Abstract
The potential of superparamagnetic iron oxide nanoparticles (SPIONs) in various biomedical applications, including magnetic resonance imaging (MRI), sensing, and drug delivery, requires that their surface be derivatized to be hydrophilic and biocompatible. We report here the design and synthesis of a compact and water-soluble zwitterionic dopamine sulfonate (ZDS) ligand with strong binding affinity to SPIONs. After ligand exchange, the ZDS coated SPIONs exhibit small hydrodynamic diameters (HD), and stability with respect to time, pH, and salinity. Furthermore, small ZDS coated SPIONs were found to have a reduced non-specific affinity (compared to negatively charged SPIONs) towards serum proteins; streptavidin/dye functionalized SPIONs were bioactive and thus specifically targeted biotin receptors.
Keywords: superparamagnetic iron oxide nanoparticles, zwitterionic dopamine sulfonate, hydrodynamic diameter, stability, serum binging test, biotin labeling
Superparamagnetic iron oxide nanoparticles (SPIONs) have been used in-vitro and in-vivo as contrast agents to improve the sensitivity of magnetic resonance imaging (MRI).1 Their defining characteristics are a high saturation magnetic moment, their relative chemical stability, and their potentially minimized toxicity. Synthetic schemes for SPIONs with the most uniform size distributions usually involve the use of organic solvents and result in SPIONs that are hydrophobic as synthesized. Exploring ligands that render these SPIONs hydrophilic and bio-compatible has been essential for demonstrating their potential uses in various biomedical applications.2 Xu et al demonstrated that dopamine could serve as an attractive ligand to render SPIONs hydrophilic,3 due to the strong interaction between vicinal diol groups and iron oxide as well as the hydrophilicity of amide and carboxyl groups. Coupling polyethylene glycol (PEG) groups to dopamine derivatives4 can improve the stability and reduce the surface charge of water-soluble SPIONs, which in turn alleviates nonspecific binding to proteins. Nevertheless, PEG-based ligands can significantly increase the effective hydrodynamic diameter (HD) of bio-compatible nanoparticles (NPs), which can restrict their access to confined spaces and prevent their renal elimination.5 PEG-coated NP dispersions can also be unstable in high-salinity buffers, resulting in aggregation.6 There remains a need for novel ligands that have a strong binding affinity to SPIONs, yet minimize their effective HD while retaining high aqueous solubility, biocompatibility with minimal non-specific interactions, and long-term stability.
We report here the design and synthesis of a compact and water-soluble zwitterionic dopamine sulfonate (ZDS) ligand with strong binding affinity to SPIONs. This ligand results in bio-compatible SPIONs with minimized HDs, minimal non-specific interactions, and stability with respect to time, pH and salinity. The ZDS ligand (Compound 2, Scheme 1a) was designed with the following considerations in mind: (1) the dopamine moiety provides strong coordination to the iron oxide surface, (2) the sulfonate group conveys high water solubility, and (3) the combination of a quaternary amine group and the sulfonate group provides the ligand with a zwitterionic character, enabling pH stability and minimizing non-specific interactions with proteins. As shown in scheme 1, the ZDS ligand was synthesized from commercially available dopamine via a simple two step reaction: first, the sulfonation of dopamine was accomplished by ring opening of the 1,3-propane sultone, followed by methylation of the amino group by addition of iodomethane (supporting information). Hydrophobic SPOINs were synthesized from the thermal decomposition of Fe(CO)5 in a dioctyl ether solvent in the presence of native oleic acid ligands and trimethylamine N-oxide oxidizing reagent.7,8
Scheme 1.
a) Chemical structure and synthesis route of DS and ZDS Ligand and b) Chemical structure of TD ligand (M.W.: ~850 g/mol, synthesis route in supporting information)
Water soluble SPIONs were obtained by a two step ligand exchange process. The native hydrophobic oleic acid ligand was first exchanged by 2-[2-(2-methoxyethoxy)ethoxy]acetic acid (MEAA) ligand in methanol. The purpose of this first exchange is to increase the solubility of the SPIONs in the solvent mixture used in the second step. Next, in a dimethylformamide/water mixed solvent, the MEAA ligand was replaced by ZDS, dopamine sulfonate (DS, Compound 1, Scheme 1a), or mixtures of ZDS with thiol-terminated catechol-derivative (TD, Compound 3, Scheme 1b) for bio-conjugations. The DS ligand was utilized as a control with a charge similar to other small but negatively charged ligands, such as 2,3-dimercaptosuccinic acid.9
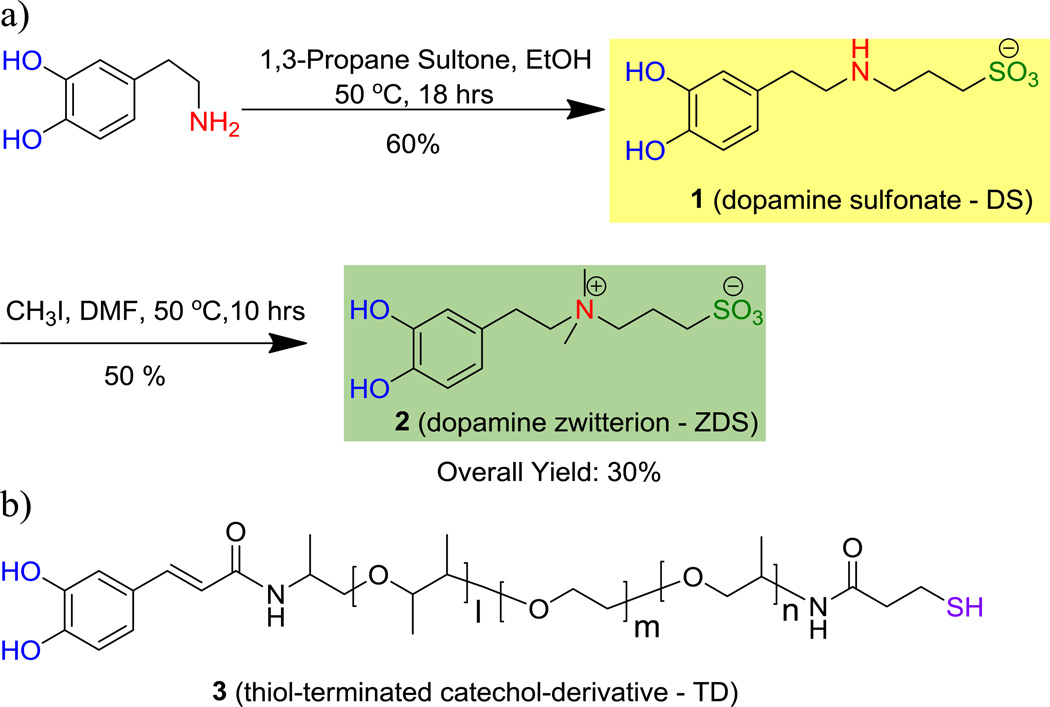
The resulting water soluble ZDS ligand-exchanged SPIONs (ZDS-NPs) were stable and well dispersible at high NP concentrations in phosphate buffered saline (PBS 1X, inset of Figure 1a). Transmission electron microscopy (TEM) further showed that the ZDS-NPs were nearly monodisperse in PBS 1X with an inorganic particle diameter of 8.0 nm (Figure 1a). Moreover, dynamic light scattering (DLS) measurement revealed that the ZDS-NPs had a narrow size distribution (inset of Figure 1b) with a hydrodynamic diameter (HD) of ~10 nm, indicating that the ZDS ligand contributes less than 1 nm to the overall radius. This size increment is significantly smaller than for dextran10 or PEG-coupled ligands,4a which can be on the order of 30 to 200 nm. In addition, the HD of ZDS-NPs was insensitive to pH over the pH range of 6.0 – 8.5, indicating good colloidal stability over physiological pHs (Figure 1b).
Figure 1.
a) TEM images of hydrophilic ZDS-NPs (Inset: photograph of ZDS-NPs dispersed in PBS 1X), b) Hydrodynamic Size of ZDS-NPs vs. pH(Inset: Size distribution of ZDS-NPs at pH=7.5).
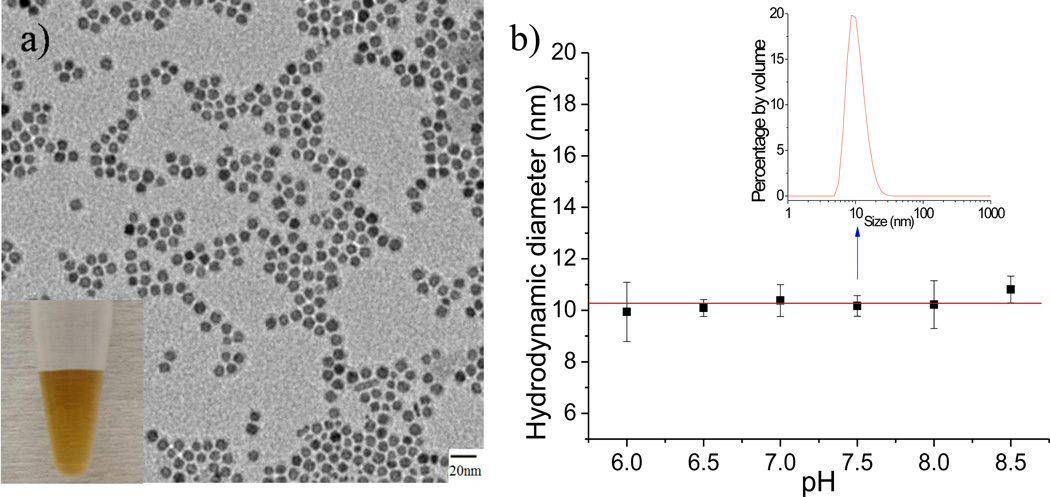
To study the in-vivo imaging potential of these NPs, we performed a serum binding test, which compares the sizes of NPs incubated with fetal bovine serum (FBS) and PBS 1X, respectively. We used high-performance liquid chromatography (HPLC) with a size-exclusion column, with the retention times of NPs having an inverse relationship with the sizes of NPs.11 In PBS 1X, both DS-NPs (Figure 2a, red lines) and ZDS-NPs (Figure 2a, blue lines) were nearly monodisperse with a retention time of ~34 min. The size of DS-NPs after incubation with 10% FBS was larger than the size of DS-NPs after incubation with PBS 1X, showing a continuous distribution between 20 and 37 min; in contrast, the size distribution of ZDS-NPs after incubation of 10% FBS was similar to the size distribution of ZDS-NPs after incubation with PBS 1X. When the serum-to-NP ratio increased, DS-NPs after incubation with 20% FBS strongly aggregated and thus showed a retention time at ~18 min (corresponding to ~9 mL, approximately the void volume of the column);12 in contrast, although a small portion of ZDS-NPs after incubation with 20% FBS showed a retention time at ~18 min (presumably caused by minor biological species of FBS that can bind to the nearly neutral ZDS-NPs),13 most of the ZDS-NPs showed a continuous distribution between 20 and 37 min. These data indicate that the negatively charged DS-NPs have a high non-specific affinity towards serum proteins; particularly, DS-NPs showed a strong aggregation in 20% FBS. Therefore, although DS ligands also give rise to water-soluble and small iron oxide NPs, the negative charge from the sulfonate group on the DS ligands electrostatically interacts with some of the proteins in FBS, and electrostatic interactions are thought to be important for the binding between iron oxide NPs and bovine serum albumin, a major component of FBS.14 In comparison with DS-NPs, ZDS-NPs showed a reduced non-specific affinity towards serum proteins. ZDS ligands not only provide good solubility and a small size to iron oxide NPs but also ensure their nearly neutral charge, which in turn decreases the non-specific interactions between NPs and serum proteins. These data therefore suggest that zwitterionic ZDS-NPs are more suitable than DS-NPs for in-vivo experiments and that their electrically neutral (e.g. zwitterionic) nature is critical to their design.15
Figure 2.
a) Chromatograms of SPIONs in serum binding test (absorbance vs. retention time), b) UV-Vis spectrum of ZDS-NPs with the increase of storage time
The stability of NPs is also an important parameter for their biomedical applications. As a result, these new NPs were studied by measuring the UV-Vis absorption over extended periods of time. We found that the UV-Vis absorbance curves (Figure 2b), which reflects the concentration of SPIONs, were nearly identical over at least a 2 week period. Carpenter and coworkers proposed16 that if SPIONs were only ligand exchanged by the simple dopamine molecule, rapid degradation of SPIONs may occur, thereby forming iron(III) oxyhydroxide. However, we did not observe this phenomenon for either ZDS or DS coated SPIONs. In addition, ZDS-NPs were stable for at least one month in PBS 1X at 4 °C (within the error range of DLS measurements, all ZDS-NPs retained the same HD). In addition ZDS-NPs were soluble even in saturated NaCl solutions, a consequence of combining the strong o-dihydroxybenzene anchor group and the highly water-soluble sulfonate group. This is in contrast to PEG-coated or single charge-stabilized nanoparticles which aggregate in saturated NaCl solutions.6, 17
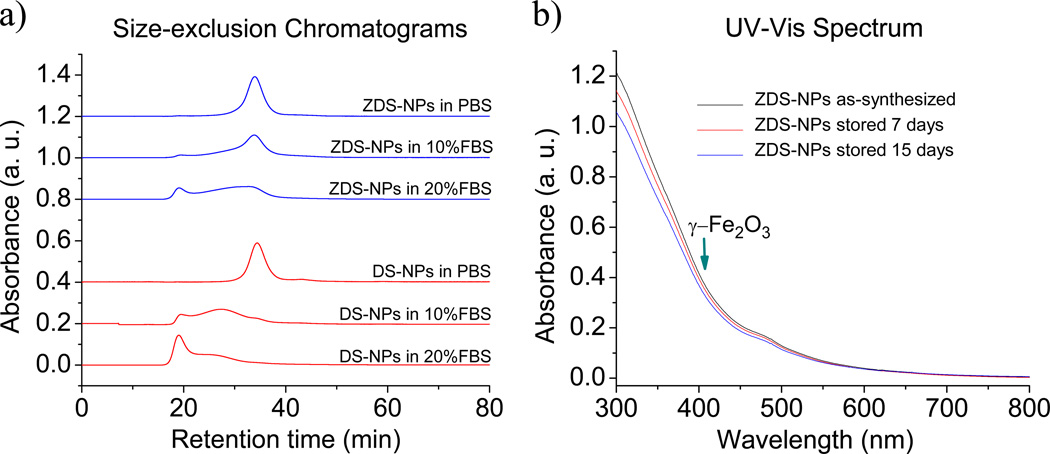
Finally, our iron oxide NPs were functionalized with streptavidin and fluorescent dyes for targeting and fluorescence imaging, respectively. In prior studies,18 long-chain ligands such as organic polymers were used for the water solubilization of iron oxide NPs (short-chain ligands were generally not hydrophilic enough), hence the hydrodynamic diameter (HD) of those biocompatible NPs was significantly larger than their inorganic diameter. In our studies, a binary coating was designed, in which ZDS ligands provided water-solubility and short-chain ligands offered functionality. As shown in Scheme 1b, this short-chain ligand (TD ligand) consists of a catechol, a polyalkylene glycol, and a thiol. After ligand exchange with a mixture of 85% ZDS ligand and 15% TD ligand (mol%), the resulting TD/ZDS-NPs were conjugated by Alexa Fluor 488 maleimide dye (Dye, absorption peak = 488 nm, emission peak = 520 nm) and streptavidin-maleimide (SA) via a standard thiol-maleimide conjugation scheme. To demonstrate the functionalization of the TD/ZDS-NPs with streptavidin, biotin-coated wells on strip plate were incubated with either pre-biotin-saturated Dye-SA-TD/ZDS-NPs or Dye-SA-TD/ZDS-NPs (Figure 3a and b). Afterwards, the sample solutions were removed and the biotin-coated wells were rinsed with PBS. By measuring the resulting fluorescence of the wells, we found the signal intensity of Dye-SA-TD/ZDS-NPs to be ~11 times higher than that of the pre-biotin-saturated Dye-SA-TD/ZDS-NPs (Figure 3c). This data showed that the peptide-functionalized TD/ZDS-NPs may be appropriate for targeting cell receptors for specific labeling.
Figure 3.
a) Schematic show of the incubation of pre biotin-saturated Dye-SA-TD/ZDS-NPs in a biotin-plated well, b) Schematic show of the incubation of Dye-SA-TD/ZDS-NPs in a biotin-plated well, and c) Fluorescent spectra of both biotin-plated wells
In conclusion, by using a compact zwitterionic dopamine sulfonate ligand coating on robust superparamagnetic iron oxide nanoparticles, we have developed aqueous iron oxide nanoparticles which are water-soluble, compact, and easily functionalized. Due to their zwitterionic nature, the ZDS-NPs have reduced nonspecific binding (compared to negatively charged SPIONs) to serum proteins; moreover, the streptavidin functionalized TD/ZDS-NPs labeled biotin specifically. These properties render the functionalized iron oxide nanoparticles suitable for in-vivo and in-vitro applications, where antibodies, peptides, or aptamers could be conjugated to TD/ZDS-NPs for targeting and imaging,19 and when combined with metal-binding proteins, TD/ZDS-NPs could serve as MRI-based metal ion sensors.20
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the MIT-Harvard NIH Center for Cancer Nanotechnology Excellence Grant (1U54-CA119349), an NIH National Cancer Institute Grant (R01-CA126642), the ARO through the Institute for Soldier Nanotechnologies (W911NF-07-D-0004), and the NSF through a Collaborative Research in Chemistry Program (CHE-0714189). H. W. thanks Robert T. Haslam presidential fellowship from Massachusetts Institute of Technology. H. W. also thanks Jongnam Park and Jie Bao for thoughtful discussions as well as Yong Zhang for his assistance with acquiring TEM images.
REFERENCES
- 1.Park Y, Piao YZ, Lee N, Yoo B, Kim BH, Choi SH, Hyeon T. J. Mater. Chem. 2011;21:11472. [Google Scholar]
- 2.(a) Na HB, Song IC, Hyeon T. Adv. Mater. 2009;21:2133. [Google Scholar]; (b) Latham AH, Williams ME. Accounts of Chemical Research. 2008;41:411. doi: 10.1021/ar700183b. [DOI] [PubMed] [Google Scholar]; (c) Jun YW, Lee JH, Cheon J. Angew. Chem. Int. Ed. 2008;47:5122. doi: 10.1002/anie.200701674. [DOI] [PubMed] [Google Scholar]
- 3.Xu C, Xu K, Gu H, Zheng R, Liu H, Zhang X, Guo Z, Xu B. J. Am. Chem. Soc. 2004;126:9938. doi: 10.1021/ja0464802. [DOI] [PubMed] [Google Scholar]
- 4.(a) Amstad E, Gillich T, Bilecka I, Textor M, Reimhult E. Nano Lett. 2009;9:4042. doi: 10.1021/nl902212q. [DOI] [PubMed] [Google Scholar]; (b) Peng S, Wang C, Xie J, Sun SH. J Am Chem Soc. 2006;128:10676. doi: 10.1021/ja063969h. [DOI] [PubMed] [Google Scholar]
- 5.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Kandapallil B, Bawendi MG, Frangioni JV. Nature Biotechnology. 2007;25:1165. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radziuk D, Skirtach A, Sukhorukov G, Shchukin D, Mohwald H. Macromol Rapid Comm. 2007;28:848. [Google Scholar]
- 7.(a) Woo K, Hong J, Choi S, Lee HW, Ahn JP, Kim CS, Lee SW. Chem Mater. 2004;16:2814. [Google Scholar]; (b) Hyeon T, Lee SS, Park J, Chung Y, Bin Na H. J Am Chem Soc. 2001;123:12798. doi: 10.1021/ja016812s. [DOI] [PubMed] [Google Scholar]
- 8.Insin N, Tracy JB, Lee H, Zimmer JP, Westervelt RM, Bawendi MG. ACS Nano. 2008;2:197. doi: 10.1021/nn700344x. [DOI] [PubMed] [Google Scholar]
- 9.(a) Lee JH, Huh YM, Jun Y, Seo J, Jang J, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Nature Medicine. 2007;13:95. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]; (b) Yoon TJ, Lee H, Shao HL, Weissleder R. Angew Chem Int Edit. 2011;50:4663. doi: 10.1002/anie.201100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Jung CW, Jacobs P. Magn. Reson. Imaging. 1995;13:661. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]; (b) Wang YX, Hussain SM, Krestin GP. Eur. J. Radiol. 2001;11:2319. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 11.Tromsdorf UI, Bruns OT, Salmen SC, Beisiegel U, Weller H. Nano Lett. 2009;9:4434. doi: 10.1021/nl902715v. [DOI] [PubMed] [Google Scholar]
- 12.Wong C, Stylianopoulos T, Cui JA, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. P Natl Acad Sci USA. 2011;108:2426. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simberg D, Park JH, Karmali PP, Zhang WM, Merkulov S, McCrae K, Bhatia SN, Sailor M, Ruoslahti E. Biomaterials. 2009;30:3926. doi: 10.1016/j.biomaterials.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang QQ, Liang JG, Han HY. J Phys Chem B. 2009;113:10454. doi: 10.1021/jp904004w. [DOI] [PubMed] [Google Scholar]
- 15.Liu WH, Choi HS, Zimmer JP, Tanaka E, Frangioni JV, Bawendi M. J Am Chem Soc. 2007;129:14530. doi: 10.1021/ja073790m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shultz MD, Reveles JU, Khanna SN, Carpenter EE. J. Am. Chem. Soc. 2007;129:2482. doi: 10.1021/ja0651963. [DOI] [PubMed] [Google Scholar]
- 17.Bakandritsos A, Psarras GC, Boukos N. Langmuir. 2008;24:11489. doi: 10.1021/la801901j. [DOI] [PubMed] [Google Scholar]
- 18.(a) Boyer C, Priyanto P, Davis TP, Pissuwan D, Bulmus V, Kavallaris M, Teoh WY, Amal R, Carroll M, Woodward R, St Pierre T. J Mater Chem. 2010;20:255. [Google Scholar]; (b) Eberbeck D, Kettering M, Bergemann C, Zirpel P, Hilger I, Trahms L. J Phys D Appl Phys. 2010:43. [Google Scholar]; (c) Chen HW, Wang LY, Yeh J, Wu XY, Cao ZH, Wang YA, Zhang MM, Yang L, Mao H. Biomaterials. 2010;31:5397. doi: 10.1016/j.biomaterials.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao JH, Gu HW, Xu B. Accounts of Chemical Research. 2009;42:1097. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 20.Atanasijevic T, Jasanoff A. Nature Protocols. 2007;2:2582. doi: 10.1038/nprot.2007.377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.