Abstract
Background: Skeletal abnormalities have been reported in HIV-infected children and adolescents. Although the etiology is not well understood, vitamin D deficiency may be involved.
Objective: The study objective was to evaluate the effect of vitamin D and calcium supplementation on bone mass accrual in HIV-infected youth.
Design: Perinatally HIV-infected children were randomly assigned to receive vitamin D (100,000 IU cholecalciferol given every 2 mo) and calcium (1 g/d) (supplemented group) or double placebo (placebo group) for 2 y. The total-body bone mineral content (TBBMC), total-body bone mineral density (TBBMD), spine bone mineral content (SBMC), and spine bone mineral density (SBMD) were assessed by using dual-energy X-ray absorptiometry at baseline and at 2 annual follow-up visits.
Results: Fifty-nine participants, aged 6–16 y, were randomly assigned to either the supplemented (n = 30) or the placebo (n = 29) group. At enrollment, supplemented and placebo groups did not differ with respect to age, sex, dietary intakes of vitamin D and calcium, mean baseline serum 25-hydroxyvitamin D [25(OH)D] concentration, TBBMC, TBBMD, SBMC, or SBMD. Significant increases in serum 25(OH)D were observed in the supplemented group but not in the placebo group. TBBMC, TBBMD, SBMC, and SBMD increased significantly at 1 and 2 y in both groups. No between-group differences were observed at any time before or after adjustment for stage of sexual maturation by mixed linear model analysis.
Conclusion: One gram of calcium per day and oral cholecalciferol at a dosage of 100,000 IU every 2 mo administered to HIV-infected children and adolescents did not affect bone mass accrual despite significant increases in serum 25(OH)D concentrations. This trial was registered at clinicaltrials.gov as NCT00724178.
INTRODUCTION
Skeletal abnormalities, including decreased BMC4 and BMD, occur in children and adolescents with HIV infection (1–6). Although adverse effects of antiretroviral medication (ART) on bone metabolism, HIV-associated T cell activation, and elaboration of proresorptive cytokines and osteoblast apoptosis have been implicated, the cause or causes of altered bone mass acquisition and bone loss are not completely understood, nor has effective management for children and adolescents been determined (7–9).
During the time when bone mineral accrual is normally maximal, HIV-infected adolescents have worsening deficits in bone mass (3). Reduced bone accrual during late childhood and adolescence may result in low peak bone mass and thereby increase the risk of osteoporotic fractures later in life (10–13). Lower areal BMC and BMD are also associated with increases in childhood fractures (14–16).
Vitamin D, a calciotropic hormone that is critical for normal bone mineralization, is suboptimal in many children and adolescents with HIV, as well as in otherwise healthy, urban-dwelling, racial- and ethnic-minority adolescents who live in the US Northeast. This appears to be a result of limited sunlight exposure and dietary intake, although the disruption of vitamin D synthesis by certain antiretroviral medications may also play a role (17–21). Supplementation with vitamin D is an effective strategy for improving bone mass acquisition in a number of childhood illnesses that adversely affect bone health, including cerebral palsy and epilepsy, nephrotic syndrome, and juvenile rheumatoid arthritis. However, to our knowledge, vitamin D supplementation has not been evaluated in children or adolescents with HIV (22–24).
The aim of this study was to determine whether supplementation with vitamin D and calcium for 2 y results in increased bone mass accrual. Our hypothesis was that bone accrual would be enhanced by supplementation with vitamin D and calcium.
SUBJECTS AND METHODS
In a 24-mo randomized, placebo-controlled multicenter clinical trial at 4 hospitals in New York City, eligible participants were randomly assigned to receive 100,000 IU oral cholecalciferol every 2 mo and 1 g Ca/d or double placebo. The dose of vitamin D was approximately equivalent to 1600 IU/d and was selected because it was used safely and successfully by Guillemant et al (25) as a single dose for the prevention of wintertime vitamin D deficiency in healthy adolescent males with 25(OH)D concentrations comparable to those observed in our pilot study of HIV-infected children and adolescents. Subjects were recruited from patients enrolled in the following 4 pediatric HIV-treatment programs: St Luke's-Roosevelt Hospital Center, Harlem Hospital Center, Bronx-Lebanon Hospital Center, and Metropolitan Hospital Center. Individuals who had previous low-trauma fractures; had known renal or liver disease, malabsorption syndrome, or inflammatory bowel disease; used tenofovir, corticosteroids, or anticonvulsant drugs; or with daily cigarette smoking or alcohol use were excluded from study participation.
Randomization was performed by the study statistician (DJM) by using computer-generated random numbers (SAS version 8.2 for Windows; SAS Institute) stratified by sex, age (ie, >12 or ≤12 y), and study site. Allocation was communicated to the research coordinator who initiated and dispensed the masked study medications (active or placebo). Other study personnel and participants were blinded to treatment allocation. During the first year of the trial, as previously reported, safety monitoring included serum albumin–corrected calcium, spot urinary calcium:creatinine ratio, and serum 25(OH)D concentrations obtained monthly immediately before administration of the cholecalciferol dose or placebo and 1 mo after dosing (19). In year 2, safety monitoring was conducted every other month.
Oral cholecalciferol (100,000 IU; Tishcon Laboratories Inc) or placebo was administered by study personnel every 2 mo during study visits. The content of the active ingredient was analyzed in Tishcon Laboratories by using HPLC and US Pharmacopeia methods (26). Calcium as the carbonate salt in the form of chocolate-flavored chewable supplements, each containing 500 mg Ca, or a placebo (Lifesmart Nutrition Technologies) was dispensed monthly by study personnel. Participants and caregivers were instructed to take 2 chewable supplements daily and to return all uneaten chewable supplements. Adherence to daily calcium or the calcium placebo was estimated by reconciliation of dispensed and returned chewable supplements.
Dietary intakes of vitamin D and calcium were assessed by using a food-frequency questionnaire (Block Kids Questionnaire; NutritionQuest, Block Dietary Data Systems), estimated weekly sunlight exposure in hours was determined by self-report, and pubertal status using the method of Tanner was determined at entry and at 2 annual follow-up visits. Tanner stage was assigned by breast stage for girls and by testicular volume for boys by study investigators (MH and SMA) (27, 28). HIV disease was classified by using CDC criteria (29).
Bone densitometry was performed at the St Luke's Pediatric Body Composition Unit by using dual X-ray absorptiometry with a single Hologic Delphi A model bone densitometer (Hologic Inc) with the manufacturer's software for pediatrics (Hologic version 12.3; Hologic). Scans were performed according to the manufacturer's protocol for whole-body and posterior-anterior lumbar spine (L1–L4, fast array). Scanner calibration and long-term stability were monitored weekly by using anthropomorphic spine and whole-body phantoms (Hologic). Precision for BMD and BMC was <1% for the spine phantom and <2.5% for the whole-body phantom. Age-, race-, and sex-specific percentiles were calculated for bone mass variables by using references from healthy American children (30).
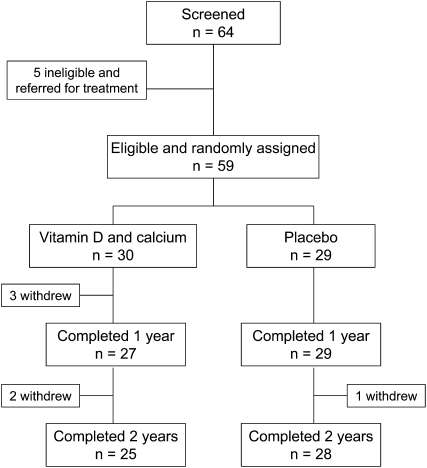
The flow of study participants is presented in Figure 1. Sixty-four perinatally HIV-infected children and adolescents, aged 6–16 y, underwent screening. Five (12.8%) subjects were ineligible because of an initial 25(OH)D concentration <12 ng/mL, which was an indication of moderately severe vitamin D deficiency (31). All 5 subjects were referred to their usual place of medical care for treatment. Fifty-nine children with screening serum 25(OH)D concentrations ≥12 ng/mL were randomly assigned to receive vitamin D and calcium supplementation (n = 30) or double placebo (n = 29). Fifty-six participants completed 1 y, and 53 participants completed 2 y.
FIGURE 1.
Flow of study participants.
Before hypothesis testing, the Kolomogrov-Smirnov normality test was applied to continuous variables; no variables required transformation to achieve normality. Treatment-group differences in baseline characteristics were assessed by using Fisher's exact test for categorical variables and independent t tests for continuous variables. The study was designed to address the primary hypothesis that the group randomly assigned to receive vitamin D plus calcium supplementation would preferentially increase TBBMC at 1 and 2 y relative to the double-placebo group by using an intention-to-treat, repeated-measures ANCOVA. One- and 2-y treatment-related differences in TBBMD and SBMC and SBMD were secondary outcomes.
When the study was designed, published data for adolescent females during early to midpubertal development suggested a 61-g/y difference in total body mineral content between groups was possible with calcium supplementation alone with a standardized effect size of 0.74 (32). In addition, pilot data from our center (The Pediatric Rosetta Project National Institute of Diabetes and Digestive and Kidney Diseases–037352) showed that 262 healthy African American and Hispanic children (aged 7–14 y) gained 195 g TBBMC/y, whereas a study in 30 HIV-infected children showed a TBBMC accrual of 120 ± 84 g/y (S Arpadi, H Horlick, unpublished data, 2004). We hypothesized that vitamin D3 treatment would normalize mineral accrual. To achieve normalization, a 0.89 standardized effect size was proposed for sample-size determination. A 2-group comparison of 1-y difference in accrued TBBMC with 80% power and 5% α and an effect size of 0.89 required 21 subjects per group. We increased the sample size to 30 subjects per group for anticipated loss to follow-up. Differences in the rate of sexual maturation among subjects were anticipated, and the Tanner stage was proposed as the covariate to adjust for possible group imbalances in maturation for the primary outcome analysis.
LMMs for repeated measures (SAS version 9.3 Proc MIXED; SAS Institute) were used to assess primary and secondary outcomes in separate models sharing the same structure of fixed effects for treatment group (supplemented compared with placebo), time (baseline and 1 and 2 y), the group-by-time interaction, the continuous time-independent covariate for the baseline value of the outcome, and a random effect for the time-dependent covariate of the number of Tanner stages advanced since baseline. The repeated factor of time used a compound-symmetry covariance structure empirically determined to best fit the pattern of within-subject autocorrelation among times before analysis. The random effect of Tanner-stage advancement used an unstructured covariance structure blocked within treatment group. Model estimated least-square means and SEs and P values for differences between means calculated by using the method of simultaneous CIs are reported. P values <0.05 was considered significant and were required for fixed effects before within-treatment or within-time differences were examined. No adjustment was made for multiple comparisons across the 4 models used for primary and secondary analyses.
To examine the influence of additional covariates on the robustness of the finding of the primary analysis, secondary analyses with other baseline variables were performed by using the same LMM method. In addition, primary outcomes were evaluated in the subset of subjects who completed 2 y of treatment. In addition to the intention-to-treat analysis, we assessed for possible differences in the change in measures of bone mass between subjects in whom more than one-half of the 25(OH)D-concentration results were >30 ng/mL and subjects in whom more than one-half of 25(OH)D-concentration results were <20 ng/mL with and without adjustment for confounding by advancement of stage of sexual maturation (33).
This study was approved by the institutional review boards of all participating institutions. Informed consent and assent were obtained before enrollment of participants.
RESULTS
The characteristics of the study sample are presented in Table 1. Groups were similar with respect to age, sex, race, weight-for-age, and level of sexual maturation. Dietary intakes of vitamin D and calcium were well below RDAs in both groups. The proportion of participants with vitamin D deficiency [ie, the baseline 25(OH)D concentration between ≥12 and <20 ng/mL] was similar in the 2 groups (approximately one-third) at baseline as was the reported use of a protease inhibitor–containing regimen. Nelfinavir and lopinavir/ritonavir were the most commonly used drugs of the protease inhibitor class in both groups; 17 (56.7%) and 21 (72.4%) participants received either a nelfinavir- or lopinavir/ritonavir-containing regimen in the supplemented and placebo groups, respectively (data not shown). Many of the participants were well controlled with respect to HIV disease; a substantial proportion of subjects in both groups had viral load concentrations below the amount of detection. In addition, 43% and 56% of subjects had CD4 counts >500 cells/mL in the supplemented and placebo groups, respectively, and the mean CD4 count was normal (ie, >500 cells/mL) in both groups. Most participants in both groups were classified with mild or moderate HIV disease at enrollment.
TABLE 1.
Characteristics of subjects who received cholecalciferol and calcium and subjects who received double placebo at the time of enrollment1
| Vitamin D and calcium (n = 30) | Placebo (n = 29) | P2 | |
| Age (y) | 10.2 ± 2.83 | 11.0 ± 2.3 | 0.29 |
| Weight (kg) | 38.0 ± 10.8 | 42.9 ± 12.1 | 0.12 |
| Weight-for-age (percentile) | 56.6 ± 28.9 | 67.6 ± 28.2 | 0.15 |
| Height (cm) | 142 ± 16 | 145 ± 13 | 0.47 |
| Height-for-age (percentile) | 47.8 ± 24.5 | 52.2 ± 30.0 | 0.55 |
| BMI (kg/m2) | 19.5 ± 5.7 | 20.2 ± 3.7 | 0.61 |
| BMI (percentile) | 59.2 ± 29.4 | 71.9 ± 24.9 | 0.09 |
| Male sex [n (%)] | 13 (43) | 13 (45) | 0.91 |
| Black [n (%)] | 18 (60) | 20 (69) | 0.48 |
| Hispanic [n (%)] | 12 (40) | 10 (34) | 0.67 |
| Tanner stage [n (%)] | 0.48 | ||
| 1–2 | 17 (56.7) | 12 (41.4) | |
| 3–4 | 9 (30.0) | 10 (34.5) | |
| ≥5 | 4 (13.3) | 7 (24.1) | |
| Antiretroviral medications [n (%)] | 0.27 | ||
| None | 0 (0.0) | 1 (3.5) | |
| RTIs only | 8 (26.7) | 3 (10.3) | |
| NNRTI containing | 7 (23.3) | 4 (13.8) | |
| PI containing | 10 (33.3) | 15 (51.7) | |
| NNRTI + PI containing | 5 (16.7) | 6 (20.7) | |
| CD4 (%) | 31.7 ± 11.8 | 30.2 ± 10.2 | 0.63 |
| CD4 count (cells/mm3) | 800 ± 377 | 759 ± 387 | 0.68 |
| CD4 >500 cells/mm3 [n (%)] | 24 (80) | 21 (72) | 0.50 |
| CDC class [n (%)] | 0.71 | ||
| A | 13 (43) | 10 (34) | |
| B | 12 (40) | 12 (42) | |
| C | 5 (17) | 7 (24) | |
| Viral load log10 (RNA copies/mL) | 2.7 ± 1.0 | 2.9 ± 1.1 | 0.48 |
| Viral load > level of detection [n (%)] | 16 (53) | 19 (66) | 0.35 |
| Sunlight exposure (h/wk) | 10.4 ± 12.2 | 7.9 ± 7.2 | 0.49 |
| Dietary calcium (mg/d) | 657 ± 290 | 654 ± 302 | 0.98 |
| Dietary calcium (% of RDA) | −34.3 ± 29.1 | −34.6 ± 30.2 | 0.98 |
| Dietary vitamin D (IU/d) | 148 ± 84 | 147 ± 85 | 0.97 |
| Dietary vitamin D (% of RDA) | −63.0 ± 21.0 | −63.3 ± 21.2 | 0.97 |
| Serum 25(OH)D (ng/mL) | 24.1 ± 9.1 | 23.6 ± 10.2 | 0.87 |
| Serum 25(OH)D between 12 and 20 ng/mL [n (%)] | 11 (37) | 10 (34) | 0.70 |
NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RDA, Recommended Dietary Allowance; RTI, reverse transcriptase inhibitor; 25(OH)D, 25-hydroxyvitamin D.
For Student's t test or Fisher's exact test.
Mean ± SD (all such values).
The groups did not differ with respect to the number of doses of vitamin D or vitamin D placebo that were given; the average adherence equaled 95% in both groups, and no treatment month showed <84% adherence. With the exception of the last study visit, at which the supplemented group reported an average adherence of 69% with calcium chewable supplements, both groups met or exceeded 80% compliance with calcium supplementation at each scheduled study visit.
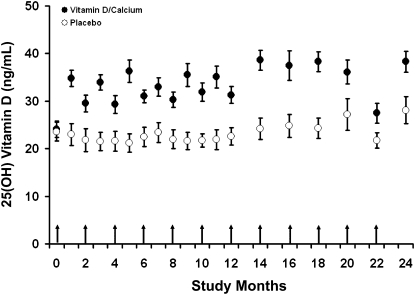
Mean 25(OH)D concentrations (Figure 2) differed significantly between intervention and control groups at 1 y (34.9 ± 8.9 compared with 22.4 ± 9.8 ng/mL, respectively; P < 0.001) as previously reported (19). In addition, differences in mean 25(OH)D concentrations were also observed during the second study year (38.6 ± 10.6 compared with 26.2 ± 11.9 ng/mL, respectively; P < 0.001). Fewer subjects in the supplemented group had any 25(OH)D concentration ≤20 ng/mL during the study than in the placebo group (30% compared with 75%, respectively; P = 0.0004). In contrast, more subjects in the supplemented group achieved 25(OH)D concentrations >30 ng/mL at any time during the study than in the placebo group (69% compared with 42%, respectively; P = 0.06).
FIGURE 2.
Mean (±SE) concentrations of 25(OH)D in subjects who received 100,000 IU cholecalciferol given bimonthly and 1 g Ca/d (Vitamin D/Calcium) and in subjects who received double placebo (Placebo) by month of study. Blood sampling was performed monthly during year 1 and bimonthly during year 2. Blood was sampled immediately before administration of cholecalciferol. Arrows indicate the time of administration of cholecalciferol or placebo, and time −1 indicates the baseline screening. 25(OH) Vitamin D, 25-hydroxyvitamin D.
The results of baseline bone mass measurements by dual X-ray absorptiometry and results of the LMMs for repeated measures of time-by-group interactions are presented in Table 2. At baseline, mean TBBMC, TBBMD, SBMC, and SBMD were similar between the 2 study groups. TBBMC and TBBMD age-, race-, and sex-adjusted percentiles were within the normal range for age in both the intervention and control groups. All bone mass indexes increased at 1 and 2 y in both groups. Although the mean TBBMC and SBMC tended to be lower, the mean age-, race-, and sex-adjusted TBBMC and SBMC percentiles tended to be higher in the supplemented group than in the placebo group at 1 and 2 y. In addition, the mean SBMD tended to be lower, and the mean race- and sex-adjusted SBMC percentiles tended to be higher in the supplemented group than in the placebo group at 1 and 2 y. However, none of these differences were significant.
TABLE 2.
Comparison of total-body bone mineral content, total-body bone mineral density, spine bone mineral content, and spine bone mineral density between subjects who received cholecalciferol and calcium and subjects who received double placebo at entry and at 1 and 2 y1
| Entry | 1 y | 2 y | P | |
| Vitamin D and calcium (n) | 30 | 27 | 25 | — |
| Placebo (n) | 29 | 29 | 28 | — |
| Total-body bone mineral content (g) | ||||
| Vitamin D and calcium | 1330 ± 495 (1145, 1515) | 1486 ± 507 (1284, 1683) | 1561 ± 485 (1362, 1761) | 0.54 |
| Placebo | 1433 ± 428 (1270, 1596) | 1581 ± 450 (1410, 1753) | 1743 ± 485 (1553, 1928) | |
| Total-body bone mineral content (percentile) | ||||
| Vitamin D and calcium | 50 ± 35 (37, 63) | 55 ± 35 (42, 69) | 55 ± 35 (40, 70) | 0.26 |
| Placebo | 47 ± 38 (33, 62) | 51 ± 37 (37, 64) | 51 ± 35 (38, 65) | |
| Total-body bone mineral density (g/cm2) | ||||
| Vitamin D and calcium | 0.814 ± 0.123 (0.768, 0.860) | 0.810 ± 0.126 (0.760, 0.860) | 0.835 ± 0.123 (0.784, 0.885) | 0.48 |
| Placebo | 0.826 ± 0.100 (0.788, 0.864) | 0.828 ± 0.110 (0.786, 0.870) | 0.863 ± 0.123 (0.815, 0.910) | |
| Total-body bone mineral density (percentile) | ||||
| Vitamin D and calcium | 26 ± 29 (15, 36) | 18 ± 24 (9, 27) | 17 ± 22 (7, 26) | 0.61 |
| Placebo | 24 ± 28 (13, 34) | 14 ± 21 (6, 22) | 17 ± 27 (5, 26) | |
| Spine bone mineral content (g) | ||||
| Vitamin D and calcium | 29.1 ± 13.3 (24.1, 34.0) | 32.9 ± 14.9 (27.0, 38.7) | 34.7 ± 13.3 (29.2, 40.2) | 0.55 |
| Placebo | 30.9 ± 10.9 (26.7, 35.0) | 34.8 ± 12.7 (30.0, 39.7) | 39.1 ± 13.6 (33.8, 44.3) | |
| Spine bone mineral content (percentile) | ||||
| Vitamin D and calcium | 44 ± 34 (31, 56) | 45 ± 33 (32, 58) | 42 ± 32 (29, 56) | 0.51 |
| Placebo | 40 ± 34 (27, 53) | 37 ± 33 (25, 50) | 38 ± 31 (26, 50) | |
| Spine bone mineral density (g/cm2) | ||||
| Vitamin D and calcium | 0.683 ± 0.176 (0.617, 0.749) | 0.732 ± 0.191 (0.656, 0.807) | 0.765 ± 0.171 (0.695, 0.836) | 0.46 |
| Placebo | 0.706 ± 0.167 (0.643, 0.770) | 0.750 ± 0.182 (0.681, 0.820) | 0.812 ± 0.196 (0.735, 0.887) | |
| Spine bone mineral density (percentile) | ||||
| Vitamin D and calcium | 47 ± 36 (33, 60) | 50 ± 34 (36, 64) | 51 ± 34 (36, 66) | 0.26 |
| Placebo | 44 ± 33 (31, 57) | 42 ± 32 (30, 54) | 44 ± 31 (32, 56) |
All values are means ± SEMs; 95% CIs in parentheses. There were no significant differences between groups at entry. P < 0.05 indicates the slope of the change in outcome differed between treatment groups by using linear mixed models for repeated measures.
The repeated-measures analysis of change from baseline after initial Tanner stage was controlled for showed no significant treatment-group differences for TBBMC or TBBMD in the intent-to-treat analysis (P < 0.13 and P < 0.59, respectively) or for the per-protocol analysis (same P values). The addition to the model of a covariate for the number of Tanner stages advanced (ie, 0, 1, or 2 Tanner stages) over the 2-y study did not change the pattern of significance across these comparisons. When the same LMM was performed for Tanner-stage advancement during the 2-y study, which was coded as any (1) compared with none (0), treatment-group differences approached significance for TBBMC (P for time × any variable of Tanner-stage advancement = 0.06) and SBMC (P for time × any variable of Tanner-stage advancement = 0.07) in subjects with Tanner-stage advancement; this result appeared to be primarily attributable to the supplemented group outperforming the placebo group during the first year.
No differences in changes in TBBMC, TBBMD, SBMC, and SBMD after adjustment for advancement of stage of sexual maturation were detected between subjects with greater than one-half of 25(OH)D concentrations >30 ng/mL and subjects with greater than one-half of 25(OH)D concentrations <20 ng/mL; this result was independent of the treatment arm to which subjects were randomly allocated (P > 0.01 for adequacy variable).
DISCUSSION
The results of this study showed that a 2-y course of supplementation with vitamin D (100,000 IU every 2 mo) and calcium (1000 mg/d) of a group of HIV-infected children and adolescents with baseline concentrations of serum 25(OH)D <30 ng/mL was associated with significant and sustained increases in mean serum 25(OH)D. In the group assigned to vitamin D and calcium supplementation, baseline mean serum 25(OH)D concentrations increased from 24 to ≥35 ng/mL, whereas they remained unchanged in the placebo group. Despite the increase of serum 25(OH)D into a range considered optimal for bone health at the time the study was designed (>30 ng/mL), we did not detect a significant effect of treatment on bone mass accrual either at the total body or spine. In the subset of subjects who progressed through puberty during the study, as assessed by advancing Tanner stage, there was a larger increase in TBBMC and SBMC in subjects who were randomly assigned to receive vitamin D and calcium; however, the difference was not significant.
To our knowledge, there are no previous studies of vitamin D supplementation in HIV-infected children or adolescents with which we could compare our results. Previous studies conducted in HIV-infected adults with osteoporosis showed moderate but not significant improvements in BMD with supplementation with vitamin D and calcium (34, 35). The lack of effect of the study intervention raises a number of issues with respect to the therapeutic potential of vitamin D supplementation and bone health, especially in minority children and adolescents with chronic diseases such as HIV/AIDS.
There is great controversy regarding the appropriate daily intake of vitamin D intake and serum concentrations of 25(OH)D that are optimal for the acquisition and maintenance of bone mass throughout childhood and adolescence (36, 37). Some investigators consider that a minimum serum concentration of 30 ng/mL is necessary, whereas other investigators suggest that the calciotropic functions of vitamin D are enhanced with higher concentrations of serum 25(OH)D within the current reference range (38–40). Heaney et al (40) showed that intestinal absorption of calcium correlated positively with serum 25(OH)D concentrations across the current reference range and that calcium absorption was significantly reduced at a mean serum 25(OH)D concentration of 20 ng/mL relative to that at a mean of 34 ng/mL, which suggested that intestinal calcium absorption might be suboptimal at lower levels within the current reference range. In NHANES III, which is a population-derived sample of >13,000 participants, BMD was strongly and positively associated with serum 25(OH)D across the reference range (41). Moreover, it is widely considered that high intakes of vitamin D are required to maintain serum concentrations >30 ng/mL and to combat the global problem of vitamin D deficiency (42–45). In contradistinction to these viewpoints, the US Institute of Medicine, which stated that there is insufficient evidence that higher serum 25(OH)D concentrations benefit bone and mineral metabolism, has recently recommended that a lower serum concentration of 25(OH)D (20 ng/mL) is consistent with vitamin D sufficiency and that vitamin D intake does not need to exceed 600 IU/d (46). With the use of the Institute of Medicine criteria, the mean baseline serum concentration of 25(OH)D of our subjects was already in the sufficient range. Moreover, only 37% of the supplemented subjects and 34% of the placebo subjects had baseline concentrations between 12 and 20 ng/mL.
In the current study, participants in the intervention arm achieved mean serum concentrations of >30 ng 25(OH)D /mL during both years 1 and 2; however, 75% of participants in the active arm had at least one monthly 25(OH)D concentration <30 ng/mL, and 30% of participants had at least one monthly 25(OH)D concentration <20 ng/mL. Thus, one possible explanation for the lack of effect of vitamin D and calcium to increase bone mass accrual is a failure to sustain adequate concentrations of 25(OH)D. Recently published studies of the kinetics of orally administered cholecalciferol provide additional support for the inadequacy of bimonthly dosing (47–49). Sustained concentrations may be necessary to improve bone mass accrual during childhood and adolescence, particularly during phases of rapid skeletal growth. This may be especially important in diseases with chronic infection and ongoing immune activation such as in the HIV-infected subjects in this study.
Calcium intake is also critical to bone accrual, and inadequate intakes in our intervention group could also have contributed to the lack of effectiveness of our study intervention. We sought to ensure an adequate calcium intake by providing supplements with 1000 mg elemental calcium, which was slightly higher than the 800-mg RDA for children in the study who were <8 y old but slightly lower than the 1300-mg RDA for children aged ≥9 y. However, because the calcium supplements were administered at home, the actual adherence was uncertain. It has been shown in a number of clinical trials performed in a variety of populations and geographic settings that calcium supplementation increases bone accrual in healthy children and adolescents (32, 50–53).
The cause of alterations in bone metabolism in HIV is unclear. Multiple factors appear to be involved, possibly related to HIV infection of osteoblasts and osteocytes that leads to decreased activity and apoptosis, adverse effects of chronic inflammation on bone metabolism, and effects of ART (54–60). Studies of bone loss in HIV-infected adults receiving ART have been inconsistent. Although some investigators reported bone loss associated with combination ART, other investigators observed increases in bone mass (54, 55). In vitro studies suggested that effects may be drug-specific because even agents within the same class appear to affect osteoclastogenesis and osteoblast activity differently (56–60). Bone resorption predominates early, whereas improved BMD is observed later in the course of ART (57, 59). Relevant to the current study, Mora et al (1) and Zuccotti et al (9) observed lower bone mass measurements in HIV-infected children and adolescents receiving ART but not in ART-naive, HIV-infected children. One possible explanation for our findings is that the constellation of factors that underlie poor bone accrual in HIV-infected youth on ART may not be responsive to improvements in vitamin D and calcium status. When compared with available data from healthy children, measures of bone mass in the study subjects appeared, on average, to be within the normal range (30). It was not possible to determine whether this result was due to the use of new reference data, a reflection of particular ART regimens in study participants, enrollment of healthy study subjects, or other factors.
Our sample size was inadequate for discerning complex interactions in age, sexual maturation, and bone mass accrual in children and adolescents who were undergoing sexual maturation. When subjects who failed to complete were accounted for, the study retained >80% power to detect annual differences of 61 g in the gain of TBBMC. We selected this difference in magnitude because it approximated the annual gain in bone mass attributed to calcium supplementation alone in adolescent females during early to midpuberty (32). However, because a substantial proportion of the participants did not change Tanner stage, either because of HIV-associated delays in sexual maturation or because they had either not yet entered, or had already completed, the most rapid phase of pubertal growth, our subjects may not have accrued bone mass at the expected rate (61). This effect may have reduced the statistical power to detect between-group treatment differences.
In conclusion, in this randomized clinical trial of perinatally HIV-infected youths, we detected no effect of vitamin D and calcium supplementation on 2-y bone mass accrual. Future studies should consider a greater frequency of vitamin D dosing to achieve more sustained concentrations of 25(OH)D. Because there was a suggestion of benefit in children whose Tanner stage advanced during the course of the study, future studies should also be of sufficient size to account for variability in bone acquisition related to sexual maturation or should focus on the most rapid phase of bone acquisition.
Acknowledgments
We acknowledge Robert Warford, Thomas Carpenter, Lenore L Levine, Jack Moye, Emma Stuard, and the study participants and their families.
The authors’ responsibilities were as follows—SMA, EJA, MP, MB, and MH: data collection; and all authors: participation in study design, aspects of data analysis and interpretation of study results, and preparation of the manuscript. None of the authors had a personal or financial conflict of interest.
Footnotes
Abbreviations used: ART, antiretroviral therapy; BMC, bone mineral content; BMD, bone mineral density; LMM, linear mixed model; RDA, Recommended Dietary Allowance; SBMC, spine bone mineral content; SBMD, spine bone mineral density; TBBMC, total-body bone mineral content; TBBMD, total-body bone mineral density; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Mora S, Sala N, Bricalli D, Chiuimello G, Zuin G, Chiumella G, Vigano A. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. AIDS 2001;15:1823–9 [DOI] [PubMed] [Google Scholar]
- 2.O'Brien KO, Razavi M, Henderson R, Caballero B, Ellis K. Bone mineral content in girls perinatally infected with HIV. Am J Clin Nutr 2001;73:821–6 [DOI] [PubMed] [Google Scholar]
- 3.Arpadi SM, Horlick M, Thornton J, Wang J, Kotler D. Bone mineral content is lower in HIV-infected children. J Acquir Imm Def Syndr 2002;29:450–4 [DOI] [PubMed] [Google Scholar]
- 4.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Viganò A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab 2004;89:24–8 [DOI] [PubMed] [Google Scholar]
- 5.Rojo Conejo P, Ramos Amador JT, García Piñar L, Ruano Fajardo C, Sánchez Granados JM, González Tomé MI, Ruiz Contreras J. Decreased bone mineral density in HIV-infected children receiving highly active antiretroviral therapy. Anal Pediatr (Barc) 2004;60:249–53 [DOI] [PubMed] [Google Scholar]
- 6.Jacobson DL, Spiegelman D, Duggan C, Weinberg GA, Bechard L, Furuta L, Nicchitta J, Gorbach SL, Miller TL. Predictors of bone mineral density in human immunodeficiency virus-1 infected children. J Pediatr Gastroenterol Nutr 2005;41:339–46 [DOI] [PubMed] [Google Scholar]
- 7.Fakruddin JM, Laurence J. HIV-1 Vpr enhances production of receptor of activated NF-kb ligand (RANKL) via potentiation of glucocorticoid receptor activity. Arch Virol 2005;150:67–78 [DOI] [PubMed] [Google Scholar]
- 8.Gibellini D, Crignis E, Ponti C, Cimatti L, Borderi M, Tschon M, Giardino R, Re MC. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFα activation. J Med Virol 2008;80:1507–14 [DOI] [PubMed] [Google Scholar]
- 9.Zuccotti G, Viganò A, Gabiano C, Giacomet V, Mignone F, Stucchi S, Manfredini V, Marinacci F, Mora S. Antiretroviral therapy and bone mineral measurements in HIV-infected youths. Bone 2010;46:1633–8 [DOI] [PubMed] [Google Scholar]
- 10.Fassler ALC, Bonjour JP. Osteoporosis as a pediatric problem. Pediatr Nutr 1996;2:811–23 [DOI] [PubMed] [Google Scholar]
- 11.Seeman E. From density to structure: growing up and growing old on the surfaces of bone. J Bone Miner Res 1997;12:509–21 [DOI] [PubMed] [Google Scholar]
- 12.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan bone mineral accrual study. J Bone Miner Res 1999;14:1672–9 [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 2000;17:1–45 [PubMed] [Google Scholar]
- 14.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: A dual-energy x-ray absorptiometry study. J Pediatr 2001;139:509–15 [DOI] [PubMed] [Google Scholar]
- 15.Jones IE, Taylor RW, Williams SM, Manning PJ, Goulding A. Four-year gain in bone mineral in girls with and without past forearm fractures: a DXA study. J Bone Miner Res 2002;17:1065–72 [DOI] [PubMed] [Google Scholar]
- 16.Goulding A, Grant A, Williams S. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res 2005;20:2090–6 [DOI] [PubMed] [Google Scholar]
- 17.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among health adolescents. Arch Pediatr Adolesc Med 2004;158:531–7 [DOI] [PubMed] [Google Scholar]
- 18.Stephensen CB, Marquis GS, Kruzich LA, Douglas SD, Aldrovandi GM, Wilson CM. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr 2006;83:1135–41 [DOI] [PubMed] [Google Scholar]
- 19.Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, Horlick M, Shane E. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics 2009;123:e121–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE. Dusso A. HIV-protease inhibitors impair vitamin D bioactivation to 1, 25-dihydroxyvitamin D. AIDS 2003;17:513–20 [DOI] [PubMed] [Google Scholar]
- 21.Welz T, Childs K, Ibrahim F, Poulton M, Taylor CB, Moniz CF, Post FA. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS 2010;24:1923–8 [DOI] [PubMed] [Google Scholar]
- 22.Jekovec-Vrhovsek M, Kocijancic A, Preselj J. Effect of vitamin D and calcium on bone mineral density in children with CP and epilepsy in full-time care. Dev Med Child Neurol 2000;42:403–5 [PubMed] [Google Scholar]
- 23.Bak M, Serdaroglu E, Guclu R. Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr Nephrol 2006;21:350–4 [DOI] [PubMed] [Google Scholar]
- 24.Lovell DJ, Glass D, Ranz J, Kramer S, Huang B, Sierra RI, Henderson CJ, Passo M, Graham B, Bowyer S, et al. A randomized controlled trial of calcium supplementation to increase bone mineral density in children with juvenile rheumatoid arthritis. Arthritis Rheum 2006;54:2235–42 [DOI] [PubMed] [Google Scholar]
- 25.Guillemant J, Le HT, Maria A, Allemandu A, Peres G, Guillemant S. Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D3 supplements. Osteoporos Int 2001;12:875–9 [DOI] [PubMed] [Google Scholar]
- 26.US Pharmacopeia Rockville, MD: US Pharmacopeial Convention Inc, 2002 [Google Scholar]
- 27.Marshall WA, Tanner JM. Variations in patterns of pubertal changes in boys. Arch Dis Child 1970;45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall WA, Tanner JM. Variations in patterns of pubertal changes in girls. Arch Dis Child 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR 43(RR-12);1–10 [Google Scholar]
- 30.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 2007;92:2087–99 [DOI] [PubMed] [Google Scholar]
- 31.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc 2003;78:1463–70 [DOI] [PubMed] [Google Scholar]
- 32.Lloyd T, Martel JK, Rollings N, Andon MB, Kulin H, Demers LM, Eggli DF, Kieselhorst K, Chinchilli VM. The effect of calcium supplementation and Tanner stage on bone density, content and area in teenage women. Osteoporos Int 1996;6:276–83 [DOI] [PubMed] [Google Scholar]
- 33.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28 ((Published erratum appears in Am J Clin Nutr 2006;84:1253 and Am J Clin Nutr 2007;86:809.) [DOI] [PubMed] [Google Scholar]
- 34.Mondy K, Powderly WG, Claxton SA, Yarasheski KH, Royal M, Stoneman JS, Hoffmann ME, Tebas P. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr 2005;38:426–31 [DOI] [PubMed] [Google Scholar]
- 35.McComsey GA, Kendall MA, Tebas P, Swindells S, Hogg E, Alston-Smith B, Suckow C, Gopalakrishnan G, Benson C, Wohl DA. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007;21:2473–82 [DOI] [PubMed] [Google Scholar]
- 36.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005;135:317–22 [DOI] [PubMed] [Google Scholar]
- 37.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998;351:805–6 [DOI] [PubMed] [Google Scholar]
- 38.Heaney RP. The vitamin D requirements in health and disease. J Steroid Biochem Mol Biol 2005;97:13–9 [DOI] [PubMed] [Google Scholar]
- 39.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005;16:713–6 [DOI] [PubMed] [Google Scholar]
- 40.Heaney RP, Dowell M, Hale C, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 2003;22:142–6 [DOI] [PubMed] [Google Scholar]
- 41.Bischoff-Ferrari HA, Dietrich T, Orav E, Dawson-Hughes B. Positive association between 25(OH)D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 2004;116:634–9 [DOI] [PubMed] [Google Scholar]
- 42.Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol 2008;624:55–71 [DOI] [PubMed] [Google Scholar]
- 43.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother 2008;9:107–18 [DOI] [PubMed] [Google Scholar]
- 44.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–20 [DOI] [PubMed] [Google Scholar]
- 45.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007;85:649–50 [DOI] [PubMed] [Google Scholar]
- 46.Institute of Medicine Dietary reference for intake of calcium and vitamin D. 2010 Report Brief, Institute of Medicine. Available from: http://iom.edu/∼/media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20Calcium%202010%20Report%20Brief.pdf (cited 7 March 2011)
- 47.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr 2008;87:1738–42 [DOI] [PubMed] [Google Scholar]
- 48.Leventis P, Kiely PD. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol 2009;38:149–53 [DOI] [PubMed] [Google Scholar]
- 49.Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr 2008;87:688–91 [DOI] [PubMed] [Google Scholar]
- 50.Bonjour J-P, Carrie A-L, Ferrari S, Clavien H, Slosman D, Theintz G, Rizzoli R. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest 1997;99:1287–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnston CC, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC, Peacock M. Calcium supplementation and increases in bone mineral density in children. N Engl J Med 1992;327:82–7 [DOI] [PubMed] [Google Scholar]
- 52.Lee WTK, Leung SSF, Leung DMY, Tsang HSY, Lau J, Cheng JCY. A randomized double-blind controlled calcium supplementation trial, and bone and height acquisition in children. Br J Nutr 1995;74:125–39 [DOI] [PubMed] [Google Scholar]
- 53.Lloyd T, Andon MB, Rollings N, Martel JK, Landis JR, Demers LM, Eggli DF, Kieselhorst K, Kulin HE. Calcium supplementation and bone mineral density in adolescent girls. JAMA 1993;270:841–4 [PubMed] [Google Scholar]
- 54.Aukrust P, Haug CJ, Ueland T, Lien E, Müller F, Espevik T, Bollerslev J, Frøland SS. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab 1999;84:145–50 [DOI] [PubMed] [Google Scholar]
- 55.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, Hoffmann M, Tebas P. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis 2003;36:482–90 [DOI] [PubMed] [Google Scholar]
- 56.Wang MW, Wei S, Faccio R, Takeshita S, Tebas P, Powderly WG, Teitelbaum SL, Ross FP. The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J Clin Invest 2004;114:206–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malizia AP, Cotter E, Chew N, Powderly WG, Doran PP. HIV protease inhibitors selectively induce gene expression alterations associated with reduced calcium deposition in primary human osteoblasts. AIDS Res Hum Retroviruses 2007;23:243–50 [DOI] [PubMed] [Google Scholar]
- 58.Fakruddin JM, Laurence J. HIV envelope gp-120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kb ligand (RANKL) decretion and its modulation by certain HIV protease inhibitors through interferon-γ /RANKL cross-talk. J Biol Chem 2003;278:48251–8 [DOI] [PubMed] [Google Scholar]
- 59.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK; 903 Study Group Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004;292:191–201 [DOI] [PubMed] [Google Scholar]
- 60.van Vonderen MG, Lips P, van Agtmael MA, Hassink EA, Brinkman K, Geerlings SE, Sutinen J, Ristola M, Danner SA, Reiss P. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS 2009;23:1367–76 [DOI] [PubMed] [Google Scholar]
- 61.de Martino M, Tovo PA, Galli L, Gabiano C, Chiarelli F, Zappa M, Gattinara GC, Bassetti D, Giacomet V, Chiappini E, et al. Puberty in perinatal HIV-1 infection: a multicentre longitudinal study of 212 children. AIDS 2001;15:1527–34 [DOI] [PubMed] [Google Scholar]