Abstract
Background
Baker's Law states that colonization by self-compatible organisms is more likely to be successful than colonization by self-incompatible organisms because of the ability for self-compatible organisms to produce offspring without pollination agents. This simple model has proved very successful in plant ecology and has been applied to various contexts, including colonizing or ruderal species, islands colonizers, invasive species or mating system variation across distribution ranges. Moreover, it is one of the only models in population biology linking two traits of major importance in ecology, namely dispersal and mating system. Although Baker's Law has stimulated a large number of empirical studies reporting the association of self-fertilization and colonizing ability in various contexts, the data have not established a general pattern for the association of traits.
Scope
In this paper, a critical position is adopted to discuss and clarify Baker's Law. From the literature referring to Baker's Law, an analysis made regarding how mating success is considered in such studies and discrepancies with population genetics theory of mating systems are highlighted. The data reporting the association of self-fertilization and colonizing ability are also briefly reviewed and the potential bias in interpretation is discussed. Lastly, a recent theoretical model analysing the link between colonizing ability and self-fertilization is considered.
Conclusions
Evolutionary predictions are actually more complex than Baker's intuitive arguments. It appears that Baker's Law encompasses a variety of ecological scenarios, which cannot be considered a priori as equivalent. Questioning what has been considered as self-evident for more than 50 years seems a reasonable objective to analyse in-depth dispersal and mating system traits.
Keywords: Self-fertilization, dispersal, syndrome, Baker's Law, empirical data, models
INTRODUCTION
To move and to reproduce are two major characteristics in ecology. Because reproduction often requires more than one individual, individual movements (dispersal) and reproduction will not a priori be independent. The interference between these two biological functions may be especially important in sessile organisms such as plants where dispersal is concentrated over a short episode of the life cycle. Moreover, plant reproduction and dispersal are intrinsically linked, as the primary mode of dispersal (seeds) is the product of sexual reproduction. In plants, variations in traits affecting mating system and dispersal have been widely documented (Barrett, 2002). These traits are known to be evolutionarily labile and may respond to various ecological circumstances. Several studies have indeed demonstrated adaptation on these traits on a short time scale (Cheptou et al., 2008; Fishman and Willis, 2008). The adaptive significance of mating system and dispersal has been an important issue in plant population biology (Baker and Stebbins, 1965). In population genetics, the evolution of mating system is often considered on is own without considering other traits (but see Morgan et al., 1997) and results from three major selective forces (Lloyd, 1979). Self-fertilization is selected because of the 3/2 transmission advantage of selfing genes over outcrossing genes (Fisher, 1941). Also, the ability of selfers to produce seeds in the absence of pollination agents produces a selective advantage of selfers in pollen-limited environments, a process known as reproductive assurance (Darwin, 1876; Lloyd, 1979). However, selfing and more generally inbreeding is disadvantageous because of the reduced fitness for inbred progeny, a process termed inbreeding depression (Knight, 1799).
In plant ecology, the association between mating system and dispersal was soon realized and has lead to various and sometimes conflicting predictions. Stebbins (1957) proposed that inbreeding should be associated with colonizing ability. The rationale was that inbreeding allows the rapid fixation of successful genotypes, adapted to the new environment. In another perspective, Grant (1967) predicted that short-lived, ruderal colonizers should outcross more because of the heterotic advantage given to out-crossers in the face of landscape heterogeneity.
In a seminal paper, Baker (1955) proposed a verbal model linking colonizing ability and mating system that was to become highly influential. Analysing several species of the family Plumbaginaceae, he observed that species at the centre of the distribution range were more likely to be out-crossers than species at the periphery of the family distribution. This pattern, which he assumed to be general (Baker, 1959), led him to propose a very general and simple model (known as ‘Baker's Law’) stating that uniparental reproduction, particularly selfing, should be advantageous in colonizing populations where pollinators or partners for mating are scarce. According to this model, selfing should be predominant among colonizers. This model has been very influential in ecology given its potential wide application to various ecological situations. Moreover, it is not restricted to plants, and Baker (1955) discussed the applications of his principles to animals, specifically the case of freshwater shrimps (Nostraca) inhabiting ephemeral pools.
The central argument of Baker's model assumes that pollen limitation is variable and may be an important force driving mating systems, which more recent studies tend to support. First, empirical studies have indeed shown that pollen limitation exists in most natural populations (Burd, 1994). Second, self-fertilization provides reproductive assurance under uncertain pollen availability (Herlihy and Eckert, 2002; Kalisz et al., 2004). Interestingly, empirical studies have demonstrated that under pollen limitation self-fertilization decreases the risk of extinction relative to outcrossing (Groom, 1998; Lennartsson, 2002), which means that selfing affects population dynamics. By capturing fundamental factors of natural populations and proposing intuitive and appealing evolutionary predictions, Baker's model had the ingredients for an influential law in ecology. This verbal model has led to numerous empirical studies reporting the association between colonizing ability and self-fertilization, in various contexts: invasive species, island colonization, weeds or mating system variation across distribution range. Theoretical investigations analysing Baker scenarios have, however, been slower to develop. The major interest in Baker's Law has been to propose an integrative model for the association of traits (or a syndrome) between dispersal and mating system. In this respect, Baker's Law must be clearly distinct from the reproductive assurance hypothesis (Lloyd, 1979), which considers the advantage of selfing in pollen-limited environments but does not consider the role of colonization as a determinant of pollen limitation.
Fifty years after its publication, Baker's model remains an unresolved question in ecology and there seems to be no general consensus about it (Busch, 2011; Massol and Cheptou, 2011b). In my opinion, Baker's Law can be analysed from two different perspectives. First, the empirical perspective asks whether the association between selfing and dispersal can be found in various ecological systems. Secondly, from a theoretical perspective, it is important to study Baker's arguments using mathematical models to evaluate the validity of the verbal predictions and if the predictions are sensitive to the ecological scenarios considered. In natural populations, pollen limitation may be driven either by mate or by pollinator limitation of seed-set, and these mechanisms have important and non-trivial impacts on the putative association and on the veracity of Baker's arguments.
Here I attempt to provide an analytical overview of Baker's Law by discussing empirical data and concepts underlying the literature on Baker's Law.
WHAT IS BAKER'S LAW?
Baker's original arguments
In his seminal paper, Baker (1955) proposes two types of arguments that, according to him, should lead to an association between self-fertilization and dispersal. First, he wrote: ‘with a self-compatible individual a single propagule is sufficient to start a sexually reproducing colony, making its establishment much more likely than if the chance growth of two self-incompatible yet cross compatible individuals sufficiently close together spatially and temporally is required’. In the following sentence, he argued that ‘self-compatible flowering plants are usually able to form some seed in the absence of visits from specialized pollinating insects, which may be absent from the new situations’. Although these two lines of arguments are often given without distinction, it is important to realize that the two hypotheses refer to distinct processes. The first statement relies on intrinsic population characteristics such as number of colonizers sent by a plant or population growth rate. To a certain extent, this argument may be assimilated to a form of an Allee effect on seed production that selfing allows the plant to cope with. The second statement relies on a pollination agent, i.e. on extrinsic factors. I show below that the selective role of these two factors differs.
Baker's Law and the advantage of selfers over out-crossers
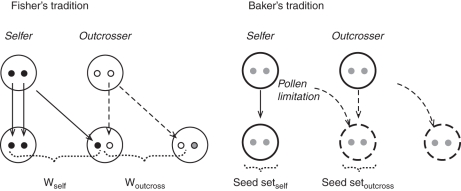
Baker's arguments seem intuitive but it may be informative to analyse conceptually the way mating success is considered. There are in fact discrepancies between classical mating system theory (Lloyd, 1979; Lande and Schemske, 1985) and Baker's arguments. Specifically, it is fundamental to realize that the way the advantage of a selfer is considered in Baker's arguments does not match with the population genetics framework. Basically, Baker's Law is founded on the demographic advantage of selfing (seed production) under low population densities or colonization episodes. In the population genetic framework, the advantage of selfing is based on the transmission of genes via paternal and maternal pathways resulting in an automatic advantage of selfing (Fisher, 1941) if the selfer can still outcross ovules in the population. In the same vein, a recent theoretical formulation by Pannell and Barrett (1998) analysed the demographic consequences of outcrossing and the minimum number of colonizers in a metapopulation to maintain outcrossing. In this model, the advantage of selfing is measured in terms of seed production. The interpretation is similar in many experimental studies. It is often noted that an out-crosser will be at a disadvantage when pollinators are scarce because it produces few seeds (Klips and Snow, 1997; Herrera et al., 2001; Anderson et al., 2003; Schueller, 2004; Ward et al., 2005) but it is seldom mentioned that low pollination implies low pollen export for an individual. In a Fisherian perspective, it is important to consider that low pollination implies not only low seed production but also an alteration in the transmission of gene copies via siring success, both resulting in a reduction of fitness for an out-crosser. Clearly, in the majority of studies, selfing advantages in Baker's arguments do not match with fitness metrics in classical mating system theory (Lloyd, 1979). Behind this discrepancy, there is a tendency to equate selfing advantage to seed set (i.e. maternal contribution only) in Baker's arguments whereas fitness in the population genetics model is the result of male and female function (Fig. 1). In the same logic, there is an implicit interpretation that selfing should maximize seed production or population growth rate in Baker's scenarios (optimality criteria) whereas classical mating system arguments do not maximize fitness (invasion criteria), as is the rule in evolution (Maynard Smith, 1978). Taking the classical interpretation of Baker's Law as an evolutionary model may therefore be misleading. Considering Baker's Law as an evolutionary model requires interpreting it in the classical mating system framework.
Fig. 1.
Measurements of mating success. Left: the self-fertilization allele is shown as closed circles and outcrossing allele as open circles. Transmission pathways from parent to offspring are shown as arrows; solid arrows represent gene transmission to progeny by the parent capable of self-fertilization, while dashed arrows represent transmission pathways for the outcrossing parent. Fitness is measured as the sum of gene copies transmitted by pollen and ovules. Assuming that the number of pollen grains produced is large relative to the number of ovules and that all the ovules are fertilized, a selfing genotype enjoys a 50 % advantage in gene transmission relative to a outcrossing genotype. This results in the cost of outcrossing (Fisher, 1941). Right: the large circles represent individual parents and their progeny. Arrows represent the production of discrete offspring by parents, with solid arrows representing pollinator-independent offspring production, and dashed arrows representing pollinator-dependent offspring production. Mating system is often equated with the number of seeds produced. Selfing ensures offspring production when lack of cross-pollinators limits seed-set (Darwin, 1876).
This confusing view of fitness in mating system studies emphasizes the need to consider the correct metrics when studying evolutionary processes, i.e. the number of genes copies per individual transmitted to the next generation. In an empirical perspective, it is thus fundamental to measure selfing advantage in terms of both maternal (seed production) and paternal contribution (siring success) in populations and the latter can be estimated using molecular markers.
EMPIRICAL ELEMENTS FOR BAKER'S LAW
Baker's Law has long been debated, generally with regard to data and predictions. Carlquist (1966) argued that ‘if dioecious stocks immigrated to the [Hawaiian] islands, Baker's Law must be in part abandoned’, which led Baker to clarify his view in a famous paper published in the pages of Evolution (Baker, 1967). My goal in this section is to give an overview of the empirical data regarding Baker's Law. The aim is not to be exhaustive but to give a general survey in the various contexts in which it has been discussed, and to point out several ambiguities in the interpretation.
Mating system variation across species ranges
The idea that selfing populations should be more peripheral than outcrossing populations is at the heart of Baker's seminal arguments because of a latitudinal gradient of pollination services. The tendency for higher outcrossing in central populations has been reported in various taxa (Stebbins, 1957). As examples, self-fertilizing morphs have been found preferentially at the periphery of the distribution for Leavenworthia alabamica (Busch, 2005), Eichornia paniculata (Barrett et al., 1989) and Clarkia xantiana (Moeller and Geber, 2005). Although this pattern is consistent with Baker's predictions, direct estimation of mating system in natural populations has sometimes revealed discrepancies with morph patterns. For example, Herlihy and Eckert (2005) demonstrated in Aquilegia canadensis that in spite of the apparent adaptation for autogamy established in peripheral populations (low herkogamy), central and peripheral populations did not differ in their realized outcrossing rates as measured by molecular markers. Also, Busch (2005) showed in Leavenworthia alabamica that not only were peripheral populations pollen-limited but so too were central populations, which casts doubt about the role of pollen limitation in shaping geographical patterns. In the context of altitudinal gradient, a recent study has revealed that outcrossing actually increased with altitude in the Alpine Boraginaceae species Erithrichum nanum (Wirth et al., 2010), contrary to the predictions that lower pollinator presence with altitude should select for selfing at higher altitudes.
Because selfers or more generally plants reproducing uniparentally are independent of pollen limitation heterogeneity across distribution ranges, it has been hypothesized that the distribution width should be larger in uniparental reproducing species than in outcrossing species. Based on floral morphology, Randle et al. (2009) found that more autogamous Collinsia species had significantly larger range sizes than their less autogamous sister-taxa. However, Johnson et al. (2010) revealed that species ranges for asexual species were not larger than for sexual taxa in section Oenantera. No clear differentiation of traits was found except heavier seeds in asexual Oenantera taxa, which would suggest, contrary to the hypothesis, a lower colonizing ability of asexual populations.
Mating systems in island colonizers
Mating system in island flora was of great interest during the 1960s. The data revealed contrasting patterns. Consistent with the idea that a few selfing plants are able to develop viable colonies on islands, empirical data have shown that the proportion of self-incompatible taxa was lower on islands (Barrett, 1996). Island flora have, however, revealed a paradoxical pattern. Several islands, such as Hawaii (Bawa, 1980), Réunion (cited in Humeau et al., 1999) and the Ogasawara (Bonin) Islands (Abe, 2006), have revealed a higher incidence of dioecious plants than on continents. Because dioecious species are complete out-crossers, they are not expected to be over-represented on islands. To explain this paradox, several authors have argued that leaky dioecy may be the reason for the high incidence of dioecy (Barrett, 1996). The underlying rationale is that leaky dioecy allows a few seeds to be produced, which may be a determinant for successful colonization on islands, a strategy that would be advantageous for further adaptation on islands. Logically, by emphasizing the fact that a few seeds is enough to establish a viable population, the leaky dioecy hypothesis is not in line with Baker's Law. Indeed, if selfing strategy is a fundamental trait during colonization, then a full selfer will be demographically much more advantageous and should out-compete an out-crosser that is able to produce a few selfing seeds. From this perspective, the leaky hypothesis tends to attenuate the demographic advantage of selfing and emphasize other aspects of the mating system (e.g. the role of recombination in adaptation) or on other traits to explain a successful colonization.
Another important question is whether patterns observed on islands result from evolutionary processes on the continent (before colonization) or on islands (after colonization). If the patterns observed have developed after colonization, they tell us little about the association between dispersal and mating system. Under this scenario, dispersing organisms colonize islands, whatever their mating system, and island conditions (few mates, few pollinators) select for autogamy via the reproductive assurance mechanism. Concerning the high incidence of dioecy on islands, some studies tend to support the suggestion that colonizers were biased towards more dioecy (Bawa, 1980; Renner and Ricklefs, 1995; Sakai et al., 1995a, b), which could be explained by the presence of well-developed dispersing structures (fleshy fruits). In the context of the Hawaiian archipelago, Sakai et al. (1995b) used phylogenetics to infer colonizer status. According to these authors, the high incidence of dioecy would in part be due to the fact that colonists were biased towards dioecious taxa.
In a recent paper published in Science, Alsos et al. (2007) demonstrated long-distance dispersal by identifying continental sources for nine species on the remote Svalbard Archipelago in the arctic. Using genetic data, they were able to identify continental sources and give a minimum number of colonizers for the observed genetic patterns. Their results showed that most of the colonization events originated from Russia, although it was not the closest continent. Using bibliographic resources and the BiolFlor database (http://www.ufz.de/biolflor/), I mapped the mating system (measured on the continent) for those nine species for which we know that long-distance colonization has occurred (Table 1). Among the nine species studied, five were either dioecious or self-incompatible and the others were mixed selfers or autogamous. Although the sample is small, the data do not support higher colonization rates for selfers in island systems.
Table 1.
Mating system in long-distance colonizing plants in the remote Svalbard Archipelago in the arctic
| Empetrum nigrum | Vaccinium uliginosum | Rubus chamaemorus | Betula nana | Dryas octopela | Salix herbacea | Cassiope tetragona | Arabis alpine | Saxifraga rivularis | |
|---|---|---|---|---|---|---|---|---|---|
| Main dispersal vector | Bird | Bird | Bird | Wind | Wind | Wind | Wind? | Wind? | Wind? |
| Minimum no. of propagules | 7 | 12 | 6 | 11 | 38 | 20 | 14 | 1 | 22 |
| Mating system | Dioecy | Selfer | Dioecy | Allogamous (self-incompatible) | Allogamous (self-incompatible) | Dioecious | Allogamous (?) | Mixed selfer | Autogamous (occasional outcrossing) |
| Reference | Bell and Tallis (1973) | Jacquemart and Thompson (1996) | Taylor (1971) | BiolFlor database | BiolFlor database | BiolFlor database | http://www.binran.ru/ | BiolFlor database | Senstad Guldahl et al. (2005) |
Long-distance past colonization was estimated by inferring the most likely sources of colonizers on the continent using DNA fingerprinting. The predominant source is north-western Russia, i.e. the most distant regions. The minimum number of colonizers was calculated as the smallest sample needed to observe genetic diversity on Svalbard. I mapped mating system for the nine species from the literature (see Reference).
Ruderal (colonizing) species
Baker (1965) suggested that ruderal species were associated with selfing. This statement was defended by Stebbins (1965), although he did not detect any evidence for this pattern in weeds from California. Price and Jain (1981) have analysed the mating system in about 400 species in the British Isles. Their survey tends to support the suggestions that weeds or colonizers are more likely to be selfers, although they note that their conclusions should be taken with caution because of possible confounding factors. It is true that some life-history traits are correlated with mating strategies. Thus, it has been established that selfing is preferentially associated with annuality (Duminil et al., 2009), a trait that is likely to be associated with ruderality. Some weed species have sometimes been classified as selfers a priori but we clearly need detailed studies to characterize mating systems. As an example, the aggressive weed Ambrosia artemisiifolia (Asteraceae) has long been considered capable of selfing (Jones, 1936) but a recent study has demonstrated that it is actually self-incompatible (Friedman and Barrett, 2008).
Studying the association between selfing and colonizing ability in ruderal species is interesting because this association is a priori shaped by metapopulation dynamics at the regional scale (Pannell and Barrett, 1998) and does not involve more complex scenarios such as rare long-distance dispersal (e.g. islands).
BAKER'S LAW ENCOMPASSES VARIOUS ECOLOGICAL SCENARIOS
Baker's model was inspired by mating system variation across species ranges. However, the success of Baker's Law lies in part in its ability to fit with various ecological contexts such as weeds, invasive species or flora colonizing islands. If the various ecological scenarios may seem similar, they actually encompass important specificities regarding population dynamics and particularly with regard to the nature of dispersal. The various scenarios may be characterized with regard to gradients of dispersal asymmetry among habitats (Massol and Cheptou, 2011b).
In the island/continent context, dispersal is purely one sided. Although islands deprived of pollinators may act as a filter for self-compatible taxa, the asymmetry of dispersal implies that evolutionary processes will probably result from continental selection pressures rather than from the island/continent system. The contribution to the next generation originates from continental populations, which implies that evolutionary trajectories will be driven by continental selection pressure. Following the terminology of Holt and Gomulkiewicz (1997), islands may be considered as black hole sinks without effect on evolutionary trajectories. In this context, Baker's Law may be considered more as an ecological law than an evolutionary law. Importantly, there is still the possibility for evolution towards autogamy on islands if gene flow with the continent is rare. Post-colonization evolution requires genetic variance of mating system traits, which may be impeded by bottlenecks during colonization. However, in this context, dispersal is of little interest and post-colonization evolution on islands is driven by local reproductive assurance only, without concern for dispersal strategy.
From the perspective of distribution range, dispersal is likely to be asymmetric, from central to peripheral populations. The long-term stability of a species distribution implies that dispersal patterns, although asymmetric, may be stationary. Dispersal asymmetry is expected to carry genes from the centre of the distribution to the periphery (Kirkpatrick and Barton, 1997), which may theoretically prevent population differentiation. The fact that population differentiation has often been found in empirical studies shows that differentiation is in practice possible along distribution ranges. Although some empirical results exhibit patterns consistent with Baker's model, it is important to bear in mind that alternative explanation may provide a parsimonious explanation. As an alternative and simple explanation, one may argue that selfing traits may favour adaptation at various environments by preventing gene flow (Kirkpatrick and Barton, 1997; Levin, 2010), which opens the possibility of the species extending spatially.
The case of colonizing organisms such as weeds provides an example in which dispersal may be considered as symmetric at the regional scale. Schematically, such organisms have a patchy distribution in spatially or temporally heterogeneous landscapes. As a consequence, dispersal and mating strategy will be the result of heterogeneous selection pressure on the whole metapopulation. Thus, patterns of dispersal are essential to understand and predict the consequences of heterogeneous pollination services in natural populations.
The ecological scenarios discussed in this section are summarized in Table 2.
Table 2.
Ecological scenarios considered in Baker's Law and their variation with regard to dispersal patterns
| Ecological scenario | Dispersal frequency | Among-habitat dispersal | Importance of dispersal in mating system evolution |
|---|---|---|---|
| Island/continent (or exotic invader) | Rare | Highly asymmetric | Little |
| Range limits | Regular | Asymmetric | Middle |
| Metapopulation dynamics (e.g. weeds) | Regular | Symmetric | High |
As a consequence of heterogeneous among-habitat dispersal, the heterogeneity of pollination environment is expected to influence more or less the outcome of mating system evolution.
EVOLUTIONARY PREDICTIONS IN A POLLEN-LIMITED METAPOPULATION
I now discuss a few theoretical results related to Baker's Law. The models are generally concerned with simpler ecological scenarios than Baker's initial verbal model. Interestingly, they show that evolutionary outcomes are sometimes more complex than a simple selfing/dispersal association.
Evolution under density-dependant pollination (Allee effect)
Two theoretical studies (Pannell and Barrett, 1998; Dornier et al., 2008) have modelled the advantage of selfing caused by the limited number of mates during colonization. In their models, pollination is related to intrinsic properties of populations and their assumption is close to Baker's first argument: ‘with a self-compatible individual a single propagule is sufficient to start a sexually reproducing colony, making its establishment much more likely than if the chance growth of two self-incompatible yet cross compatible individuals sufficiently close together spatially and temporally is required’. Pannell and Barrett (1998) considered a number of colonists to be the result of dispersal from occupied patches and analysed the advantage of colonization by a single individual allowed by selfing. Dornier et al. (2008) considered a metapopulation model with an explicit Allee effect function and random extinction of patches. In their model, the success of an out-crosser depends on the local density after the colonization episode until extinction while selfers suffer from inbreeding depression. Dornier et al. (2008) showed that metapopulation viability is dependent on the selfing rate. Whereas full out-crossers can form a viable metapopulation, only partial selfers are able to recover from very low density at the regional scale and the minimum selfing rate can be derived analytically.
The two models demonstrate the intuitive conclusion that when the number of colonizers is low, selfing is favoured. However, when fertility (number of seeds) is high, this effect may be small and does not impede the maintenance of outcrossing. Interestingly, the models revealed that the advantage of selfing during colonization is particularly significant when patch occupancy is low so that the number of colonizers is small. By contrast, when patch occupancy is high, the number of colonizers is sufficient to overcome the Allee effect during colonization. This may explain why common weeds such as Centaurea solstitialis (Sun and Ritland, 1998) and Crepis sancta (Cheptou et al., 2002) can maintain a high outcrossing rate, in spite of recurrent colonization. Also, Dornier et al. (2008) showed that the number of colonizers also depends on mating strategy. Assuming the same parameters (fertility, dispersal rates, etc.), out-crossers can even have a demographic advantage and thus can produce many more colonizers than selfers. The reason for this is that inbreeding depression may lower fertility and thus the density in colonizing sites. While Baker assumed that ‘a single propagule is sufficient [for a selfer] making its establishment much more likely than […] two self-incompatible individuals’, Dornier et al. (2008) demonstrated that because of the demographic effect of inbreeding depression, two colonizers for an out-crosser or one colonizer for a selfer may have the same probability of arriving in a colonizing area. Interestingly, for the same set of parameters, the number of colonizers can increase with outcrossing rate, thus favouring outcrossing, so that mating strategy tends to self-reinforce itself (positive demographic feedback).
Using an explicit demographic argument, these models have the advantage of disentangling the various forces at work in a metapopulation and show that the expectations are not necessarily as straightforward as implied by the verbal arguments in Baker's Law.
Evolution under pollination heterogeneity
In another context, Cheptou and Massol (2009) developed an analytical evolutionary stable strategy (ESS) model to study the joint evolution of dispersal and self-fertilization. Contrary to most models for the evolution of selfing, both dispersal and selfing are free to evolve. The main hypothesis of the model is not density-dependent pollination but extrinsic pollination heterogeneity in the landscape. To a certain extent, their hypothesis is close to the Baker's second argument: ‘self-compatible flowering plants are usually able to form some seed in the absence of visits from specialized pollinating insects, which may be absent from the new situations’. Basically, they assume that pollination fluctuates temporally in patches, as may be the case for among-year insect activities (Kalisz et al., 2004). The details of the model are shown in Fig. 2. Their results are simple but may seem counterintuitive. They found that, depending on the parameters, two types of association evolve: either complete outcrossing associated with dispersal (the ‘dispersal/outcrossing’ syndrome) or complete or mixed selfing associated with the absence of dispersal (the ‘no-dispersal/selfing’ syndrome). In short, the syndromes are the exact opposite of Baker's predictions. Although this may appear paradoxical from the mating system point of view, the theory for the evolution of dispersal provides a comprehensive view of the results. For an out-crosser, pollination heterogeneity in the landscape creates fitness variation among a patch. In such a context, among-patch fitness variation creates a positive selection for dispersal (Comins et al., 1980; Olivieri et al., 1995). By contrast, for a selfer, pollination heterogeneity in the landscape does not translate into fitness variation among a patch. As a consequence, pollination heterogeneity does not select for dispersal in selfers, as observed in the field. Interestingly, Massol and Cheptou (2011a) generalized the model by allowing various types of pollination heterogeneity in the landscape: from temporal variation to spatial variation. Their conclusions indicate that the association of traits does not change. As a consequence, whatever the pollination heterogeneity, the selfing-disperser is never selected.
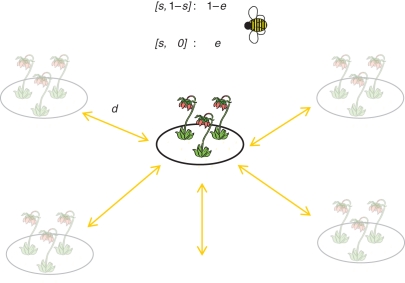
Fig. 2.
Joint evolution of dispersal and self-fertilization in a temporally heterogeneous pollination environment (from Cheptou and Massol, 2009; Massol and Cheptou, 2011). The model assumes an infinite island model where each patch is prone to temporally heterogeneous pollination because of the stochastic presence of pollinator (see Kalisz et al., 2004, for empirical support). We consider a basic mechanism of self-fertilization: plants self-fertilize a proportion, s, of their ovules while the proportion 1 – s can be fertilized by outcrossing only if pollinators are present, which occur with a probability (1 – e). Otherwise, the fraction 1 – s is left unfertilized (probability e). Seeds produced disperse at random among patch at rate d but only a fraction q survive to dispersal. Progeny produced by self-fertilization suffer from inbreeding depression (δ). Model outcome: the mathematical analysis (invasion analysis) reveals that only two syndromes of traits are possible: the ‘dispersal/outcrossing’ syndrome (d* = (e/[1 – q(1 – e)], s* = 0) and the ‘no-dispersal/selfing’ syndrome (d* = 0, s* = (2e/[2δ + e – 1])) (see Cheptou and Massol, 2009, for the influence of parameters). Pollination fluctuations suffered by out-crossers create among-patch variance and thus favour dispersal (see Comins et al., 1982). In contrast, selfers do not suffer from pollination heterogeneity, thus cancelling selection pressure for dispersal.
Although the first metapopulation models mitigated Baker's arguments, Cheptou and Massol (2009) showed that outcrossing can in fact favour dispersal. Although simple in its formulation, this provides an explanation for paradoxical patterns such as the high incidence of dioecy. According to Cheptou and Massol (2009), dioecious plants evolving in heterogeneous pollination landscapes on the continents are conformed to the outcrossing/dispersing syndrome. As good dispersers, they may be over-represented on islands.
Overall, although modelling Baker's Law in a metapopulation scenario may be limited and does not encompass all the scenarios classically envisaged, these models have revealed unexpected and counterintuitive results. They showed that intuition may sometimes be misleading. Indeed, reasoning at the metapopulation level allows us to capture the colonization process as the result of the whole set of patches, which is not trivial without using a mathematical model.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
By articulating concepts, models and empirical data, I have tried to give a synthetic overview of Baker's Law. Detailed analysis of the arguments shows that this model is not totally compatible with the mating system model from the population genetic tradition. At the heart of the problem is the definition of fitness. Whereas the population genetic framework considers the number of genes transmitted as the fitness metric, Baker's arguments as generally used tend to adopt a demographic view of mating success which does not totally fit with the fitness metric (Cheptou, 2007). This question must be clarified when studying Baker's arguments in natural populations, and conceptual tools to clarify this point already exist (Lloyd, 1979). Specifically, empiricists should be careful in interpreting pollen limitation data in natural populations, and the use of molecular markers (e.g. microsatellites) coupled with parentage analysis may help to estimate individual siring success and thus to get an unbiased estimate of fitness.
Examining the few mathematical models that have tried to model Baker's Law indicates that evolutionary predictions are more complex than the intuitive verbal predictions. Ecological scenarios and in particular the patterns of dispersal among heterogeneous pollination habitats are key elements to analysing in more depth the way selfing and dispersal traits interact. Beyond the simplified classification of ecological scenarios given above, real systems are likely to behave as a continuum of scenarios, from highly asymmetric gene flow to symmetric gene flow. This points to the need to characterize colonization/extinction dynamics, i.e. metapopulation scenarios, to interpret selection processes. Although Baker's Law is concerned with the interaction between dispersal and mating system, mating system biologists interested in such questions have probably placed too much emphasis on local selection (population-centred) caused by reproductive assurance but too little on among-population processes at the metapopulation level. Statistical tools to model metapopulation dynamics from field surveys are now available (Moilanen, 2004). Although little used in plant metapopulation systems, a study by Dornier et al. (2011) succeeded in differentiating island/continent dynamics from ‘true’ metapopulation dynamics in Crepis sancta.
Fifty years after its publication, the simplicity of Baker's law and its diverse interpretations are still a source of inspiration in mating system studies. Although theoretical and conceptual models have little influenced the debate on Baker's Law, we should expect much progress based on sound articulation of models with empirical data.
LITERATURE CITED
- Abe T. Threatened pollination systems in native flora of the Ogasawara (Bonin) Islands. Annals of Botany. 2006;98:317–334. doi: 10.1093/aob/mcl117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsos IG, Eidesen PB, Ehrich D, et al. Frequent long-distance plant colonization in the changing Arctic. Science. 2007;316:1606–1609. doi: 10.1126/science.1139178. [DOI] [PubMed] [Google Scholar]
- Anderson B, Midgley JJ, Stewart BA. Facilitated selfing offers reproductive assurance: a mutualistism between a hemipteran and carnivorous plant. American Journal of Botany. 2003;90:1009–1015. doi: 10.3732/ajb.90.7.1009. [DOI] [PubMed] [Google Scholar]
- Baker HG. Self-compatibility and establishment after ‘long distance’ dispersal. Evolution. 1955;9:347–348. [Google Scholar]
- Baker HG. Reproductive methods as factors in speciation in flowering plants. Cold Spring Harbor Symposia on Quantitative Biology. 1959;24:177–191. doi: 10.1101/sqb.1959.024.01.019. [DOI] [PubMed] [Google Scholar]
- Baker HG. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The genetics of colonizing species. New York: Academic Press; 1965. [Google Scholar]
- Baker HG. Support for Baker's law as a rule. Evolution. 1967;21:853–856. doi: 10.1111/j.1558-5646.1967.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Baker HG, Stebbins GL. The genetics of colonizing species. New York: Academic Press; 1965. pp. 147–172. [Google Scholar]
- Barrett SCH. The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society B: Biological Sciences. 1996;351:725–733. [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Morgan MT, Husband BC. The dissolution of a complex genetic polymorphism: the evolution of self-fertilisation in tristylous Echornia paniculata (Pontedariaceae) Evolution. 1989;43:1398–1416. doi: 10.1111/j.1558-5646.1989.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Bawa KS. Evolution of dioecy in flowering plants. Annual Review of Ecology and Systematics. 1980;11:15–39. [Google Scholar]
- Bell JN, Tallis JH. Biological Flora of the British Isles: Empetrum nigrum L. Journal of Ecology. 1973;61:289–305. [Google Scholar]
- Burd M. Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. The Botanical Review. 1994;60:83–139. [Google Scholar]
- Busch JW. The evolution of self-compatibility in geographically peripheral populations of Leavenworthia alabamica (Brassicaceae) American Journal of Botany. 2005;92:1503–1512. doi: 10.3732/ajb.92.9.1503. [DOI] [PubMed] [Google Scholar]
- Busch JW. Demography, pollination, and Baker's law. Evolution. 2011;65:1511–1513. doi: 10.1111/j.1558-5646.2011.01224.x. [DOI] [PubMed] [Google Scholar]
- Carlquist S. The biota of long-distance dispersal. IV. Genetic systems in the floras of oceanic islands. Evolution. 1966;20:433–455. doi: 10.1111/j.1558-5646.1966.tb03379.x. [DOI] [PubMed] [Google Scholar]
- Cheptou PO. Why should mating system biologists be demographers? Trends in Ecology & Evolution. 2007;22:562–563. doi: 10.1016/j.tree.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Cheptou PO, Massol F. Pollination fluctuations drive evolutionary syndromes linking dispersal and mating system. American Naturalist. 2009;174:46–55. doi: 10.1086/599303. [DOI] [PubMed] [Google Scholar]
- Cheptou PO, Lepart J, Escarre J. Mating system variation along a successional gradient in the allogamous and colonizing plant Crepis sancta (Asteraceae) Journal of Evolutionary Biology. 2002;15:753–762. [Google Scholar]
- Cheptou PO, Carrue O, Rouifed S, Cantarel A. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proceedings of the National Academy of Sciences of the USA. 2008;105:3796–3799. doi: 10.1073/pnas.0708446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comins HN, Hamilton WD, May RM. Evolutionarily stable dispersal strategies. Journal of Theoretical Biology. 1980;82:205–230. doi: 10.1016/0022-5193(80)90099-5. [DOI] [PubMed] [Google Scholar]
- Darwin CR. The effects of cross and self fertilization in the vegetable kingdom. London: Murray; 1876. [Google Scholar]
- Dornier A, Munoz F, Cheptou PO. Allee Effect and self-fertilization in hermaphrodites: reproductive assurance in a structured metapopulation. Evolution. 2008;62:2558–2569. doi: 10.1111/j.1558-5646.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- Dornier A, Pons V, Cheptou P-O. Colonization and extinction dynamics of an annual plant metapopulation in an urban environment. Oikos. 2011 (in press). doi:10.1111/j.1600-0706.2010.18959.x. [Google Scholar]
- Duminil J, Hardy OJ, Petit RJ. Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evolutionary Biology. 2009;9:177. doi: 10.1186/1471-2148-9-177. doi:10.1186/1471-2148-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Average excess and average effect of a gene substitution. Annals of Eugenics. 1941;11:53–63. [Google Scholar]
- Fishman L, Willis JH. Pollen limitation and natural selection on floral characters in the yellow monkeyflower. Mimulus guttatus New Phytologist. 2008;177:802–810. doi: 10.1111/j.1469-8137.2007.02265.x. [DOI] [PubMed] [Google Scholar]
- Friedman J, Barrett SCH. High outcrossing in the annual colonizing species Ambrosia artemisiifolia (Asteraceae) Annals of Botany. 2008;101:1303–1309. doi: 10.1093/aob/mcn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WF. Cytogenetic factors associated with the evolution of weeds. Taxon. 1967;16:283–293. [Google Scholar]
- Groom MJ. Allee effects limit population viability of an annual plant. American Naturalist. 1998;151:487–496. doi: 10.1086/286135. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plants. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. Evolution of self-fertilisation at geographical range margins? A comparison of demographica, floral, and mating system variables in central versus peripheral populations of Aquilegia canadensis (Ranunculaceae) American Journal of Botany. 2005;92:744–751. doi: 10.3732/ajb.92.4.744. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Sanchez-Lafuente M, Medrano M, Guitian J, Cerda X, Rey P. Geographical variation in autonomous self-pollination levels unrelated to pollinator service in Helleborus foetidus (Ranunculaceae) American Journal of Botany. 2001;88:1025–1032. [PubMed] [Google Scholar]
- Holt RD, Gomulkiewicz R. The influence of immigration on local adaptation: a re-examination of a familiar paradigm. The American Naturalist. 1997;149:563–572. [Google Scholar]
- Humeau L, Pailler T, Thompson JD. Cryptic dioecy and leaky dioecy in endemic species of Dombeya (Sterculiaceae) on La Reunion. American Journal of Botany. 1999;86:1437–1447. [PubMed] [Google Scholar]
- Johnson MTJ, Smith SD, Rausher MD. Effects of plant sex on range distributions and allocation to reproduction. New Phytologist. 2010;186:769–779. doi: 10.1111/j.1469-8137.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- Jacquemart A-L, Thompson JD. Floral and pollination biology of three sympatric Vaccinium (Ericaceae) species in the Upper Ardenne, Belgium. Canadian Journal of Botany. 1996;74:210–221. [Google Scholar]
- Jones KL. The inheritance of floral types in the ragweed, Ambriosia elatior L. American Midland Naturalist. 1936;17:673–699. [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependant autonomous self-fertilisation yields reproductive assurance and mixed mating. Nature. 2004;430:884–886. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton NH. Evolution of a species' range. American Naturalist. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- Klips RA, Snow AA. Delayed autonomous self-pollination in Hibiscus laevis (Malvaceae) American Journal of Botany. 1997;84:48–53. [Google Scholar]
- Knight T. Experiments on the fecundation of vegetables. Philosophical Transactions of the Royal Society of London. 1799;89:195–204. [Google Scholar]
- Lande R, Schemske DW. The evolution of self fertilization and inbreeding depression in plants. I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Lennartsson T. Extinction threshold and disrupted plant–pollinator interactions in fragmented plant populations. Ecology. 2002;83:3060–3072. [Google Scholar]
- Levin DA. Environment-enhanced self-fertilization: implications for niche shifts in adjacent populations. Journal of Ecology. 2010;98:1276–1283. [Google Scholar]
- Lloyd DG. Some reproductive factors affecting the selection of self-fertilization in plants. American Naturalist. 1979;113:67–79. [Google Scholar]
- Massol F, Cheptou PO. Evolutionary syndromes linking dispersal and mating system: the effect of autocorrelation in pollination conditions. Evolution. 2011a;65:591–98. doi: 10.1111/j.1558-5646.2010.01134.x. [DOI] [PubMed] [Google Scholar]
- Massol F, Cheptou PO. When should we expect the evolutionary association of self-fertilisation and dipsersal? Evolution. 2011b;65:1217–1220. doi: 10.1111/j.1558-5646.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. The evolution of sex. London: Cambridge University Press; 1978. [Google Scholar]
- Moeller DA, Geber MA. Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution. 2005;59:786–799. doi: 10.1554/04-656. [DOI] [PubMed] [Google Scholar]
- Moilanen A. SPOMSIM: software for stochastic patch occupancy models of metapopulation dynamics. Ecological Modelling. 2004;179:533–550. [Google Scholar]
- Morgan MT, Schoen DJ, Bataillon TM. The evolution of self fertilization in perennials. American Naturalist. 1997;150:618–638. doi: 10.1086/286085. [DOI] [PubMed] [Google Scholar]
- Olivieri I, Michalakis Y, Gouyon P-H. Metapopulation genetics and the evolution of dispersal. American Naturalist. 1995;146:202–228. [Google Scholar]
- Pannell JR, Barrett CH. Bakers' law: reproductive assurance in a metapopulation. Evolution. 1998;52:657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Price SC, Jain SK. Are inbreeders better colonizers? Oecologia. 1981;49:283–86. doi: 10.1007/BF00349202. [DOI] [PubMed] [Google Scholar]
- Randle AM, Slyder JB, Kalisz S. Can differences in autonomous selfing ability explain differences in range size among sister-taxa pairs of Collinsia (Plantaginaceae)? An extension of Baker's Law. New Phytologist. 2009;183:618–629. doi: 10.1111/j.1469-8137.2009.02946.x. [DOI] [PubMed] [Google Scholar]
- Renner SS, Ricklefs RE. Dioecy and its correlates in the flowering plants. American Journal of Botany. 1995;82:596–606. [Google Scholar]
- Sakai AK, Wagner WL, Ferguson DM, Herbst DR. Origins of dioecy in the Hawaiian flora. Ecology. 1995a;76:2517–2529. [Google Scholar]
- Sakai AK, Wagner WL, Ferguson DM, Herbst DR. Biogeographical and ecological correlates of dioecy in the Hawaiian flora. Ecology. 1995b;76:2530–2543. [Google Scholar]
- Schueller SK. Self-pollination in island and mainland populations of the introduced hummingbird-pollinated plant, Nicotinia glauca (Solanaceae) American Journal of Botany. 2004;91:672–681. doi: 10.3732/ajb.91.5.672. [DOI] [PubMed] [Google Scholar]
- Senstad Guldahl A, Gabrielsen TM, Scheen AC, et al. The Saxifraga rivularis complex in Svalbard: molecules, ploidy and morphology. Flora (Germany) 2005;200:207–221. [Google Scholar]
- Stebbins GL. Self-fertilization and population variability in higher plants. American Midland Naturalist. 1957;91:337–354. [Google Scholar]
- Stebbins GL. Colonizing species of native California flora. In: Baker HG, Stebbins GL, editors. The genetics of colonizing species. New York: Academic Press; 1965. pp. 173–192. [Google Scholar]
- Sun M, Ritland K. Mating system of yellow starthistle (Centaurea solstitialis), a successfull colonizer in North America. Heredity. 1998;80:225–232. [Google Scholar]
- Taylor K. Biological Flora of the British Isles: Rubus chamaemorus L. Journal of Ecology. 1971;59:293–306. [Google Scholar]
- Ward M, Dick CW, Gribel R, Lowe AJ. To self, or not to self: a review of outcrossing and pollen-mediated gene flow in neotropical trees. Heredity. 2005;95:246–254. doi: 10.1038/sj.hdy.6800712. [DOI] [PubMed] [Google Scholar]
- Wirth LR, Graf R, Gugerli F, Landergott U, Holderegger R. Lower selfing rate at higher altitudes in the alpine plant Eritrichium nanum (BORAGINACEAE) American Journal of Botany. 2010;97:899–901. doi: 10.3732/ajb.0900297. [DOI] [PubMed] [Google Scholar]