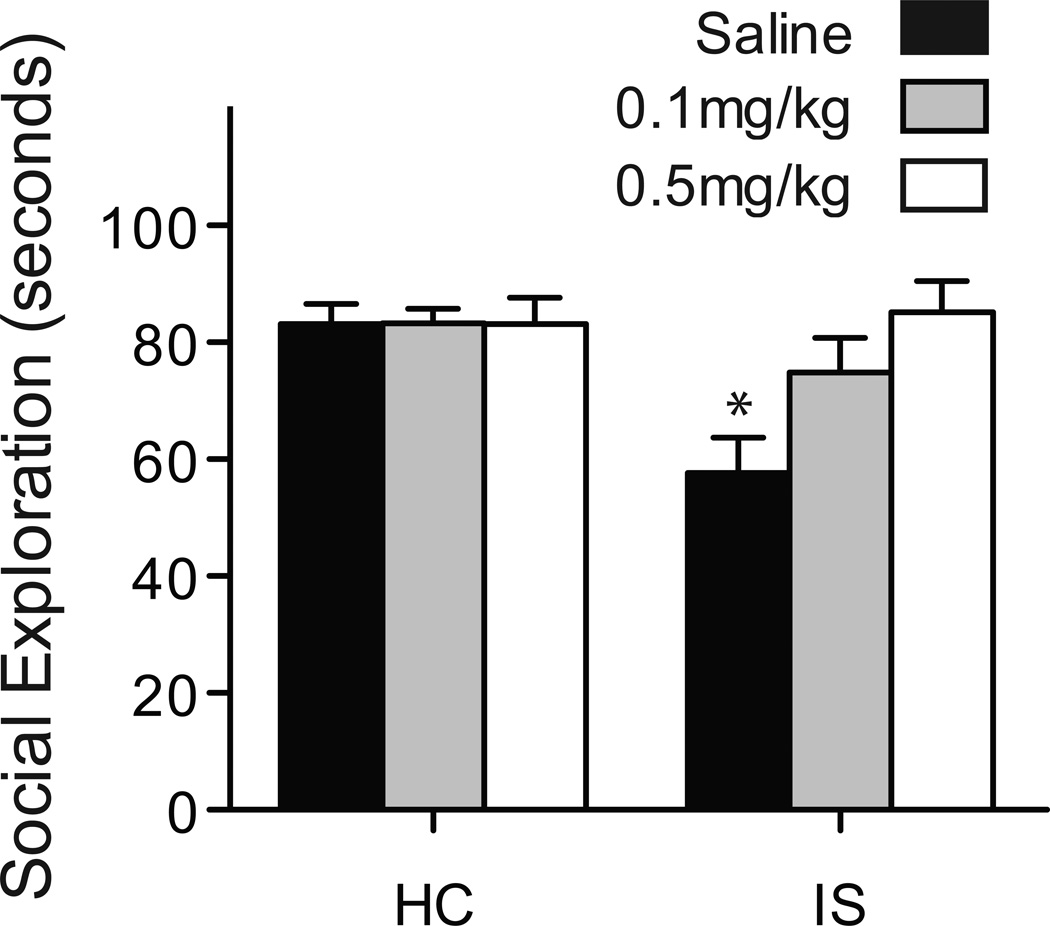
Mean JSE time is depicted in Figure 3, ns = 9–10. Systemic administration of the 5-HT2C antagonist dose-dependently blocked the effect of prior IS on social exploration. A 2 (Stress: IS or HC) by 3 (Drug: saline, 0.1, 0.5mg/kg SB) ANOVA revealed significant main effects for Stress: F(1, 53) = 7.211, p < 0.01, Drug: F(2, 53) = 4.084, p < 0.05, and a significant Stress by Drug interaction: F(2, 53) = 4.032, p < 0.05. Exploration was significantly reduced in the IS-saline group compared to all HC groups, and to the IS-0.5 mg/kg SB group. The IS-0.1 mg/kg SB group was greater than the IS-saline group, p <0.05, but did not differ from the IS-0.5 mg/kg SB group. These data indicate that 0.5mg/kg SB completely blocked the expression of stress-induced anxiety without affecting JSE per se.
Figure 3.
Mean(+ S.E.M.) time spent in exploration during a 3 m juvenile social encounter. Prior IS resulted in a significant reduction in exploration compared to HC treated controls, p < 0.05. Systemic administration of the 5-HT2C antagonist SB 242084 45 m before the encounter dose-dependently blocked the effect of IS with 0.5 mg/kg resulting in complete reversal compared to IS-saline, p < 0.05. SB 242084 was without effect in the HC treated groups. *p<0.05 compared to HC-Saline.
Intra-BLA 5-HT2C receptor antagonism
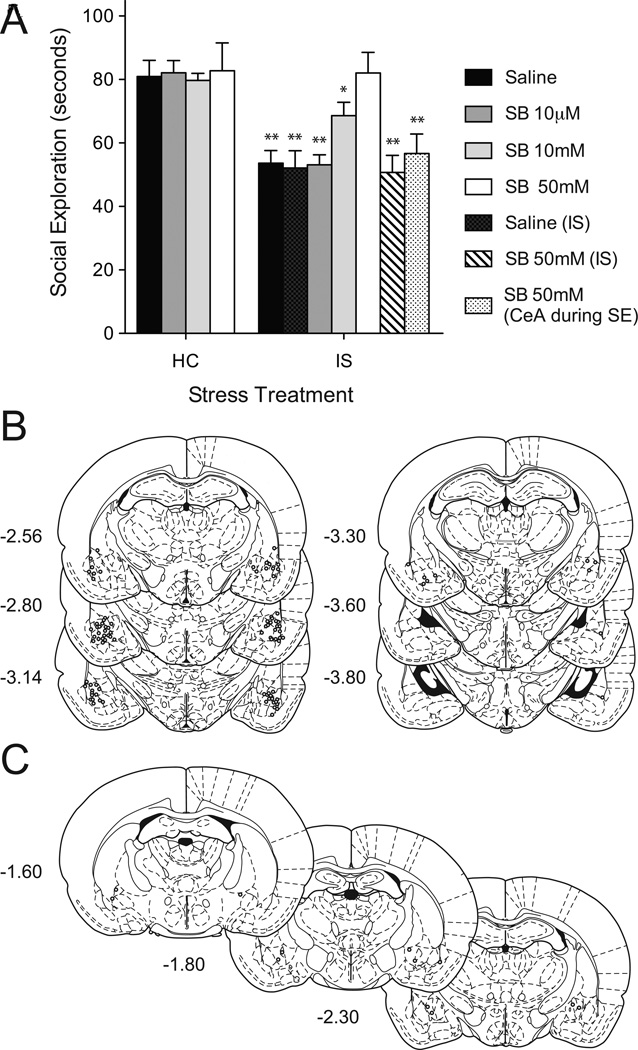
Mean JSE time is shown in Figure 4. After removing rats with misplaced cannulae ns = 7–9. The results indicate a clear effect of IS and a dose-dependent reversal of the effect by SB. The primary experimental groups were compared using a 2 (Stress: IS or HC) by 4 (Drug: saline, 10µM, 10mM, or 50mM) ANOVA that revealed significant main effects of Stress: F(1, 63) = 45.69, p < 0.001; Drug: F(3, 63) = 8.153, p < 0.001; and a Stress by Drug interaction: F (3, 63) = 7.308, p < 0.001. IS significantly reduced exploration in the IS-saline and IS-10µM SB groups compared to HC and the IS-10mM and IS-50mM SB groups (ps < 0.05). The IS-50mM SB group did not differ from any HC groups illustrating a complete reversal of the IS effect (ps > 0.05). The control groups that received drug injections to the CeA before JSE or into the BLA prior to IS were significantly different from the HC-saline group (unrestricted t-tests, ps < 0.05) but did not differ significantly from the IS-saline group (ps > 0.05). Although not all rats with CeA placements fell strictly within the borders of the CeA the purpose of this group was not to establish a role for the CeA but to verify specificity to the BLA. No rats were included in the CeA group if the cannulae tips were found to be in or near the BLA.
Figure 4.
(A) Mean (+ S.E.M.) time spent in exploration during a 3 m juvenile social encounter. Prior IS resulted in a significant reduction in exploration compared to HC controls, p < 0.05. Intra-BLA injection of the 5-HT2C antagonist SB 242084 45 m before the social encounter dose-dependently blocked the effect of prior IS. 10 µm was without effect, 10mM exhibited a partial effect, and 50mM completely blocked the effect of prior IS, ps < 0.05 when compared to IS-Saline. Intra-BLA administration of SB prior to IS had no effect [SB 50mM (IS)]. Furthermore, intra-central amygdala (CeA) injection of SB prior to JSE had no effect suggesting a site-specific effect of SB in the BLA. *p<0.05 compared to HC-Saline, IS-Saline and IS-SB 242084 50mM. **p<0.05 compared to HC-Saline. (B) Graphic reconstruction of microinjector tip placements within the BLA or (C) CeA. Illustrations were adapted from the atlas of Paxinos and Watson (51) and are listed in relationship to bregma in mm.
DISCUSSION
Here we explored the role of the DRN, 5-HT, and BLA 5-HT2C receptors in uncontrollable stress-induced anxiety-like behavior in the JSE test. Consistent with our prior work and that of others, exposure to an uncontrollable stressor (IS) reduced the rat’s tendency to investigate a juvenile conspecific (12, 13, 37), whereas equal controllable stress did not (12, 36). The hypothesis has been that IS produces an intense activation of DRN 5-HT neurons, leading to sensitization of these neurons via down-regulation of DRN 5-HT1A autoreceptors. The consequence of this sensitization would be exaggerated release of 5-HT in DRN projection regions to subsequent stimuli such as those used in behavioral testing (e.g., the footshock in shuttlebox escape testing) and that excessive 5-HT is the proximate cause of the behavioral changes (6). However, there has been little evidence that uncontrollable stress could sensitize DRN 5-HT neurons and that activation of those neurons at the time of behavioral testing is necessary for the occurrence of behavioral effects.
With regard to sensitization, there is only the study of Amat et al. (30) who reported that 2 footshocks was sufficient to increase 5-HT efflux in the BLA in subjects that had received IS 24 h earlier, but that no change in 5-HT to 2 footshocks was found after ES or HC treatment. Here a 5 min exposure to a juvenile conspecific had no effect on 5-HT efflux in the BLA in naïve subjects, as would be expected because DRN 5-HT neurons generally are not activated by minor stressors (38). Prior IS, but not ES, led the 5 min juvenile encounter to produce a large and sustained increase in 5-HT efflux. It should be recognized that the IS and juvenile conditions were distinct; the test environment: retraining tubes versus dialysis bowls with wood chip bedding, the lighting conditions: dark sound-attenuation chambers versus a brightly lit room, and the handling conditions: immediate handling and restraint before IS versus none during dialysis were all quite different. Thus, the sensitization that was revealed here is quite remarkable. In rats that received IS DRN 5-HT neurons responded to a seemingly innocuous event as if it were a potent stressor.
By preventing DRN 5-HT neuronal activity with 8-OH-DPAT, we demonstrate that DRN activity at the time of JSE testing is critical to the expression of a stress-induced anxiety-like effect. The role of the DRN here is consistent with observations from other research. Ethanol withdrawl decreases social interaction (39) which is prevented by intra-DRN buspirone a, 5-HT1A partial agonist (28). Social defeat activates DRN neurons, downregulates 5-HT1A receptor mRNA (40) and increases later fear-like behavior which can by blocked by DRN inhibition (41). Finally, humans with social anxiety disorder have significantly less in vivo DRN 5-HT1A binding compared to controls (42). In each of these examples, DRN hyperactivity is a correlate of the expression of anxiety.
The DRN is not viewed to be part of the circuit that mediates “normal” fear or anxiety behaviors, but BLA-projecting 5-HT neurons are situated to be potent modulators of anxiety (43). That only IS would affect BLA 5-HT levels suggests that 5-HT activity in the BLA is involved in the divergent anxiety outcomes observed after uncontrollable versus controllable stress. 5-HT receptors in the BLA have received attention as a site-of-action for numerous compounds with potential as anxiolytic therapeutics. We focused on the 5-HT2C receptor for several reasons. First, 5-HT2C receptors are involved in the potentiation of fear produced by 5-HT. The increase in the expression of conditioned fear by acute SSRI treatment is blocked by systemic administration of SB (20, 21). Second, 5-HT2C knock-out mice display an anxiolytic phenotype and reduced neuronal activation in BLA projection regions in response to anxiogenic stimuli (44). Third, non-specific systemic 5-HT2 agonists activate the amygdala and limbic structures associated with anxiety-like behaviors (45). Lastly, systemic 5-HT2C antagonists block the shuttlebox escape deficit produced by IS (34).
Using the selective and brain-penetrant 5-HT2C antagonist SB 242084 (SB), we observed a dose-dependent and site-specific effect on the expression of anxiety. That is, SB administered before JSE testing, either systemically or intra-BLA, blocked the effects of IS. When injected during IS, SB was without effect indicating that the 5-HT2C receptor in the BLA is not involved in the induction or acquisition of stress effects. The BLA appears to be the critical site of action because injections to the central amygdala before testing were without effect. Furthermore, BLA 5-HT2C binding is sufficient to reduce social exploration (See supplemental materials). The involvement of the 5-HT2C receptor in the expression of anxiety is consistent with the expression of ethanol-withdrawal induced anxiety which is also prevented by intra-BLA 5-HT2C antagonism (28). Although we have reported that systemic 5-HT2C antagonism prevents the expression of learned helplessness in the shuttle box (34), future work is required to determine if, in fact, the BLA is critical to that effect.
Anxiety- and fear-like behaviors are correlated with an increase in output of the BLA complex to limbic structures that mediate specific behaviors (25, 26, 43, 46). The anxiogenic effect of IS in later JSE may be mediated by an enhancement of BLA output by 5-HT action at the 5-HT2C receptor. Unfortunately, the mechanism by which 5-HT2C receptors modulate BLA neurotransmission is poorly understood and there are conflicting reports. For example, activation of the 5-HT2 receptor with nonselective agonists increases BLA -aminobutyric acid (GABA) release (47, 48), an effect that would be anxiolytic in the JSE test (22). However, 5-HT2 agonists exert a dose-dependent effect, with low doses promoting, and higher doses reducing, net GABAergic activity (48). In contrast, 5-HT2C receptors co-localized with GABAA receptors are able to reduce GABAA inhibitory currents (49) and may account fort the dose-dependent effect of 5-HT2 agonists. Since 5-HT2C agonists enhance excitability in a majority of BLA neurons (50) one would predict that high levels of 5-HT2C stimulation, akin to those observed after IS, would cause a net excitation of BLA neurons including projection neurons. Clearly, the precise mechanism(s) by which 5-HT2C activation in the BLA potentiates anxiety-like behavior awaits further study.
To conclude, early treatment of ASD is an important step in the prevention of PTSD (5). Here the expression of anxiety after stress was correlated with BLA 5-HT during a JSE test, was prevented by 5-HT2C antagonism, and was mimicked by 5-HT2C agonism. Hyperactivity at BLA 5-HT2C receptors may be central to anxiety expression in ASD and PTSD and 5-HT2C antagonism may be a relevant early intervention for victims exhibiting ASD after trauma.
Supplementary Material
ACKNOWLEDGEMENT
Support for this research was provided by National Institute of Mental Health Grants MH050479 and MH082453, the University of Colorado Undergraduate Research Opportunities Program and the Irene and Eric Simon Brain Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Davis LL, English BA, Ambrose SM, Petty F. Pharmacotherapy for posttraumatic stress disorder: a comprehensive review. Expert Opin Pharmacother. 2001;2:1583–1595. doi: 10.1517/14656566.2.10.1583. [DOI] [PubMed] [Google Scholar]
- 3.Association AP. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, D.C.: 2000. [Google Scholar]
- 4.Bryant RA. Early predictors of posttraumatic stress disorder. Biol Psychiatry. 2003;53:789–795. doi: 10.1016/s0006-3223(02)01895-4. [DOI] [PubMed] [Google Scholar]
- 5.Bryant RA, Mastrodomenico J, Felmingham KL, Hopwood S, Kenny L, Kandris E, et al. Treatment of acute stress disorder: a randomized controlled trial. Arch Gen Psychiatry. 2008;65:659–667. doi: 10.1001/archpsyc.65.6.659. [DOI] [PubMed] [Google Scholar]
- 6.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 8.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 9.Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, et al. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process. 1990;16:137–149. [PubMed] [Google Scholar]
- 11.Minor TR, Dess NK, Ben-David E, Chang WC. Individual differences in vulnerability to inescapable shock in rats. J Exp Psychol Anim Behav Process. 1994;20:402–412. [PubMed] [Google Scholar]
- 12.Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, et al. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacol Biochem Behav. 1993;45:827–835. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- 14.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- 17.Lipinski JF, Jr, Mallya G, Zimmerman P, Pope HG., Jr Fluoxetine-induced akathisia: clinical and theoretical implications. J Clin Psychiatry. 1989;50:339–342. [PubMed] [Google Scholar]
- 18.Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- 19.Handley SL, McBlane JW. 5HT drugs in animal models of anxiety. Psychopharmacology (Berl) 1993;112:13–20. doi: 10.1007/BF02247358. [DOI] [PubMed] [Google Scholar]
- 20.Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwood BN, Strong PV, Brooks L, Fleshner M. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berl) 2008;199:209–222. doi: 10.1007/s00213-008-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 23.Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, et al. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 24.Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- 25.Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- 26.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 27.Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- 28.Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology (Berl) 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strong PV, Greenwood BN, Fleshner M. The effects of the selective 5-HT(2C) receptor antagonist SB 242084 on learned helplessness in male Fischer 344 rats. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1413-3. [DOI] [PubMed] [Google Scholar]
- 30.Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- 31.Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, et al. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. J Neurosci. 2008;28:13703–13711. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matos FF, Urban C, Yocca FD. Serotonin (5-HT) release in the dorsal raphe and ventral hippocampus: raphe control of somatodendritic and terminal 5-HT release. J Neural Transm. 1996;103:173–190. doi: 10.1007/BF01292626. [DOI] [PubMed] [Google Scholar]
- 33.Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 34.Strong PV, Greenwood BN, Fleshner M. The effects of the selective 5-HT(2C) receptor antagonist SB 242084 on learned helplessness in male Fischer 344 rats. Psychopharmacology (Berl) 2009;203:665–675. doi: 10.1007/s00213-008-1413-3. [DOI] [PubMed] [Google Scholar]
- 35.Navailles S, Moison D, Ryczko D, Spampinato U. Region-dependent regulation of mesoaccumbens dopamine neurons in vivo by the constitutive activity of central serotonin2C receptors. J Neurochem. 2006;99:1311–1319. doi: 10.1111/j.1471-4159.2006.04188.x. [DOI] [PubMed] [Google Scholar]
- 36.Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety. Stress. doi: 10.1080/10253890802510302. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haller J, Bakos N. Stress-induced social avoidance: a new model of stress-induced anxiety? Physiol Behav. 2002;77:327–332. doi: 10.1016/s0031-9384(02)00860-0. [DOI] [PubMed] [Google Scholar]
- 38.Rueter LE, Fornal CA, Jacobs BL. A critical review of 5-HT brain microdialysis and behavior. Rev Neurosci. 1997;8:117–137. doi: 10.1515/revneuro.1997.8.2.117. [DOI] [PubMed] [Google Scholar]
- 39.Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 44.Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 45.Hackler EA, Turner GH, Gresch PJ, Sengupta S, Deutch AY, Avison MJ, et al. 5-Hydroxytryptamine2C receptor contribution to mchlorophenylpiperazine and N-methyl-beta-carboline-3-carboxamide-induced anxiety-like behavior and limbic brain activation. J Pharmacol Exp Ther. 2007;320:1023–1029. doi: 10.1124/jpet.106.113357. [DOI] [PubMed] [Google Scholar]
- 46.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 47.Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- 49.Huidobro-Toro JP, Valenzuela CF, Harris RA. Modulation of GABAA receptor function by G protein-coupled 5-HT2C receptors. Neuropharmacology. 1996;35:1355–1363. doi: 10.1016/s0028-3908(96)00084-6. [DOI] [PubMed] [Google Scholar]
- 50.Stein C, Davidowa H, Albrecht D. 5-HT(1A) receptor-mediated inhibition and 5-HT(2) as well as 5-HT(3) receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38:328–337. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 51.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th Ed. New York: Academic Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.