Abstract
Background: Previous studies have reported that green tea consumption is associated with a lower risk of diseases that cause functional disability, such as stroke, cognitive impairment, and osteoporosis. Although it is expected that green tea consumption would lower the risk of incident functional disability, this has never been investigated directly.
Objective: The objective was to determine the association between green tea consumption and incident functional disability in elderly individuals.
Design: We conducted a prospective cohort study in 13,988 Japanese individuals aged ≥65 y. Information on daily green tea consumption and other lifestyle factors was collected via questionnaire in 2006. Data on functional disability were retrieved from the public Long-term Care Insurance database, in which subjects were followed up for 3 y. We used Cox proportional hazards regression analysis to investigate the association between green tea consumption and functional disability.
Results: The 3-y incidence of functional disability was 9.4% (1316 cases). The multiple-adjusted HR (95% CI) of incident functional disability was 0.90 (0.77, 1.06) among respondents who consumed 1–2 cups green tea/d, 0.75 (0.64, 0.88) for those who consumed 3–4 cups/d, and 0.67 (0.57, 0.79) for those who consumed ≥5 cups/d in comparison with those who consumed <1 cup/d (P-trend < 0.001).
Conclusion: Green tea consumption is significantly associated with a lower risk of incident functional disability, even after adjustment for possible confounding factors.
INTRODUCTION
Tea is the most frequently consumed beverage in the world. Three billion kilograms of tea are produced worldwide annually. Because of the high rates of tea consumption in the global population, even small effects on an individual could have a large impact on public health.
The health effects of green tea have been extensively investigated by prospective cohort studies. We have found that green tea consumption is significantly associated with a lower risk of mortality due to stroke (1) and pneumonia (2) and a lower risk of cognitive impairment (3), depression (4), and psychological distress (5). These results have been confirmed by other researchers (6–9). In addition, other epidemiologic studies have indicated that green tea consumption is associated with a lower risk of osteoporosis (10, 11), and randomized controlled trials have indicated that green tea is effective for cardiovascular risk factors (12, 13). Because all of the above conditions are major causes of functional disability (14–16), it is expected that green tea consumption would contribute to disability prevention. To our knowledge, however, no study has yet investigated the relation between green tea consumption and the incident risk of functional disability.
We therefore conducted the present analysis to test the hypothesis that green tea consumption is associated with a lower risk of developing functional disability.
SUBJECTS AND METHODS
Study cohort
The design of the Ohsaki Cohort 2006 Study has been described in detail elsewhere (17). In brief, the source population for the baseline survey comprised 31,694 men and women aged ≥65 y who were living in Ohsaki City, northeastern Japan, on 1 December 2006.
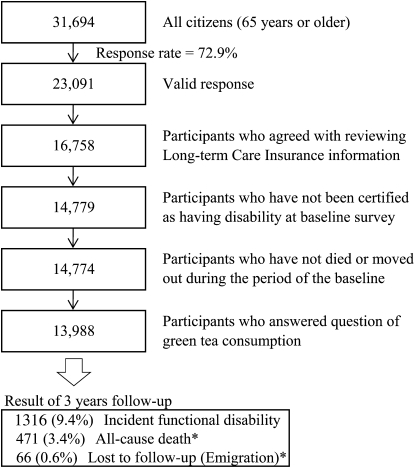
The baseline survey was conducted between 1 December and 15 December 2006. A questionnaire was distributed by the heads of individual administrative districts to individual households and then collected by mail. In this analysis, 23,091 persons who provided valid responses formed the study cohort (Figure 1). We excluded 6333 persons who did not provide written consent for review of their Long-term Care Insurance (LTCI) information, 1979 persons who had already been certified as having disability by the LTCI at the time of the baseline survey, 5 persons who had died or moved out of the district during the period of the baseline survey, and 786 persons who missed answering the questions on green tea consumption. Thus, 13,988 responses were analyzed for the purposes of this study.
FIGURE 1.
Flowchart of study participants: the Ohsaki Cohort 2006 Study. *Without experiencing incident functional disability.
During the 3-y period, only 66 persons were lost to follow-up because of moving from the study area, without developing incident functional disability, which provided a follow-up rate of 99.5%. Among 38,660 person-years, incident functional disability was determined in 1316 persons and the number of all-cause deaths without incident functional disability was 471.
We also analyzed the association between consumption of black tea, oolong tea (Chinese tea), or coffee and incident functional disability. In these analyses, we excluded individuals for whom data on consumption of these beverages were missing (n = 2539 for black tea, n = 2626 for oolong tea, and n = 1105 for coffee).
Exposure data
The survey included questions about the frequency of recent average consumption of green tea, oolong tea, black tea, coffee, and 36 food items, as well as items on history of disease, blood pressure, educational level, smoking, alcohol drinking, body weight, height, cognitive activity score (18), psychological distress score (K6) (19, 20), time spent walking per day, and motor function score of the Kihon Checklist (21). The frequency of green tea consumption was categorized as never, occasionally, or 1–2, 3–4, or ≥5 cups/d. Within the study region, the volume of a typical cup of green tea is 100 mL.
We conducted a validation study of the food-frequency questionnaire in which 113 respondents provided four 3-d food records within 1 y and subsequently responded to the questionnaire. The Spearman rank correlation coefficient between green tea consumption according to the questionnaire and that according to the food records was 0.71 for men and 0.53 for women; the correlation between consumption measured by the 2 questionnaires administered 1 y apart was 0.63 for men and 0.64 for women (22).
BMI was calculated as the self-reported body weight (in kg) divided by the square of the self-reported body height (in m). The degree of social support available to each individual was assessed by asking the following questions (23): Do you have someone 1) with whom you can talk when you are in trouble, 2) whom you can consult when you do not feel well, 3) who can help you with your daily housework, 4) who can take you to a hospital when you feel ill, and 5) who can take care of you if you become bedridden? This social support questionnaire consisted of 5 questions, each requiring a “yes” or “no” answer. This questionnaire was available only in Japanese. The validity and reliability of the questionnaire had not been evaluated. We also assessed participation in community activities. We asked about how often each respondent participated in the following activities: 1) neighborhood associations; 2) sports, exercise, or hobbies; 3) volunteering for activities related to nonprofit organizations; and 4) any other type of social gatherings. The frequency of these activities was assessed as never, a few times each year, monthly, 2–3 times/mo, 1 time/wk, 2–3 times/wk, and ≥4 times/wk. The motor function score of the Kihon Checklist has been previously evaluated and has shown predictive validity of functional disability (21).
The LTCI system in Japan
In this study, we defined incident functional disability as certification for LTCI in Japan, which uses a nationally uniform standard of functional disability. LTCI is mandatory social insurance to assist daily activities in the frail and the elderly (24–28). Everyone aged ≥40 y pays premiums, and everyone aged ≥65 y is eligible for formal caregiving services. When a person applies to the municipal governments for benefits, a care manager visits his or her home and assesses the degree of functional disability by using a questionnaire developed by the Ministry of Health, Labor, and Welfare. Then, the municipal governments calculate the standardized scores for physical and mental functions on the basis of the questionnaire and classify whether the applicant is eligible for LTCI benefits (certification). If a person is judged as eligible for benefits, the Municipal Certification Committee decides on 1 of 7 levels of support, ranging from Support Level 1, Support Level 2, and Care Level 1 to Care Level 5. In brief, LTCI certification levels are defined as follows: Support Level 1 is defined as “limited in instrumental activities of daily living but independent in basic activities of daily living (ADLs)”, Care Level 2 is defined as “requiring assistance in at least one basic ADL task,” and Care Level 5 is defined as “requiring care in all ADL tasks.” A community-based study has shown that levels of LTCI certification are well correlated with ability to perform ADLs, and with Mini Mental State Examination scores (29). A prospective study has also indicated that levels of LTCI certification are significantly associated with mortality risk (30). LTCI certification was used as a measure of incident functional disability in the elderly (31–33).
Follow-up and case ascertainment
Incident functional disability was set as our endpoint, which was defined as LTCI certification. The primary outcome was LTCI certification (Support Level 1 or higher), in which deaths without LTCI certification were treated as censored. In the subanalysis, we set the criteria of disability toward a more severe level, ie, Care Level 2 (requiring assistance with one basic ADL task) or higher.
We obtained information on the date of LTCI certification, death, or moving from Ohsaki City. With regard to LTCI certification, information on care level was also provided. All data were transferred from the Ohsaki City Government under the agreement related to Epidemiologic Research and Privacy Protection yearly each December.
Ethical issues
We considered the return of completed questionnaires to imply consent to participate in the study involving the baseline survey data and subsequent follow-up of death and emigration. We also confirmed information regarding LTCI certification status after obtaining written consent from the subjects. The Ethics Committee of Tohoku University Graduate School of Medicine (Sendai, Japan) reviewed and approved the study protocol.
Statistical analysis
We counted the person-years of follow-up for each subject from 16 December 2006 until the date of incident functional disability, date of moving from Ohsaki City, date of death, or the end of the study period (30 November 2009), whichever occurred first.
Baseline characteristics were evaluated by using ANOVA for continuous variables and the chi-square test for categorical variables. We used the multiple adjusted Cox proportional hazards model to calculate HRs and 95% CIs for incidence of functional disability according to amounts of green tea consumption.
We defined respondents who consumed <1 cup green tea/d as the reference category, and examined the relation between green tea consumption and incident functional disability by using the following models. Model 1 was sex- and age-adjusted. To examine whether the association between green tea consumption and incident functional disability could be explained as resulting from healthy physical status or other lifestyle factors, model 2 was further adjusted for history of stroke, myocardial infarction, hypertension (individuals with self-measured systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg were also defined as hypertensive), arthritis, osteoporosis and fracture, educational level, smoking status, alcohol consumption, BMI, tertile categories of cognitive activity score, psychological distress score, and time spent walking per day. Because green tea consumption was thought to be especially related to a healthy dietary pattern, model 3 was further adjusted for 3 tertile groups of consumption volume of rice, miso soup, meat, fish, green and yellow vegetables, potatoes, soy products, fruit, and sweets. Model 4 was fully adjusted and included answers to questions about social support, participation in community activities, and motor function score.
Because green tea is the beverage most frequently served at social activities in Japan, its consumption might be merely a surrogate marker of social support or participation in community activity (5, 34). Therefore, we further stratified the responses according to social support and community activity. Those who did not answer any questions about social support or participation in community activities were excluded from these stratified analyses. For analysis of social support and participation in community activities, neither of these was used as the respective covariate.
We also analyzed the consumption of black tea, oolong tea, and coffee as independent variables by using the fully adjusted model (model 4). In the analyses for black tea, oolong tea, or coffee as a main exposure, persons with missing data were excluded (n = 11,449 for black tea, n = 12,883 for oolong tea, and n = 11,362 for coffee).
All data were analyzed by using SAS version 9.1 (SAS Institute Inc). All statistical tests described here were 2-sided, and differences at P < 0.05 were accepted as significant.
RESULTS
The baseline characteristics of the participants according to green tea consumption category are shown in Table 1. Subjects who consumed larger amounts of green tea were less likely to be men, to suffer from psychological distress, to have <16 y of education, to have shown a weight reduction of >2 kg compared with 1 y ago, to be current smokers, to be current alcohol drinkers, and to have a history of stroke, myocardial infarction, or hepatic disease. More frequent consumption of green tea was associated with significantly higher consumption of meat, fish, green and yellow vegetables, soy products, fruits, and sweets; greater intake of energy and protein; better cognitive activity; better perception of support for all 5 social support categories; and greater participation in the 4 community activities categories. Conversely, subjects who more frequently consumed green tea included a higher proportion of individuals with arthritis and a lower proportion of individuals who walked ≥1 h/d.
TABLE 1.
Relation between green tea consumption and characteristics of the participants
| Green tea consumption |
|||||
| <1 cup/d | 1–2 cups/d | 3–4 cups/d | ≥5 cups/d | P value1 | |
| n | 2318 | 3141 | 3978 | 4551 | |
| Male sex (%) | 57.0 | 48.9 | 42.5 | 36.0 | <0.001 |
| Age (y) | 73.7 ± 6.22 | 73.9 ± 6.1 | 73.9 ± 5.9 | 74.0 ± 5.8 | 0.152 |
| BMI (kg/m2) | 23.7 ± 3.8 | 23.6 ± 3.4 | 23.5 ± 3.2 | 23.6 ± 3.3 | 0.319 |
| Psychological distress (%)3 | 6.8 | 4.6 | 4.4 | 4.1 | <0.001 |
| Educational level <16 y (%) | 35.1 | 31.1 | 26.4 | 28.0 | <0.001 |
| Past history of (%) | |||||
| Stroke | 4.1 | 3.4 | 2.4 | 2.0 | <0.001 |
| Myocardial infarction | 6.3 | 5.2 | 5.1 | 4.2 | 0.003 |
| Hypertension | 43.3 | 44.3 | 44.0 | 43.0 | 0.662 |
| Dyslipidemia | 6.6 | 8.8 | 9.4 | 8.6 | 0.002 |
| Diabetes | 12.5 | 12.0 | 12.0 | 11.5 | 0.646 |
| Arthritis | 14.1 | 15.1 | 16.0 | 17.3 | 0.003 |
| Osteoporosis | 9.8 | 10.2 | 11.4 | 11.4 | 0.091 |
| Fracture | 16.1 | 16.7 | 15.9 | 15.3 | 0.404 |
| Cancer | 8.8 | 8.1 | 9.2 | 8.6 | 0.437 |
| Hepatic disease | 7.3 | 6.0 | 4.5 | 4.6 | <0.001 |
| Gastric and duodenal ulcer | 16.7 | 15.2 | 15.7 | 15.1 | 0.323 |
| Body pain ≥moderate (%) | 31.1 | 28.6 | 28.9 | 26.7 | <0.001 |
| Been in bed for >1 wk (%) | 5.9 | 3.7 | 3.2 | 2.9 | <0.001 |
| Weight reduction of ≥2 kg compared with 1 y ago (%) | 14.0 | 13.5 | 12.2 | 12.0 | 0.001 |
| Current smoker (%) | 18.4 | 14.1 | 11.4 | 11.4 | <0.001 |
| Current alcohol drinker (%) | 43.9 | 39.9 | 36.8 | 32.8 | <0.001 |
| Frequent cognitive activity (%)4 | 34.2 | 40.2 | 45.1 | 44.8 | <0.001 |
| Social support (%) | |||||
| To consult when you are in trouble | 85.5 | 89.3 | 91.5 | 92.7 | <0.001 |
| To consult when you are in poor physical condition | 91.3 | 93.9 | 94.1 | 95.1 | <0.001 |
| To help with your daily housework | 82.8 | 85.2 | 86.2 | 86.9 | <0.001 |
| To take you to a hospital | 90.3 | 92.8 | 93.2 | 93.7 | <0.001 |
| To take care of you | 84.9 | 88.2 | 87.0 | 86.8 | <0.001 |
| Participation in community activities (%) | |||||
| Activities in neighborhood association | 41.4 | 49.1 | 51.0 | 50.8 | <0.001 |
| Sports or exercise | 39.7 | 47.9 | 49.4 | 50.3 | <0.001 |
| Volunteering | 28.4 | 32.4 | 33.7 | 34.0 | 0.001 |
| Social gathering | 40.9 | 49.3 | 52.4 | 53.0 | <0.001 |
| Time spent walking ≥1 h/d (%) | 39.0 | 36.9 | 35.4 | 32.5 | <0.001 |
| Better motor function (%)5 | 75.4 | 76.1 | 78.5 | 79.2 | <0.001 |
| Intake of (g/d) | |||||
| Rice | 434 ± 220 | 429 ± 228 | 425 ± 197 | 421 ± 186 | 0.078 |
| Miso soup | 19.7 ± 9.7 | 20.2 ± 10.3 | 20.4 ± 8.6 | 21.7 ± 74.3 | 0.233 |
| Meat | 21.2 ± 15.7 | 22.4 ± 16.7 | 23.0 ± 16.2 | 23.6 ± 16.4 | <0.001 |
| Fish | 57.0 ± 32.5 | 59.1 ± 31.5 | 62.2 ± 30.8 | 65.7 ± 31.2 | <0.001 |
| Green and yellow vegetables | 79.8 ± 46.6 | 89.5 ± 47.5 | 96.2 ± 45.9 | 105.4 ± 47.5 | <0.001 |
| Potatoes | 21.2 ± 16.4 | 23.1 ± 16.2 | 25.4 ± 16.1 | 28.3 ± 16.6 | <0.001 |
| Soy products | 57.6 ± 29.9 | 62.7 ± 28.3 | 66.0 ± 26.5 | 68.8 ± 25.5 | <0.001 |
| Fruit | 113.6 ± 89.8 | 132.1 ± 92.0 | 145.8 ± 91.0 | 160.6 ± 92.0 | <0.001 |
| Sweets | 14.6 ± 15.7 | 16.6 ± 15.9 | 18.2 ± 16.2 | 20.3 ± 17.3 | <0.001 |
| Black tea consumption of <1 cup/d (%) | 95.5 | 86.6 | 91.6 | 90.7 | <0.001 |
| Oolong tea consumption of <1 cup/d (%) | 95.0 | 89.2 | 93.2 | 92.1 | <0.001 |
| Coffee consumption of <1 cup/d (%) | 50.4 | 40.2 | 48.2 | 55.2 | <0.001 |
| Energy intake (kcal/d)6 | 1355 ± 423 | 1402 ± 417 | 1445 ± 394 | 1495 ± 374 | <0.001 |
| Protein intake (g/d) | 48.9 ± 14.8 | 51.3 ± 14.5 | 53.9 ± 13.8 | 56.8 ± 13.7 | <0.001 |
Obtained by using chi-square test for variables of proportion and 1-factor ANOVA for continuous variables.
Mean ± SD (all such values).
Kessler 6-item psychological distress scale score ≥13.
Cognitive activity score ≥23.
Motor function score of the Kihon Checklist <3.
Excluding alcohol.
The relation between green tea consumption and incident functional disability with HRs and associated 95% CIs are shown in Table 2. We found that green tea consumption was inversely associated with incident functional disability in model 1 (P-trend < 0.001). Even with the addition of the several adjustment items, these associations remained significant. In model 4, the multivariate HRs were 1.00 (reference) for <1 cup/d, 0.90 (95% CI: 0.77, 1.06) for 1–2 cups/d, 0.75 (95% CI: 0.64, 0.88) for 3–4 cups/d, and 0.67 (95% CI: 0.57, 0.79) for ≥5 cups/d. This inverse association was significant for both sexes (P = 0.384 for interaction with sex).
TABLE 2.
Relation between green tea consumption and incident functional disability1
| Green tea consumption |
||||||
| Incident functional disability | <1 cup/d | 1–2 cups/d | 3–4 cups/d | ≥5 cups/d | P-trend | P-interaction |
| All (n = 13,988) | ||||||
| No. of participants | 2318 | 3141 | 3978 | 4551 | ||
| Primary outcome events [no. (%)] | 296 (12.8) | 343 (10.9) | 339 (8.5) | 338 (7.4) | ||
| Model 1 | 1.00 (reference)2 | 0.79 (0.68, 0.93) | 0.60 (0.51, 0.70) | 0.51 (0.44, 0.60) | <0.001 | |
| Model 2 | 1.00 (reference) | 0.86 (0.74, 1.01) | 0.70 (0.60, 0.82) | 0.61 (0.52, 0.72) | <0.001 | |
| Model 3 | 1.00 (reference) | 0.88 (0.75, 1.03) | 0.72 (0.61, 0.85) | 0.63 (0.54, 0.75) | <0.001 | |
| Model 4 | 1.00 (reference) | 0.90 (0.77, 1.06) | 0.75 (0.64, 0.88) | 0.67 (0.57, 0.79) | <0.001 | |
| Men (n = 6186) | ||||||
| No. of participants | 1320 | 1536 | 1691 | 1639 | ||
| Primary outcome events [no. (%)] | 140 (10.6) | 138 (9.0) | 140 (8.3) | 108 (6.6) | ||
| Model 1 | 1.00 (reference) | 0.80 (0.63, 1.01) | 0.71 (0.56, 0.89) | 0.55 (0.42, 0.70) | <0.001 | |
| Model 2 | 1.00 (reference) | 0.90 (0.71, 1.15) | 0.87 (0.68, 1.10) | 0.64 (0.50, 0.83) | <0.001 | |
| Model 3 | 1.00 (reference) | 0.90 (0.70, 1.14) | 0.85 (0.66, 1.08) | 0.64 (0.49, 0.83) | 0.001 | |
| Model 4 | 1.00 (reference) | 0.88 (0.69, 1.13) | 0.86 (0.68, 1.10) | 0.67 (0.52, 0.88) | 0.005 | 0.384 |
| Women (n = 7802) | ||||||
| No. of participants | 998 | 1605 | 2287 | 2912 | ||
| Primary outcome events [no. (%)] | 156 (15.6) | 205 (12.8) | 199 (8.7) | 230 (7.9) | ||
| Model 1 | 1.00 (reference) | 0.78 (0.64, 0.96) | 0.53 (0.43, 0.66) | 0.49 (0.40, 0.60) | <0.001 | |
| Model 2 | 1.00 (reference) | 0.83 (0.67, 1.02) | 0.61 (0.50, 0.76) | 0.58 (0.47, 0.71) | <0.001 | |
| Model 3 | 1.00 (reference) | 0.84 (0.68, 1.04) | 0.64 (0.52, 0.80) | 0.62 (0.50, 0.77) | <0.001 | |
| Model 4 | 1.00 (reference) | 0.87 (0.70, 1.07) | 0.67 (0.54, 0.83) | 0.65 (0.53, 0.81) | <0.001 | |
Model 1 was adjusted for age (65–69, 70–74, 75–79, 80–84, or ≥85 y) and sex (among all participants). Model 2 was adjusted as for model 1 plus history of disease [stroke, myocardial infarction, hypertension, arthritis, osteoporosis, or fracture (yes, no)], educational level (age at last school graduation: <16 y, 16–18 y, ≥19 y, or missing), smoking (never, former, current, or missing), alcohol drinking (never, former, current, or missing), BMI (in kg/m2; <18.5, 18.5–24.9, ≥25.0, or missing), cognitive activity score (<19, 19–23, ≥23, or missing), psychological distress score (<13, ≥13, or missing), and time spent walking (<30 min/d, 30 min to 1 h/d, ≥1 h/d, or missing). Model 3 was adjusted as for model 2 plus 3 tertile groups of consumption volume of rice, miso soup, meat, fish, green and yellow vegetables, potatoes, soy products, fruit, and sweets. Model 4 was adjusted as for model 3 plus social support (whether subject perceived that he or she was supported for all 5 categories), participation in community activities (whether subject participated in any of 4 categories), and motor function score(<3, ≥3, or missing).
HR; 95% CI in parentheses (all such values).
Even if we set stricter criteria for disability (LTCI certification for Care Level 2 or higher), the results did not change. The multivariate HRs (model 4) were 1.00 (reference) for <1 cup/d, 0.92 (95% CI: 0.72, 1.17) for 1–2 cups/d, 0.71 (95% CI: 0.55, 0.91) for 3–4 cups/d, and 0.68 (95% CI: 0.53, 0.88) for ≥5 cups/d (data not shown).
To examine possible reverse causality, we analyzed whether the association would be different by excluding participants whose event of disability occurred in the first year of follow-up. When we excluded 577 such participants, the results did not change substantially. The multivariate HRs (model 4) were 1.00 (reference) for <1 cup/d, 0.91 (95% CI: 0.75, 1.10) for 1–2 cups/d, 0.81 (95% CI: 0.66, 0.98) for 3–4 cups/d, and 0.71 (95% CI: 0.58, 0.87) for ≥5 cups/d (data not shown). In addition, when we excluded participants with any history of diseases that cause functional disability (stroke, myocardial infarction, hypertension, arthritis, osteoporosis, or fracture), the results also did not change substantially. The multivariate HRs (model 4) were 1.00 (reference) for <1 cup/d, 0.89 (95% CI: 0.66, 1.20) for 1–2 cups/d, 0.69 (95% CI: 0.51, 0.94) for 3–4 cups/d, and 0.72 (95% CI: 0.53, 0.98) for ≥5 cups/d (n = 4954; data not shown).
To confirm whether there was a relation between green tea consumption and incident functional disability, irrespective of social support or participation in community activities, we also conducted stratified analyses for these 2 factors (see Table 3). The inverse association was observed irrespective of social support or participation in community activities (P = 0.103 for interaction with social support, P = 0.585 for interaction with community activities).
TABLE 3.
Relation between green tea consumption and incident functional disability stratified by social support and community activity subgroup1
| Green tea consumption |
||||||
| <1 cup/d | 1–2 cups/d | 3–4 cups/d | ≥5 cups/d | P-trend | P-interaction | |
| Social support | ||||||
| No lack | ||||||
| No. of participants | 1570 | 2252 | 2947 | 3392 | ||
| Primary outcome events [no. (%)] | 208 (13.3) | 248 (11.0) | 235 (8.0) | 239 (7.1) | ||
| Age-and sex-adjusted HR (95% CI)2 | 1.00 (reference) | 0.75 (0.63, 0.90) | 0.54 (0.45, 0.65) | 0.46 (0.38, 0.56) | <0.001 | |
| Multiple-adjusted HR (95% CI)3 | 1.00 (reference) | 0.89 (0.73, 1.07) | 0.68 (0.56, 0.83) | 0.61 (0.50, 0.75) | <0.001 | 0.103 |
| Any lack | ||||||
| No. of participants | 624 | 710 | 867 | 979 | ||
| Primary outcome events [no. (%)] | 74 (11.9) | 75 (10.6) | 81 (9.3) | 83 (8.5) | ||
| Age-and sex-adjusted HR (95% CI)2 | 1.00 (reference) | 0.86 (0.62, 1.19) | 0.65 (0.48, 0.90) | 0.59 (0.43, 0.81) | <0.001 | |
| Multiple-adjusted HR (95% CI)3 | 1.00 (reference) | 0.95 (0.68, 1.33) | 0.78 (0.56, 1.09) | 0.74 (0.53, 1.04) | 0.047 | |
| Participation in community activities | ||||||
| Participated | ||||||
| No. of participants | 1114 | 1669 | 2297 | 2542 | ||
| Primary outcome events [no. (%)] | 80 (7.2) | 106 (6.4) | 122 (5.3) | 115 (4.5) | ||
| Age-and sex-adjusted HR (95% CI)2 | 1.00 (reference) | 0.80 (0.60, 1.08) | 0.61 (0.46, 0.82) | 0.52 (0.39, 0.70) | <0.001 | |
| Multiple-adjusted HR (95% CI)3 | 1.00 (reference) | 0.84 (0.62, 1.13) | 0.73 (0.54, 0.97) | 0.65 (0.48, 0.88) | 0.003 | 0.585 |
| Did not participate | ||||||
| No. of participants | 781 | 802 | 951 | 1066 | ||
| Primary outcome events [no. (%)] | 162 (20.7) | 164 (20.5) | 139 (14.6) | 142 (13.3) | ||
| Age-and sex-adjusted HR (95% CI)2 | 1.00 (reference) | 0.86 (0.69, 1.07) | 0.62 (0.49, 0.78) | 0.55 (0.44, 0.70) | <0.001 | |
| Multiple-adjusted HR (95% CI)3 | 1.00 (reference) | 0.90 (0.72, 1.13) | 0.69 (0.55, 0.88) | 0.64 (0.50, 0.81) | <0.001 | |
Any lack, participants who perceived that they were not supported for at least one social support category; Did not participate, participants who did not participate in any community activities; No lack, participants who perceived that they were supported for all 5 social support categories; Participated, participants who participated in at least one community activity.
Adjusted as for model 1 in Table 2.
Adjusted as for model 4 in Table 2.
The multiple-adjusted HRs for the primary outcome event according to frequency of consumption of oolong tea, black tea, and coffee are compared in Table 4. We observed a weak association between coffee consumption and incident functional disability in age- and sex-adjusted models (P-trend= 0.023). However, there were null associations for consumption of oolong tea, black tea, or coffee in multiple-adjusted models.
TABLE 4.
Relation between consumption of other beverages and incident functional disability
| Beverage consumption |
|||||
| <1 cup/d | 1–2 cups/d | 3–4 cups/d | ≥5 cups/d | P-trend | |
| Oolong tea (Chinese tea) | |||||
| No. of participants | 10,482 | 502 | 225 | 153 | |
| Primary outcome events [no. (%)] | 925 (8.8) | 45 (9.0) | 11 (4.9) | 13 (8.5) | |
| Age- and sex-adjusted HR (95% CI)1 | 1.00 (reference) | 1.12 (0.83, 1.52) | 0.58 (0.32, 1.05) | 0.94 (0.54, 1.63) | 0.387 |
| Multiple-adjusted HR (95% CI)2 | 1.00 (reference) | 1.47 (1.07, 2.03) | 0.77 (0.42, 1.40) | 1.25 (0.71, 2.18) | 0.354 |
| Black tea | |||||
| No. of participants | 10,408 | 785 | 190 | 66 | |
| Primary outcome events [no. (%)] | 914 (8.8) | 73 (9.3) | 11 (5.8) | 4 (6.1) | |
| Age- and sex-adjusted HR (95% CI)1 | 1.00 (reference) | 1.11 (0.87, 1.41) | 0.61 (0.34, 1.11) | 0.65 (0.24, 1.74) | 0.323 |
| Multiple-adjusted HR (95% CI)2 | 1.00 (reference) | 1.23 (0.96, 1.59) | 0.82 (0.45, 1.51) | 1.01 (0.37, 2.75) | 0.567 |
| Coffee | |||||
| No. of participants | 6317 | 4997 | 1031 | 538 | |
| Primary outcome events [no. (%)] | 701 (11.1) | 357 (7.1) | 62 (6.0) | 41 (7.6) | |
| Age- and sex-adjusted HR (95% CI)1 | 1.00 (reference) | 0.83 (0.73, 0.94) | 0.82 (0.63, 1.07) | 0.92 (0.67, 1.27) | 0.023 |
| Multiple-adjusted HR (95% CI)2 | 1.00 (reference) | 0.90 (0.79, 1.03) | 0.93 (0.72, 1.22) | 1.02 (0.74, 1.41) | 0.408 |
DISCUSSION
In this study, we found significant inverse dose-response associations between green tea consumption and incident functional disability. To our knowledge, this is the first reported study to have proved the relation between green tea consumption and incident risk of functional disability.
Our study had a number of strengths: 1) it was a large population-based cohort study in 13,988 persons, 2) it had a follow-up rate of almost 100%, 3) the study subjects lived in an area in which green tea is widely consumed, and 4) many confounding factors were taken into account.
Because green tea consumption is associated a variety of health behavior or social factors, we used several approaches to control for these effects. First, we adjusted the effect of dietary habit, because green tea is usually consumed with a Japanese-style diet such as fish and soy bean products (Table 1). Consumption of fish and soy products has been reported to reduce the risk of stroke, fracture, and dementia (35–40). However, our results indicated that the association between green tea consumption and incident functional disability did not alter, even when dietary covariates were adjusted for.
Second, we also considered the confounding effect of social support or community activities. Previous studies have shown that these factors are associated with a lower risk of functional disability (41, 42). However, we found that the inverse association between green tea consumption and incident functional disability persisted even after adjustment for social support and participation in community activities.
Because our follow-up period was only 3 y, the effects of reverse causality could not be fully avoided. However, the strong inverse relation between green tea consumption and incident functional disability persisted even after excluding individuals who experienced incident functional disability in the first year of follow-up. The above findings suggest that the present results are unlikely to be explained by reverse causality.
We thus considered that the inverse relation between green tea consumption and functional disability risk would be attributable to the preventive effect of green tea consumption on disabling diseases such as stroke, cognitive impairment, and osteoporosis. These diseases are major causes of functional disability in Japanese elderly individuals, with prevalence as follows: 23.3% for stroke, 14.0% for dementia, 12.2% for articular disease, and 9.3% for bone fracture (43). As we noted before, green tea consumption was associated with lower risks of stroke, dementia, and bone fracture. This survey reported that the third most common cause of functional disability was “frailty” (13.6%), which is mostly associated with sarcopenia and lower muscle strength. More recently, green tea polyphenols have been reported to improve leg strength (44). Furthermore, depression is also known to pose a risk of functional disability in the elderly (45). Our previous study indicated that green tea consumption was associated with a lower risk of depression. All of these findings provide a biological basis for the effect of green tea in preventing or postponing the onset of functional disability in the elderly.
In contrast to green tea, we observed no association between black tea, oolong tea, or coffee consumption and incident functional disability, which is consistent with previous epidemiologic studies (1, 3–5). This discrepancy among beverages suggests that the effect of green tea cannot be explained by fluid intake but rather by some component in the beverage. As compared with black tea and oolong tea, green tea contains a large amount of polyphenols such as epigallocatechin gallate, which reduce oxidative damage to DNA and lipid concentrations (46–48). Randomized controlled trials of green tea polyphenol have indicated that it exerts antiatherosclerotic effects by reducing the level of oxidative stress (49).
This study had several limitations. First, we did not investigate the causes of functional disability in subjects who received LTCI certification. Thus, the mechanism responsible for functional disability reduction by green tea remained unidentified.
Second, among the source population of 31,694, the valid response rate (72.9%, n = 23,091) in the present study was not high. In addition, among the number of valid responses (n = 23,091), the number of subjects included in the present study was 13,988 (60.6%) and the number of those who were not included was 9103 (39.4%). Three-year follow-up indicated that mortality was higher in the nonstudy subjects (13%) than in the study subjects (5%). Thus, the present study would have been biased toward the healthier people in the community. However, this bias did not explain to affect the internal validity of association between green tea consumption and incident functional disability.
Third, not all potential confounding factors were considered, because we used only indirect measures of physical and cognitive function for adjustment. Furthermore, addition of income to the multivariate analysis might have been an appropriate indicator of socioeconomic status.
Fourth, because not all candidates applied for LTCI certification, this study may not have been completely free from detection bias. The degree of this bias remains to be verified.
In conclusion, this cohort study indicates that green tea consumption is inversely associated with incident functional disability. Clinical trials are ultimately be necessary to confirm the protective effect of green tea against functional disability.
Acknowledgments
We thank Yoshiko Nakata, Mika Wagatsuma, and Yuko Kikuchi for their technical assistance.
The authors’ responsibilities were as follows—YT, MK, SK, AH, and IT: study concept and design; YT, MK, NN, SK, AH, and IT: acquisition of data; YT, MK, TT, SK, AH, and IT: analysis and interpretation of data; YT: drafting of the manuscript; YT, MK, NN, TT, TS,SK, AH, and IT: critical revision of the manuscript for important intellectual content; YT, MK, AH, and IT: statistical analysis; and IT: study supervision. None of the authors declared a conflict of interest.
REFERENCES
- 1.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 2006;296:1255–65 [DOI] [PubMed] [Google Scholar]
- 2.Watanabe I, Kuriyama S, Kakizaki M, Sone T, Ohmori-Matsuda K, Nakaya N, Hozawa A, Tsuji I. Green tea and death from pneumonia in Japan: the Ohsaki cohort study. Am J Clin Nutr 2009;90:672–9 [DOI] [PubMed] [Google Scholar]
- 3.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr 2006;83:355–61 [DOI] [PubMed] [Google Scholar]
- 4.Niu K, Hozawa A, Kuriyama S, Ebihara S, Guo H, Nakaya N, Ohmori-Matsuda K, Takahashi H, Masamune Y, Asada M, et al. Green tea consumption is associated with depressive symptoms in the elderly. Am J Clin Nutr 2009;90:1615–22 [DOI] [PubMed] [Google Scholar]
- 5.Hozawa A, Kuriyama S, Nakaya N, Ohmori-Matsuda K, Kakizaki M, Sone T, Nagai M, Sugawara Y, Nitta A, Tomata Y, et al. Green tea consumption is associated with lower psychological distress in a general population: the Ohsaki Cohort 2006 Study. Am J Clin Nutr 2009;90:1390–6 [DOI] [PubMed] [Google Scholar]
- 6.Arab L, Liu W, Elashoff D. Green and black tea consumption and risk of stroke: a meta-analysis. Stroke 2009;40:1786–92 [DOI] [PubMed] [Google Scholar]
- 7.Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, Yamamoto A, Kikuchi S, Inaba Y, Toyoshima H, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health 2011;65:230–40 [DOI] [PubMed] [Google Scholar]
- 8.Tanabe N, Suzuki H, Aizawa Y, Seki N. Consumption of green and roasted teas and the risk of stroke incidence: results from the Tokamachi-Nakasato cohort study in Japan. Int J Epidemiol 2008;37:1030–40 [DOI] [PubMed] [Google Scholar]
- 9.Ng TP, Feng L, Niti M, Kua EH, Yap KB. Tea consumption and cognitive impairment and decline in older Chinese adults. Am J Clin Nutr 2008;88:224–31 [DOI] [PubMed] [Google Scholar]
- 10.Wu CH, Yang YC, Yao WJ, Lu FH, Wu JS, Chang CJ. Epidemiological evidence of increased bone mineral density in habitual tea drinkers. Arch Intern Med 2002;162:1001–6 [DOI] [PubMed] [Google Scholar]
- 11.Muraki S, Yamamoto S, Ishibashi H, Oka H, Yoshimura N, Kawaguchi H, Nakamura K. Diet and lifestyle associated with increased bone mineral density: cross-sectional study of Japanese elderly women at an osteoporosis outpatient clinic. J Orthop Sci 2007;12:317–20 [DOI] [PubMed] [Google Scholar]
- 12.Nantz MP, Rowe CA, Bukowski JF, Percival SS. Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition 2009;25:147–54 [DOI] [PubMed] [Google Scholar]
- 13.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88:38–50 [DOI] [PubMed] [Google Scholar]
- 14.Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, Jacob KS, Jotheeswaran AT, Rodriguez JJ, Pichardo GR, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet 2009;374:1821–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiers NA, Matthews RJ, Jagger C, Matthews FE, Boult C, Robinson TG, Brayne C. Diseases and impairments as risk factors for onset of disability in the older population in England and Wales: findings from the Medical Research Council Cognitive Function and Ageing Study. J Gerontol A Biol Sci Med Sci 2005;60:248–54 [DOI] [PubMed] [Google Scholar]
- 16.Wolff JL, Boult C, Boyd C, Anderson G. Newly reported chronic conditions and onset of functional dependency. J Am Geriatr Soc 2005;53:851–5 [DOI] [PubMed] [Google Scholar]
- 17.Kuriyama S, Nakaya N, Ohmori-Matsuda K, Shimazu T, Kikuchi N, Kakizaki M, Sone T, Sato F, Nagai M, Sugawara Y, et al. The Ohsaki Cohort 2006 Study: design of study and profile of participants at baseline. J Epidemiol 2010;20:253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–8 [DOI] [PubMed] [Google Scholar]
- 19.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, Walters EE, Zaslavsky AM. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002;32:959–76 [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, Howes MJ, Normand SL, Manderscheid RW, Walters EE, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry 2003;60:184–9 [DOI] [PubMed] [Google Scholar]
- 21.Tomata Y, Hozawa A, Ohmori-Matsuda K, Nagai M, Sugawara Y, Nitta A, Kuriyama S, Tsuji I. Validation of the Kihon Checklist for predicting the risk of 1-year incident long-term care insurance certification: the Ohsaki Cohort 2006 Study. Nippon Koshu Eisei Zasshi 2011;58:3–13 (in Japanese) [PubMed] [Google Scholar]
- 22.Ogawa K, Tsubono Y, Nishino Y, Watanabe Y, Ohkubo T, Watanabe T, Nakatsuka H, Takahashi N, Kawamura M, Tsuji I, et al. Validation of a food-frequency questionnaire for cohort studies in rural Japan. Public Health Nutr 2003;6:147–57 [DOI] [PubMed] [Google Scholar]
- 23.Muraoka Y, Ikichi A, Ihara K. The physical and psychological and social background factor of elderly depression in the community. Ronen Seishin Igaku Zasshi 1996;7:397–407 (in Japanese) [Google Scholar]
- 24.Imai H, Fujii Y, Fukuda Y, Nakao H, Yahata Y. Health-related quality of life and beneficiaries of long-term care insurance in Japan. Health Policy 2008;85:349–55 [DOI] [PubMed] [Google Scholar]
- 25.Ikegami N. Public long-term care insurance in Japan. JAMA 1997;278:1310–4 [PubMed] [Google Scholar]
- 26.Tsutsui T, Muramatsu N. Care-needs certification in the long-term care insurance system of Japan. J Am Geriatr Soc 2005;53:522–7 [DOI] [PubMed] [Google Scholar]
- 27.Imahashi K, Kawagoe M, Eto F, Haga N. Clinical status and dependency of the elderly requiring long-term care in Japan. Tohoku J Exp Med 2007;212:229–38 [DOI] [PubMed] [Google Scholar]
- 28.Ministry of Health, Labor, and Welfare. Long-term care insurance in Japan. Tokyo, Japan: Ministry of Health, Labor, and Welfare, 2008. Available from: http://www.mhlw.go.jp/english/topics/elderly/care/index.html (cited 20 October 2011).
- 29.Arai Y, Zarit SH, Kumamoto K, Takeda A. Are there inequities in the assessment of dementia under Japan's LTC insurance system? Int J Geriatr Psychiatry 2003;18:346–52 [DOI] [PubMed] [Google Scholar]
- 30.Takeda S. Two-year survival and changes in the level of care for the elderly patients recognized as in need of long-term care in the public nursing-care insurance scheme. Nippon Koshu Eisei Zasshi 2004;51:157–67 (in Japanese) [PubMed] [Google Scholar]
- 31.Kondo N, Kawachi I, Hirai H, Kondo K, Subramanian SV, Hanibuchi T, Yamagata Z. Relative deprivation and incident functional disability among older Japanese women and men: prospective cohort study. J Epidemiol Community Health 2009;63:461–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nitta A, Hozawa A, Kuriyama S, Nakaya N, Ohmori-Matsuda K, Sone T, Kakizaki M, Ebihara S, Ichiki M, Arai H. doi: 10.5551/jat.5389. Relationship between peripheral arterial disease and incident disability among elderly Japanese: the Tsurugaya project. J Atheroscler Thromb 2010;26(17):1290–6. [DOI] [PubMed] [Google Scholar]
- 33.Hozawa A, Sugawara Y, Tomata Y, Kakizaki M, Ohmori-Matsuda K, Nakaya N, Kuriyama S, Fukao S, Tsuji I. Relationships between N-terminal pro B-type natriuretic peptide and incident disability and mortality in older community-dwelling adults: the Tsurugaya study. J Am Geriatr Soc 2010;58:2439–41 [DOI] [PubMed] [Google Scholar]
- 34.Saito M, Kobayashi A, Hattori Y. The Effects of “OCHANOMI” on elderly individuals, aged 65 to 74, in relation to social support, subjective well-being, and results of social exchanges. J Jpn Acad Com Health Nurs 2005;7:41–7 (in Japanese) [Google Scholar]
- 35.Shimazu T, Kuriyama S, Hozawa A, Ohmori K, Sato Y, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Dietary patterns and cardiovascular disease mortality in Japan: a prospective cohort study. Int J Epidemiol 2007;36:600–9 [DOI] [PubMed] [Google Scholar]
- 36.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, Davey Smith G, et al. doi: 10.1002/14651858.CD003177.pub2. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev 2004. 18;4:CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim WS, Gammack JK, Van Niekerk J, Dangour AD. doi: 10.1002/14651858.CD005379.pub2. Omega 3 fatty acid for the prevention of dementia. Cochrane Database Syst Rev 2006. 25;1:CD005379. [DOI] [PubMed] [Google Scholar]
- 38.Carlson S, Peng N, Prasain JK, Wyss JM. doi: 10.1016/j.genm.2008.03.008. Effects of botanical dietary supplements on cardiovascular, cognitive, and metabolic function in males and females. Gend Med 2008;5(suppl A):S76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J Nutr Biochem 2005;16:641–9 [DOI] [PubMed] [Google Scholar]
- 40.Atmaca A, Kleerekoper M, Bayraktar M, Kucuk O. doi: 10.1097/gme.0b013e31815c1e7f. Soy isoflavones in the management of postmenopausal osteoporosis. Menopause 2008;15(4):748–57. [DOI] [PubMed] [Google Scholar]
- 41.Mendes de Leon CF, Gold DT, Glass TA, Kaplan L, George LK. Disability as a function of social networks and support in elderly African Americans and Whites: the Duke EPESE 1986-1992. J Gerontol B Psychol Sci Soc Sci 2001;56:S179–90 [DOI] [PubMed] [Google Scholar]
- 42.Jung Y, Gruenewald TL, Seeman TE, Sarkisian CA. Productive activities and development of frailty in older adults. J Gerontol B Psychol Sci Soc Sci 2010;65B:256–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ministry of Health, Labor, and Welfare. 19. Percentage distribution of major causes due to need of assistance or care by sex, 2007. Comprehensive Survey of Living Conditions. Available from: http://www.mhlw.go.jp/english/database/db-hss/cslc-tables.html (cited 20 October 2011)
- 44.Shen CL, Chyu MC, Yeh JK, Zhang Y, Pence BC, Felton CK, Brismée JM, Arjmandi BH, Doctolero S, Wang JS. doi: 10.1007/s00198-011-1731-x. Effect of green tea and Tai Chi on bone health in postmenopausal osteopenic women: a 6-month randomized placebo-controlled trial. Osteoporos Int (Epub ahead of print 16 July 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenze EJ, Schulz R, Martire LM, Zdaniuk B, Glass T, Kop WJ, Jackson SA, Reynolds CF III. The course of functional decline in older people with persistently elevated depressive symptoms: longitudinal findings from the Cardiovascular Health Study. J Am Geriatr Soc 2005;53:569–75 [DOI] [PubMed] [Google Scholar]
- 46.Cabrera C, Giménez R, López MC. Determination of tea components with antioxidant activity. J Agric Food Chem 2003;51:4427–35 [DOI] [PubMed] [Google Scholar]
- 47.Han KC, Wong WC, Benzie IF. A genoprotective effects of green tea (Camellia sinensis) in human subjects: results of a controlled supplementation trial. Br J Nutr 2011;105:171–9 [DOI] [PubMed] [Google Scholar]
- 48.Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem 2005;16:144–9 [DOI] [PubMed] [Google Scholar]
- 49.Oyama J, Maeda T, Kouzuma K, Ochiai R, Tokimitsu I, Higuchi Y, Sugano M, Makino N. Green tea catechins improve human forearm endothelial dysfunction and have antiatherosclerotic effects in smokers. Circ J 2010;74:578–88 [DOI] [PubMed] [Google Scholar]