Abstract
Dietary fatty acids (FA) are increasingly recognized as major biologic regulators and have properties that relate to health outcomes and disease. The longer chain, more bioactive (n-6) (or omega-6) FA and (n-3) (or omega-3) FA share similar elongation and desaturation enzymes in their conversion from the essential (n-6) FA, linoleic acid, and (n-3) FA, α-linolenic acid (ALA). Conversion from these essential FA is very inefficient. However, now for the (n-3) FA series, soy oil can be enriched with (n-3) stearidonic acid (SDA) to allow for much more efficient conversion to longer chain EPA. EPA and the longer chain DHA possess distinct physical and biological properties that generally impart properties to cells and tissue, which underlie their ability to promote health and prevent disease. Although active in a number of areas of human biology, mechanisms of action of EPA and DHA are perhaps best defined in cardiovascular disease. There is concern that to reach the intake recommendations of EPA and DHA, their supply from cold water fish will be insufficient. Gaps in understanding mechanisms of action of (n-3) FA in a number of health and disease areas as well as optimal sources and intake levels for each need to be defined by further research. Because of the inefficient conversion of ALA, the appearance of SDA in enriched soy oil offers a biologically effective and cost effective approach to providing a sustainable plant source for (n-3) FA in the future.
Synthesis of Omega-3 and Omega-6 FA
Dietary FA are increasingly recognized as major biologic regulators and have properties that relate to both health outcomes and disease (1–3). The (n-6) (or omega-6) FA are defined by having their first double bond six carbons from the methyl terminal of the FA6 and the (n-3) (or omega-3) series have the first double bond three carbons from the methyl terminal. Mammals require the two essential FA, (n-3) ALA [18:3 (n-3)] and (n-6) LA [18:2 (n-6)] for elongation and desaturation to their more bioactive derivatives. The longer chain (n-3) FA bioactive derivatives (n-3) EPA [20:5 (n-3)] and (n-3) DHA [22:6 (n-3)] as well as (n-6) arachidonic acid [20:4 (n-6)] can also be acquired by oral intake. During elongation and desaturation, similar enzymes are used by both series so that possibilities of substrate competition exist. However, under most dietary conditions, the amounts of each essential FA, EPA, and DHA in the diet are most relevant in determining (n-6) and (n-3) FA status (4). LA is elongated and desaturated to its major bioactive derivative, arachidonic acid, a FA that in general leads to the production of proinflammatory cytokines and eicosanoids but can also lead to the production of proresolution and antiinflammatory lipoxins (5). Elongation and desaturation of the (n-3) FA series lead to the production of EPA, which generally produces cytokines and other derivatives that are antiinflammatory and reduce proinflammatory effects. Continued elongation and desaturation of EPA lead to the synthesis of DHA. DHA is a major component of brain phospholipids and retinal membranes and also has antiinflammatory effects. Membrane EPA and DHA can be further metabolized by the initial action of phospholipases into very potent antiinflammatory and antiapoptotic docosanoids, the resolvins and protectins (5). Generally, conversion of the essential FA, LA and ALA, to their more unsaturated, bioactive, long-chain derivatives is very inefficient, with most of these FA being utilized for energy (1, 6). Recent studies show that (n-3) SDA (SDA,18:4), which is now being synthesized at high levels in genetically modified soy beans, leads to much more efficient conversion to EPA than does ALA (7), although SDA is only one FA further down the synthetic pathway from ALA.
(n-3) FA in Cell Biology and Human Health
The (n-3) FA have a number of roles in cell function and biology. The (n-3) FA, EPA and DHA, have different biological effects compared to other FA. This likely derives not only from their longer chain length but also the high number and positioning of double bonds, which could impart unique physical and biochemical properties distinct from the more common (n-6) and (n-9) FA and SFA. These physical differences could underlie specific properties of (n-3) FA-rich TG and phospholipids in metabolic pathways (1, 8).
As an example, our laboratory has reported that “classical” mechanisms used for (n-6) FA TG-rich lipoprotein blood clearance, e.g., LpL-mediated TG hydrolysis and LDL receptor or apoprotein E-dependent cell binding are much less important for (n-3) FA-rich particles (9). Rather, (n-3) FA-rich particles use direct lipid-lipid and proteoglycan interactions at the cell surface for blood clearance and cell uptake (10, 11). The long chain FA EPA and DHA can alter cell membrane structure and function generally by increasing fluidity as well as decreasing the amount of membrane occupied by lipid rafts and changing their properties (12). The (n-3) FA and their derivatives are potent molecules important in chemotaxis and other aspects of the immune and inflammatory response. The (n-3) FA decrease blood pressure and alter vascular resistance (13). They are important in a large number of metabolic signaling pathways relating to cell proliferation and differentiation and receptor expression (1). Recent evidence shows that DHA can interact directly with other transcription factors and specific cell receptors (14, 15). Thus, the (n-3) FA are potential regulators of a large number of genes (8). Given the large number of cellular pathways affected by (n-3) FA, it is clear that they have the ability to regulate cell metabolism through multiple mechanisms (as reviewed in more detail in this supplement in the article of Calder et al.).
The (n-3) FA have important roles relating to human health and disease. In infancy, they improve cognitive development and learning as well as visual development. Again, long-chain EPA and DHA have been reported to be beneficial in terms of decreasing the risk of depression and suicide and delaying the onset of the neurological degeneration of aging (1, 16, 17). It has been suggested that these FA decrease the risk of certain forms of cancer (1). Although not recognized to improve manifestations of type 2 diabetes once developed, these FA have beneficial effects on some of the parameters associated with metabolic syndrome (18).
The (n-3) FA are reported in a number of studies to have beneficial effects in decreasing stroke and coronary heart disease (13, 19). A number of intervention trials have shown positive effects from supplementation with (n-3) FA after cardiac events. For example, the Gissi-Prevenzione (20) study showed ~20% improved survival after a cardiac event with substantial benefit evident shortly after 3 mo of (n-3) FA supplementation. However, some intervention studies have not shown benefit of supplementation with (n-3) FA. For example, in the recently reported Alpha Omega Trial, no significant reduction of cardiovascular events after myocardial infarction was reported (21). Of interest, the median time of intervention with (n-3) EPA and DHA supplementation was 3.7 y after the cardiac event compared to Gissi-Prevenzione, where intervention began within the first month after the event (12). Also, in the Alpha Omega Trial, the relatively low dose of EPA plus DHA was provided (~400 mg/d) in margarine and the margarine itself provided ~6000 mg of LA daily. Because (n-6) PUFA have also been proven effective in lowering the risk of heart disease (22) and in the Alpha Omega Trial (n-6) FA were provided to both the control and intervention groups, these (n-6) FA may have confounded the effects of the relatively low levels of (n-3) FA administered.
A number of reasons could contribute to different cardiovascular disease study outcomes in (n-3) FA trials (23). Baseline intakes of both (n-3) and (n-6) FA may not be considered. Populations with high intakes of (n-3) FA may have fewer effects noted from additional (n-3) FA. The duration of the interventions and study endpoints differ substantially between different studies. Also, baseline medications can be additional confounders. Results may vary in primary and secondary preventive studies with (n-3) FA and, as stated above, the timing of the (n-3) intervention after the event is likely important. Finally, the effects of ALA rather than those of EPA and/or DHA are often compared with wide variations in dosages for the different FA.
Mechanisms of Action of (n-3) FA in Cardiovascular Diseases
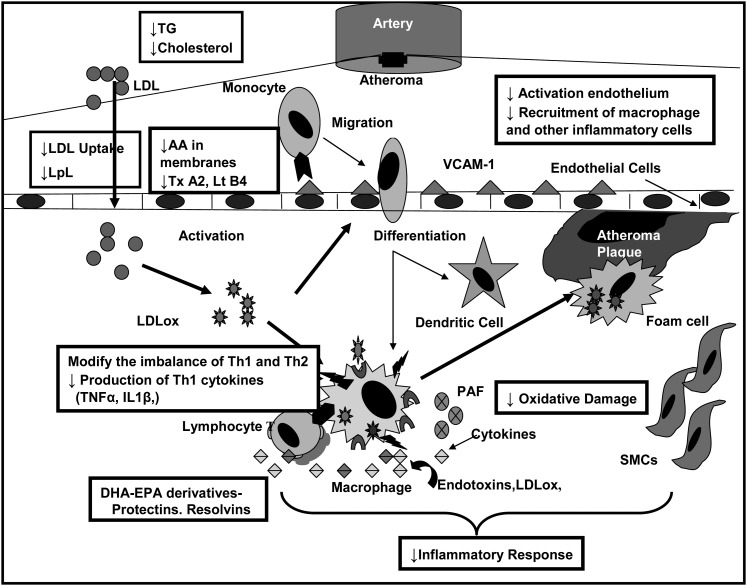
Specific cardiovascular benefits have been reported for (n-3) FA in terms of reducing cardiac death from arrhythmias, providing TG-lowering effects, and providing antithrombotic and antiinflammatory as well as antihypertensive effects (13). Our laboratory, in published (1, 24–26) and unpublished data (C.L. Chang, C. Torrejon, and R.J. Deckelbaum), has shown in a number of animal models that high-fat diets rich in (n-3) FA, especially compared with SFA, decrease dyslipidemia, cholesterol delivery to the arterial wall, and arterial wall proinflammatory pathways, and increase arterial wall antiinflammatory markers. Arterial LDL uptake can be affected by the concentration of circulating LDL and is modulated by different types of dietary FA ingested. Adding to its catalytic activities in hydrolyzing blood TG, LpL has marked effects on cell binding of LDL through its bridging or anchoring abilities and, as an example, binding LDL to the macrophage cell surface (27). In addition to whole particle uptake, selective uptake, which is the uptake of cholesterol esters from LDL without uptake of the whole LDL particle, can also lead to deposition of cholesterol within cells and tissues such as the arterial wall and thereby contribute to atherogenesis. In mouse models, a high-saturated fat diet increased arterial cholesterol delivery via both markedly increased total LDL and selective uptake (>4-fold) and this was associated with increases in arterial wall LpL and LpL activity (25). In contrast, high-fat diets rich in (n-3) FA essentially negated selective uptake and very much decreased whole-particle LDL uptake, with accompanying decreases of LpL in the aortic media (24, 26). Nevertheless, there was a redistribution of LpL to the intima, which also had increased endothelial binding of LDL but with no LDL penetration into the arterial wall (24, 26). Thus, it is possible that (n-3) FA decrease arterial cholesterol deposition by actually anchoring LDL at the intima surface, thus decreasing its penetration into deeper layers to inhibit atherogenesis. In a recent review, we detailed many of the mechanisms associated with (n-3) FA effects at the level of the arterial wall (13). Figure 1 shows a number of potential targets where EPA and DHA can be bioactive antiatherogenic molecules.
FIGURE 1.
EPA and DHA: potential pathways for antiatherogenesis. The boxes emphasize mechanisms of action where EPA/DHA may decrease the development of atherosclerosis. LDLox, oxidized LDL; LpL, lipoprotein lipase; Lt, leukotriene; SMC, smooth muscle cell; Tx, thromboxane; VCAM-1, vascular cell adhesion molecule 1.
(n-3) FA and Stroke
Evidence is mixed as to the possible benefits (n-3) FA-rich diets or (n-3) FA supplements on stroke outcomes in humans (19). Most studies have focused on comparing levels of fish intake in populations that have not previously had a stroke. Of interest, results seem to be more positive for stroke prevention in populations that have low (n-3) FA intakes, such as U.S. populations, but do not show positive results in populations with high baseline (n-3) intakes, such as those in Japan or Sweden (19). Of interest however, in the Japan EPA Lipid Intervention Study where the primary endpoints were cardiac events, supplemental EPA decreased stroke incidence in the secondary prevention group but did not affect primary prevention participants without a previous stroke (28). Thus, although dietary intake of (n-3) FA likely has some protective effects against stroke in humans, the effects are generally weaker than those that have been observed with cardiac morbidity and mortality. In contrast, our laboratory now has early, unpublished data showing that acute injection of TG emulsions rich in EPA and DHA are very effective in markedly reducing brain death when administered after a hypoxic ischemic insult (stroke) in four different rodent models (J.J. Williams, K. Mayurasakorn, V.S. Ten, and R.J. Deckelbaum, unpublished data). This approach has the significant advantage of demonstrated efficacy when administered after the traumatic event, which is the time point when intervention is commonly made.
In summary, although (n-3) FA show positive benefits in a number of health areas and diseases, basic mechanisms underlying these benefits in cardiovascular diseases are probably better defined compared to other health areas and diseases. More research is required to better define the pathways whereby (n-3) FA may or may not be a useful adjunct at a number of periods throughout the lifecycle.
Sources of (n-3) FA
Current sources of (n-3) FA include fish, algae, krill, and plants (29). Table 1 compares the FA composition of a number of sources of (n-3) FA. It is largely the cold water fish that serve as the major sources of EPA and DHA and even these show marked variability in their EPA and DHA content (29). Of note, even fish acquire EPA and DHA from preformed ingestion and from converting some ALA found in krill and algae to the longer, more unsaturated bioactive forms. Seasonal variations of EPA and DHA in a single fish species can be substantial according to the season of catch and the geographic areas where harvested, with variations of as much as 1.5-fold (29).
TABLE 1.
FA composition of different oils1
|
Typical weight % of total FA |
||||||||
| FA | SBO | SDA-SBO | Fish oil | Algal oil | Krill oil | Flax oil | Canola oil | |
| Palmitic acid | 16:0 | 10–11 | 11–13 | 19 | 18–28 | 13 | 3–6 | 0–4 |
| Stearic acid | 18:0 | 4–5 | 3–5 | 3 | 0–2 | 1 | 2–6 | 2–7 |
| Oleic acid | 18:1 (n-9) | 21–23 | 19–21 | 13 | 0–8 | 5 | 9–14 | 50–62 |
| LA | 18:2 (n-6) | 52–53 | 23–25 | 2 | 0–2 | 1 | 9–12 | 20–30 |
| γ-LA | 18:3 (n-6) | <0.1 | 5–7 | 0 | 0 | 0 | 0 | 0 |
| ALA | 18:3 (n-3) | 6–8 | 8–11 | 2 | 0 | 1 | 50–75 | 7–10 |
| SDA | 18:4 (n-3) | 0 | 20–22 | 4 | 0 | 0 | 0 | 0 |
| EPA | 20:5 (n-3) | 0 | 0 | 14 | 0–3 | 14 | 0 | 0 |
| DHA | 22:6 (n-3) | 0 | 0 | 12 | 35 | 8 | 0 | 0 |
Adapted from available literature sources; note columns may not total 100% as some FA are not listed and/or ranges are given for some. ALA, α-linolenic acid; FA, fatty acid; LA, linoleic acid; SBO, soybean oil; SDA, enriched soybean oil.
The (n-3) FA-rich oils are also obtained from sources other than fish (29). Marine microalgae that produce TG very rich in DHA can be selectively cultivated and help solve the problem of seasonal variation and can provide a continuous supply of (n-3) with consistent levels of quality and context. Still, obtaining (n-3) DHA from microalgae presently involves high production costs. In addition, success in cultivating algae to produce high levels of EPA has been quite limited. Some species of krill, however, do have high levels of EPA and DHA, but production costs are also high (29). Thus, SDA-enriched plant sources are potentially a sustainable and cost-effective source of bioactive (n-3) FA.
A few plants provide (n-3) FA. These include canola, soy, and flaxseed. Still, plant oils mainly provide ALA and contain no or only very low levels of EPA and DHA. With the inefficient conversion of ALA to EPA and DHA, overall there is weak evidence for higher dietary intakes of ALA on decreasing risk of heart disease and stroke compared to higher intakes of EPA and DHA. Obtaining (n-3) FA from plants such as flaxseed is unlikely to have a major impact on improving cardiovascular disease outcomes as well as the other health benefits of the longer chain EPA and DHA. Another factor to be considered is that the current supply of cold water fish is likely insufficient to provide European and North American populations with the recommended levels of intake for EPA and DHA. Thus, the availability in the near future of SDA-enriched soy oil is very promising in terms of improving the EPA or DHA status of populations with low intake levels of these bioactive, long chain FA, such as in the US. A number of papers in this supplement will provide evidence for SDA as a cost-efficient, efficacious source of longer chain (n-3) FA. SDA-enriched soy oil is likely to be considered now as one of the “major” sources of (n-3) FA intake, joining ALA, EPA, and DHA.
Although evidence is growing for a role of increasing intakes of (n-3) FA as a major contributor to improving health and reducing the burden of certain diseases, a number of questions regarding (n-3) FA require further inquiry and research. For example, should (n-3) intake be different in different health/disease areas? Are the recommended intakes for cardiovascular disease going to be similar or different than what is recommended for optimizing reproductive outcomes, brain development, mental health, or immune/inflammatory disorders? What are the amounts, dosages, and ratios of (n-3):(n-6) FA that are needed for different health and disease conditions? Will the sources of (n-3) FA be important in terms of whether they are derived from TG or phospholipids or as ethyl esters? How will individual and population baseline (n-3) and (n-6) FA intakes and levels affect results of intervention studies? What is the timing and duration needed for (n-3) FA to have optimal effects? Finally, which (n-3) FA are needed for most beneficial effects—ALA, EPA, and/or DHA? SDA now also needs to be considered and evaluated more as an important (n-3) FA in addressing how best to utilize (n-3) FA for health promotion and disease prevention and therapy. These questions, along with the likely utility of SDA as a sustainable source of bioactive (n-3) FA, can certainly be approached in the next few years by appropriate research studies. In addition, there is a need for better understandings on the underlying mechanisms whereby specific (n-3) FA may benefit different areas of normal and abnormal human biology.
Acknowledgments
R.J.D. conceptualized and wrote the manuscript with contributions from C.J. on the figure and concepts related to (n-3) FA and cardiovascular disease. Both authors read and approved the final manuscript.
Footnotes
Supported by NIH grant no. HL40404 (R.J.D.) and a fellowship from the International Nutrition Foundation/Ellison Medical Foundation (C.T.).
Abbreviations used: ALA, α-linolenic acid; FA, fatty acid; LA, linoleic acid; LpL, lipoprotein lipase; (n-3), omega-3 fatty acid; (n-6), omega-6; SDA, stearidonic acid.
Literature Cited
- 1.Seo T, Blaner WS, Deckelbaum RJ. Omega-3 fatty acids: molecular approaches to optimal biological outcomes. Curr Opin Lipidol. 2005;16:11–8 [DOI] [PubMed] [Google Scholar]
- 2.Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A, et al. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med. 1995;24:308–15 [DOI] [PubMed] [Google Scholar]
- 3.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30 [DOI] [PubMed] [Google Scholar]
- 4.Deckelbaum RJ. n-6 and n-3 Fatty acids and atherosclerosis: ratios or amounts? Arterioscler Thromb Vasc Biol. 2010;30:2325–6 [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, Hong S, Lu Y. Lipid mediator informatics-lipidomics: novel pathways in mapping resolution. AAPS J. 2006;8:E284–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45:581–97 [DOI] [PubMed] [Google Scholar]
- 7.Lemke SL, Vicini JL, Su H, Goldstein DA, Nemeth MA, Krul ES, Harris WS. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: randomized, double-blind clinical study of efficacy and safety. Am J Clin Nutr. 2010;92:766–75 [DOI] [PubMed] [Google Scholar]
- 8.Deckelbaum RJ, Worgall TS, Seo T. n-3 Fatty acids and gene expression. Am J Clin Nutr. 2006;83:S1520–5 [DOI] [PubMed] [Google Scholar]
- 9.Qi K, Seo T, Al-Haideri M, Worgall TS, Vogel T, Carpentier YA, Deckelbaum RJ. Omega-3 triglycerides modify blood clearance and tissue targeting pathways of lipid emulsions. Biochemistry. 2002;41:3119–27 [DOI] [PubMed] [Google Scholar]
- 10.Densupsoontorn N, Carpentier YA, Racine R, Murray FM, Seo T, Ramakrishnan R, Deckelbaum RJ. CD36 and proteoglycan-mediated pathways for (n-3) fatty acid enriched triglyceride-rich particle blood clearance in mouse models in vivo and in peritoneal macrophages in vitro. J Nutr. 2008;138:257–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray-Taylor FM, Ho YY, Densupsoontorn N, Chang CL, Deckelbaum RJ, Seo T. n-3, But not n-6 lipid particle uptake requires cell surface anchoring. Biochem Biophys Res Commun. 2010;392:135–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaqoob P, Shaikh SR. The nutritional and clinical significance of lipid rafts. Curr Opin Clin Nutr Metab Care. 2010;13:156–66 [DOI] [PubMed] [Google Scholar]
- 13.Sudheendran S, Chang CC, Deckelbaum RJ. N-3 vs. saturated fatty acids: effects on the arterial wall. Prostaglandins Leukot Essent Fatty Acids. 2010;82:205–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–4 [DOI] [PubMed] [Google Scholar]
- 15.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–70 [DOI] [PubMed] [Google Scholar]
- 17.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–2 [DOI] [PubMed] [Google Scholar]
- 18.Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. Am J Clin Nutr. 2006;83:S1499–504 [DOI] [PubMed] [Google Scholar]
- 19.Mayurasakorn K, Williams JJ, Ten VS, Deckelbaum RJ. Docosahexaenoic acid: brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Curr Opin Clin Nutr Metab Care. 2011;14:158–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903 [DOI] [PubMed] [Google Scholar]
- 21.Kromhout D, Giltay EJ, Geleijnse JM. n-3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–26 [DOI] [PubMed] [Google Scholar]
- 22.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–7 [DOI] [PubMed] [Google Scholar]
- 23.Deckelbaum RJ, Akabas SR. n-3 Fatty acids and cardiovascular disease: navigating toward recommendations. Am J Clin Nutr. 2006;84:1–2 [DOI] [PubMed] [Google Scholar]
- 24.Chang CL, Seo T, Du CB, Accili D, Deckelbaum RJ. n-3 Fatty acids decrease arterial low-density lipoprotein cholesterol delivery and lipoprotein lipase levels in insulin-resistant mice. Arterioscler Thromb Vasc Biol. 2010;30:2510–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo T, Qi K, Chang C, Liu Y, Worgall TS, Ramakrishnan R, Deckelbaum RJ. Saturated fat-rich diet enhances selective uptake of LDL cholesteryl esters in the arterial wall. J Clin Invest. 2005;115:2214–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CL, Seo T, Matsuzaki M, Worgall TS, Deckelbaum RJ. n-3 Fatty acids reduce arterial LDL-cholesterol delivery and arterial lipoprotein lipase levels and lipase distribution. Arterioscler Thromb Vasc Biol. 2009;29:555–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J Clin Invest. 1992;90:1504–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, Hishida H, et al. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke. 2008;39:2052–8 [DOI] [PubMed] [Google Scholar]
- 29.Racine RA, Deckelbaum RJ. Sources of the very-long-chain unsaturated omega-3 fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Curr Opin Clin Nutr Metab Care. 2007;10:123–8 [DOI] [PubMed] [Google Scholar]