Abstract
In this contribution, we report in vitro folding of the archaebacterial voltage gated K+ channel, KvAP. We show that in vitro folding of the KvAP channel from the extensively unfolded state requires lipid vesicles and that the refolded channel is biochemically and functionally similar to the native channel. The in vitro folding process is slow at room temperature and the folding yield depends on the composition of the lipid bilayer. The major factor influencing refolding is temperature and almost quantitative refolding of the KvAP channel is observed at 80 °C. In order to differentiate between insertion into the bilayer and folding within the bilayer, we developed a cysteine protection assay. Using this assay, we demonstrate that insertion of the unfolded protein into the bilayer is relatively fast at room temperature and independent of lipid composition suggesting that temperature and bilayer composition influence folding within the bilayer. Further, we demonstrate that in vitro folding provides an effective method for obtaining high yields of the native channel. Our studies suggest that the KvAP channel provides a good model system to investigate the folding of a multi-domain integral membrane protein.
Voltage gated K+ (Kv) channels are integral membrane proteins (IMPs) that are critical for the generation of electrical impulses by excitable cells (1). Kv channels are tetrameric proteins with each subunit consisting of six transmembrane segments that are arranged in two distinct domains (2, 3) (Fig. 1A). The first four transmembrane segments form the voltage sensor domain (VSD) while the last two transmembrane segments form the pore domain (Fig. 1B). The pore domain contains the K+ translocation pathway while the VSD couples the opening and closing of the pore domain to changes in electrical potential across the membrane. Extensive investigations have been carried out on Kv channels resulting in a wealth of information on the structure and functional mechanisms of these channels. One aspect of Kv channels that is not well understood is the process of folding to the native state. Understanding the folding of Kv channels is important as folding defects, mainly due to mutations, leads to diseases (4, 5). Studies on the folding of Kv channels reported until now have focused on the integration of individual transmembrane helices into the lipid bilayer, the association of channel subunits and the trafficking of Kv channels to the cell surface (6–10). These studies were carried out using in vitro translation or by expression in Xenopus oocytes or cultured cells. However, there have been no reports of the in vitro folding of a Kv channel from the unfolded state. The advantage of in vitro folding is that it allows us to precisely manipulate the reaction conditions and to thereby identify the factors important for folding.
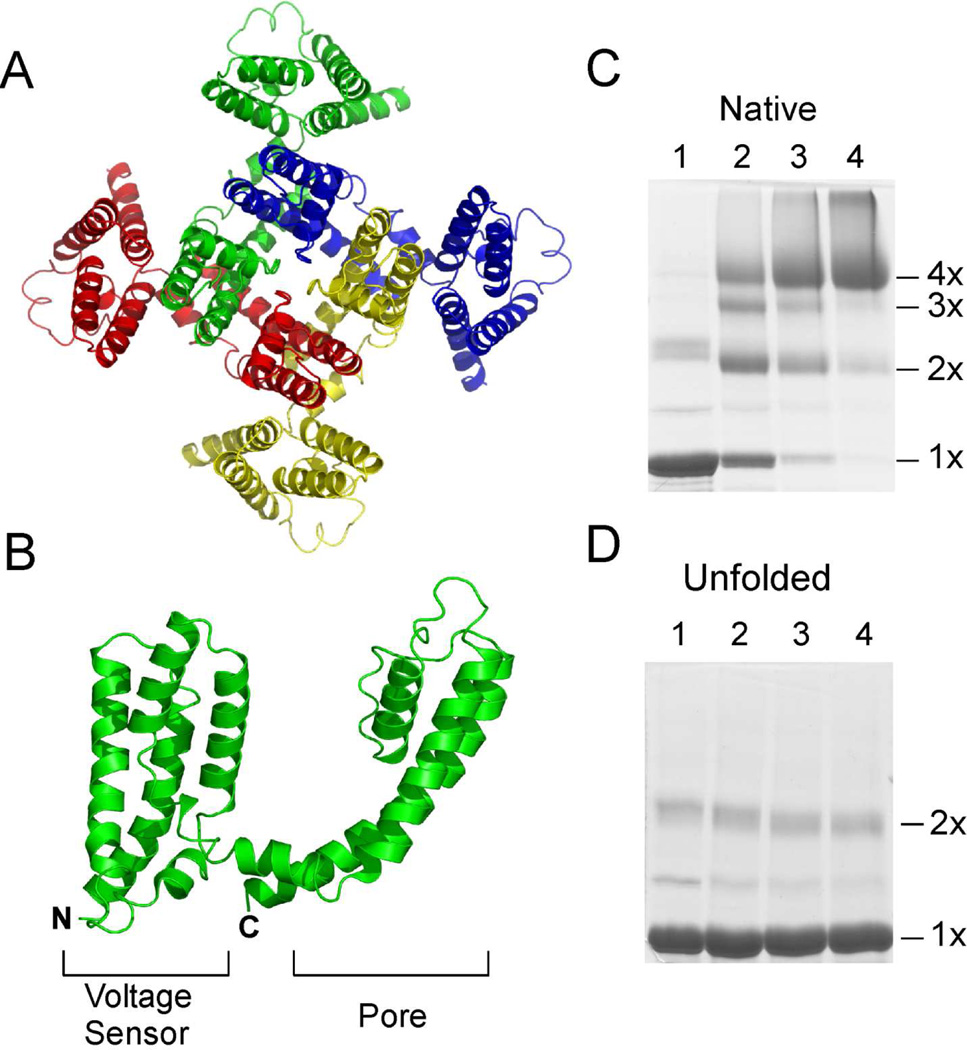
Fig. 1. Structure of the KvAP channel.
(A) Top view of the tetrameric KvAP channel. The structural model of the KvAP channel as described in (24) is shown. (B) Structure of a single subunit of the KvAP channel. Each subunit consists of two domains, the sensor domain and the pore domain. SDS-PAGE gel showing glutaraldehdye crosslinking of the native KvAP channel (C) and the unfolded protein (D). Lanes 1) without crosslinker; 2) 0.025%; 3) 0.05% and 4) 0.1% glutaraldehyde. The oligomeric nature (1×, 2×, 3× & 4×) of the crosslinked bands is indicated.
There are a number of technical challenges encountered during the in vitro folding of IMPs. IMPs require the anisotropic environment provided by the cell membrane for their structural and functional integrity (11). Therefore, a major challenge during in vitro folding is defining the appropriate detergents or lipids to serve as a mimic for the cell membrane (12). Another challenge is the lack of a suitable denaturant as those commonly used for soluble proteins, such as urea or guanidine hydrochloride, have limited ability to unfold IMPs (13, 14). Yet another challenge, particularly in the case of ion channels, is the lack of a suitable assay to monitor the folding process. Due to these challenges, there are only a few reports of the in vitro folding of IMPs (15). In the family of ion channels, in vitro folding has been extensively investigated only for the KcsA channel (16–18). In addition, in vitro folding has been reported for the NaK and MscL channels as a part of the chemical synthesis of these channels and refolding has been demonstrated for a thermally denatured (but not chemically denatured) sodium channel, (19–21).
Here, we investigate the in vitro folding of the Kv channel KvAP, from the archaebacteria, Aeropyrum pernix. The KvAP channel though archaebacterial in origin, is functionally very similar to an eukaryotic Kv channel (22). The KvAP channel was the first Kv channel for which a crystal structure was reported (2). Subsequently, crystal structures were reported for the eukaryotic Kv1.2 and the Kv1.2–2.1 chimera channels (3, 23). In the crystal structures of the KvAP channel, the VSD is distorted, which was demonstrated to be due to the absence of a lipid bilayer (24). A structural model for the native state of the KvAP channel has been proposed based on the structure of the isolated VSD, structural homology to the Kv1.2 channel along with disulfide crosslinking and EPR spectroscopic data (24, 25). KvAP has been used as a model Kv channel for biophysical and spectroscopic investigations due to the structural and functional similarity to eukaryotic Kv channels and the relative ease of overexpression and purification of KvAP compared to eukaryotic Kv channels (26, 27).
In this study, we demonstrate that the KvAP channel can be folded in vitro from the extensively unfolded state. We show that lipid bilayers are required for refolding and that the refolded KvAP channel is biochemically and functionally similar to the native channel. We investigate the factors that are important and identify that refolding depends upon the composition of the lipid bilayer. Interestingly, we observe that refolding of the KvAP channel is strongly dependent on temperature, with very efficient refolding at elevated temperatures. Using a Cys-protection assay we demonstrate that lipid bilayer composition and temperature does not affect the insertion of the unfolded polypeptide into the bilayer suggesting that these factors influence the folding of KvAP within the bilayer. We demonstrate that the efficient in vitro folding coupled with overexpression as inclusion bodies provides a facile approach for obtaining high yields of the KvAP channel.
EXPERIMENTAL PROCEDURES
Native expression and purification of the KvAP channel
A His6 tagged KvAP channel gene sub-cloned into the pQE60 plasmid (Qiagen) was kindly provided by Dr. Roderick MacKinnon (The Rockefeller University) (22). The native KvAP channel was expressed in Escherichia coli XL10 (Stratagene) cells as previously described (22). Following expression, cells were pelleted, resuspended in 50 mM Tris-HCl pH 7.5, 150 mM KCl, 0.25 M sucrose, 1 mM MgCl2, DNAse (5 µg/mL) and lysed by sonication. Unlysed cells and cell debris present was removed by centrifugation at 7500g, following which the cell membranes were pelleted by centrifugation at 100000g. The membranes were solubilized by decyl-β-D-maltoside (DM, 2% w/v) and the His6 tagged KvAP channel was purified by metal affinity chromatography (Talon, Clontech) and size exclusion chromatography (22). Size exclusion chromatography was carried out on a Superdex S200 column (GE Healthcare) using a running buffer composed of 50mM HEPES-KOH pH 7.5, 150mM KCl and 0.25% DM.
Expression of KvAP in inclusion bodies
The Glutathione-S-transferase (GST)-KvAP fusion used for expression as inclusion bodies consists of the KvAP channel linked to the C-terminus of GST with a linker that consists of a thrombin site, His6 and a Factor Xa site. Initial expression studies showed premature translation terminations in the N-terminal region of the KvAP channel. The KvAP gene consists of a number of rare amino acid codons in the N-terminal region, which could account for the premature terminations. To ensure translation of the complete gene, the rare codons present within the N-terminal 20 amino acids were substituted by the most frequently used codons for the corresponding amino acids. The single native Cys at position 247 was substituted with Ser in the fusion construct to prevent disulfide crosslinkinig.
The GST-KvAP fusion was expressed in Escherichia coli Rosetta2 (DE3) cells (EMD Biosciences) using the autoinduction procedure (28). For isolation of the inclusion bodies, cells were pelleted and resuspended in 50 mM Tris-HCl pH 7.5, 0.2 M NaCl, 1 mM MgCl2, DNAse (5 µg/mL). Lysozyme (0.1 mg/mL) was added and the cells were incubated at room temperature with gentle stirring. After 30 min, phenylmethanesulfonyl fluoride was added to 1 mM and the cells were lysed by sonication. Triton X-100 (Tx-100) was added (1%, v/v) and the cell lysate was stirred at room temperature for 30 min. The soluble and insoluble fractions were separated by centrifugation at 12000g for 10 min. The insoluble fraction, which contains the inclusion bodies, was washed 3 times with 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Tx-100. The inclusion bodies were resuspended in 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% N-Lauryl Sarcosine (NLS) and digested with thrombin (Roche, 1U/L of culture) overnight, to cleave the KvAP polypeptide from GST.
For purification of the KvAP polypeptide, 1 vol of 50 mM Tris-HCl pH 7.5, 150 mM NaCl was added to the thrombin cleavage mixture. Tx-100 was added to 2% (v/v) and the KvAP polypeptide was purified using metal affinity chromatography. The KvAP polypeptide obtained was unfolded using the protocol described, prior to use in the refolding studies.
Unfolding of the KvAP channel
A multi-step protocol was used for unfolding the KvAP channel. The protocol is based on the unfolding procedure described for bacteriorhodopsin (13). In the first step, Tx-100 was added to 2% (v/v) and the protein was precipitated by 15% trichloroacetic acid (TCA, w/v) for 15 min at 4 °C. The protein precipitate was collected by centrifugation at 3000g, washed twice with acetone + 0.1% trifluoroacetic acid (TFA), solubilized in 50% trifluoroethanol (TFE) + 0.1% TFA and lyophilized to provide the unfolded KvAP channel used for the refolding studies.
Refolding of the KvAP channel
The lyophilized KvAP polypeptide was dissolved in 100 mM sodium phosphate pH 7.5, 1% sodium dodecylsulfate (SDS), 10 mM β-mercaptoethanol. The concentration of the unfolded protein was determined by comparing the band intensities on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) of the unfolded protein to known concentrations of the native KvAP channel. The lipids used: Asolectin (Soy Total Lipid Extract, Aso), 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-glycero-3-phosphoglycerol (POPG), 1-palmitoyl-2-oleoyl-glycero-3-phosphoethanolamine (POPE) and 1, 2-diphytanoyl-glycero-3-phosphocholine (DPhPC) were obtained from Avanti Polar Lipids. Lipids solutions in chloroform were dried under argon, re-dissolved in cyclohexane, and lyophilized. Lyophilized lipids were hydrated for 1 hour at a concentration of 5 mg/ml using 50 mM HEPES-KOH pH 7.5, 150 mM KCl, 10 mM β-mercaptoethanol and then sonicated for 5 × 30 sec pulses in a water bath sonicator to obtain lipid vesicles. For refolding experiments at room temperature, the unfolded KvAP polypeptide was diluted 10 fold into lipids or detergents and the mixture sonicated briefly (~5 sec pulse). For refolding at elevated temperatures, dilution without sonication was used. A protein concentration of 0.8 – 1.0 mg/ml was used for the refolding experiments. Refolding of the KvAP channel was assayed by glutaraldehyde crosslinking for 15 min at room temperature and the reaction was quenched by addition of SDS sample buffer that contained 100 mM Tris.
The unfolded KvAP polypeptide has a strong tendency to aggregate, which complicated analysis of the refolding reactions using glutaraldehyde crosslinking. We observed that in dodecylphosphocholine [Fos-12, 2% (w/v)], the native KvAP channel is stable while non-specific aggregates dissociate into monomers. The glutaraldehyde crosslinking reaction was therefore carried out after the addition of 2% Fos-12, to distinguish the folded tetrameric species from the non specific aggregates. This step was also beneficial as the addition of Fos-12 served to quench the refolding reaction. The refolding samples (15–20 µgs of protein) were separated on a 10% SDS-PAGE gel and stained with coomassie blue. Following destaining, the gels were dried, scanned using a flat-bed scanner and the protein bands were quantified using Scion Image (http://www.scioncorp.com). Protein bands corresponding to the monomer, dimer, trimer and tetramer in each lane were quantified and background subtracted. The refolding yield was calculated as the fraction of the KvAP polypeptide in each lane that was present as the tetrameric species.
To determine the time course of the refolding of the KvAP channel, the folding reaction was quenched by Fos-12, followed by GA crosslinking and SDS-PAGE to determine the refolding yield at the various time points. The time course of the refolding showed mono-exponential behavior and a single exponential fit was used to obtain the half time (t1/2) to maximal yield under the different refolding conditions.
For purification of the refolded KvAP channel, lipid vesicles used for refolding were solubilized with DM and the refolded channel was purified using metal affinity chromatograph followed by size exclusion chromatography, as described for the native channel.
Electrophysiological measurements on the refolded KvAP channel
Prior to electrophysiological measurements, the native and the refolded KvAP channels were reconstituted into lipid vesicles composed of DPhPC, 10 mg/ml as previously described (22, 29). Functional measurements on the KvAP channel were carried out using planar lipid bilayers composed of DPhPC. The recording solutions used for channel measurements consisted of 10 mM HEPES-KOH pH 7.5, 150 mM KCl on both the cis and trans sides. Voltage dependence of the native and refolded KvAP channel was determined from a holding potential of −120 mV. Tail currents were measured at −100 mV after a test potential of 200 ms duration. Normalized tail current amplitude (I/Imax) was plotted against the test potential and fitted with a Boltzmann function I/Imax = 1/(1+exp(−zF(V−V0.5)/RT)) to obtain values of V0.5 and z. Slow inactivation was recorded by a 5 sec depolarization to 100 mV from a holding potential of −150 mV. The inactivation time constant (τinact) was determined by fitting the decay in current after peak activation to a single exponential equation, i = A exp(−t/τinact) + C, where i is the current at time t.
Cys protection assay using PEG maleimide
Single Cys substitutions at residues 101, 127, 139, 161, 175, and 214 were generated using QuickChange (Stratagene) in a KvAP channel gene in which the native Cys (Cys247) was substituted with Ser. The Cys mutants were expressed and purified as described for the native protein and unfolded using the protocol previously described.
Refolding of the unfolded Cys mutant proteins was carried out at room temperature as described previoulsy, except that 1 mM DTT was used insted of 10 mM beta-mercaptoethanol. The unfolded Cys mutant proteins were diluted into lipids or into 2% Fos-12 (as a control). Immediately following dilution (< 30 sec), an aliquot of the refolding reaction was incubated with 8 mM PEG-2K-mal (Creative PEGWorks) directly or after solubilization of the lipid vesicles with 2% Fos-12. The control reaction was also similarly treated with PEG-2K-mal. PEGylation was carried out for 30 min at room temperature and quenched by addition of 200mM DTT followed by SDS-PAGE loading buffer. The extent of labeling was determined by electrophoresis on a 12% SDS-PAGE gel to separate the labeled and the unlabelled proteins.
RESULTS
Unfolding of the KvAP channel
Two different approaches were used to obtain the unfolded KvAP polypeptide that was required for the in vitro folding experiments. In the first approach, the KvAP polypeptide was obtained by unfolding the native channel. The native KvAP channel was expressed and purified as previously described (22). Unfolding of the native KvAP channel takes place on treatment with SDS (1% w/v), precipitation with TCA or with organic solvents (data not shown). To ensure extensive unfolding, the KvAP channel was TCA precipitated, washed with acetone, dissolved in 50% TFE + 0.1% TFA and lyophilized. The lyophilized precipitate was dissolved in SDS (1% w/v) to provide the unfolded KvAP used for the in vitro folding experiments. Unfolding of the KvAP channel was confirmed by chemical crosslinking using glutaraldehyde. The native KvAP channel is a tetramer and glutaraldehyde crosslinking of the native channel gives a protein band that migrates corresponding to a tetramer on SDS-PAGE (Fig. 1C). Unfolding results in loss of the native tetrameric state and glutaraldehyde crosslinking of the unfolded protein only gives a protein band that corresponds to the monomer on SDS-PAGE (Fig. 1D).
The second approach used to obtain the unfolded KvAP polypeptide was to express the KvAP channel as a fusion with GST. Appending GST at the N-terminus of KvAP results in directing protein expression to inclusion bodies. Following expression, the inclusion bodies were isolated and the KvAP polypeptide was released from GST by proteolysis. The KvAP polypeptide was then purified by metal affinity chromatography and subjected to the extensive unfolding protocol described for native KvAP prior to in vitro folding experiments. The yield of the unfolded KvAP polypeptide using this approach was 10 – 12 fold higher per L compared to native expression followed by unfolding.
Refolding of the KvAP channel
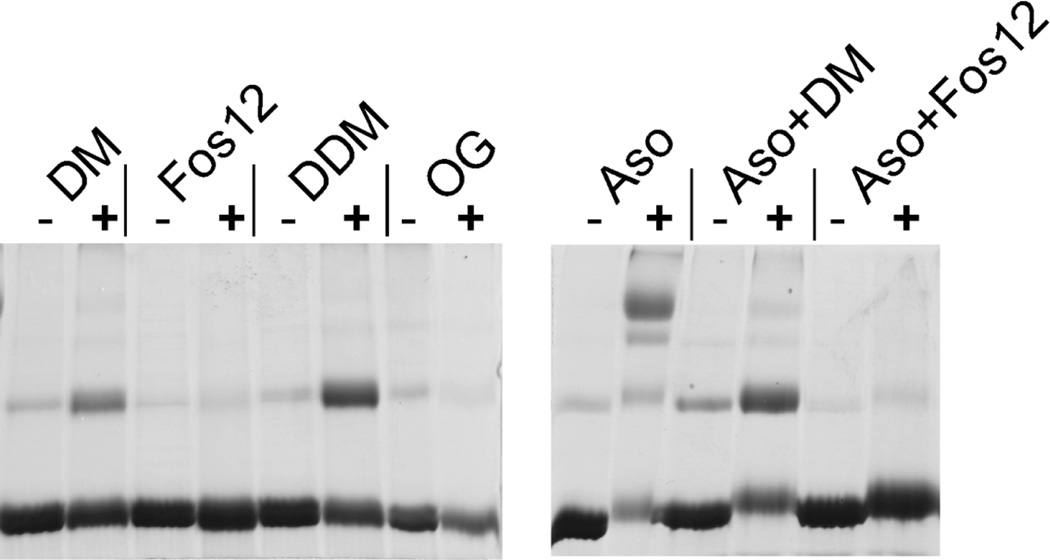
For assembly of the native tetrameric KvAP channel from the unfolded polypeptide in SDS, we initially tested refolding by dilution of the denaturant (SDS) with mild detergents. The detergents: DM, n-Dodecyl-β-D-Maltopyranoside (DDM), n-Octyl-β-D-Glucopyranoside (OG) and Fos-12 (2% w/v) were selected based on their prior use in structural studies of the KvAP channel (2, 24, 30, 31). Glutaraldehyde crosslinking was used to check for refolding, which was indicated by the presence of a tetrameric species on SDS-PAGE. Refolding by dilution of the unfolded polypeptide into a detergent solution was not successful as the tetrameric species was not observed with any of the detergents tested (Fig. 2). A dimeric species was observed in the case of DM and DDM. Dimerization of the isolated VSD in DM has been previously reported and was suggested to take place through the S4 segment (32). We anticipate that the dimerization of the KvAP polypeptide in DM and DDM is similarly mediated through the S4 segment. Next, we investigated lipid vesicles for refolding of the KvAP channel. Lipid vesicles have successfully been used for refolding of the KcsA and NaK channels from the extensively unfolded state (14, 21). On dilution of the unfolded KvAP polypeptide into asolectin vesicles, refolding was observed as indicated by the presence of a tetrameric species on glutaraldehdye crosslinking (Fig. 2). Refolding of the KvAP channel was however not observed when the asolectin vesicles were solubilized by detergents, indicating that the refolding process requires an intact lipid bilayer.
Fig. 2. In vitro folding of the KvAP channel.
Refolding was tested after dilution of the unfolded protein into detergents (DM, Fos-12, DDM and OG; 2% w/v), asolectin lipid vesicles (Aso) or asolectin lipid vesicles solubilized with DM or Fos-12 (2% w/v). Glutaraldehyde crosslinking followed by SDS-PAGE was used to determine formation of tetrameric species. (−) without crosslinker and (+) 0.1% glutaraldehyde.
Characterization of the refolded KvAP channel
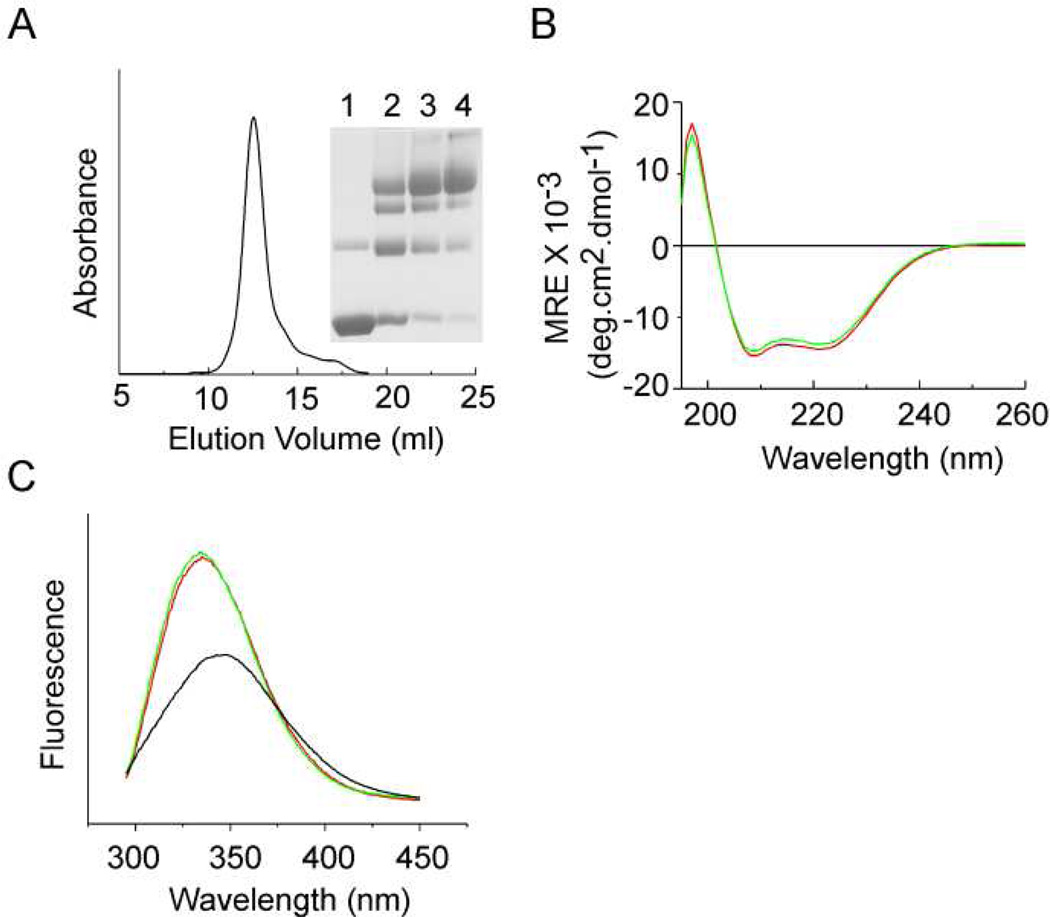
The KvAP channel obtained after in vitro folding was purified by metal affinity chromatography followed by size exclusion chromatography. The refolded KvAP channel had a similar retention time on size exclusion chromatography compared to the native protein and glutaraldehyde crosslinking confirmed the tetrameric nature of the refolded KvAP channel (Fig. 3A).
Fig. 3. Characterization of the refolded KvAP channel.
(A) Size exclusion chromatography of the refolded KvAP channel. Elution volume observed = 12.5 ml. Inset: Glutaraldehyde crosslinking of the peak fraction. Lanes 1) without crosslinker; 2) 0.025%; 3) 0.05% and 4) 0.1% glutaraldehyde. (B) CD spectra of the refolded KvAP channel. CD spectra reported as mean residue ellipiticity (MRE) for the native (red) and the refolded KvAP channel (green) in 10mM sodium phosphate buffer pH 7.5, 150mM KCl, 0.25% DM. A protein concentration of 450 µg/ml was used. (C) Fluorescence spectra of the refolded KvAP channel. Intrinsic fluorescence spectra (excitation = 280 nm) for the native (red), refolded (green) recorded in 50 mM HEPES pH 7.5, 150 mM KCl and 0.25% DM and unfolded KvAP in 100 mM sodium phosphate pH 7.5, 1% SDS (black). A protein concentration of 25 µg/ml was used.
The CD spectrum of the refolded KvAP channel is similar to the native channel (Fig. 3B). Calculation of the helical content using the k2d3 algoritham (http://www.ogic.ca/projects/k2d3/orainaldia.html) gave similar values, 40% for the native and 37% for the refolded KvAP channel. In contrast, the KvAP polypeptide has a helical content of 70% in 50% TFE + 0.1% TFA and 47% in 1% SDS (data not shown). These CD experiments therefore indicate a similar secondary structure for the native and refolded KvAP channels and that there are changes in the secondary structure during the refolding process.
The intrinsic fluorescence spectrum of the native KvAP channel shows an emission maxima at 335 nm (Fig. 3C). Unfolding of the KvAP channel results in a red-shift of the emission maxima to 345 nm and is accompanied by a 50% decrease in the fluorescence intensity (at 335 nm). The fluorescence spectrum of the refolded KvAP channel is similar to that of the native protein. The KvAP channel consists of 3 Trp residues, 2 in the pore domain and 1 in the voltage sensor domain. The similar fluorescence spectra, indicates a similar environment for the Trp residues in the native and the refolded KvAP channels pointing to the structural similarity of these proteins.
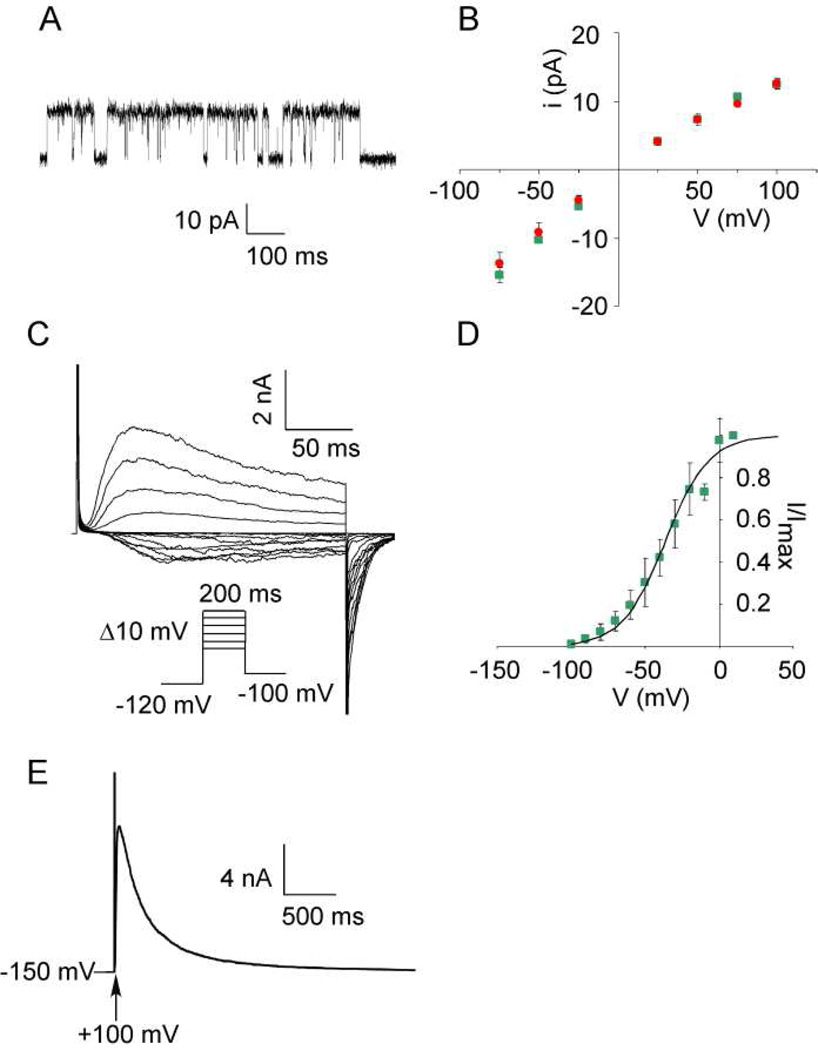
The purified refolded KvAP channel was reconstituted into DPhPC planar lipid bilayers for measurement of channel activity. Single channel openings for the refolded KvAP channel are shown in Fig. 4A. The single channel conductance (124 ± 6 pS for refolded vs 125 ± 8 pS for the native channel at +100 mV in 150 mM K+) and the single channel current-voltage curve for the refolded KvAP channel is similar to the native channel (Fig. 4B). Voltage dependence of channel opening for the refolded KvAP channel was measured using macroscopic (multi-channel) currents (Fig. 4C). KvAP is a depolarization activated K+ channel and channel openings increase sharply with depolarization (22). The data for channel opening versus voltage was fit to a two state Boltzmann distribution, to obtain the values for V0.5, the voltage for half maximal opening and z, the apparent gating charge. The values for the refolded KvAP channel (V0.5 = −36.1 ± 4.3, z = 1.86 ± 0.36) were similar to the corresponding values (V0.5 = −40.7 ± 5.2, z = 1.81 ± 0.25) for the native channel indicating similar gating behavior in these channels (Fig. 4D). Slow inactivation of the refolded KvAP channel (287 ± 7 msec−1, Fig. 4E) was also similar to the native channel (285 ± 20 msec−1). The electrophysiological behavior of the refolded KvAP channel is therefore essentially similar to the native channel.
Fig. 4. Functional characterization of the refolded KvAP channel.
(A) Single channel trace for the refolded KvAP channel recorded at +100 mV. (B) Single channel current as a function of voltage for the native (red) and refolded (green) KvAP channels. (C) Voltage activated macroscopic currents from the refolded KvAP channel recorded using the voltage protocol shown (inset) (D) Voltage gating of the refolded KvAP channel. The fraction of the maximal current observed was plotted as a function of the test potential (see methods). The smooth line corresponds to a Boltzmann function with a V0.5 = −36.1 ± 4.3 and z =1.86 ± 0.36. (E) Slow inactivation of the refolded KvAP channel. KvAP currents were elicited from a holding potential of −150 mV by a depolarization to +100 mV. The inactivation time constant was determined by fitting the decay in current after peak activation to a single exponential function. The recordings were carried out in 150 mM KCl, 10 mM HEPES-KOH pH 7.5. For Panels B and D, the error bars indicate the standard deviation for 3 or more experiments.
The similar biochemical behavior, spectroscopic and electrophysiological characteristics all suggest that the refolded KvAP is similar to the native channel.
Effect of lipid bilayer composition on the refolding of the KvAP channel
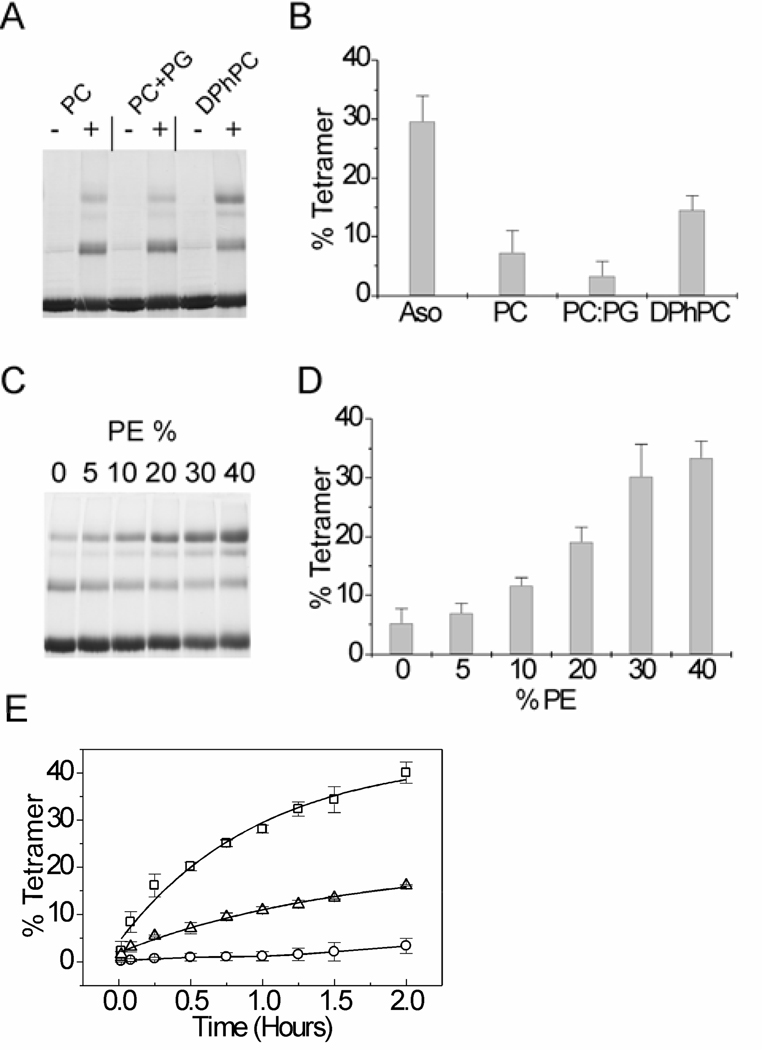
In experiments described until now, asolectin vesicles were used for the refolding of the KvAP channel. Asolectin is a heterogeneous mixture of soybean lipids. To investigate if specific lipids are required, we tested in vitro folding of the KvAP channel using lipid vesicles of defined composition. Unfolded KvAP was diluted into lipid vesicles and the refolding yields were evaluated after a 2 hour incubation at room temperature by glutaraldehdye crosslinking (Fig. 5A, B). In POPC lipid vesicles, the extent of refolding was very low compared to asolectin vesicles (7 ± 3% versus 30 ± 4%). Negatively charged lipids have been shown to be important for the structure and function of certain membrane proteins, for examples see (33, 34). To determine if negatively charged lipids are required, we tested in vitro folding of the KvAP channel in a mixture of POPC and POPG. We observed that the inclusion of POPG did not improve the extent of refolding (3 ± 2%) indicating that the low extent of refolding observed in the POPC lipid vesicles is not due to the absence of negatively charged lipids. As the KvAP channel is archaeal in origin, we tested refolding in DPhPC lipid vesicles. DPhPC contains isoprenyl tails similar to archaeal lipids (35, 36). Our tests indicated a higher extent of folding in the DPhPC lipid vesicles (15 ± 2%) compared to POPC vesicles. POPC and DPhPC have the same head group, therefore the differences in refolding of the KvAP channel in these lipids must arise either due to a specific requirement for a lipid with an isoprenyl tail (similar to archaeal lipids) or due to differences in the bilayer properties of POPC and DPhPC lipids.
Fig. 5. Refolding of the KvAP channel in various lipids.
(A) SDS-PAGE gel showing glutaraldehyde crosslinking of the KvAP channel refolded in POPC, POPC: POPG (75: 25) and DPhPC lipid vesicles for 2 hours at room temperature (−, without crosslinker and +, 0.1% glutaraldehdye) and (B) bar graph comparing the refolding yields in the various lipids. Refolding yields of KvAP in asolectin vesicles (Aso) under similar refolding conditions is also shown. (C) SDS-PAGE gel and (D) bar graph showing the effect of increasing the ratio of POPE in POPC: POPE lipid vesicles on refolding of the KvAP channel. The extent of refolding after 2 hours at room temperature is shown. (E) Time course of refolding of the KvAP channel in POPC (circles), DPhPC (triangles) and POPC: POPE (60: 40, squares) lipid vesicles. The smooth lines for DPhPC and POPC: POPE corresponds to a single exponential fit while a line joining points is shown for POPC. For Panels B, C and E, the error bars indicate the standard deviation for 3 or more experiments.
Lipid bilayer properties such as the bilayer curvature stress can strongly influence the folding of membrane proteins (37, 38). The curvature stress of the lipid bilayer can influence the folding of a membrane protein either by an effect on the insertion into the lipid bilayer or through an effect on the packing of the transmembrane helices during folding. The curvature stress of the bilayer can be altered by varying the ratio of phosphoethanolamine lipids (PE) in a PC: PE lipid bilayer (37). To determine how the curvature stress of the bilayer influences the refolding of the KvAP channel, we carried out the refolding using lipid vesicles with varying ratios of POPC and POPE (Fig. 5C, D). We observed a steady increase in the refolding yield with an increase in the POPE ratio. We could not determine the effect of POPE percentages greater than 40% due to the interference of POPE with the glutaraldehyde crosslinking reaction. We established that POPE, at percentages greater than 40%, interferes with the glutaraldehyde reaction by evaluating the crosslinking of the native KvAP channel in lipid vesicles with varying ratios of POPC and POPE (data not shown). In the presence of 40% POPE, the extent of refolding was higher than the extent of refolding observed in DPhPC lipids and equals the extent of refolding observed in asolectin vesicles.
The kinetics of in vitro folding of the KvAP channel was determined by quenching the folding reaction at various time points by the addition of Fos-12 (2% w/v), followed by glutaraldehyde crosslinking. The time course of folding of KvAP in POPC, DPhPC and POPC: POPE (6: 4) lipids are shown in Fig. 5E. The rate of folding of the KvAP channel in POPC is very slow while a higher rate and extent of folding is observed in DPhPC and in POPC: POPE (6: 4) lipid vesicles. In these vesicles, the time course of folding was well fit by a single exponential and these fits were used to obtain the half time (t1/2) for the reaction. The higher rate of folding observed in POPC: POPE (6: 4) lipid vesicles (t1/2 = 40.8 min) compared to DPhPC (t1/2 = 65.8 min) suggests that lipids with isoprenyl tails are not essential for the refolding of the KvAP channel. The increase in the rate of refolding with the ratio of POPE in a POPC: POPE lipid bilayer suggests that increasing the membrane curvature stress of the lipid bilayer favors the refolding of the KvAP channel.
Effect of temperature on the refolding of the KvAP channel
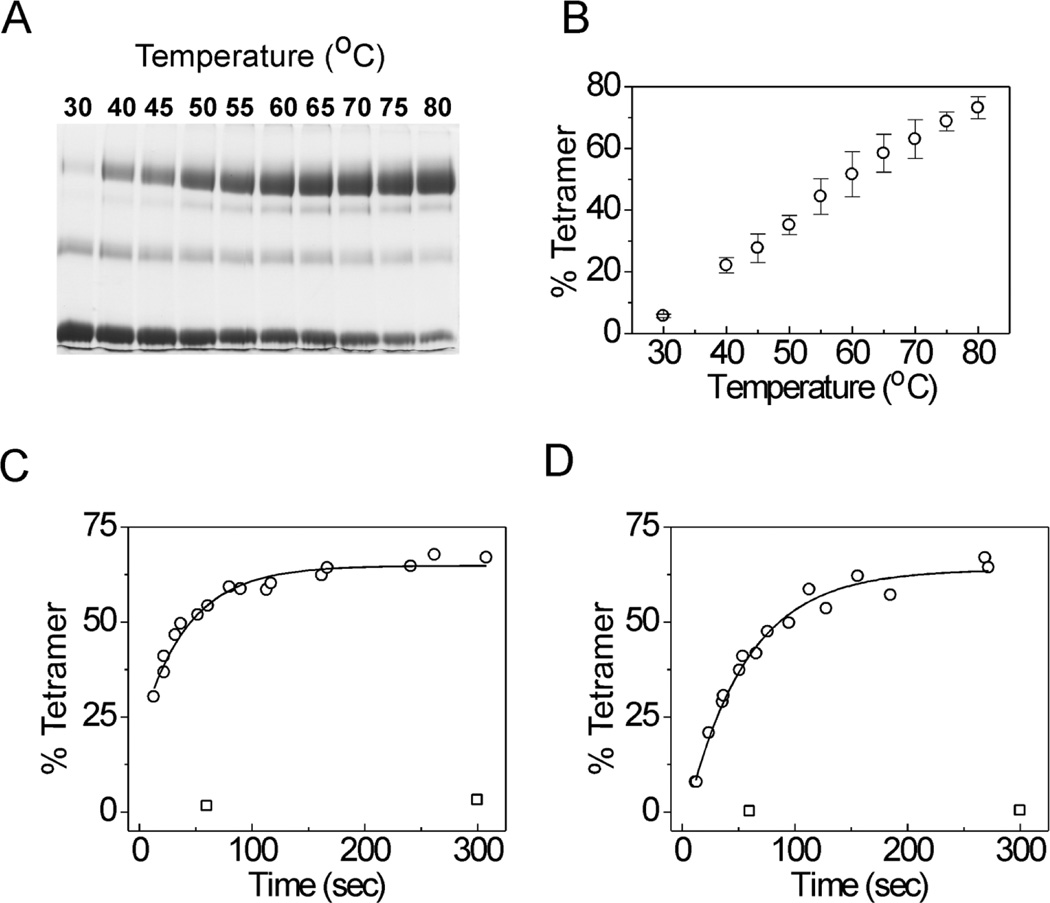
As the KvAP channel was cloned from a hyperthermophilic archaeon (Aeropyrum pernix) (39), we investigated the effect of elevated temperatures on the in vitro folding process. Refolding of KvAP was carried out at temperatures ranging from 30 to 80 °C for 10 min and the folding yields were determined by quenching the folding reaction with Fos-12 (2% w/v), followed by glutaraldehdye crosslinking (0.1% w/v at room temperature for 15 min). We used DPhPC lipid vesicles for our initial experiments as DPhPC lipid bilayers do not show a phase transition over this temperature range (35). With an increase in temperature, we observed a dramatic improvement in the extent of folding (Fig. 6A, B). At the highest temperature tested, the refolding yield was 73 ± 4% after 10 minutes compared to a yield of 15 ± 2% after a 2 hour incubation at room temperature. The time course of folding of KvAP in DPhPC vesicles at room temperature and 80 °C is shown in Fig 6C. Single exponential fits to the data indicate that the half time (t1/2) for the reaction changes from 65.8 min at room temperature to 0.48 min at 80 °C.
Fig. 6. Effect of temperature on refolding of the KvAP channel.
(A) SDS-PAGE gel showing refolding of KvAP in DPhPC lipid vesicles after 10 min incubation at the indicated temperatures. (B) The refolding yields after 10 min are plotted as a function of temperature. Error bars indicate standard deviation (n=3). Time course of refolding of the KvAP channel in DPhPC (C) and POPC (D) at 25°C (squares) and 80°C (circles). The solid lines represent single exponential fits to the data.
A similar influence of temperature was also observed for the refolding of KvAP in POPC lipid vesicles. The extent of refolding of the KvAP channel in POPC lipid vesicles was 63 ± 5 % after 10 min at 80 °C compared to a yield of 7 ± 3 % after 2 hours at room temperature. The rate of refolding in POPC lipid vesicles at 80 °C (t1/2 = 0.61 min) approaches the rate of refolding observed for DPhPC lipid vesicles (Fig. 6D). These observations indicate that temperature has a major influence on the in vitro folding of the KvAP channel.
Membrane insertion of the KvAP channel during refolding
One mechanism by which the composition of lipid vesicles and temperature can alter refolding rates of the KvAP channel is through an effect on the insertion of the unfolded polypeptide into the lipid bilayer. To investigate this possibility, we developed an assay to monitor the incorporation of the KvAP polypeptide into lipid bilayers. Our assay is based on the approach developed by Deutsch et. al. (40). In our assay, we introduced Cys residues into various segments of the KvAP channel and determined the accessibility of these Cys residues to modification by PEG-2K-mal in the presence and absence of lipid vesicles. If the Cys containing segment of the KvAP polypeptide is incorporated into the lipid bilayer, then it is protected from modification by PEG-2K-mal while a Cys residue in a segment that is not incorporated into the lipid bilayer is accessible to modification. The difference in modification by PEG-2K-mal arise because Cys residues within the lipid bilayer show a lower reactivity compared to aqueous exposed Cys residues. Further, the modifying reagent used, PEG-2K-mal, is highly water soluble and therefore concentrations within the lipid bilayer are expected to be extremely low. Modification by PEG-2K-mal is detected by a shift in the mobility on SDS-PAGE and the extent of modification reports on the degree of incorporation of that segment of the KvAP polypeptide into the lipid bilayer.
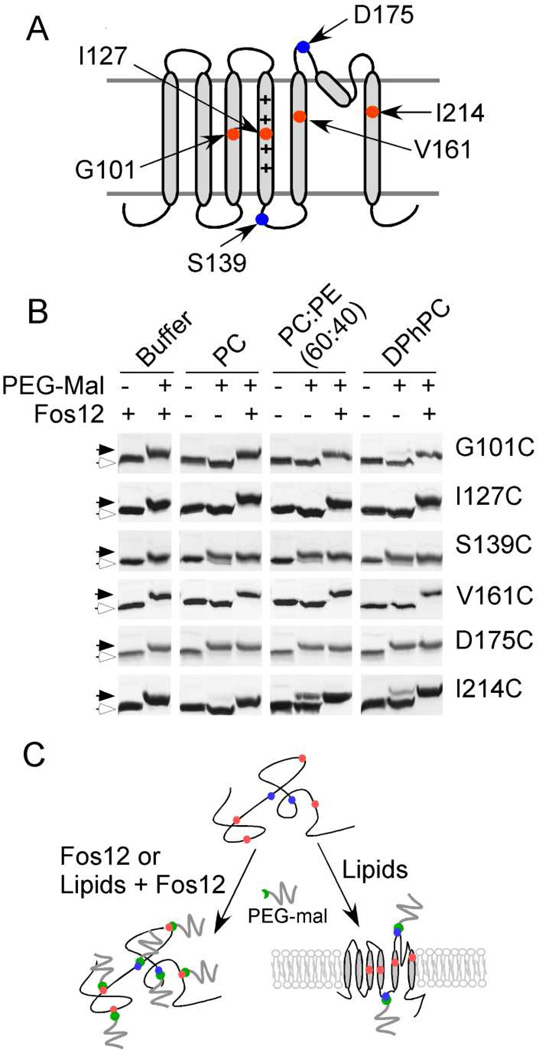
We generated Cys substitutions at residues 101 in the S3 helix and 127 in the S4 helix to monitor the incorporation of the VSD into the lipid bilayer while Cys substitutions at residues 161 in the S5 helix and 214 in the S6 helix were generated to monitor the incorporation of the pore domain (Fig. 7A). Cys substitutions at residues 139 in the S4-S5 loop and at residue 175 in the S5-pore helix loop were generated to serve as markers for the aqueous exposed segments of the KvAP channel. The Cys mutants of the KvAP channel were purified and unfolded as previously described. The unfolded KvAP polypeptides were diluted into lipid vesicles for refolding at room temperature and an aliquot was immediately withdrawn (~30 sec) and treated with PEG-2K-mal to test incorporation of the KvAP polypeptide into the lipid bilayer.
Fig. 7. Insertion of the KvAP polypeptide into the lipid bilayer during refolding.
(A) Cartoon depicting the location of Cys residues introduced into the KvAP channel for the PEGylation experiment. (B) Accessibility of Cys residues to PEGylation immediately after dilution of unfolded protein into lipid vesicles or into buffer containing 2% (w/v) Fos12 at room temperature. SDS-PAGE gels showing modification of the single Cys mutants of KvAP by PEG-2K-mal. The slow migrating PEGylated protein (solid arrows) and fast migrating protein without modification (open arrows) are indicated. (C) Summary of the PEGylation experiment. Upon dilution of the unfolded KvAP polypeptide into lipid vesicles, the Cys residues in the transmembrane segments (red) became inaccessible to PEGylation while the Cys residues in the loops (blue) remain accessible. In contrast, all the Cys residues are accessible to PEGylation when the unfolded KvAP polypeptide is diluted into detergent micelles or into lipid vesicles solubilized by Fos12 (2%, w/v).
On addition to lipid vesicles, Cys residues in the transmembrane segments (Cys 101 in S3, Cys 127 in S4, Cys 161 in S5 and Cys 214 in S6) were immediately protected from reaction with PEG-2K-mal (Fig. 7B). This protection was not observed when the KvAP polypeptides were diluted into buffer (in the absence of lipid vesicles) or when the lipid vesicles were solubilized with detergent. The composition of the lipid bilayer does not influence the protection of the Cys residues and almost complete protection of these transmembrane Cys residues from modification was seen for POPC, POPC: POPE (6:4) or DPhPC lipid vesicles. In contrast, the Cys139 and Cys 175 in the aqueous exposed regions were not protected from modification by PEG-2K-mal on the addition of the KvAP polypeptide to lipid vesicles. A similar extent of modification was observed for these Cys residues in the presence of lipid vesicles compared to the absence of lipid vesicles or when the lipid vesicles were solubilized with detergent.
These experiments indicate that upon addition of the unfolded KvAP polypeptide to lipid vesicles, the transmembrane segments are immediately incorporated into the lipid bilayer while the loop regions stay external to the membrane (Fig. 7C). We observe complete labeling of Cys139 and Cys 175, residues in loop regions that are expected to be on opposite sides of the membrane. This observation suggests that PEG-2K-mal is able to permeate into the lipid vesicles, probably due to the residual detergent (SDS, 3.5 mM) during refolding. The similar labeling of Cys 139 and Cys 175 indicates that we cannot differentiate between Cys residues exposed to the exterior and the lumen of the lipid vesicles by using the PEG-2K-mal reagent.
Our results suggest that during refolding, the unfolded KvAP polypeptide rapidly incorporates into lipid vesicles with a “native-like” topology. The extent of incorporation is independent of the composition of the lipid bilayer as similar incorporation is seen in the case of POPC, POPC: POPE (6:4) or DPhPC lipid vesicles. This rapid incorporation of the KvAP polypeptide into lipid vesicles is observed at room temperature, at which the rate of refolding is very low. These Cys protection experiments therefore suggest that the two factors, composition of the lipid bilayer and the temperature that are important for the in vitro folding of the KvAP channel do not influence the insertion of the KvAP polypeptide into the lipid bilayer but instead influence a later folding step that takes place within the lipid bilayer.
High level expression of the KvAP channel
A major challenge in investigations of membrane proteins is obtaining sufficient quantities of the native protein for structural and biochemical analysis. The general approach used is expression in the native state in a heterologous system. In the case of the KvAP channel, expression in the native state in E. coli membranes provides ~1 mg of the purified protein per L of the culture medium. In vitro folding of the KvAP channel provides an alternate to native expression. The GST fusion approach provides high level expression of the KvAP polypeptide as inclusion bodies, which using the optimal refolding conditions identified, can be efficiently folded to the native state. Using this approach, we were able to obtain 8 mgs of the purified refolded KvAP channel per L of the culture medium, ~8 fold higher than the protein yield obtained by native expression.
DISCUSSION
In this study, we demonstrate that the KvAP channel can be folded in vitro from the extensively unfolded state. Biochemical and functional characterization of the refolded KvAP channel indicate that it is similar to the native channel. While we have established that the unfolded KvAP polypeptide is monomeric, the extent of residual structure in this state is not known. We anticipate that the folding process described corresponds to rearrangement of secondary structure and the formation of the tertiary and quaternary structure. We show that in vitro folding of the KvAP channel requires lipid bilayers. A lipid bilayer requirement has been demonstrated for the refolding of the KcsA and NaK ion channels (14, 21). The similar architecture of the pore domain in these channels and the KvAP pore suggests that the lipid bilayer requirement for the folding, probably originates from the pore domain.
The refolding of the KvAP channel in lipid vesicles is influenced by the curvature stress of the lipid bilayer. An increase in the curvature stress of the lipid bilayer increases the refolding yield of the KvAP channel. The major factor influencing the refolding of the KvAP channel is temperature and at elevated temperatures, close to quantitative refolding of the KvAP channel is observed. The KvAP channel was cloned from a hyperthermophile and so it is expected that the channel can fold at an elevated temperature. However, to our knowledge, this is the first report of the necessity of elevated temperatures for the efficient folding of a membrane protein. The increase in efficiency of in vitro folding with temperature is probably a feature unique to hyperthermophilic membrane proteins like KvAP. The efficient in vitro folding of the KvAP channel combined with high level expression of the KvAP polypeptide as inclusion bodies provides an easy means to obtain high yields of the KvAP channel. The higher yields obtained using the in vitro folding approach will be useful in isotopic labeling of the KvAP channel for NMR studies. This approach will also be useful in investigating the specific lipid effects on structural and functional properties of the channel as unlike the protein purified from Escherichia coli membranes, the refolded KvAP channel is devoid of any lipid contaminations of unknown nature (29, 41, 42). Further, in vitro folding also sets the stage for semisynthesis of the KvAP channel, an important technique that enables the incorporation of unnatural amino acids and peptide backbone modifications for structure-function investigations (43).
The in vitro folding of the KvAP channel can be considered to consist of two stages with the first stage corresponding to the insertion of the unfolded polypeptide into the lipid bilayer and the second stage comprising of channel folding and assembly within the bilayer. Insertion of the KvAP polypeptide into the lipid bilayer was probed using the Cys protection assay. The assay demonstrates that the insertion of the unfolded KvAP polypeptide into lipid bilayers is quite rapid and the insertion takes place with a “native-like” topology. The rapid insertion of the KvAP polypeptide into the lipid bilayer is probably related to the presence of residual detergent (SDS, 3.5 mM) during refolding, which may facilitate the insertion process.
As membrane insertion is relatively fast, the rate limiting step in KvAP refolding must be the channel assembly within the lipid bilayer. Channel assembly within the lipid bilayer consists of the folding of the VSD, the folding and tetramerization of the pore domain, and the inter subunit association of the VSD and the pore domain. Further experiments will be necessary to determine the order of these steps in the in vitro folding of the KvAP channel and to pinpoint the specific step that is enhanced by elevated temperatures and the composition of the lipid bilayer. The efficient in vitro folding of the KvAP channel and the ability to manipulate the folding rate by experimental conditions such as temperature or lipid bilayer composition makes the KvAP channel a good model system for investigating the assembly pathway of a multi-domain membrane protein.
Acknowledgments
The research was supported by grants to FIV from the NIH (GM087546), a Scientist Development Grant from the American Heart Association (0835166N) and a Pew Scholar Award.
REFERENCES
- 1.Hille B. Ion Channels of Excitable Membranes. Third ed. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- 2.Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 3.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 4.Kullmann DM. The neuronal channelopathies. Brain. 2002;125:1177–1195. doi: 10.1093/brain/awf130. [DOI] [PubMed] [Google Scholar]
- 5.Abbott GW. Molecular mechanisms of cardiac voltage-gated potassium channelopathies. Curr. Pharm. Des. 2006;12:3631–3644. doi: 10.2174/138161206778522065. [DOI] [PubMed] [Google Scholar]
- 6.Tu L, Wang J, Helm A, Skach WR, Deutsch C. Transmembrane biogenesis of Kv1.3. Biochemistry. 2000;39:824–836. doi: 10.1021/bi991740r. [DOI] [PubMed] [Google Scholar]
- 7.Manganas LN, Wang Q, Scannevin RH, Antonucci DE, Rhodes KJ, Trimmer JS. Identification of a trafficking determinant localized to the Kv1 potassium channel pore. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14055–14059. doi: 10.1073/pnas.241403898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson JM, Kosolapov A, Deutsch C. Tertiary and quaternary structure formation of voltage-gated potassium channels. Methods Mol. Biol. 2006;337:41–52. doi: 10.1385/1-59745-095-2:41. [DOI] [PubMed] [Google Scholar]
- 9.Schwappach B. An overview of trafficking and assembly of neurotransmitter receptors and ion channels. Mol. Membr. Biol. 2008;25:270–278. doi: 10.1080/09687680801960998. [DOI] [PubMed] [Google Scholar]
- 10.Gajewski C, Dagcan A, Roux B, Deutsch C. Biogenesis of the pore architecture of a voltage-gated potassium channel. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3240–3245. doi: 10.1073/pnas.1017097108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White SH, Wimley WC. Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 12.Booth PJ, Curran AR, Templer RH, Lu H, Meijberg W. Manipulating the folding of membrane proteins: using the bilayer to our advantage. Biochem. Soc. Symp. 2001;68:27–33. doi: 10.1042/bss0680027. [DOI] [PubMed] [Google Scholar]
- 13.Huang KS, Bayley H, Liao MJ, London E, Khorana HG. Refolding of an integral membrane protein. Denaturation, renaturation, and reconstitution of intact bacteriorhodopsin and two proteolytic fragments. J. Biol. Chem. 1981;256:3802–3809. [PubMed] [Google Scholar]
- 14.Valiyaveetil FI, Zhou Y, MacKinnon R. Lipids in the structure, folding, and function of the KcsA K+ channel. Biochemistry. 2002;41:10771–10777. doi: 10.1021/bi026215y. [DOI] [PubMed] [Google Scholar]
- 15.Booth PJ, Curnow P. Membrane proteins shape up: understanding in vitro folding. Curr. Opin. Struct. Biol. 2006;16:480–488. doi: 10.1016/j.sbi.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Barrera FN, Renart ML, Molina ML, Poveda JA, Encinar JA, Fernandez AM, Neira JL, Gonzalez-Ros JM. Unfolding and refolding in vitro of a tetrameric, alpha-helical membrane protein: the prokaryotic potassium channel KcsA. Biochemistry. 2005;44:14344–14352. doi: 10.1021/bi050845t. [DOI] [PubMed] [Google Scholar]
- 17.Barrera FN, Renart ML, Poveda JA, de Kruijff B, Killian JA, Gonzalez-Ros JM. Protein self-assembly and lipid binding in the folding of the potassium channel KcsA. Biochemistry. 2008;47:2123–2133. doi: 10.1021/bi700778c. [DOI] [PubMed] [Google Scholar]
- 18.Raja M. The potassium channel KcsA: a model protein in studying membrane protein oligomerization and stability of oligomeric assembly? Arch. Biochem. Biophys. 2011;510:1–10. doi: 10.1016/j.abb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Clayton D, Shapovalov G, Maurer J, Dougherty D, Lester H, Kochendoerfer G. Total chemical synthesis and electrophysiological characterization of mechanosensitive channels from Escherichia coli and Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S. A. 2004;101:4764–4769. doi: 10.1073/pnas.0305693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charalambous K, O'Reilly AO, Bullough PA, Wallace BA. Thermal and chemical unfolding and refolding of a eukaryotic sodium channel. Biochim. Biophys. Acta. 2009;1788:1279–1286. doi: 10.1016/j.bbamem.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linn KM, Derebe MG, Jiang Y, Valiyaveetil FI. Semisynthesis of NaK a Na(+) and K(+) conducting ion channel. Biochemistry. 2010;49:4450–4456. doi: 10.1021/bi100413z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruta V, Jiang Y, Lee A, Chen J, MacKinnon R. Functional analysis of an archaebacterial voltage-dependent K+ channel. Nature. 2003;422:180–185. doi: 10.1038/nature01473. [DOI] [PubMed] [Google Scholar]
- 23.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Lee A, Chen J, MacKinnon R. Structure of the KvAP voltage-dependent K+ channel and its dependence on the lipid membrane. Proc. Natl. Acad. Sci U.S.A. 2005;102:15441–15446. doi: 10.1073/pnas.0507651102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarov-Yarovoy V, Baker D, Catterall WA. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K(+) channels. Proc. Natl. Acad. Sci. U.S. A. 2006;103:7292–7297. doi: 10.1073/pnas.0602350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuello L, Cortes D, Perozo E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science. 2004;306:491–495. doi: 10.1126/science.1101373. [DOI] [PubMed] [Google Scholar]
- 27.Richardson J, Blunck R, Ge P, Selvin PR, Bezanilla F, Papazian DM, Correa AM. Distance measurements reveal a common topology of prokaryotic voltage-gated ion channels in the lipid bilayer. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15865–15870. doi: 10.1073/pnas.0607532103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt D, Cross SR, MacKinnon R. A gating model for the archeal voltage-dependent K(+) channel KvAP in DPhPC and POPE:POPG decane lipid bilayers. J. Mol. Biol. 2009;390:902–912. doi: 10.1016/j.jmb.2009.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butterwick JA, MacKinnon R. Solution structure and phospholipid interactions of the isolated voltage-sensor domain from KvAP. J. Mol. Biol. 2010;403:591–606. doi: 10.1016/j.jmb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shenkarev ZO, Paramonov AS, Lyukmanova EN, Shingarova LN, Yakimov SA, Dubinnyi MA, Chupin VV, Kirpichnikov MP, Blommers MJ, Arseniev AS. NMR structural and dynamical investigation of the isolated voltage-sensing domain of the potassium channel KvAP: implications for voltage gating. J. Am. Chem. Soc. 2010;132:5630–5637. doi: 10.1021/ja909752r. [DOI] [PubMed] [Google Scholar]
- 32.Chakrapani S, Cuello LG, Cortes DM, Perozo E. Structural dynamics of an isolated voltage-sensor domain in a lipid bilayer. Structure. 2008;16:398–409. doi: 10.1016/j.str.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heginbotham L, Kolmakova-Partensky L, Miller C. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 1998;111:741–749. doi: 10.1085/jgp.111.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seddon AM, Lorch M, Ces O, Templer RH, Macrae F, Booth PJ. Phosphatidylglycerol lipids enhance folding of an alpha helical membrane protein. J. Mol. Biol. 2008;380:548–556. doi: 10.1016/j.jmb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Lindsey H, Petersen NO, Chan SI. Physicochemical characterization of 1,2-diphytanoyl-sn-glycero-3-phosphocholine in model membrane systems. Biochim. Biophys. Acta. 1979;555:147–167. doi: 10.1016/0005-2736(79)90079-8. [DOI] [PubMed] [Google Scholar]
- 36.De Rosa M, Gambacorta A, Gliozzi A. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 1986;50:70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth PJ. Sane in the membrane: designing systems to modulate membrane proteins. Curr. Opin. Struct. Biol. 2005;15:435–440. doi: 10.1016/j.sbi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Miller D, Charalambous K, Rotem D, Schuldiner S, Curnow P, Booth PJ. In vitro unfolding and refolding of the small multidrug transporter EmrE. J. Mol. Biol. 2009;393:815–832. doi: 10.1016/j.jmb.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 39.Kawarabayasi Y, Hino Y, Horikawa H, Yamazaki S, Haikawa Y, Jin-no K, Takahashi M, Sekine M, Baba S, Ankai A, Kosugi H, Hosoyama A, Fukui S, Nagai Y, Nishijima K, Nakazawa H, Takamiya M, Masuda S, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Kikuchi H, et al. Complete genome sequence of an aerobic hyper-thermophilic crenarchaeon, Aeropyrum pernix K1. DNA Res. 1999;6:83–101. doi: 10.1093/dnares/6.2.83. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Deutsch C. Pegylation: a method for assessing topological accessibilities in Kv1.3. Biochemistry. 2001;40:13288–13301. doi: 10.1021/bi0107647. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt D, Jiang QX, MacKinnon R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444:775–779. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]
- 42.Zheng H, Liu W, Anderson LY, Jiang QX. Lipid-dependent gating of a voltage-gated potassium channel. Nat. Commun. 2011;2:250. doi: 10.1038/ncomms1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Focke PJ, Valiyaveetil FI. Studies of ion channels using expressed protein ligation. Curr. Opin. Chem. Biol. 2010;14:797–802. doi: 10.1016/j.cbpa.2010.09.014. [DOI] [PubMed] [Google Scholar]