Abstract
Background
Gynodioecy is a reproductive system of interest for evolutionary biologists, as it poses the question of how females can be maintained while competing with hermaphrodites that possess both male and female functions. One necessary condition for the maintenance of this polymorphism is the occurrence of a female advantage, i.e. a better seed production or quality by females compared with hermaphrodites. Theoretically, its magnitude can be low when sterility mutations are cytoplasmic, while a 2-fold advantage is needed in the case of nuclear sterility. Such a difference is often thought to be due to reduced inbreeding depression in obligatory outcrossed females. Finally, variation in sex ratio and female advantage occur among populations of some gynodioecious species, though the prevalence of such variation is unknown.
Scope
By reviewing and analysing the data published on 48 gynodioecious species, we examined three important issues about female advantage. (1) Are reduced selfing and inbreeding depression likely to be the major cause of female advantage? (2) What is the magnitude of female advantage and does it fit theoretical predictions? (3) Does the occurrence or the magnitude of female advantage vary among populations within species and why?
Conclusions
It was found that a female advantage occurred in 40 species, with a magnitude comprised between 1 and 2 in the majority of cases. In many species, reduced selfing may not be a necessary cause of this advantage. Finally, female advantage varied among populations in some species, but both positive and negative correlations were found with female frequency. The role of reduced selfing in females for the evolution of gynodioecy, as well as the various processes that affect sex ratios and female advantage in populations are discussed.
Keywords: Female advantage, female compensation, male sterility, gynodioecy, inbreeding depression, mating system, resource allocation, sex ratio, self-incompatibility
INTRODUCTION
Gynodioecy, the co-occurrence of females and hermaphrodites within the same species, is a relatively common sexual system in flowering plants (reviewed in Webb, 1999) and has been a model of interest for evolutionary biologists for several reasons. First, like any other polymorphism, it poses the question of its appearance and its maintenance within populations. Because this particular polymorphism involves the occurrence of female individuals that have lost one of their sexual functions with hermaphrodites that possess both, its maintenance at equilibrium is particularly intriguing. Secondly, because gynodioecy is sometimes viewed as an intermediate step in the evolution of dioecy (co-occurrence of female and male plants), its understanding appears crucial for the study of the evolution of separate sexes, which is a common transition in the evolutionary history of Angiosperms (Charlesworth, 1999; Barrett, 2002). Thirdly, since gynodioecy results in many cases from interactions between cytoplasmic and nuclear genes (see below), it constitutes a choice model for the study of nuclear–cytoplasmic conflicts (Saumitou-Laprade et al., 1994).
Two main sex determination systems in gynodioecious species have been investigated theoretically and documented in nature: (1) mutations responsible for male sterility are nuclear (Godley, 1955; Kohn, 1989) or (2) mutations of male sterility are cytoplasmic (CMS, for cytoplasmic male sterility) and their effect can be counteracted by nuclear alleles, named restorers, that restore pollen production (Cosmides and Tobby, 1981; reviewed in Chase, 2007). In the latter case, because they are purely maternally inherited, the loss of pollen production does not reduce the transmission of cytoplasmic genes, while it clearly constitutes a cost for nuclear genes that are bi-parentally transmitted, leading to the so-called nuclear–cytoplasmic conflict. For both kinds of sex determination, theory predicts that females should benefit from a better female fitness than hermaphrodites, in order for mutations of male sterility to invade a population. Such a fitness difference has been called female advantage (FA) or female compensation in the literature (Darwin, 1877) and is a central parameter of the evolution of gynodioecy. In a meta-analysis conducted on 29 different gynodioecious species, Shykoff et al. (2003) found indeed that females produced more flowers, set more fruits and produced more seeds that were larger and germinated better than those of hermaphrodites from the same populations, thus showing that the occurrence of a FA is a general trend in gynodioecious species.
Practically, FA, when it occurs, can have several proximal causes including (a) reallocation of resources from the male towards the female function; (b) sex differences in interactions with herbivores (hermaphrodite-biased predation); and (c) reduced selfing and associated inbreeding depression (ID) in females. The latter process has been investigated in theoretical models (e.g. Charlesworth and Charlesworth, 1978) and is sometimes considered to be the main process responsible for the fitness advantage of females, and thus for gynodioecy (e.g. Kubota and Ohara, 2009); however, the strength and the generality of this effect remain poorly known.
Besides the occurrence of FA, both the among-population variation and the magnitude of this effect are potentially important for the evolutionary dynamics of gynodioecy. First, although most theoretical models – even those that consider species within a meta-population context (Frank, 1989; Pannell, 1997; Couvet et al., 1998; Dufay and Pannell, 2010) – generally assume the FA to be a constant parameter of the species, it is thought possibly to vary among populations, as a function of a biotic or abiotic component of the environment and/or of the population sex ratio. Such a variation in the FA among populations has been shown to facilitate the maintenance of the sexual polymorphism in some cases (McCauley and Taylor, 1997; Frank and Barr, 2001). Secondly, although a FA is theoretically needed in all gynodioecious species, its magnitude should vary according to the details of genetic determination of male sterility. On the one hand, the invasion and the maintenance of nuclear-transmitted male sterility requires a 2-fold FA, that compensates the loss of pollen production for nuclear alleles of male sterility (Lewis, 1941; Lloyd, 1976). On the other hand, a FA comprised between 1 and 2 (i.e. females are better, but not necessarily twice better, than hermaphrodites) should be sufficient for CMS to invade a population (Gouyon et al., 1991; Bailey et al., 2003), although other conditions (i.e. a cost of restorer alleles and/or recurrent local extinctions and invasions of demes in a metapopulation context) are necessary for the stable maintenance of the sexual polymorphism in that case (Gouyon et al., 1991; Couvet et al., 1998; Bailey et al., 2003). To date, no clear synthesis is available regarding the effective magnitude of FA that has been empirically measured in gynodioecious plants nor about its possible variation among the populations of a given species.
This work reviews experimental studies that have investigated the occurrence of FA in 48 different gynodioecious species. The main purpose of the current study was not to verify the overall occurrence of a FA (already shown by Shykoff et al., 2003), but rather to examine other questions related to this fitness effect. (a) Is reduced selfing and associated ID likely to be the major cause of FA? (b) What is the magnitude of the FA, how does it vary among species and does it fit with theoretical predictions? (c) Does the occurrence or the magnitude of the FA vary among populations within species and why?
METHODS
Literature search and data extraction
We searched the bibliographic database Web of Science up to July 2009 for all papers containing the words ‘gynodioecy’, ‘male sterility’, ‘female advantage’ or ‘female compensation’ in the title or keywords. We retained all studies that showed a statistical comparison of at least one of the reproductive traits listed below, between females and hermaphrodites, either in natural populations or in controlled conditions (greenhouse or experimental garden). The present work reviews the data published in 82 studies, based on 48 gynodioecious species, belonging to 39 different genera and 27 families (Table 1).
Table 1.
Main characteristics of the 48 gynodioecious species and occurrence of female advantage
| Fitness traits |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Family | SI/SC | Poll | Life cycle | Genetics | FA? | Females are better | Females are not better | No differences | References | |
| Beta vulgaris | Amaranthaceae | SI | W | P | NC | NO | NFL, SS, NS, G | Boutin-Stadler (1987); Dufay et al. (2009)* | |||
| Gingidia flabellata | Apiaceae | P | FA | FS | NFL | Webb (1981) | |||||
| Cirsium chikushiense | Asteraceae | SI | E | P | NO | NFL | Kawabuko (1994) | ||||
| Echium vulgare | Boraginaceae | SC | E | Bisa | NC | FA | FS, SS, NS | NFL, SM, G | Klinkhamer et al. (1991*, 1994) | ||
| Eritrichum aretioides | Boraginaceae | pSC | E | P | FA | SM, G | NFL, SS | Puterbaugh et al. (1997) | |||
| Phacelia linearis | Boraginaceae | SC | E | A | N | FA | NS | SM, SS, G, Surv | Eckhart (1992a, b) | ||
| Raphanus sativus | Brassicaceae | SI | E | A | NC | NO | NFL, NS, G | SM | Miyake et al. (2009) | ||
| Opuntia quimilo | Cactaceae | SC | E | P | FA | FS | NFL,SS | Diaz and Cocucci, 2003 | |||
| Lobelia siphilitica | Campanulaceae | SC | E | P | NC | FA | NFL, NS | SS, NFL | Caruso and Yakobowski (2008); Caruso and Case (2007*); Caruso et al. (2003) | ||
| Dianthus sylvestris | Caryophyllaceae | SC | E | P | FA | NFL, SM | SS, G | Collin et al. (2002) | |||
| Moehringia lateriflora | Caryophyllaceae | pSC | E | P | VAR | FS, SS | FS, SS | Sugawara (1993) | |||
| Schiedea adamantis | Caryophyllaceae | SC | W/E | P | N | FA | FS, SM, SS | NFL, G, Surv-Ad, | Sakai et al. (1997) | ||
| Schiedea salicaria | Caryophyllaceae | SC | W/E | P | N | VAR | G, SM, SS | NFL, FS, SM, SS, G | Weller and Sakai (2005) | ||
| Silene acaulis | Caryophyllaceae | SC | E | P | NC | FA | FS | NFL | Shykoff (1992*); Hermanutz and Innes (1994) | ||
| Silene italica | Caryophyllaceae | SC | E | P | NC | FA | FS, SM | NFL | NFL | Maurice (1999); Lafuma and Maurice (2006) | |
| Silene nutans | Caryophyllaceae | SC | E | P | NC | NO | NFL | Dufay et al. (2010) | |||
| Silene stockenii | Caryophyllaceae | SC | E | A | NC | NO | FS, SS | Talavera et al. (1996) | |||
| Silene vulgaris | Caryophyllaceae | SC | E | P | NC | FA | FS, SM, SS | SS, G | Dulberger and Horovitz (1984); Olson et al. (2006); Andersson et al. (2008*) | ||
| Clusia nemorosa | Clusiaceae | SC | E | P | FA | FS | Lopes and Machado (1998) | ||||
| Wurmbea biglandulosa | Colchicaceae | SC | E | P | VAR | NFL, SS, NS, G | SM, NS | SS, Surv | Ramsey and Vaughton (2002); Vaughton and Ramsey (2004) | ||
| Cucurbita foetidissima | Cucurbitaceae | SC | E | P | N | FA | NFL,NS | SS | FS,SM | Kohn (1989) | |
| Erythroxylum havanense | Erythroxylaceae | SI | E | P | FA | FS, Surv-Ad | Avila-Sakar and Dominguez (2000); Rosas et al. (2005) | ||||
| Geranium maculatum | Geraniaceae | SC | E | P | FA | NFL, FS, SM, SS, NS, G, Surv | NFL, FS, G, Surv | Agren and Willson (1991); Chang (2006) | |||
| Geranium sylvaticum | Geraniaceae | SC | E | P | VAR | FS, SS, NS | NFL,SM,G,Surv | Asikainen and Mutikainen (2003, 2005); Ramula and Mutikainen (2003) | |||
| Nemophila menziesii | Hydrophyllaceae | SC | E | A | NC | FA | NS | NFL | Barr (2004) | ||
| Iris douglasiana | Iridaceae | SC | E | P | Amb. | SS | SS | Uno (1982) | |||
| Glechoma longituba | Lamiaceae | SC | E | P | VAR | SM, SS | SS | Zhang et al. (2008) | |||
| Lycopus maackianus | Lamiaceae | . | E | P | FA | NS | Hong and Moon (2003) | ||||
| Thymus vulgaris | Lamiaceae | SC | E | P | NC | FA | NS, G | Assouad et al. (1978) | |||
| Limnanthes douglasii | Limnanthaceae | SC | E | A | NC | FA | NFL | Kesseli and Jain (1984, 1985) | |||
| Nototriche compacta | Malvaceae | SC | E | P | NO | SS | FS, SS | Garcia-Franco and Arroyo (1995) | |||
| Sidalcea hendersonii | Malvaceae | SC | E | P | N | VAR | SS | FS, SS | Marshall and Ganders (2001) | ||
| Sidalcea malviflora | Malvaceae | SC | E | P | NC | FA | SS | Graff (1999) | |||
| Chionographis japonica | Melianthaceae | SC | E | P | FA | NS | NFL | Maki (1993) | |||
| Satyrium ciliatum | Orchidaceae | SC | E | P | FA | FS | Huang et al. (2009) | ||||
| Hebe subalpina | Plantaginaceae | SC | E | P | FA | FS | NFL | Delph (1993) | |||
| Plantago coronopus | Plantaginaceae | SC | W | A | NC | Amb. | NFL, SM | SS | SS, Surv | Koelewijn (1996, 1998); Koelewijn and VanDamme (1996*) | |
| Plantago lanceolata | Plantaginaceae | SI | W | P | NC | FA | NFL, NS, SM, Surv-Ad | SM, SS, G, Surv | Poot et al. (1996); Vandamme and Vandelden, (1982*, 1984) | ||
| Plantago maritima | Plantaginaceae | Mainly SI | W | P | NC | VAR | NFL, SM, NS | FS, SS, NS | Nilsson and Agren (2006) | ||
| Chionochloa bromoides | Poaceae | SC | W | NO | NFL, SS | Connor (1990) | |||||
| Cortaderia richardii | Poaceae | SC | W | P | N | FA | SM, G | Connor (1965) | |||
| Fragaria virginiana | Rosaceae | SC | E | P | N | FA | FS, NFL, NS | NFL | Ashman (1999, 2003); Case and Ashman (2007) | ||
| Prunus mahaleb | Rosaceae | pSC | E | P | FA | NFL, SM, NF | FS | G | Jordano (1993) | ||
| Bequaertiodendron magalismontanum | Sapotaceae | E | P | FA | FS | Steyn and Robbertse (1990) | |||||
| Saxifraga granulata | Saxifragaceae | SC | E | P | NC | Amb. | SM, G | SS | NFL | Stevens and Richards (1985*); Stevens (1988) | |
| Daphne laureola | Thymelaeceae | SC | E | P | NO | NFL | FS | SM, FS | Alonso and Herrera (2001); Alonso (2005); Alonso et al. (2007) | ||
| Gnidia wikstroemiana | Thymelaeceae | SC | E | P | FA | NFL | FS | Beaumont et al. (2006); Smith (2009*) | |||
| Kallstroemia grandiflora | Zygophyllaceae | SC | E | A | Amb. | FS | SS | NS, SM | Cuevas et al. (2008) | ||
For each species is listed: whether it is self-compatible (SC), partially self-compatible (pSC) or self-incompatible (SI); its pollination mode (Poll), if it is either wind pollinated (W) or entomophilous (E); whether it is perennial (P), bisannual (Bisa) or annual (A) and, where known, the determination of sex (NC, nuclear–cytoplasmic; N, nuclear). This table reports the traits for which studies have found a female advantage, a female disavadvantage or no difference between females and hermaphrodites. When the same trait is found in several categories for a given species, it means that either different studies found contradictory results or that results vary according to the population or year. Abbreviation for the traits are the followings: NFL, number of flowers; FS, fruit set; SS, seed set; NS, number of seeds per plant; NF, number of fruits per plant; SM, seed mass or size; G, germination; Surv, survival of offspring; Surv-Ad, adult survival. Only results obtained on open-pollinated flowers are reported here. The column ‘FA’ summarizes all reported results, by indicating whether a female advantage has been statistically demonstrated by the original studies for at least one trait (FA), ambiguous cases (Amb.), species for which female advantage varies with populations (VAR) and species for which no female advantage has been found (NO). References tagged with an asterisk are those that were used for reporting data on sex ratios but not on fitness traits.
For all investigated species, we collected the results of statistical comparisons between open-pollinated females and open-pollinated hermaphrodites for the following reproductive traits: number of flowers (NFL), fruit set (FS), defined as the fruit/flower ratio, seed set or number of seeds per fruit (SS), seed size or seed mass (SM), total number of fruits per plant (NF), total number of seeds per plant (NS) and seed germination rate (G). Female–hermaphrodite comparisons were also recorded for offspring or adult survival rate (Surv). For each of these traits, it was noted whether the reviewed study found a statistical advantage for females, a statistical advantage for hermaphrodites or a non-significant difference. In a second step, the results obtained for the different reproductive traits for each species were summarized: a FA was considered to occur in a given species as soon as a significantly higher value was found in females for at least one of the traits. When only female disadvantages or non-significant differences were found on the several investigated traits, it was considered that no FA effectively occurred. Species for which different studies found contradictory results were classified in a separate category (see Results).
For traits related to fruit or seed production and quality (FS, SS, NS, NF, G and SM), the results of other types of female–hermaphrodite comparisons (experimentally cross-pollinated females vs. experimentally cross-pollinated hermaphrodites; experimentally cross-pollinated females vs. experimentally self-pollinated hermaphrodites) were also recorded when available, because they could provide some information on the proximal causes of the FA (Table 2). Indeed, a FA that has been found by comparing seed production or quality between open-pollinated female flowers and open-pollinated hermaphroditic flowers reflects an advantage that females should experience in natural conditions, and was subsequently reported in Table 1, but such a FA may be attributable to different factors, including differences in the number of ovules per flower, in the ability to set and mature seeds, in the quantity of resources that can be invested in seeds and in the average quantity of pollen deposited on stigmas. The proportion of self-pollen deposited on stigmas and the overall quality of selfed/outcrossed seeds in hermaphrodites are other important factors that can influence various components of female reproduction. Thus, when only open-pollinated flowers had been compared between females and hermaphrodites, we could assess whether a FA effectively occurred, but it was not possible to assess which of these factors was responsible for it. However, comparisons performed on hand-pollinated (outcrossed) flowers could provide additional information, because one can expect that (a) the quantity of pollen deposited on stigmas was similar between female and hermaphroditic flowers and that (b) only outcrossed pollen had been deposited, consistently leading to an inbreeding level that should be similar between seeds produced by the two sexes. Consequently, if a FA was found in such a case, it could not be attributable to a difference in the level of pollen limitation nor to a reduced selfing and associated ID in females (Table 2). We also extracted from the reviewed articles any additional information that would help to clarify this (e.g. measurements of the selfing rate occurring in populations; measurements of the magnitude of ID on fitness traits).
Table 2.
How to interpret different types of comparison between females and hermaphrodites for a given species
| Comparison of open-pollinated flowers |
|||
|---|---|---|---|
| Comparison of outcrossed flowers | F < H | F = H | F > H |
| F < H | FD | – | – |
| F = H | FD due to pollen limitation | No FA | FA mainly due to the avoidance of ID |
| F > H | FD due to pollen limitation. FA may be pollinator dependent* | FA may be pollinator dependent* | FA not (entirely) due to the avoidance of ID |
When female (F) vs. hermaphrodite (H) comparisons were performed in both open-pollinated and artificially outcrossed flowers, several combinations of results could be found for a given trait. Whereas comparisons of open-pollinated flowers highlight whether females benefit from a female advantage (FA) or disadvantage (FD) in natural conditions, comparisons based on hand-pollinated (outcross) flowers provide information on whether the avoidance of self-pollination and associated inbreeding depression (ID) may be one proximal explanation of this fitness advantage, as soon as they were performed in the same ecological conditions as open pollinations.
A dash indicates situations that have not been reported in this review. In cases marked by an asterisk, a female advantage could occur (probably due to resource reallocation, rather than avoidance of ID) in non-pollen-limited situations.
In order to investigate the possible variation of occurrence of a FA among populations, within a given species, we then focused on studies in which at least one of the fitness traits listed above was measured on plants originating from different populations. Based on these studies, we recorded whether the statistical analyses showed a significant FA in all populations or whether a variation of FA occurrence was found within the same study. Any details that could explain such among-populations variation were also recorded.
Finally, for each species, the following reproductive characteristics were also reported, when information was available: the sex ratio in natural populations (average and/or minimum and maximum female frequencies), mode of pollination (entomophilous vs. anemophilous), sex determination (nuclear vs. nuclear–cytoplasmic), life cycle (annual vs. perennial) and potential for self-pollination in hermaphrodites (self-compatible vs.self-incompatible species). Such information can be useful for the understanding of the dynamics of gynodioecy since (a) sex differences in interactions with pollinators may lead to sex differences in reproductive success in entomophilous species; (b) the magnitude of FA is expected to differ according to the details of sex determination; (c) sex differences in reproductive success may be caused by differences in terms of survival in perennial species; and (d) information on self-(in)compatibility will help us in investigating the possible role of reduced selfing and ID in the expression of FA (see below).
Quantifying female advantage
It was possible to estimate the magnitude of the FA for 28 gynodioecious species. For this analysis, species for which either the number of fruits or the number of seeds per plant had been measured or estimated for both females and hermaphrodites were retained. This was done in order to (a) build a homogeneous data set and (b) obtain reliable estimates of the female reproductive success. Then, the FA was computed as the ratio of the average value reported in females divided by the average value in hermaphrodites. We recorded either the number of seeds or fruits that was directly measured by the study, or an estimation of this number, by computing data measured on other reproductive traits (number of flowers × fruit set or number of flowers × number of seeds per fruit). We only used data that had been recorded on open-pollinated flowers, in order to obtain values as close as possible to the effective variation in female fitness in natural populations.
In a few species (Wurmbea biglandulosa, Geranium maculatum, Geranium sylvaticum and Fragaria virginiana), several values were available, from different studies. In such cases, we retained the study that had been performed using the highest number of populations (i.e. Asikainen and Mutikainen, 2005 for G. sylvaticum; and Vaughton and Ramsey, 2004 for W. biglandulosa). In G. maculatum and F. virginiana, for which such criteria could not help in choosing one of the studies, we retained the study that provided an average value for the FA (i.e. Agren and Willson, 1991, instead of Chang, 2006 that provided a range of values for G. maculatum; and Ashman, 1999 instead of Case and Ashman, 2007 for F. virginiana).
Finally, in order to investigate possible variation in the magnitude of FA, we focused on studies that contained such data measured on plants from different populations, and recorded the different values of the numbers of seeds or fruits per plant, corresponding to each studied population. In comparing populations, we preferred to rely only on data from single studies, rather than combining data from different studies, which may have used different methods.
RESULTS
Occurrence of a female advantage
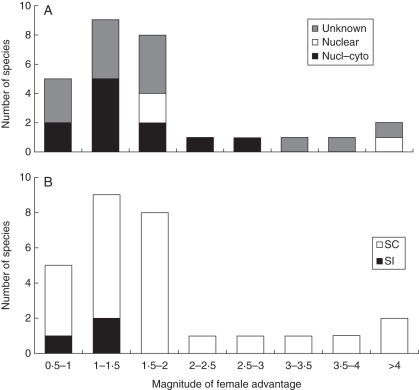
Out of the 48 investigated species, a FA was found with no ambiguity on at least one fitness-related trait in 29 different species. In a few cases among these 29 species (Cucurbita foetidissima, F. virginiana, Gingidia flabellata, Prunus mahaleb and Silene italica), one other trait, either the number of flowers, fruit set or seed set, was found to favour hermaphrodites; however, the total number of seeds or fruits per plant was found in all cases to be larger in females, showing that the advantage for females was not cancelled out by their disadvantage in other fitness components. For all of these 29 species, we thus considered that a FA occurred (Tables 1 and 3).
Table 3.
Summary of the different situations in terms of female advantage: occurrence (FA), variation in female advantage among populations (VAR), ambiguous cases (Amb.) and no female advantage (NO), according to the genetic determination of sex and the mating system
| FA | VAR | Amb. | NO | Total | |
|---|---|---|---|---|---|
| Self-compatible (SC) species | |||||
| Nuclear | 5 | 2 | 0 | 0 | 7 |
| Nuclear–cytoplasmic | 9 | 0 | 2 | 2 | 13 |
| Unknown | 10 | 4 | 2 | 3 | 19 |
| Total | 24 | 6 | 4 | 5 | 39 |
| Self-incompatible (SI) species | |||||
| Nuclear | 0 | 0 | 0 | 0 | 0 |
| Nuclear–cytoplasmic | 1 | 1 | 0 | 2 | 4 |
| Unknown | 1 | 0 | 0 | 1 | 2 |
| Total | 2 | 1 | 0 | 3 | 6 |
| No information on SI/SC or on determination of genetics | 3 | 0 | 0 | 0 | 3 |
| Total | 29 | 7 | 4 | 8 | 48 |
In four additional species, although a FA was found on one or several traits, it was difficult to reach a firm conclusion. In Iris douglasiana, hermaphrodites experienced stronger pre-dispersal seed predation by caterpillars, but the quantitative consequence on female seed set relative to hermaphrodites was not detailed (Uno, 1982). In Plantago coronopus, one study found a FA (Koelewijn, 1996), while another found the opposite result (Koelewijn, 1998). Finally, in the two last species, a FA was found for one trait, but a hermaphroditic advantage was found for at least one other. In Saxifraga granulata, females produced heavier seeds with a higher germination rate, but showed a lower seed set than hermaphrodites, and no quantitative measurement of these contradictory effects was available (Stevens, 1988). In Kallstroemia grandiflora, females showed a higher fruit set but a lower seed set than hermaphrodites, apparently leading to a globally similar seed number among females and hermaphrodites (Cuevas et al., 2008). These four species were scored as ‘ambiguous’ cases for the occurrence of a FA (‘Amb.’ in Tables 1 and 3).
In 25 species, at least one study was carried out on the reproductive success of females and hermaphrodites originating from more than one population. Among them, seven species were found to show a variation in the occurrence of FA, a statistical advantage being found for females in some data sets but no significant difference (or a significant disadvantage) being detected in other data sets generated by the same study (‘VAR’ category, Tables 1 and 3; details provided in Table 4). In some of these cases, the occurrence of FA seemed to be related to the sex ratio, either at the patch or at the population level, but both positive and negative correlations with female frequency were reported (Table 4).
Table 4.
Variation of female advantage among populations
| Species | No. of populations | Measure | Results | Details | References |
|---|---|---|---|---|---|
| Qualitative variation | |||||
| Moehringia lateriflora | 2 | Seed set | 1·02; 1·46* | FA in patches with low female frequency | Sugawara (1993) |
| Wurmbea biglandulosa | 3 | Nb seeds | 0·79; 1·07; 1·63* | Variation in the magnitude of pollen limitation, which affects females more than hermaphrodites | Ramsey and Vaugthon (2002) |
| Geranium sylvaticum | 2 | Nb seeds | 1·2; 3·3 * | FA in populations with high female frequency | Ramula and Mutikainen (2003) |
| Glechoma longituba | 6 | Seed set | FA in 3 populations; FD in 3 populations | FA in populations with low female frequencies | Zhang et al. (2008) |
| Sidalcea hendersonii | 6 | Seed set | 0·4; 0·8; 1; 1·15; 1·16*; 1·21* | FA in populations with high female frequencies | Marshall and Ganders (2001) |
| Plantago maritima | 12 | Nb seeds | From 0·4 to 2·2* | FA in populations with high female frequencies | Nilsson and Agren (2006) |
| Schiedea salicaria | 2 × 5 years | Germination | From 1·24 to 2·3* | Strong ID reduces seed germination in hermaphrodites; selfing rate varies among populations and years | Weller and Sakai (2005) |
| Quantitative variation | |||||
| Gingidia flabellata | 4 | Nb fruits | From 1·23* to 2·07* | Altitude and abiotic conditions could affect fruit set of hermaphrodites, while females always show a very high fruit set | Webb (1981) |
| Phacelia linearis | 6 | Nb seeds | From 1·31* to 2·52* | No correlation between FA and female frequency; FA may increase with water availability | Eckhart (1992b) |
| Silene vulgaris | 2 | Nb seeds | From 1·7* to 2·7* | Local female frequency and magnitude of FA are negatively correlated | Olson et al. (2006) |
| Nemophila menziesii | 23 | Nb seeds | From 1·31* to 2·3* | FA is higher in dry sites compared with sites with higher moisture | Barr (2004) |
| Thymus vulgaris | 7 | Nb seeds | From 1·5* to 8·5* | Plant size varies among populations and affects seed production; the highest FA was found in the populations with the highest density | Assouad et al. (1978) |
| Plantago coronopus | 5 | Nb seeds | From 1·4* to 2·5* | The highest FA was found in the populations with the highest female frequency | Koelewijn (1996) |
The first part of the table includes the seven species for which a significant female advantage was found in some but not all the data sets within a given study (‘VAR’ category). When available, the different numbers for the magnitude of female advantage are reported, and numbers tagged with an asterisk indicate populations/years for which female advantage was found to be significant. The second part of the table includes species for which a female advantage was always found to be significant but with a varying magnitude (defined as the number of fruits or seeds per plant) among populations. FA, female advantage; FD, female disadvantage (females <hermaphrodites); ID, inbreeding depression.
Finally, in the eight remaining species, no FA could be found (Tables 1 and 3) and, in some cases, females even seemed to be overall disadvantaged in terms of their female fitness compared with hermaphrodites. In some of these species (Cirsium chikushiense, Silene nutans and Chionochloa bromoides), such a result was not very informative since very few traits were compared between females and hermaphrodites. Some others (Raphanus sativus, Beta vulgaris and Daphne laureola) constitute intriguing cases, in which no benefit could be found in favour of females, when comparing sex types for many different traits, in several populations.
Only six species were defined as self-incompatible (B. vulgaris spp. maritima, C. chikushiense, R. sativus, Erythroxylum havanense and Plantago lanceolata) or mainly self-incompatible (Plantago maritima). For three other species, we could not find any information on self-compatibility/incompatibility. All the other (39) species were defined as self-compatible or at least partially self-compatible (Table 1), meaning that hermaphrodites have the potential for self-pollination, producing some inbred seeds that might suffer from ID. Because of the very low number of self-incompatible species and also because these data are not phylogenetically independent from each other, a statistical comparison of self-incompatible and self-compatible species would be difficult to interpret and would probably lack statistical power. However, as shown in Table 3, it is interesting to note that species for which no FA was found represent half of the self-incompatible species, but only 13 % of the self-compatible species.
Proximal causes of the female advantage
Among the 40 species in which a FA was detected on at least one fitness trait, in at least one population (categories ‘FA’, ‘Amb’ and ‘VAR’ in Tables 1 and 3), we could hypothesize on the proximal cause of such a fitness difference in 21 cases (Table 5). In 14 of these species, the FA could not be entirely explained by reduced self-pollination and associated ID: (a) when the species was self-incompatible (three cases); (b) when a FA was also clearly found when comparing outcrossed seeds or fruits between females and hermaphrodites (eight cases); and (c) when the selfing rate of hermaphrodites was found to be naturally low in populations or when the magnitude of ID was found to be low, at least for the trait on which a FA had been found (three cases). In contrast, in seven other species, the FA could be mainly attributed to reduced self-pollination and associated ID, either by using direct evidence (no FA when comparing outcrossed seeds or fruits; one case) or by using other information, based on the selfing rate or occurrence of ID (six cases; Table 5).
Table 5.
When is female advantage likely to be due to an avoidance of inbreeding depression?
| Species | FA due to ID avoidance? | Type of evidence |
|---|---|---|
| Cortaderia richardii | No | FA found on outcrossed flowers |
| Erythroxylum havanense | No | SI species |
| Fragaria virginiana | No | FA found on outcrossed flowers |
| Gnidia wikstroemiana | Unlikely | No difference between females and self-pollinated hermaphrodites; FA occurs through a higher number of flowers |
| Kallstroemia grandiflora | Unlikely | No ID found on fruit set in another study1 |
| Phacelia linearis | Unlikely | Selfing rate was found to be low; females have more resources than hermaphrodites |
| Plantago coronopus | No | FA found on outcrossed flowers |
| Plantago lanceolata | No | SI species |
| Plantago maritima | No | Mainly SI species |
| Prunus mahaleb | No | FA found on outcrossed flowers |
| Sidalcea hendersonii | No | FA found on outcrossed flowers |
| Silene acaulis | No | FA found on outcrossed flowers2 |
| Thymus vulgaris | No | FA found on outcrossed flowers3 |
| Satyrium ciliatum | No | FA found on outcrossed flowers |
| Cucurbita foetidissima | Likely | High selfing rate, strong ID measured on hermaphrodites4 |
| Limnanthes douglasii | Likely | Selfing rate of hermaphrodites high in gynodioecious populations; ID reduces number of flowers and survival |
| Lobelia siphilitica | Likely | Strong ID5 |
| Opuntia quimilo | Yes | FA found in open but not outcrossed flowers |
| Schiedea adamantis | Likely | Strong ID measured on self-pollinated flowers |
| Schiedea salicaria | Likely | High selfing rate, strong ID, which reduces – among others – germination rate |
| Silene vulgaris | Likely | Strong ID, affects all stages of life cycle6 |
FA, female advantage; ID, inbreeding depression; SI, self-incompatible.
When some evidence was found in published studies other those listed in Table 1, the additional reference is noted: 1Cuevas et al. (2005); 2Keller and Schwaegerle (2006); 3Thompson and Tarayre (2000); 4Kohn and Biardi (1995); 5Mutikainen and Delph (1998); 6Glaettli and Goudet (2006).
Geranium maculatum and Schiedea adamantis were two additional species in which females had a higher female fecundity compared with open-pollinated hermaphrodites but not with outcrossed hermaphrodites. However, in both these species, the result of open pollinations was observed in natural populations (Agren and Wilson, 1991; Sakai et al., 1997; Chang, 2006) whereas outcrossed pollinations had been performed in greenhouses (Sakai et al., 1997; Van Etten et al., 2008). Thus, the difference in the occurrence of FA could be explained not only by a difference in the pollination treatment, but also by a difference in ecological conditions, such as soil fertility, moisture or light availability. It was thus decided not to include these inconclusive results in Table 5.
The fact that FA was, or was not, due to reduced ID seemed not to affect the type of reproductive trait on which the FA was detected. For instance, a FA was found through a higher seed mass or germination regardless of whether reduced ID had apparently played a role (e.g. S. adamantis, Schiedea salicaria and Silene vulgaris) or not (e.g. Silene acaulis, E. havanense, Thymus vulgaris, P. coronopus, P. lanceolata, P. maritima, Cortaderia richardii, P. mahaleb). Similarly, fruit set was often found to be higher in females that apparently benefited from reduced ID (e.g. Opuntia quimilo, S. adamantis, S. vulgaris), but also when female advantage had other proximal causes (e.g. S. acaulis, E. havanense, Kallstroemia grandiflora).
Finally, some of the studies reported sex differences in biotic interactions that could possibly affect the occurrence of a FA. In four cases, hermaphrodites were reported to be more affected by herbivory or parasitism than females. In Sidalcea hendersonii, sex-biased seed predation led to higher seed production in females, at least in populations that contained many females (Marshall and Ganders, 2001), while in the other three examples [more nectar robbing in hermaphrodites of Glechoma longituba (Zhang et al., 2009) and more pre-dispersal predation in hermaphrodites of Iris douglasiana (Uno, 1982) and Dianthus sylvestris (Collin et al., 2002)]; the consequences on the reproductive success of females relative to hermaphrodites was not quantified. Regarding interactions with pollinators, it was possible to integrate the results obtained in some of the studies on both open- and outcross-pollinated flowers and reach conclusions about differences in the magnitude of pollen limitation between females and hermaphrodites (as explained in Table 2). However, in all cases (D. laureola, P. mahaleb, S. granulata, S. hendersonii and G. longituba) pollen limitation affected female plants more strongly than hermaphroditic plants. This means that when a FA occurred in these particular species, this was not because of but rather in spite of these differences in pollinator interactions.
Magnitude of the female advantage
The values of FA, estimated as the average ratio between females and hermaphrodites in the number of seeds or fruits produced per plant, varied from 0·64 (D. laureola) to 37·7 (Lycopus maackianus). These values were mostly comprised between 1 and 2 (17 species), and females producing more than twice the number of seeds or fruits compared with hermaphrodites were found in only six cases (Table 6; Fig. 1). Because the magnitude of the FA is theoretically expected to vary with sex determination, it would have been interesting to compare these values among species with nuclear vs. cytoplasmic male sterility. Unfortunately, (a) the genetic system is only known for a very low number of species and statistical comparisons would be meaningless and (b) for some of the few species with nuclear determination of male sterility, no data on the number of seeds or fruits were available. However, one can note that (a) among the species that could be included in this study, no species with nuclear male sterility were found having a FA <1·5 and (b) the second strongest advantage (6·68) was found for F. virginiana in which male sterility has a nuclear determination (see also Fig. 1A). For L. maackianus, which had the strongest FA, no information was available on sex determination. Finally, among the three self-incompatible species for which this could have been estimated, the female–hermaphrodite ratio of the number of seeds was <1 in one species (no FA) and <1·5 in all three cases (Fig. 1B).
Table 6.
Magnitude of the female advantage in the 28 species for which information was available
| Species | SI/SC | Genetics | FA | Measurement/calculation | Reference |
|---|---|---|---|---|---|
| Beta vulgaris | SI | NC | 0·93 | NS | Boutin-Stadler (1987) |
| Gingidia flabellata | 1·49 | NFL × FS* | Webb (1981) | ||
| Echium vulgare | SC | NC | 1·42 | NS | Klinkhamer et al. (1994) |
| Phacelia linearis | SC | N | 1·68 | NS | Eckhart (1992b) |
| Opuntia quimilo | SC | 0·88 | NS | Diaz and Cocucci (2003) | |
| Lobelia siphilitica | SC | NC | 1·31 | NS | Caruso and Yakobowski (2008) |
| Dianthus sylvestris | SC | 1·62 | NFL × NSF* | Collin et al. (2002) | |
| Silene italica | SC | NC | 1·22 | NS | Lafuma and Maurice (2006) |
| Silene vulgaris | SC | NC | 2·17 | FS × NSF | Olson et al. (2006) |
| Wurmbea biglandulosa | SC | 1·77 | NS | Vaughton and Ramsey (2004) | |
| Cucurbita foetidissima | SC | N | 1·50 | NS | Kohn (1989) |
| Geranium maculatum | SC | 1·60 | NS | Agren and Willson (1991) | |
| Geranium sylvaticum | SC | 1·68 | NS | Asikainen and Mutikainen (2005) | |
| Nemophila menziesii | SC | NC | 1·67 | NS | Barr (2004) |
| Lycopus maackianus | 37·73 | NS | Hong and Moon (2003) | ||
| Thymus vulgaris | SC | NC | 2·5 | NS | Assouad et al. (1978) |
| Chionographis japonica | SC | 1·4 | NS | Maki (1993) | |
| Hebe subalpina | SC | 3·44 | NFL × FS* | Delph (1993) | |
| Plantago coronopus | SC | NC | 1·72 | NS# | Koelewijn (1996) |
| Plantago lanceolata | SI | NC | 1·38 | NS | Vandamme and Vandelden (1984) |
| Plantago maritima | SI | NC | 1·01 | NS | Nilsson and Agren (2006) |
| Chionochloa bromoides | SC | . | 0·91 | NFL × NSF* | Connor (1990) |
| Fragaria virginiana | SC | N | 6·68 | NFL × FS * | Ashman (1999) |
| Prunus mahaleb | pSC | 1·40 | NF | Jordano (1993) | |
| Saxifraga granulata | SC | NC | 0·72 | NFL × NSF* | Stevens (1988) |
| Daphne laureola | SC | 0·65 | NS | Alonso et al. (2007) | |
| Gnidia wikstroemiana | SC | 3·58 | NFL × FS* | Beaumont et al. (2006) | |
| Kallstroemia grandiflora | SC | 1·29 | NS | Cuevas et al. (2008) |
The table shows the average FA, defined as the female fitness of females relative to hermaphrodites. The fourth column indicates whether this value was obtained by directly measuring the number of seeds or fruits per plant (NS and NF, respectively) or by estimating seed or fruit production through a calculation. NFL, number of flowers; NSF, number of seeds per fruits; FS, fruit set. Lines tagged with an asterisk show cases for which we performed the calculation ourselves, based on values available in the original study, and a hash sign refers to a study in which the number of seeds was estimated through the number of spikes per plant.
For each species is listed: whether it is self-compatible (SC), partially self-compatible (pSC) or self-incompatible (SI) and, where known, the determination of sex (NC, nuclear–cytoplasmic; N, nuclear).
Fig. 1.
Distribution of the magnitude of female advantage among 28 gynodioecious species according to (A) their genetic determination of sex (nuclear–cytoplasmic, nuclear, or not known, as indicated) and (B) the ability of hermaphrodites to self-pollinate (self-incompatibility or self-compatibility, as indicated). A female advantage <1 corresponds to species in which either no female advantage or a female disadvantage was reported.
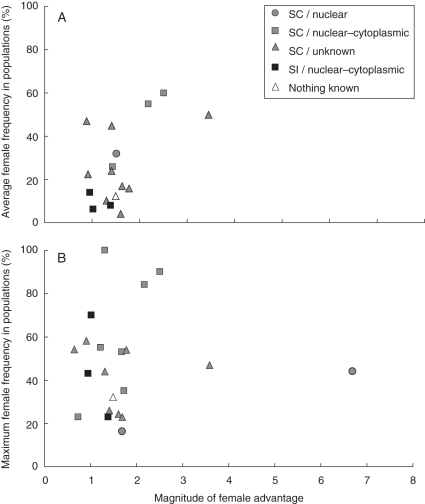
The magnitude of the FA appeared to increase significantly with the average female frequency reported in natural populations (Fig. 2A; P = 0·02; R2 = 0·3; n = 17). It must be noted, however, that the significance of the test was explained by the three species having the highest FA in this data set (2·17, 2·5 and 3·44) and being indeed associated with the highest recorded average female frequencies (0·55, 0·60 and 0·5, respectively). When these three values were removed from the data set, thus focusing on species in which the FA was <2, no further statistical correlation could be found (P = 0·46; R2 = 0·04; n = 14). Finally, the magnitude of FA was found not to be significantly dependent on the maximum female frequency reported in populations (Fig. 2B; P = 0·8; R2 = 0·02; n = 21),
Fig. 2.
Sex ratio in natural populations as a function of the magnitude of female advantage based on (A) average female frequencies and (B) the highest female frequencies reported in natural populations. Among the 28 species for which the magnitude of female advantage was estimated, data on the average sex ratio and the maximum sex ratio were available for 17 and 21 species, respectively. The colours and the shapes of symbols provide information about the self-incompatible (SI)/self-compatible (SC) status and the genetics determination of sex, respectively.
Finally, in six species, it was found that a FA always occurred but with a magnitude that varied among populations (Table 4). In some cases, this seemed to depend on environment quality or on the size or vigour of the plant, which suggests a sex difference in the ability to produce seeds according to the quantity of available resources. In two other species, the magnitude of FA apparently varied with the sex ratio: females in S. vulgaris benefitted from a lower FA when occurring at high frequencies, while in P. coronopus, the highest value of FA was found in the population with the highest female frequency. One must note that a quantitative variation in other fitness parameters (such as fruit or seed set) was frequently reported among populations in the reviewed studies. However, for the reasons explained above, this study of FA magnitude only included estimations of fruit or seed number per plant. The current study thus presents only a part of the possible variation of FA within investigated species.
DISCUSSION
Gynodioecy is a fascinating sexual polymorphism that has triggered numerous theoretical and empirical studies. A number of recently published reviews on the topic illustrate the growing interest in gynodioecy in ecology and evolution, but, to date, they mainly focused on either the genetic determination of gynodioecy (Touzet et al., 2003; Chase et al. 2007; Delph et al., 2007; McCauley and Olson, 2008) or the effect of population structure on the reproductive system (e.g. McCauley and Bailey, 2009), while only Shykoff et al. (2003) reviewed and analysed the data published on the FA. The current study extends the results highlighted by Shykoff et al. (2003) by investigating in more detail the occurrence, magnitude, possible causes and variation of this fitness effect.
A female advantage was found in most, but not all, gynodioecious species
Consistently with the meta-analysis by Shykoff et al. (2003), this review showed evidence for the occurrence of a FA, at least on one fitness trait, in a majority of the investigated gynodioecious species. This fits theoretical predictions that mutations responsible for male sterility should benefit from a better transmission through female fitness in order to invade hermaphroditic populations (Lewis, 1941; Frank, 1989; Gouyon et al., 1991; Bailey et al., 2003; Dufay et al., 2007).
In eight species, however, no FA was found. In half of the cases, because the measurements were carried out on a restricted number of fitness traits or individual plants/populations, future complementary studies involving more genotypes and/or more fitness traits may detect an advantage for females. In other species, no FA could be found even though many different traits were compared between females and hermaphrodites. In such species, it seems unlikely that females are maintained in so many different populations [as in B. vulgaris (Dufay et al., 2009), D. laureola (Alonso and Herrera, 2001) and R. sativus: Murayama et al. (2004)] without benefiting from a selective advantage, and thus relying only on mutations that would be maintained by stochastic processes. In these three species, since females were found at high frequencies in some populations, one should keep in mind that a high increase in female frequency may lead to a strong decrease in FA in some cases (see the end of the Discussion and Fig. 3). Such a phase in the evolutionary dynamics of a gynodioecious population may thus lead to a failure to detect a FA. However, while this constitutes an interesting explanation for the absence of FA in some populations of some species, it is less likely to occur in every studied population of B. vulgaris, R. sativus and D. laureola, in particular in those exhibiting a low female frequency.
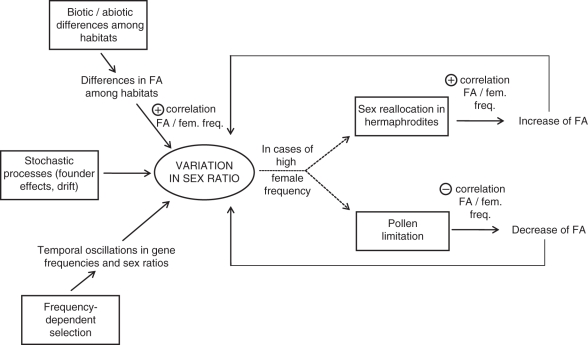
Fig. 3.
Schematic representation of the causes and consequences of sex ratio variation among gynodioecious populations, and its interplay with female advantage.
In species with a nuclear–cytoplasmic determination of sex, such as B. vulgaris and R. sativus, a FA of a very low magnitude is theoretically sufficient for the maintenance of females, possibly at high frequencies (Gouyon et al., 1991; Dufay et al., 2007) and thus may be particularly difficult to detect statistically. One other likely hypothesis is that a FA occurs on some fitness traits that have not been investigated yet (e.g. germination and seed quality in the annual R. sativus; adult survival in the perennials B. vulgaris and D. laureola). More generally, many traits, such as adult survival, probability of flowering and age at first flowering, are rarely investigated. In perennial species (which is the case in 38 of the 48 species included in the current study), better performance through one of these traits could still increase seed production by females over their lifetime (see Morris and Doak, 1998 for an example in S. acaulis). This constitutes a perspective for the study of gynodioecious species, in particular those in which FA has not yet been found.
The magnitude of female advantage varies greatly among gynodioecious species
First of all, one needs to consider these results with caution, since the magnitude of FA was estimated through the number of fruits or seeds, neglecting other traits such as differences in seed quality or adult survival. Nonetheless, we found a spectacular variation in the value of FA among species, that varied from <1 to >30. A large majority of species was found to have a FA comprised between 1 and 2, which corresponds to theoretical expectations under nuclear–cytoplasmic determination of sex (Gouyon et al., 1991; Dufay et al., 2007). Regarding the few species that seemed to have a large, or even extremely large, FA, some of them may be sub-dioecious or on the way to evolving towards dioecy (Lewis, 1941).
Although a large FA appeared to be associated with high female frequencies, no correlation was found between FA and sex ratio in the case of species having their FA comprised between 1 and 2 (Fig. 2; see also Couvet et al., 1990). As explained below, processes affecting the sex ratio are numerous and the relationships between female frequency and FA are quite complex, and undoubtedly non-linear. Moreover, in the case of nuclear–cytoplasmic determination, theory predicts that frequency-dependent selection can lead to large oscillations of female frequency within a population (and thus, also among different populations) even when the value of FA remains fixed (Gouyon et al., 1991; Bailey et al., 2003; Dufay et al., 2007). In the light of these different predictions, the absence of correlation between FA and female frequency is thus not very surprising.
Differences in biotic interactions seem not to often lead to a female advantage
Because hermaphrodites often carry larger flowers than females (this was documented in 26 of the species included in the study; see also Shykoff et al., 2003), they are likely to attract more insects, which can either be beneficial in the case of pollinators or costly in the case of natural enemies. A higher rate of predation or parasitism was reported in the hermaphrodites of four species, and was found to provide females with a fitness advantage in at least one of these cases. Sex differences in pollination efficiency were also found in some species, with females suffering more from pollen limitation than hermaphrodites (although this may not be a general trend, as suggested by Shykoff et al., 2003; see also Dudle, 1999 for an example of stronger pollen limitation in hermaphrodites). In all of the cases investigated in the current study, this led to a lower seed or fruit set in females compared with hermaphrodites; thus, when a FA occurred, this was not because of, but rather in spite of differences in interactions with pollinators.
Female advantage may be caused by reduced selfing in some cases but not all
In seven species, it was found that reduced ID could be responsible – at least partly – for the occurrence of FA, while in 14 other species, it appeared that reduced ID was not a necessary cause of the FA. This result was quite surprising, since the reduced self-pollination and associated ID is sometimes cited as the main proximal cause for FA in gynodioecious species (e.g. Kubota and Ohara, 2009). This does not mean that no ID occurs within these species (in some cases, e.g. S. hendersonii and P. mahaleb, self-pollinated flowers were shown to have a low seed set or to produce seeds of low quality), but this could mean that selfing rates are sufficiently low in these species to not disadvantage hermaphrodites, at least under the conditions examined in the reviewed studies. However, one must note that a FA possibly results from a combination of different mechanisms, and that reduced selfing and ID, while not being the only necessary cause of it, may increase the magnitude of FA in some species. This may explain why this magnitude is low in the investigated self-incompatible species, while it reaches very high values in some self-compatible species (Fig. 1B).
Our study contradicts the view that selfing and ID are a necessary process for a FA to occur and persist. Importantly, this means that gynodioecy should not be particularly difficult to evolve in self-incompatible species (in which neither females nor hermaphrodites self-pollinate), as argued sometimes in the literature (e.g. Kubota and Ohara, 2009). It is true, however, that few gynodioecious species are known to be self-incompatible, even though no phylogenetic analyses on the occurrence of these two traits have been made to confirm their negative association within Angiosperms. In addition, in the two species Trillium camschatcense and P. maritima, both exhibiting self-compatible and self-incompatible populations on the one hand, and gynodioecious and hermaphroditic populations on the other hand, gynodioecy and self-incompatibility appeared to be negatively correlated at the population level (Nilsson, 2005; Nilsson and Agren, 2006; Kubota and Ohara, 2009). However, even though reduced selfing and ID in females may help in selecting for male sterility in self-compatible species, such an explanation may not be true in all cases, as suggested by the current study. Alternatively, the model of Ehlers and Schierup (2008) has shown that the occurrence of male sterility could enhance the breakdown of self-incompatibility. Consequently, such negative association may sometimes result from a higher probability of self-incompatibly breakdown within gynodioecious lineages, rather than from a stronger selection for male sterility in self-compatible lineages.
Why does the female advantage vary within species, among populations?
Among species for which one study documented the FA in more than one population, at least half of them showed a variation in the occurrence or in the magnitude of FA. In some cases, the population variation in FA may have been due to sex-specific responses to environmental variation. In addition, in many cases, FA was found to vary with the population sex ratio, but both negative and positive correlations were highlighted among the reviewed studies. In order to understand this puzzling result, one needs to consider all processes likely to affect both FA and the sex ratio, and also to understand when female frequency is the cause vs. the consequence of the FA (Fig. 3 and see also the review by McCauley and Bailey, 2009).
First, three main processes can theoretically affect the sex ratio in a gynodioecious population: drift and founder effects that lead to non-equilibrium situations (Nilsson and Agren, 2006); frequency-dependent selection that leads to oscillations in the sex ratio over time in nuclear–cytoplasmic systems (Gouyon et al., 1991; Dufay et al., 2007, 2009); and the magnitude of FA (Dufay et al., 2007). The latter point simply means that a large FA induces a strong positive selection for male sterility, leading to high female frequencies. Thus, for this reason, positive correlations between female frequencies and FA values should be observed, at least in some cases (Fig. 3). If female frequency reaches high values in a population, two very different processes can then occur. On the one hand, as postulated by Ramula and Mutikainen (2003), a high female frequency could lead hermaphrodites to allocate more resources to male function, consequently decreasing their seed production and thus increasing the value of FA. This is another reason for observing positive correlations between FA and female frequency. Such a mechanism should theoretically have a positive feedback on female frequency and is sometimes considered as a step towards the evolution of dioecy (Charlesworth and Charlesworth, 1978; Maurice et al., 1994). On the other hand, in populations containing a lot of females, the overall availability of pollen should decrease. As already outlined, females seem sometimes to be more affected by pollen limitation than hermaphrodites, for several reasons: (a) in pollen-limited situations, self-compatible hermaphrodites have the potential to self-pollinate; (b) hermaphroditic flowers are often more attractive to pollinators than female flowers; and/or (c) because of the spatial clustering of cytoplasmic haplotypes and thus sex types within populations (e.g. McCauley, 1998; Klaas and Olson, 2006; De Cauwer et al., 2010), females have a higher risk of experiencing local depletion of pollen. In such a case, we expect female frequency to decrease in the next steps of the dynamics. Consequently, one could imagine that both positive and negative correlations between FA and female frequencies could be encountered in the same set of populations, depending on the phase of the dynamics of sex ratio oscillations. For instance, in a first step, populations in which FA is the highest should experience an increase in female frequency, but once female frequency has reached some threshold value, this could lead to a decrease of female seed production, which should ultimately lead females to be counter-selected (Fig. 3).
CONCLUSIONS AND PERSPECTIVES
One of the main results suggested by the current study is that reduced selfing may not be the major cause of the occurrence of FA, and thus of the maintenance of gynodioecy. However, this could be investigated in only a part of the reviewed species: while female–hermaphrodite comparisons of female fitness based on open-pollinated flowers are necessary to know about the possible occurrence of a FA in natural conditions, many studies lack some data based on outcrossed and selfed hand pollination, which are helpful to discriminate among the possible mechanisms responsible for the FA. We propose that future studies should document female–hermaphrodite fitness comparisons using both open-pollinated and hand-pollinated flowers, in order to assess the role of reduced selfing in the evolution of gynodioecy in a larger number of species.
One other very important type of data that were surprisingly missing from many studies was the genetic details of sex determination. It was thus not possible to test the theoretical predictions about how sex determination should be related to the sex ratio in populations and to the magnitude of FA. Indeed, many studies used these theoretical predictions to infer sex determination from data obtained on FA or the sex ratio. Although such an argument may be very useful, as proposed by Bailey and Delph (2007), direct evidence obtained from crossings would considerably enhance our knowledge of gynodioecy. Because both nuclear (Charlesworth and Charlesworth, 1978) and nuclear–cytoplasmic gynodioecy (Maurice et al., 1994) may theoretically lead to dioecy, but involving slightly different genetic and/or ecological mechanisms, this could also help us in understanding one major and common transition in the evolutionary history of plant mating systems.
ACKNOWLEDGEMENTS
We thank Camille Barr, Vince Eckhart and Keiko Miyake for sharing some of their data and answering specific questions about their study species. We also thank Jean-François Arnaud, Pascal Touzet, Sylvain Billiard, Denis Roze as well as three anonymous referees for helpful and constructive comments on various versions of this manuscript.
LITERATURE CITED
- Agren J, Willson MF. Gender variation and sexual differences in reproductive characters and seed production in gynodioecious Geranium maculatum. American Journal of Botany. 1991;78:470–480. [Google Scholar]
- Alonso C. Pollination success across an elevation and sex ratio gradient in gynodioecious Daphne laureola. American Journal of Botany. 2005;92:1264–1269. doi: 10.3732/ajb.92.8.1264. [DOI] [PubMed] [Google Scholar]
- Alonso C, Herrera CM. Neither vegetative nor reproductive advantages account for high frequency of male-steriles in southern Spanish gynodioecious Daphne laureola (Thymelaeaceae) American Journal of Botany. 2001;88:1016–1024. [PubMed] [Google Scholar]
- Alonso C, Mutikainen P, Herrera CM. Ecological context of breeding system variation: sex, size and pollination in a (predominantly) gynodioecious shrub. Annals of Botany. 2007;100:1547–1556. doi: 10.1093/aob/mcm254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Mansby E, Prentice HC. Paternal effects on seed germination: a barrier to the genetic assimilation of an endemic plant taxon? Journal of Evolutionary Biology. 2008;21:1408–1417. doi: 10.1111/j.1420-9101.2008.01554.x. [DOI] [PubMed] [Google Scholar]
- Ashman TL. Determinants of sex allocation in a gynodioecious wild strawberry: implications for the evolution of dioecy and sexual dimorphism. Journal of Evolutionary Biology. 1999;12:648–661. [Google Scholar]
- Ashman TL. Constraints on the evolution of males and sexual dimorphism: field estimates of genetic architecture of reproductive traits in three populations of gynodioecious Fragaria virginiana. Evolution. 2003;57:2012–2025. doi: 10.1111/j.0014-3820.2003.tb00381.x. [DOI] [PubMed] [Google Scholar]
- Asikainen E, Mutikainen P. Female frequency and relative fitness of females and hermaphrodites in gynodioecious Geranium sylvaticum (Geraniaceae) American Journal of Botany. 2003;90:226–234. doi: 10.3732/ajb.90.2.226. [DOI] [PubMed] [Google Scholar]
- Asikainen E, Mutikainen P. Pollen and resource limitation in a gynodioecious species. American Journal of Botany. 2005;92:487–494. doi: 10.3732/ajb.92.3.487. [DOI] [PubMed] [Google Scholar]
- Assouad MW, Dommée B, Lumaret R, Valdeyron G. Reproductive capacities in the sexual forms of the gynodioecious species Thymus vulgaris L. Botanical Journal of the Linnean Society. 1978;77:29–39. [Google Scholar]
- Avila-Sakar G, Dominguez CA. Parental effects and gender specialization in a tropical heterostylous shrub. Evolution. 2000;54:866–877. doi: 10.1111/j.0014-3820.2000.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Bailey MF, Delph LF. A field guide to models of sex-ratio evolution in gynodioecious species. Oikos. 2007;116:1609–1617. [Google Scholar]
- Bailey MF, Delph LF, Lively CM. Modeling gynodioecy: novel scenarios for maintaining polymorphism. American Naturalist. 2003;161:762–776. doi: 10.1086/374803. [DOI] [PubMed] [Google Scholar]
- Barr CM. Soil moisture and sex ratio in a plant with nuclear–cytoplasmic sex inheritance. Proceedings of the Royal Society B: Biological Sciences. 2004;271:1935–1939. doi: 10.1098/rspb.2004.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Beaumont AJ, Edwards TJ, Smith FR. The first record of gynodioecy in a species of Gnidia (Thymelaeaceae) from South Africa. Botanical Journal of the Linnean Society. 2006;152:219–233. [Google Scholar]
- Boutin-Stadler V. 1987 Sélection sexuelle et dynamique de la stérilité mâle dans les populations de betteraves sauvages, Beta maritima L. PhD thesis, Université de Lille 1, France. [Google Scholar]
- Caruso CM, Case AL. Sex ratio variation in gynodioecious Lobelia siphilitica: effects of population size and geographic location. Journal of Evolutionary Biology. 2007;20:1396–1405. doi: 10.1111/j.1420-9101.2007.01361.x. [DOI] [PubMed] [Google Scholar]
- Caruso CM, Yakobowski SJ. Selection on floral and carbon uptake traits of Lobelia siphilitica is similar in females and hermaphrodites. Journal of Evolutionary Biology. 2008;21:1514–1523. doi: 10.1111/j.1420-9101.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- Caruso CM, Maherali H, Jackson RB. Gender-specific floral and physiological traits: implications for the maintenance of females in gynodioecious Lobelia siphilitica. Oecologia. 2003;135:524–531. doi: 10.1007/s00442-003-1199-2. [DOI] [PubMed] [Google Scholar]
- Case AL, Ashman TL. An experimental test of the effects of resources and sex ratio on maternal fitness and phenotypic selection in gynodioecious Fragaria virginiana. Evolution. 2007;61:1900–1911. doi: 10.1111/j.1558-5646.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- Chang SM. Female compensation through the quantity and quality of progeny in a gynodioecious plant, Geranium maculatum (Geraniaceae) American Journal of Botany. 2006;93:263–270. doi: 10.3732/ajb.93.2.263. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. Model for evolution of dioecy and gynodioecy. American Naturalist. 1978;112:975–997. [Google Scholar]
- Charlesworth D. Theories of the evolution of dioecy. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag; 1999. pp. 33–60. [Google Scholar]
- Chase CD. Cytoplasmic male sterility: a window to the world of plant mitochondrial–nuclear interactions. Trends in Genetics. 2007;23:81–90. doi: 10.1016/j.tig.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Collin CL, Pennings PS, Rueffler C, Widmer A, Shykoff JA. Natural enemies and sex: how seed predators and pathogens contribute to sex-differential reproductive success in a gynodioecious plant. Oecologia. 2002;131:94–102. doi: 10.1007/s00442-001-0854-8. [DOI] [PubMed] [Google Scholar]
- Connor HE. Breeding systems in New Zealand grasses VI. Control of gynodioecism in Cortaderia richardii (endl.) zotov. New Zealand Journal of Botany. 1965;3:233–242. [Google Scholar]
- Connor HE. Breeding systems in New Zealand grasses XI. Gynodioecism in Chionochloa bromoides. New Zealand Journal of Botany. 1990;28:59–65. [Google Scholar]
- Cosmides LM, Tooby J. Cytoplasmic inheritance and intragenomic conflict. Journal of Theoretical Biology. 1981;89:83–129. doi: 10.1016/0022-5193(81)90181-8. [DOI] [PubMed] [Google Scholar]
- Couvet D, Atlan A, Belhassen E, Gliddon C, Gouyon PH, Kjellberg F. Co-evolution between two symbionts: the case of cytoplasmic male-sterility in higher plants. Oxford Survey of Evolutionary Biology. 1990;7:225–249. [Google Scholar]
- Couvet D, Ronce O, Gliddon C. The maintenance of nucleocytoplasmic polymorphism in a metapopulation: the case of gynodioecy. American Naturalist. 1998;152:59–70. doi: 10.1086/286149. [DOI] [PubMed] [Google Scholar]
- Cuevas E, Molina-Freaner F, Eguiarte LE, Dominguez CA. Patterns of male sterility within and among populations of the distylous shrub Erythroxylum havanense (Erythroxylaceae) Plant Ecology. 2005;176:165–172. [Google Scholar]
- Cuevas E, Parker IM, Molina-Freaner F. Variation in sex ratio, morph-specific reproductive ecology and an experimental test of frequency-dependence in the gynodioecious Kallstroemia grandiflora (Zygophyllaceae) Journal of Evolutionary Biology. 2008;21:1117–1124. doi: 10.1111/j.1420-9101.2008.01530.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. London: Murray; 1877. [Google Scholar]
- De Cauwer I, Arnaud JF, Schmitt E, Dufay M. Pollen limitation of female reproductive success at fine spatial scale in a gynodioecious and wind-pollinated species, Beta vulgaris ssp. maritima. Journal of Evolutionary Biology. 2010;23:2636–2647. doi: 10.1111/j.1420-9101.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- Delph LF. Factors affecting intraplant variation in flowering and fruiting in the gynodioecious species Hebe subalpina. Journal of Ecology. 1993;81:287–296. [Google Scholar]
- Diaz L, Cocucci AA. Functional gynodioecy in Opuntia quimilo (Cactaceae), a tree cactus pollinated by bees and hummingbirds. Plant Biology. 2003;5:531–539. [Google Scholar]
- Dudle DA. Maintenance and consequences of females in the gynodioecious plant, Lobelia siphilitica. 1999 Doctoral thesis, Indiana University. [Google Scholar]
- Dufay M, Pannell JR. The effects of pollen versus seed flow on the maintenance of nuclear–cytoplasmic gynodioecy. Evolution. 2010;64:772–784. doi: 10.1111/j.1558-5646.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- Dufay M, Touzet P, Maurice S, Cuguen J. Modelling the maintenance of male–fertile cytoplasm in a gynodioecious population. Heredity. 2007;99:349–356. doi: 10.1038/sj.hdy.6801009. [DOI] [PubMed] [Google Scholar]
- Dufay M, Cuguen J, Arnaud JF, Touzet P. Sex ratio variation among gynodioecious populations in sea beet: can it be explained by negative frequency-dependent selection? Evolution. 2009;63:1483–1497. doi: 10.1111/j.1558-5646.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- Dufay M, Lahiani E, Brachi B. Gender variation and inbreeding depression in gynodioecious–gynomonoecious Silene nutans (Caryophyllaceae) International Journal of Plant Sciences. 2010;171:53–62. [Google Scholar]
- Dulberger R, Horovitz A. Gender polymorphism in flowers of Silene vulgaris (moench) Garcke (Caryophyllaceae) Botanical Journal of the Linnean Society. 1984;89:101–117. [Google Scholar]
- Eckhart VM. The genetics of gender and the effects of gender on floral characters in gynodioecious Phacelia linearis (Hydrophyllaceae) American Journal of Botany. 1992a;79:792–800. [Google Scholar]
- Eckhart VM. Resource compensation and the evolution of gynodioecy in Phacelia linearis (Hydrophyllaceae) Evolution. 1992b;46:1313–1328. doi: 10.1111/j.1558-5646.1992.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Ehlers BK, Schierup MH. When gametophytic self-incompatibility meets gynodioecy. Genetical Research. 2008;90:27–35. doi: 10.1017/S0016672307009007. [DOI] [PubMed] [Google Scholar]
- Frank SA. The evolutionary dynamics of cytoplasmic male-sterility. American Naturalist. 1989;133:345–376. [Google Scholar]
- Frank SA, Barr CM. Spatial dynamics of cytoplasmic male sterility. In: Silvertown J, Antonovics J, editors. Integrating ecology and evolution in a spatial context. Oxford: Blackwell Science; 2001. pp. 219–243. [Google Scholar]
- Garcia-Franco JG, Arroyo MTK. Breeding system, sex ratio and individual size of the gynodioecious Nototriche compacta (Malvaceae) in the Andes of central Chile. Plant Species Biology. 1995;10:147–153. [Google Scholar]
- Glaettli M, Goudet J. Variation in the intensity of inbreeding depression among successive life-cycle stages and generations in gynodioecious Silene vulgaris (Caryophyllaceae) Journal of Evolutionary Biology. 2006;19:1995–2005. doi: 10.1111/j.1420-9101.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- Godley EJ. Monoecy and incompatibility. Nature. 1955;176:1176–1177. [Google Scholar]
- Gouyon PH, Vichot F, Vandamme JMM. Nuclear–cytoplasmic male-sterility – single-point equilibria versus limit-cycles. American Naturalist. 1991;137:498–514. [Google Scholar]
- Graff A. Population sex structure and reproductive fitness in gynodioecious Sidalcea malviflora malviflora (Malvaceae) Evolution. 1999;53:1714–1722. doi: 10.1111/j.1558-5646.1999.tb04556.x. [DOI] [PubMed] [Google Scholar]
- Hermanutz LA, Innes DJ. Gender variation in Silene acaulis (Caryophyllaceae) Plant Systematics and Evolution. 1994;191:69–81. [Google Scholar]
- Hong S-P, Moon H-K. Gynodioecy in Lycopus maackianus makino (Lamiaceae) in Korea: floral dimorphism and nutlet production. Flora – Morphology, Distribution, Functional Ecology of Plants. 2003;198:461–467. [Google Scholar]
- Huang S-Q, Lu Y, Chen Y-Z, Luo Y-B, Delph LF. Parthenogenesis maintains male sterility in a gynodioecious orchid. American Naturalist. 2009;174:578–584. doi: 10.1086/605378. [DOI] [PubMed] [Google Scholar]
- Jordano P. Pollination biology of Prunus mahaleb L. – deferred consequences of gender variation for fecundity and seed size. Biological Journal of the Linnean Society. 1993;50:65–84. [Google Scholar]
- Kawabuko N. Gynodioecy in Cirsium chikushiense koidz. (Compositae) Annals of Botany. 1994;74:357–364. [Google Scholar]
- Keller SR, Schwaegerle KE. Maternal sex and mate relatedness affect offspring quality in the gynodioecious Silene acaulis. Journal of Evolutionary Biology. 2006;19:1128–1138. doi: 10.1111/j.1420-9101.2006.01101.x. [DOI] [PubMed] [Google Scholar]
- Kesseli RV, Jain SK. An ecological genetic study of gynodioecy in Limnanthes douglasii (Limnanthaceae) American Journal of Botany. 1984;71:775–786. [Google Scholar]
- Kesseli RV, Jain SK. Breeding systems and population structure in Limnanthes. Theoretical and Applied Genetics. 1985;71:292–299. doi: 10.1007/BF00252070. [DOI] [PubMed] [Google Scholar]
- Klaas AL, Olson MS. Spatial distributions of cytoplasmic types and sex expression in Alaskan populations of Silene acaulis. International Journal of Plant Sciences. 2006;167:179–189. [Google Scholar]
- Klinkhamer PGL, Dejong TJ, Wesselingh RA. Implications of differences between hermaphrodite and female flowers for attractiveness to pollinators and seed production. Netherlands Journal of Zoology. 1991;41:130–143. [Google Scholar]
- Klinkhamer PGL, Dejong TJ, Nell HW. Limiting factors for seed production and phenotypic gender in the gynodioecious species Echium vulgare (Boraginaceae) Oikos. 1994;71:469–478. [Google Scholar]
- Koelewijn HP. Sexual differences in reproductive characters in gynodioecious Plantago coronopus. Oikos. 1996;75:443–452. [Google Scholar]
- Koelewijn HP. Effects of different levels of inbreeding on progeny fitness in Plantago coronopus. Evolution. 1998;52:692–702. doi: 10.1111/j.1558-5646.1998.tb03694.x. [DOI] [PubMed] [Google Scholar]
- Koelewijn HP, VanDamme JMM. Gender variation, partial male sterility and labile sex expression in gynodioecious Plantago coronopus. New Phytologist. 1996;132:67–76. doi: 10.1111/j.1469-8137.1996.tb04510.x. [DOI] [PubMed] [Google Scholar]
- Kohn JR. Sex-ratio, seed production, biomass allocation, and the cost of male function in Cucurbita foetidissima hbk (Cucurbitaceae) Evolution. 1989;43:1424–1434. doi: 10.1111/j.1558-5646.1989.tb02593.x. [DOI] [PubMed] [Google Scholar]
- Kohn JR, Biardi JE. Outcrossing rates and inferred levels of inbreeding depression in gynodioecious Cucurbita foetidissima (Cucurbitaceae) Heredity. 1995;75:77–83. [Google Scholar]
- Kubota S, Ohara M. Discovery of male sterile plants and their contrasting occurrence between self-compatible and self-incompatible populations of the hermaphroditic perennial Trillium camschatcense. Plant Species Biology. 2009;24:169–178. doi: 10.1007/s10265-009-0245-5. [DOI] [PubMed] [Google Scholar]
- Lafuma L, Maurice S. Reproductive characters in a gynodioecious species, Silene italica (Caryophyllaceae), with attention to the gynomonoecious phenotype. Biological Journal of the Linnean Society. 2006;87:583–591. [Google Scholar]
- Lewis D. Male-sterility in natural populations of hermaphrodite plants. New Phytologist. 1941;40:56–63. [Google Scholar]
- Lloyd DG. The transmission of genes via pollen and ovules in gynodioecious angiosperms. Theoretical Population Biology. 1976;9:299–316. doi: 10.1016/0040-5809(76)90050-2. [DOI] [PubMed] [Google Scholar]
- Lopes AV, Machado IC. Floral biology and reproductive ecology of Clusia nemorosa (Clusiaceae) in northeastern Brazil. Plant Systematics and Evolution. 1998;213:71–90. [Google Scholar]
- Maki M. Outcrossing and fecundity advantage of females in gynodioecious Chionographis japonica var. kurohimensis (Liliaceae) American Journal of Botany. 1993;80:629–634. [Google Scholar]
- Marshall M, Ganders FR. Sex-biased seed predation and the maintenance of females in a gynodioecious plant. American Journal of Botany. 2001;88:1437–1443. [PubMed] [Google Scholar]
- Maurice S. Gynomonoecy in Silene italica (Caryophyllaceae): sexual phenotypes in natural populations. Plant Biology. 1999;1:346–350. [Google Scholar]
- Maurice S, Belhassen E, Couvet D, Gouyon PH. Evolution of dioecy – can nuclear–cytoplasmic interactions select for maleness. Heredity. 1994;73:346–354. doi: 10.1038/hdy.1994.181. [DOI] [PubMed] [Google Scholar]
- McCauley DE. The genetic structure of a gynodioecious plant: nuclear and cytoplasmic genes. Evolution. 1998;52:255–260. doi: 10.1111/j.1558-5646.1998.tb05159.x. [DOI] [PubMed] [Google Scholar]
- McCauley DE, Bailey MF. Recent advances in the study of gynodioecy: the interface of theory and empiricism. Annals of Botany. 2009;104:611–620. doi: 10.1093/aob/mcp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley DE, Taylor DR. Local population structure and sex-ratio: evolution in gynodioecious plants. The American Naturalist. 1997;150:406–419. doi: 10.1086/286072. [DOI] [PubMed] [Google Scholar]
- Miyake K, Miyake T, Terachi T, Yahara T. Relative fitness of females and hermaphrodites in a natural gynodioecious population of wild radish, Raphanus sativus L. (Brassicaceae): comparison based on molecular genotyping. Journal of Evolutionary Biology. 2009;22:2012–2019. doi: 10.1111/j.1420-9101.2009.01808.x. [DOI] [PubMed] [Google Scholar]
- Morris WF, Doak DF. Life history of the long-lived gynodioecious cushion plant Silene acaulis (Caryophyllaceae), inferred from size-based population projection matrices. American Journal of Botany. 1998;85:784–793. [PubMed] [Google Scholar]
- Murayama K, Yahara T, Terachi T. Variation of female frequency and cytoplasmic male-sterility gene frequency among natural gynodioecious populations of wild radish (Raphanus sativus l.) Molecular Ecology. 2004;13:2459–2464. doi: 10.1111/j.1365-294X.2004.02231.x. [DOI] [PubMed] [Google Scholar]
- Mutikainen P, Delph LF. Inbreeding depression in gynodioecious Lobelia siphilitica: among-family differences override between-morph differences. Evolution. 1998;52:1572–1582. doi: 10.1111/j.1558-5646.1998.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Nilsson E. Breeding system evolution and pollination success in the wind-pollinated herb Plantago maritima. 2005 PhD thesis, Uppsala University, Sweden. [Google Scholar]
- Nilsson E, Agren J. Population size, female fecundity, and sex ratio variation in gynodioecious Plantago maritima. Journal of Evolutionary Biology. 2006;19:825–833. doi: 10.1111/j.1420-9101.2005.01045.x. [DOI] [PubMed] [Google Scholar]
- Olson MS, Graf AV, Niles KR. Fine scale spatial structuring of sex and mitochondria in Silene vulgaris. Journal of Evolutionary Biology. 2006;19:1190–1201. doi: 10.1111/j.1420-9101.2006.01103.x. [DOI] [PubMed] [Google Scholar]
- Pannell JR. The maintenance of gynodioecy and androdioecy in a metapopulation. Evolution. 1997;51:10–20. doi: 10.1111/j.1558-5646.1997.tb02383.x. [DOI] [PubMed] [Google Scholar]
- Poot P, Pilon J, Pons TL. Photosynthetic characteristics of leaves of male-sterile and hermaphrodite sex types of Plantago lanceolata grown under conditions of contrasting nitrogen and light availabilities. Physiologia Plantarum. 1996;98:780–790. [Google Scholar]
- Puterbaugh MN, Wied A, Galen C. The functional ecology of gynodioecy in Eritrichum aretioides (Boraginaceae), the alpine forget-me-not. American Journal of Botany. 1997;84:393–400. [PubMed] [Google Scholar]
- Ramsey M, Vaughton G. Maintenance of gynodioecy in Wurmbea biglandulosa (Colchicaceae): gender differences in seed production and progeny success. Plant Systematics and Evolution. 2002;232:189–200. [Google Scholar]
- Ramula S, Mutikainen P. Sex allocation of females and hermaphrodites in the gynodioecious Geranium sylvaticum. Annals of Botany. 2003;92:207–213. doi: 10.1093/aob/mcg125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas LF, Perez-Alquicira JP, Dominguez CA. Environmentally induced variation in fecundity compensation in the morph-biased male-sterile distylous shrub Erythroxylum havanense (Erythroxylaceae) American Journal of Botany. 2005;92:116–122. doi: 10.3732/ajb.92.1.116. [DOI] [PubMed] [Google Scholar]
- Sakai AK, Weller SG, Chen ML, Chou SY, Tasanont C. Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea adamantis (Caryophyllaceae) Evolution. 1997;51:724–736. doi: 10.1111/j.1558-5646.1997.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Saumitou-Laprade P, Cuguen J, Vernet P. Cytoplasmic male sterility in plants: molecular evidence and the nucleocytoplasmic conflict. Trends in Ecology and Evolution. 1994;9:431–435. doi: 10.1016/0169-5347(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Shykoff JA. Sex polymorphism in Silene acaulis (Caryophyllaceae) and the possible role of sexual selection in maintaining females. American Journal of Botany. 1992;79:138–143. [Google Scholar]
- Shykoff JA, Kolokotronis SO, Collin CL, Lopez-Villavicencio M. Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia. 2003;135:1–9. doi: 10.1007/s00442-002-1133-z. [DOI] [PubMed] [Google Scholar]
- Smith FR. Sex-ratio variation in the gynodioecious shrub Gnidia wikstroemiana (Thymelaeaceae) South African Journal of Botany. 2009;75:591–593. [Google Scholar]
- Stevens DP. On the gynodioecious polymorphism in Saxifraga granulata l. (Saxifragaceae) Biological Journal of the Linnean Society. 1988;35:15–28. [Google Scholar]
- Stevens DP, Richards AJ. Gynodioecy in Saxifraga granulata L. (Saxifragaceae) Plant Systematics and Evolution. 1985;151:43–54. [Google Scholar]
- Steyn EMA, Robbertse PJ. Fruit production in a morphologically gynodioecious population of Bequaertiodendron magalismontanum. South African Journal of Botany. 1990;56:6–10. [Google Scholar]
- Sugawara T. Sexual polymorphism in Moehringia lateriflora (Caryophyllaceae) Acta Phytotaxonomica et Geobotanica. 1993;44:3–10. [Google Scholar]
- Talavera S, Arista M, Salgueiro FJ. Population size, pollination and breeding system of Silene stockenii chater (Caryophyllaceae), an annual gynodioecious species of southern Spain. Botanica Acta. 1996;109:333–339. [Google Scholar]
- Thompson JD, Tarayre M. Exploring the genetic basis and proximate causes of female fertility advantage in gynodioecious Thymus vulgaris. Evolution. 2000;54:1510–1520. doi: 10.1111/j.0014-3820.2000.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Uno GE. Comparative reproductive-biology of hermaphroditic and male-sterile Iris douglasiana herb (iridaceae) American Journal of Botany. 1982;69:818–823. [Google Scholar]
- Van Etten ML, Prevost LB, Deen AC, Ortiz BV, Donovan LA, Chang SM. Gender differences in reproductive and physiological traits in a gynodioecious species, Geranium maculatum (geraniaceae) International Journal of Plant Sciences. 2008;169:271–279. [Google Scholar]
- Vandamme JMM, Vandelden W. Gynodioecy in Plantago lanceolata L. 1. Polymorphism for plasmon type. Heredity. 1982;49:303–318. [Google Scholar]
- Vandamme JMM, Vandelden W. Gynodioecy in Plantago lanceolata L. 4. Fitness components of sex types in different life-cycle stages. Evolution. 1984;38:1326–1336. doi: 10.1111/j.1558-5646.1984.tb05653.x. [DOI] [PubMed] [Google Scholar]
- Vaughton G, Ramsey M. Dry environments promote the establishment of females in monomorphic populations of Wurmbea biglandulosa (Colchicaceae) Evolutionary Ecology. 2004;18:323–341. [Google Scholar]
- Webb CJ. Gynodioecy in Gingidia flabellata (Umbelliferae) New Zealand Journal of Botany. 1981;19:111–113. [Google Scholar]
- Webb CJ. Empirical studies: evolution and maintenance of dimorphic breeding systems. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. New York: Springer-Verlag; 1999. pp. 61–96. [Google Scholar]
- Weller SG, Sakai AK. Selfing and resource allocation in Schiedea salicaria (Caryophyllaceae), a gynodioecious species. Journal of Evolutionary Biology. 2005;18:301–308. doi: 10.1111/j.1420-9101.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Wang Y, Yu Q, Zhao JM. Sex expression, female frequency, and reproductive output in a gynodioecious clonal herb, Glechoma longituba (Lamiaceae) Plant Ecology. 2008;199:255–264. [Google Scholar]
- Zhang YW, Yang CF, Zhao JM, Guo YH. Selective nectar robbing in a gynodioecious plant (Glechoma longituba) enhances female advantage. Journal of Evolutionary Biology. 2009;22:527–535. doi: 10.1111/j.1420-9101.2008.01669.x. [DOI] [PubMed] [Google Scholar]