Abstract
Background: Beverage consumption is implicated in the overweight/obesity epidemic through the weaker energy compensation response it elicits compared with solid food forms. However, plausible mechanisms are not documented.
Objective: This study assessed the cognitive and sensory contributions of differential postingestive responses to energy- and macronutrient-matched liquid (in beverage form) and solid food forms and identifies physiologic processes that may account for them.
Design: Fifty-two healthy adults [mean ± SD age: 24.7 ± 5.5 y; BMI (in kg/m2): 26.3 ± 6.3] completed this randomized, 4-arm crossover study. Participants consumed oral liquid and solid preloads that they perceived, through cognitive manipulation, to be liquid or solid in their stomach (ie, oral liquid/perceived gastric liquid, oral liquid/perceived gastric solid, oral solid/perceived gastric liquid, or oral solid/perceived gastric solid). However, all preloads were designed to present a liquid gastric challenge. Appetite, gastric-emptying and orocecal transit times, and selected endocrine responses were monitored for the following 4 h; total energy intake was also recorded.
Results: Oral-liquid and perceived gastric-liquid preloads elicited greater postprandial hunger and lower fullness sensations, more rapid gastric-emptying and orocecal transit times, attenuated insulin and glucagon-like peptide 1 release, and lower ghrelin suppression than did responses after oral-solid and perceived gastric-solid treatments (all P < 0.05). Faster gastric-emptying times were significantly associated with greater energy intake after consumption of perceived gastric-liquid preloads (P < 0.05). Energy intake was greater on days when perceived gastric-liquid preloads were consumed than when perceived gastric solids were consumed (2311 ± 95 compared with 1897 ± 72 kcal, P = 0.007).
Conclusions: These data document sensory and cognitive effects of food form on ingestive behavior and identify physical and endocrine variables that may account for the low satiety value of beverages. They are consistent with findings that clear, energy-yielding beverages pose a particular risk for positive energy balance. This study was registered at clinicaltrials.gov as NCT01070199.
INTRODUCTION
“We cannot entertain a doubt that every change in our sensations and ideas must be accompanied by some corresponding change in the organic matter of the body.” —Sir Humphry Davy
The rise in obesity and overweight closely parallels the increase in energy-yielding beverage consumption over the past 3 decades (1–3). Children and adults now consume ∼400 kcal/d of energy-yielding beverages, accounting for ∼20–25% of daily energy intake (3). The contribution of energy-yielding beverages to the promotion of positive energy balance and weight gain remains controversial. However, most studies reveal that beverages, particularly clear varieties, hold weak satiety properties and evoke limited compensatory dietary responses (ie, failure to adjust intake at subsequent eating occasions for energy supplied by the beverages) in comparison to solid food forms (4, 5). Whereas extensive available data have clearly focused concern on sweetened beverages, evidence that beverages containing different energy sources elicit weak dietary compensation (6–9) suggests that the food form (ie, liquid in beverage form compared with semisolid or solid physical state), and not the macronutrient or energy source (ie, carbohydrate, fat, or protein), is likely responsible for the association between energy-yielding beverage consumption and positive energy balance (10).
Conversely, a small body of evidence fails to support the link between energy-yielding beverages and weak appetitive or dietary responses (11, 12). Discrepant findings between studies could be attributable to variations in study design, most notably failure to isolate effects of food form on postprandial responses (11, 13, 14). Comparisons of dissimilar foods and beverages are confounded by expectations and properties such as palatability and nutrient sources. There is a need for direct comparison between responses for beverages and solids with the use of appropriately designed test loads (ie, equally palatable, isocaloric, macronutrient-matched). In addition, mechanistic explanations of why food forms elicit differential regulatory responses are lacking. Beverages require less oral processing, have more rapid gastric-emptying and orocecal transit times (15, 16), and evoke lower expected satiation values (17) (ie, the degree to which an individual expects a particular food to be satiating) (18). Short-term feeding studies show that cognitive manipulations (eg, time, energy content, food labeling, portion size) significantly influence appetitive ratings and subsequent energy intake (19–22). Indeed, the perceived energy content of a food may better predict self-reported appetitive sensations than the true energy content (23, 24). Whether expected appetitive effects differ between beverage and solid food forms or between populations potentially at risk for positive energy balance associated with beverage consumption is poorly characterized.
In comparison with lean individuals, the obese reportedly have higher beverage intake and experience greater weight loss (25) or gain (26) with reduced or increased beverage consumption, respectively. Obese individuals may have less precise regulatory systems (27) and more rapid gastrointestinal motility, leading to increased energy intake due to a rapid loss of satiety (28). These responses may specifically hold after beverage compared with solid food consumption as numerous studies have failed to document differences in energy intake after solid preload consumption (6). In addition, habitual exercisers have more accurate dietary compensation after beverage preloads than do nonexercisers (29), suggesting that exercise not only increases energy expenditure but also improves appetite control sensitivity (30).
The primary aim of the present trial was to contrast appetitive, dietary, gastric emptying, orocecal transit time, and selected endocrine (insulin, GLP-14, CCK, and ghrelin) responses to preingestive (cognitive and orosensory) properties of energy-matched liquid (in beverage form) and solid food forms to identify plausible mechanisms for beverage-specific effects on body weight. The primary hypotheses tested were that consumption of an oral liquid and expectation it would remain a liquid in the gastrointestinal tract would lead to weaker appetitive effects (ie, greater hunger and lower fullness sensations), more rapid gastric-emptying and orocecal transit times, lower satiety hormone release (ie, GLP-1, CCK), lesser orexigenic hormone suppression (ie, ghrelin), and reduced energy intake compensation compared with when a solid food was consumed and expected to remain solid in the gastrointestinal tract. Furthermore, it was predicted that beliefs about food form would have especially weak effects in obese and unfit participants compared with their lean and fit counterparts.
SUBJECTS AND METHODS
Participant eligibility
Eligibility criteria included the following: age of 18–50 y, BMI (in kg/m2) of 18–23 (lean) or 30–35 (obese), body fat percentage in the lower (lean) or upper (obese) tertile for age and sex, cardiorespiratory fitness (estimated maximum aerobic power) in the upper (fit) or lower (unfit) tertile for age and sex (31, 32), a dietary restraint score <11 (33), consistent diet and activity patterns, not pregnant or lactating, glucose tolerant, not taking medications known to influence appetite or metabolism, and a self-reported breakfast and lunch consumer. All participants signed an informed consent form approved by the Purdue University Institutional Review Board and received monetary compensation.
Experimental design and procedures
The study followed a 4-arm, randomized crossover design with a 1-wk washout period between sessions. Analyses were based on a mixed-model, repeated-measures design with cognitive information and sensory properties related to food form as within-subject factors and body fat percentage (lean compared with obese) and cardiorespiratory fitness (fit compared with unfit) as between-subject factors. Equal numbers were recruited in each participant group: lean/fit, lean/unfit, obese/fit, or obese/unfit. Participant height was measured without shoes or socks with a Holtain stadiometer (Holtain Ltd). Fasting-state body weight was measured to the nearest 0.1 kg after the participant had voided. Fasting-state whole-body density was determined by using a whole-body plethysmography system (BodPod; Life Instrument Inc). Whole-body percentage body fat was estimated from body density by using the 2-compartment Siri equation (34). To assess cardiorespiratory fitness, participants completed the YMCA Submaximal Cycle Ergometer Test (35) to estimate maximal oxygen uptake (V̇O2max) (36).
To standardize testing conditions, participants consumed their customary breakfast and reported to the laboratory at their habitual lunchtime after refraining from eating for >3 h on each test day. Appetitive sensations were rated, and testing continued if hunger was rated greater than “strong” and fasting glucose concentrations were <6.1 mmol/L. An indwelling catheter was inserted, and after a 15-min rest, breath and blood samples and subjective appetite ratings were obtained. Participants were shown the session's preload, informed of its postingestive properties through a demonstration, and allowed 10 min to consume the preload. Breath, blood, and subjective sensory and appetite ratings were collected immediately after preload consumption (time = 0) and at 15, 30, 45, 60, 90, 120, 180, and 240 min. During this time, participants were semisupine and isolated from all food-related cues. At the end of the session, participants consumed an ad libitum, weighed challenge meal of macaroni and cheese (380 kcal/100 g) and 350 mL water. They were instructed to eat as much as it took to reach a comfortable level of fullness (3 on a 9-point scale: 1 = extremely full, 9 = not full at all), and intake was recorded. Participants also completed diet records and appetite ratings for the remainder of the testing day.
Study preloads
The preloads corresponded to ~10% of individual daily energy requirements (37) (0% fat, 88.4% carbohydrate, 11.6% protein). Participants were placed into 1 of 3 groups according to their estimated energy needs: 175, 225, or 275 kcal. One test session required consumption of a clear, cherry-flavored unthickened beverage (viscosity of ∼10 mPa · s). The treatment demonstration involved pouring the preload into a clear liquid that participants were told was gastric acid but was actually tap water. This session was referred to as the “liquid to liquid” (L-L) session because participants consumed a liquid in the form of a beverage and believed it would remain liquid in their stomach.
Another session, referred to as “liquid to solid” (L-S), involved the researcher pouring a 1% alginate solution that resembled the cherry-flavored beverage into a 5% calcium chloride solution (“gastric acid”). This resulted in an instantaneous formation of a solid mass that participants were informed would occur in their stomach. However, the actual preload consumed was identical to the previously described session and only differed in the expected gastric food form (ie, liquid or solid).
A third session involved consumption of 1” × 1” × 1” cherry-flavored gelatin cubes. Texture analysis of the dense gelatin cubes measured an average peak bloom strength of 313.8 ± 12 g (TA.XTplus; Stable Microsystems Ltd). The demonstration involved placement of a cube into warm water (“gastric acid”), resulting in liquefaction in <10 s, and was referred to as the “solid to liquid” (S-L) session. Whereas the cubes were solid in the oral cavity and masticated at a fixed rate, timed to a metronome, they were isocaloric to the beverage preloads and assumed to liquefy in the stomach in seconds based on simulated gastric models.
The fourth session, “solid to solid” (S-S), involved the same gelatin cubes, but participants were informed that the consistency would remain solid in the stomach. To demonstrate this, a cube was placed into cold water (“gastric acid”) where it remained solid, thereby leading to the expectation that it would remain a gastric solid when, in fact, it would rapidly liquefy and resemble all other preloads.
To retain the rheologic properties while keeping the macronutrient composition equal between food forms, participants consumed 20–25 capsules (on the basis of energy needs) filled with unflavored gelatin or maltodextrin with 150–200 mL water. Capsules consumed on oral-liquid testing days contained gelatin present in the oral-solid food forms. Capsules consumed on oral-solid testing days contained maltodextrin that was dissolved in the oral-liquid preloads. Participants were not aware of capsule contents.
Sensory and appetitive ratings
Viscosity, hunger, fullness, and desire to eat (38) were measured on 100-mm visual analog scales with end anchors of “not at all” to “extremely.”
Gastric-emptying and orocecal transit times
Immediately after preload consumption, participants consumed 10 g liquid lactulose (39) and 1.5 g liquid acetaminophen (40) to permit estimation of orocecal transit times and gastric emptying, respectively. Serum acetaminophen was quantified via enzymatic colorimetry by using the Cobas Integra 400 Analyzer (Roche Diagnostics). Orocecal transit times were measured through hydrogen analysis of end-alveolar air samples (39, 41) (QuinTron SC MicroLyzer; QuinTron Instrument Co).
Biochemical analyses
Blood was collected into ice-cooled, evacuated EDTA-coated tubes, and the protease inhibitors dipeptidyl peptidase-IV (Millipore), 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (Roche Diagnostics), and aprotinin (Phoenix Pharmaceuticals) were added immediately per the manufacturers’ instructions to prevent GLP-1, ghrelin, and CCK degradation, respectively. Samples were centrifuged at 4°C, separated into aliquots, and frozen at −80°C until analyzed. In addition, after centrifugation plasma ghrelin was acidified with HCl (42). Commercial ELISAs were used to determine active plasma GLP-17–36 (EGLP-35K; Millipore), active n-octanoyl ghrelin (EZGRA-88K; Millipore), and CCK26–33 (FEK-069–04; Phoenix Pharmaceuticals). The limits of detection were 1.97, 7.40, and 5.21 pmol/L with intraassay CVs of 7%, 1.7%, and 10%, respectively. All samples for each participant were analyzed in duplicate on the same assay plate. Serum insulin concentrations were measured via electrochemiluminescence immunoassays by using an Elecsys 2010 analyzer (Roche Diagnostics), and serum acetaminophen and glucose concentrations were measured by enzymatic colorimetry via the Cobas Integra 400 Analyzer (Roche Diagnostics). The limits of detection were 1.38 pmol/L, 1.32 μmol/L, and 0.12 mmol/L with intraassay CVs of 1.9%, 3.1%, and 0.4%, respectively.
Dietary intake
Energy intake was recorded on test and nontest days and analyzed with the use of the University of Minnesota Nutrition Data System for Research 2009.
Statistical analysis
Statistical analyses were performed with the use of SPSS software, version 17.0 (SPSS Inc). Significance was defined as P < 0.05, 2-tailed. All data were expressed as means ± SEMs unless stated otherwise. Treatment effects were tested by repeated-measures ANOVA with a Bonferroni correction for multiple comparisons. When significant effects were noted, AUCt was determined by using the trapezoidal rule. Associations between appetite and energy intake with study outcomes were assessed via Pearson's correlation coefficients.
RESULTS
Participant characteristics
Three hundred eighty-one participants completed the initial screening questionnaire. The 81 individuals meeting initial eligibility criteria completed additional screening procedures, and 57 participants met all inclusion criteria. Five individuals discontinued participation for the following reasons: scheduling conflicts (2 individuals), initiated an exercise regimen (1 individual), and unable to set catheters (2 individuals). Contrary to the hypotheses, subgroup analyses were unremarkable, so all participant data were pooled. Fifty-two adults (23 men, 29 women; mean ± SD age: 24.7 ± 5.5 y) with a mean (±SD) BMI of 26.3 ± 6.3, body fat percentage of 26.0 ± 12%, and dietary restraint score of 6.5 ± 2.9 completed the study.
Sensory and appetitive responses
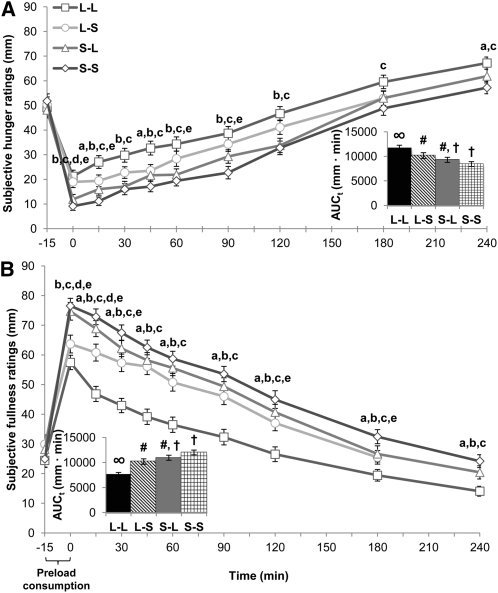
Viscosity ratings of oral liquid and solid preloads were greater when paired with expectation of a gastric solid. The S-S test load was rated as the most viscous (71.0 ± 4.0 mm) followed by S-L (55.7 ± 4.82 mm), L-S (2.2 ± 3.4 mm), and L-L (11.8 ± 2.6 mm) as the least viscous (all different from each other, P < 0.05). A main effect of treatment and a treatment-by-time interaction was observed for subjective hunger [F(3,144) = 15.3, P < 0.001; F(27,1296) = 3.18, P < 0.001] (Figure 1A), fullness [F(3,144) = 30.9, P < 0.001; F(27,1296) = 5.51, P < 0.001] (Figure 1B), and desire-to-eat ratings [F(3,144) = 13.7, P < 0.001; F(27,1296] = 2.92, P < 0.001]. L-L elicited greater hunger and desire to eat and a lower fullness AUCt compared with all other conditions (P < 0.05). Orosensory (actual food form in the oral cavity) and cognitive (expected food form in the gastrointestinal tract) contributions were observed. To further examine the orosensory (actual food form in the oral cavity) and cognitive (perceived food form in the gastrointestinal tract) effects of food form on study outcomes, oral-liquid preloads (mean of L-L and L-S) were analyzed and compared with oral-solid preloads (mean of S-L and S-S), and perceived gastric-liquid preloads (mean of L-L and S-L) were compared with perceived gastric-solid preloads (mean of L-S and S-S). Oral-liquid and perceived gastric-liquid preload consumption led to increased hunger and desire to eat and a lower fullness AUCt compared with oral-solid and perceived gastric-solid preloads (P < 0.01). The majority of participants also made unsolicited comments confirming their appetitive ratings (Table 1).
FIGURE 1.
Postprandial subjective appetite ratings. Mean (±SEM) ratings and AUCt (insets) of subjective hunger (A) and fullness (B) after ingestion of study preloads; n = 52. The bracket indicates the time allotted for preload demonstration and consumption. Comparisons were based on repeated-measures ANOVA with post hoc Bonferroni multiple-comparison tests. Significant main effects of treatment and treatment-by-time interactions were observed for hunger and fullness (both P < 0.001). Different letters indicate significant differences between treatments at a given time point: a,b,cDifferences between L-L and L-S, S-L, or S-S preloads, respectively; P < 0.05. d,eDifferences between L-S and S-L or S-S preloads, respectively; P < 0.05. Different symbols indicate significant differences between treatment AUCt: P < 0.05. AUCt, total AUC; L-L, oral liquid/perceived gastric liquid; L-S, oral liquid/perceived gastric solid; S-L, oral solid/perceived gastric liquid; S-S, oral solid/perceived gastric solid.
TABLE 1.
Subjective comments of participants after preload consumption1
| Preloads | Participant comments |
| L-L | “This didn't fill me up at all.” |
| “It went right through me, I am so hungry.” | |
| “It seems less thick than a regular drink.” | |
| “Not filling!” | |
| L-S | “It feels like I swallowed a rock.” |
| “I could barely swallow the liquid it was so thick.” | |
| “When I push on my stomach, it feels harder.” | |
| “It is very surprising—I feel like I ate a large meal.” | |
| “I am so full I can barely finish the glass.” | |
| “My stomach normally doesn't react to a lot, but I can definitely feel the liquid turning to solid.” | |
| “This is definitely thicker than regular drinks.” | |
| “It sits like a solid in your stomach.” | |
| “It came out like a solid, too.” (ie, feces appeared to be affected) | |
| S-L | “I felt full at first, but it immediately went away when the cubes turned to liquid in my stomach.” |
| “It hardly feels like I ate anything.” | |
| “It feels like I drank a bunch of liquid.” | |
| “I knew it was a solid, but my body was tricked—it felt like a liquid.” | |
| “I was afraid I would be really full, but the feeling quickly disappeared.” | |
| S-S | “I can't remember ever being so full.” |
| “My stomach feels so heavy.” | |
| “I feel like I just ate an entire buffet.” | |
| “These cubes are harder to chew than the ‘S-L’ cubes.” | |
| “It is sitting very heavy in my stomach.” | |
| “These cubes are extremely dense.” | |
| “I felt the same sensation when the liquid turned to solid in my stomach—it feels very hard.” |
The recorded comments were made freely by participants and were not solicited by the researchers. L-L, oral liquid/perceived gastric liquid; L-S, oral liquid/perceived gastric solid; S-L, oral solid/perceived gastric liquid; S-S, oral solid/perceived gastric solid.
Gastric-emptying and orocecal transit times
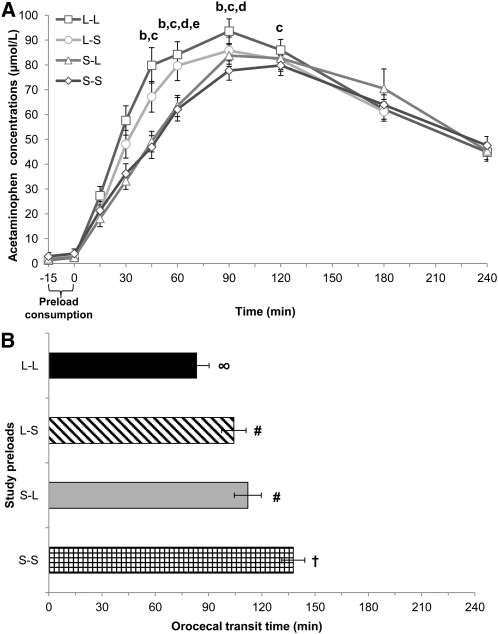
Gastric emptying was more rapid after L-L and L-S preloads than after S-L and S-S preloads on the basis of greater peak acetaminophen concentrations (Cmax) and time to Cmax (Tmax; P < 0.05) (Figure 2A). This was primarily attributable to an orosensory contribution as oral liquids (mean of L-L and L-S) elicited more rapid gastric emptying compared with oral-solid preloads (mean of S-L and S-S) (mean ± SEM Cmax: 109.4 ± 5.3 compared with 95.6 ± 5.0 μmol/L, P = 0.008; Tmax: 83.9 ± 5.6 compared with 95.6 ± 5.0 min, P = 0.005). There was a trend for a cognitive contribution (P = 0.08), and faster gastric-emptying times were associated with greater energy intake after consumption of perceived gastric-liquid preloads (L-L: r = −0.489, P < 0.001; S-L: r = −0.313, P = 0.03).
FIGURE 2.
Gastric-emptying and orocecal transit times. Mean (±SEM) acetaminophen concentrations (A) and orocecal transit times (B) after ingestion of study preloads; n = 52. Comparisons were based on repeated-measures ANOVA with post hoc Bonferroni multiple-comparison tests. The bracket indicates the time allotted for preload demonstration and consumption. Different letters indicate significant differences between treatments at a given time point: a,b,cDifferences between L-L and L-S, S-L, or S-S preloads, respectively; P < 0.05. d,eDifferences between L-S and S-L or S-S preloads, respectively; P < 0.05. Different symbols indicate significant differences in orocecal transit times between treatments: P < 0.05. L-L, oral liquid/perceived gastric liquid; L-S, oral liquid/perceived gastric solid; S-L, oral solid/perceived gastric liquid; S-S, oral solid/perceived gastric solid.
Consumption of the S-S preload resulted in delayed orocecal transit times (137 ± 6.5 min) compared with all other treatments (L-L: 83.5 ± 8.3 min, P < 0.001; L-S: 104 ± 6.9 min, P < 0.001; S-L: 112 ± 7.6 min, P = 0.047) (Figure 2B). Orosensory and cognitive effects were noted. Greater orocecal transit times resulted from consumption of an oral-solid (mean of S-L and S-S; 125 ± 5.3 min) or a perceived gastric-solid preload (mean of L-S and S-S; 121 ± 5.7 min) and compared with an oral-liquid (mean of L-L and L-S; 93.9 ± 6.1 min, P < 0.001) or perceived gastric-liquid preload (mean of L-L and S-L; 97.8 ± 5.9 min, P = 0.004). Prolonged orocecal transit times after consumption of oral-solid preloads were associated with lower hunger ratings (S-L: r = −0.331, P = 0.02; S-S: r = −0.286, P = 0.04).
Glucose and endocrine responses
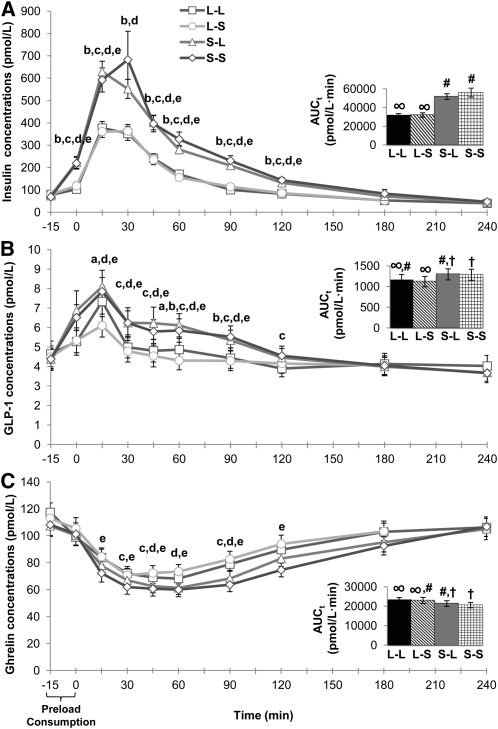
The magnitude of change in glucose Cmax (Cmax minus baselines) was greater after the L-L preload (3.02 ± 0.14 pmol/L) compared with both S-L (2.55 ± 0.13 pmol/L, P = 0.003) and S-S (2.63 ± 0.12 pmol/L, P = 0.012) preloads. Main effects of treatment and treatment-by-time interactions were observed for insulin [F(3,153) = 33.1, P < 0.001; F(27,1377) = 5.63, P < 0.001], GLP-1 [F(3,153) = 6.72, P < 0.001; F(27,1377) = 4.02, P < 0.001], and ghrelin [F(3,150) = 5.80, P = 0.001; F(27,1350) = 1.88, P = 0.004] (Figure 3, A–C). Insulin and GLP-1 AUCt were lower after L-L and L-S preloads than after S-L and S-S preloads (all P < 0.05). Opposite responses were observed for ghrelin AUCt, and nadir concentrations were lower after S-S (45.9 ± 3.4 pmol/L, P = 0.019) compared with L-L (55.0 ± 3.3 pmol/L, P = 0.001) and L-S (57.9 ± 4.4 pmol/L, P = 0.001) preloads. Higher insulin (P < 0.001) and GLP-1 (P = 0.011) and lower ghrelin AUCt (P < 0.001) were noted with oral solids (mean of S-L and S-S) compared with oral liquids (mean of L-L and L-S), which is consistent with an orosensory effect. We did not observe significant treatment effects for cholecystokinin.
FIGURE 3.
Postprandial endocrine responses. Mean (±SEM) and AUCt (inset) postprandial insulin (A), GLP-1 (B), and ghrelin (C) concentrations after ingestion of study preloads; n = 52. Comparisons were based on repeated-measures ANOVA with post hoc Bonferroni multiple-comparison tests. The bracket indicates time allotted for preload demonstration and consumption. Different letters indicate significant differences between treatments at a given time point: a,b,cDifferences between L-L and L-S, S-L, or S-S preloads, respectively; P < 0.05. d,eDifferences between L-S and S-L or S-S preloads, respectively; P < 0.05. Different symbols indicate significant differences between treatment AUCt, P < 0.05. AUCt, total AUC; GLP-1, glucagon-like peptide 1; L-L, oral liquid/perceived gastric liquid; L-S, oral liquid/perceived gastric solid; S-L, oral solid/perceived gastric liquid; S-S, oral solid/perceived gastric solid.
Energy intake
Challenge meal intake was greater after L-L (720 ± 40 kcal) compared with L-S (583 ± 35 kcal, P = 0.004) and S-S (562 ± 38 kcal, P < 0.001) preloads as well as after S-L (643 ± 44 kcal) compared with S-S (P = 0.008) preloads. The same pattern was observed for the remainder of the testing-day energy intake, which resulted in greater total testing-day intake after L-L (2370 ± 101 kcal) and S-L (2252 ± 113 kcal) preloads than after both L-S (1940 ± 77 kcal) and S-S (1853 ± 82 kcal) preloads (all P < 0.01). Consequently, energy intake was higher by ~21.8% on days when perceived gastric-liquid preloads were consumed (mean of L-L and S-L; 2311 ± 95 kcal) compared with perceived gastric solids (mean of L-S and S-S; 1897 ± 72 kcal, P = 0.007). Water intake at the challenge meal and throughout the study visit day was not significantly different between study treatments.
DISCUSSION
The high energy intake from beverages is potentially problematic; evidence from animal behavioral studies (43, 44) and short-term mechanistic human trials (17, 6, 45), suggest that fluids stimulate weak appetitive and compensatory dietary responses compared with energy-matched semisolid or solid items. The primary aim of this study was to isolate and characterize cognitive and orosensory influences stemming from consumption of liquid (in the form of a beverage) and solid food forms. These forms of input are known to evoke neurally mediated physiologic responses to food exposure (ie, cephalic phase responses), with implications for energy balance (46). Stimulation was accomplished by exposures to beverage and solid food forms orally and by providing information about the impending physical state of the test foods in the participant's gastrointestinal tract. In addition to the fact that this cognitive manipulation led to multiple objectively measured differential responses, the effectiveness of the intervention was supported by numerous confirming spontaneous subjective comments from study participants (Table 1). The findings indicate that the mere expectation that food will be in one form or another in the gastrointestinal tract produces behavioral and physiologic responses likely to contribute to lower satiety effects (Figure 1) and weaker dietary compensation after beverage ingestion.
Initially, viscosity ratings of the 2 identical oral-liquid samples and the 2 oral-solid samples were higher if participants expected the preload to transform into or remain a solid in their gut. Previous work suggests that there is a direct relation between viscosity and postprandial hunger suppression and 24-h energy intake (4, 47, 48), purportedly through increased gastric viscosity and prolonged gastric emptying. However, gastric processing may rapidly reduce viscosity and result in similar gastric-emptying times (49). Thus, perceived oral and gastric meal viscosity may be an important mediator of these effects (16). The present findings also document a strong orosensory effect because oral-liquid stimulation led to more rapid gastric-emptying and orocecal transit times (Figure 2), a smaller increase of GLP-1 and insulin, as well as a smaller reduction in ghrelin compared with oral-solid stimulation (Figure 3). Slower gastric-emptying (48) and orocecal transit (50) times are associated with enhanced satiety, whereas insulin (51) and GLP-1 (52) are purported satiety hormones, and ghrelin is reportedly an orexigenic hormone (53). Thus, all noted responses would favor the observed weaker satiety effect for the oral-liquid stimulus that also led to a greater energy intake. This could reflect differential cephalic phase activation. A weaker insulin response to a beverage compared with a food has been documented previously, and pre- and postabsorptive responses were correlated (54). Hence, the rheologic properties of beverages provide a second mechanism by which beverages may hold weaker satiety properties and facilitate greater energy intake.
Although differential responses were noted on the basis of food form, these were further modified by cognitive manipulation. Energy intake was greater after L-L preload ingestion than after L-S preload ingestion, and after S-L than after S-S preload ingestion. This is supported by the cognitive influence on gastric-emptying and orocecal transit times, which were shorter with expectations that the gastrointestinal challenge would be liquid. A sensory contribution was also present because the differences were greatest for the L-L condition and weakest for the S-S preload.
Overall, it is notable that, for the appetitive and gastrointestinal transit time responses, both oral compared with stomach (cognitive) and beverage compared with solid (sensory) differences were observed. In contrast, the gastric-emptying and hormonal responses were more closely aligned with the sensory difference with no cognitive effect. Whether this shows differences between sensory and cognitive influences on these processes is worthy of further study.
This study sought to determine the cognitive and sensory contributions of differential responses to beverage and solid food forms in groups defined according to adiposity and fitness that may have varying sensitivities to cognitive or sensory food cues. However, similar to other studies (6, 20, 47), these data showed no distinct response patterns between such groups. This suggests processes other than cognition and orosensory stimulation mediate reported response differences in these specific groups.
The measurement precision afforded by conducting this trial in a laboratory setting is perhaps the trial's primary weakness because its external validity remains to be established. However, the findings provide initial mechanistic support for the observed differential appetitive and dietary responses to beverage and solid foods (4, 17) and their likely influence on body weight.
Acknowledgments
The authors’ responsibilities were as follows—BAC: conceived and designed the study, conducted participant recruitment and testing, performed sample and data analyses, interpreted data, and generated the initial manuscript; RVC: performed hormone analyses and interpretation; and RDM: conceived and designed the study and performed data analyses and interpretation. All authors discussed the results and contributed to the final manuscript. None of the authors had a personal or financial conflict of interest.
Footnotes
Abbreviations used: AUCt, total AUC; Cmax, peak concentration; CCK, cholecystokinin; GLP-1, glucagon-like peptide 1; L-L, oral liquid/perceived gastric liquid; L-S, oral liquid/perceived gastric solid; S-L, oral solid/perceived gastric liquid; S-S, oral solid/perceived gastric solid; Tmax, time to Cmax.
REFERENCES
- 1.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 3.Popkin BM. Patterns of beverage use across the lifecycle. Physiol Behav 2010;100:4–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tournier A, Louis-Sylvestre J. Effect of the physical state of a food on subsequent intake in human subjects. Appetite 1991;16:17–24 [DOI] [PubMed] [Google Scholar]
- 5.Mattes RD, Campbell WW. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J Am Diet Assoc 2009;109:430–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mourao DM, Bressan J, Campbell WW, Mattes RD. Effects of food form on appetite and energy intake in lean and obese young adults. Int J Obes (Lond) 2007;31:1688–95 [DOI] [PubMed] [Google Scholar]
- 7.Shields DH, Corrales KM, Metallinos-Katsaras E. Gourmet coffee beverage consumption among college women. J Am Diet Assoc 2004;104:650–3 [DOI] [PubMed] [Google Scholar]
- 8.De Castro JM. The effects of the spontaneous ingestion of particular foods or beverages on the meal pattern and overall nutrient intake of humans. Physiol Behav 1993;53:1133–44 [DOI] [PubMed] [Google Scholar]
- 9.Beridot-Therond ME, Arts I, Fantino M, De La Gueronniere V. Short-term effects of the flavour of drinks on ingestive behaviours in man. Appetite 1998;31:67–81 [DOI] [PubMed] [Google Scholar]
- 10.Mattes RD. Beverages and positive energy balance: the menace is the medium. Int J Obes 2006;30:S60–5 [Google Scholar]
- 11.Almiron-Roig E, Flores SY, Drewnowski A. No difference in satiety or in subsequent energy intakes between a beverage and a solid food. Physiol Behav 2004;82:671–7 [DOI] [PubMed] [Google Scholar]
- 12.Rolls BJ, Fedoroff IC, Guthrie JF, Laster LJ. Foods with different satiating effects in humans. Appetite 1990;15:115–26 [DOI] [PubMed] [Google Scholar]
- 13.Almiron-Roig E, Chen Y, Drewnowski A. Liquid calories and the failure of satiety: how good is the evidence? Obes Rev 2003;4:201–12 [DOI] [PubMed] [Google Scholar]
- 14.Drewnowski A, Bellisle F. Liquid calories, sugar, and body weight. Am J Clin Nutr 2007;85:651–61 [DOI] [PubMed] [Google Scholar]
- 15.Glasbrenner B, Pieramico O, Brecht-Krauss D, Baur M, Malfertheiner P. Gastric emptying of solids and liquids in obesity. Clin Investig 1993;71:542–6 [DOI] [PubMed] [Google Scholar]
- 16.Hoad CL, Rayment P, Spiller RC, Marciani L, Alonso B de C, Traynor C, Mela DJ, Peters HP, Gowland PA. In vivo imaging of intragastric gelation and its effect on satiety in humans. J Nutr 2004;134:2293–300 [DOI] [PubMed] [Google Scholar]
- 17.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000;24:794–800 [DOI] [PubMed] [Google Scholar]
- 18.Brunstrom JM, Brown S, Hinton EC, Rogers PJ, Fay SH. Expected satiety changes hunger and fullness in the inter-meal interval. Appetite 2011;56:310–5 [DOI] [PubMed] [Google Scholar]
- 19.Schachter S, Gross LP. Manipulated time and eating behavior. J Pers Soc Psychol 1968;10:98–106 [DOI] [PubMed] [Google Scholar]
- 20.Wooley SC. Physiologic versus cognitive factors in short term food regulation in the obese and nonobese. Psychosom Med 1972;34:62–8 [DOI] [PubMed] [Google Scholar]
- 21.Wansink B, Painter JE, North J. Bottomless bowls: why visual cues of portion size may influence intake. Obes Res 2005;13:93–100 [DOI] [PubMed] [Google Scholar]
- 22.Mattes R. Soup and satiety. Physiol Behav 2005;83:739–47 [DOI] [PubMed] [Google Scholar]
- 23.Wooley OW, Wooley SC, Dunham RB. Can calories be perceived and do they affect hunger in obese and nonobese humans? J Comp Physiol Psychol 1972;80:250–8 [DOI] [PubMed] [Google Scholar]
- 24.Crum AJ, Corbin WR, Brownell KD, Salovey P. Mind over milkshakes: mindsets, not just nutrients, determine ghrelin response. Health Psychol 2011;30:424–9 [DOI] [PubMed] [Google Scholar]
- 25.Gillis LJ, Bar-Or O. Food away from home, sugar-sweetened drink consumption and juvenile obesity. J Am Coll Nutr 2003;22:539–45 [DOI] [PubMed] [Google Scholar]
- 26.Houchins JA, Burgess JR, Campbell WW, Daniel JR, Ferruzzi MG, McCabe GP, Mattes RD. Beverage versus solid fruits and vegetables: effects on energy intake and body weight. Obesity (Silver Spring) 2011;Jun 30. (Epub ahead of print;DOI:10.1038/oby.2011.192) [DOI] [PubMed] [Google Scholar]
- 27.Blundell JE, Gillett A. Control of food intake in the obese. Obes Res 2001;9:263S–70S [DOI] [PubMed] [Google Scholar]
- 28.Wisén O, Johansson C. Gastrointestinal function in obesity: motility, secretion, and absorption following a liquid test meal. Metabolism 1992;41:390–5 [DOI] [PubMed] [Google Scholar]
- 29.Long SJ, Hart K, Morgan LM. The ability of habitual exercise to influence appetite and food intake in response to high- and low-energy preloads in man. Br J Nutr 2002;87:517–23 [DOI] [PubMed] [Google Scholar]
- 30.King NA, Appleton K, Rogers PJ, Blundell JE. Effects of sweetness and energy in drinks on food intake following exercise. Physiol Behav 1999;66:375–9 [DOI] [PubMed] [Google Scholar]
- 31.Whaley MH, Brubaker PH, Otto RM, Armstrong LE. ACSM's guidelines for exercise testing and prescription. 7th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2006 [Google Scholar]
- 32.Kaminsky LA. ACSM's resource manual for Guidelines for exercise testing and prescription. 5th ed. Baltimore, MD: Lippincott, Williams & Wilkins, 2006 [Google Scholar]
- 33.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 34.Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition 1993;9:480–91; discussion 480, 492 [PubMed] [Google Scholar]
- 35.Golding L, Meyers CR, Sinning WE. Y's way to physical fitness: the complete guide to fitness testing and instruction. 3rd ed. Champaign, IL: Human Kinetics Publishers, 1989 [Google Scholar]
- 36.Fitchett MA. Predictability of VO2 max from submaximal cycle ergometer and bench stepping tests. Br J Sports Med 1985;19:85–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris J, Benedict F. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institute of Washington, 1919 [Google Scholar]
- 38.Hill AJ, Blundell JE. Nutrients and behaviour: research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. J Psychiatr Res 1982–1983;17:203–12 [DOI] [PubMed] [Google Scholar]
- 39.Ladas SD, Latoufis C, Giannopoulou H, Hatziioannou J, Raptis SA. Reproducible lactulose hydrogen breath test as a measure of mouth-to-cecum transit time. Dig Dis Sci 1989;34:919–24 [DOI] [PubMed] [Google Scholar]
- 40.Heading RC, Nimmo J, Prescott LF, Tothill P. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol 1973;47:415–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarkston WK, Pantano MM, Morley JE, Horowitz M, Littlefield JM, Burton FR. Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am J Physiol 1997;272:R243–8 [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 2008;93:1980–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson TL, Swithers SE. Food viscosity influences caloric intake compensation and body weight in rats. Obes Res 2005;13:537–44 [DOI] [PubMed] [Google Scholar]
- 44.West DS, Bursac Z, Quimby D, Prewitt TE, Spatz T, Nash C, Mays G, Eddings K. Self-reported sugar-sweetened beverage intake among college students. Obesity (Silver Spring) 2006;14:1825–31 [DOI] [PubMed] [Google Scholar]
- 45.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9 [DOI] [PubMed] [Google Scholar]
- 46.Mattes RD. Physiologic responses to sensory stimulation by food: nutritional implications. J Am Diet Assoc 1997;97:406–13 [DOI] [PubMed] [Google Scholar]
- 47.Mattes RD, Rothacker D. Beverage viscosity is inversely related to postprandial hunger in humans. Physiol Behav 2001;74:551–7 [DOI] [PubMed] [Google Scholar]
- 48.Marciani L, Gowland PA, Spiller RC, et al. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am J Physiol Gastrointest Liver Physiol 2001;280:G1227–33 [DOI] [PubMed] [Google Scholar]
- 49.Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, Al-Sahab S, Bush D, Wright J, Fillery-Travis AJ. Gastric response to increased meal viscosity assessed by echo-planar magnetic resonance imaging in humans. J Nutr 2000;130:122. [DOI] [PubMed] [Google Scholar]
- 50.Smits GJ, Lefebvre RA. Influence of aging on gastric emptying of liquids, small intestine transit, and fecal output in rats. Exp Gerontol 1996;31:589–96 [DOI] [PubMed] [Google Scholar]
- 51.Holt SH, Miller JB. Increased insulin responses to ingested foods are associated with lessened satiety. Appetite 1995;24:43–54 [DOI] [PubMed] [Google Scholar]
- 52.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998;101:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001;86:5992–5 [DOI] [PubMed] [Google Scholar]
- 54.Teff KL, Mattes RD, Engelman K. Cephalic phase insulin release in normal weight males: verification and reliability. Am J Physiol 1991;261:E430–6 [DOI] [PubMed] [Google Scholar]