Abstract
Background and aims
Sexually dimorphic populations are often located in drier habitats than cosexual populations. Gender plasticity (GP), whereby hermaphrodites alter female and male functions depending on resources, and sex-differential plasticity (SDP) between hermaphrodites and unisexuals are predicted to affect sexual system stability. Here, GP and SDP are evaluated in cosexual and gynodioecious Wurmbea biglandulosa and sub-dioecious and dioecious W. dioica.
Methods
GP was evaluated under two resource conditions, compared among sexual systems and assessed as to whether (1) males produced perfect flowers and (2) hermaphrodites altered investment in perfect (female function) and total (male function) flowers. SDP was assessed within sexual systems as differences between sex functions of hermaphrodites vs. unisexuals. Males and hermaphrodites were compared to assess whether size thresholds for female function differed among sexual systems. Plasticity costs were evaluated using correlations between female function and male traits in hermaphrodites, and in W. dioica by comparing hermaphrodite and male regressions between plant size and pollen production.
Key Results
In dioecious W. dioica no males exhibited GP, whereas 100 % did in gynodioecious and cosexual W. biglandulosa. In sub-dioecious W. dioica, resources affected GP (high, 66 %; low, 42 %). Hermaphrodites in all sexual systems reduced perfect but not total flowers under low resources. Unisexuals were unaffected, demonstrating SDP for female function only. Thresholds for female function were greater in sub-dioecious W. dioica than in W. biglandulosa. Plasticity costs were detected only in sub-dioecious W. dioica.
Conclusions
SDP for female function could assist female establishment in cosexual populations and maintain females in gynodioecious and sub-dioecious populations. Although the absence of male SDP should stabilize sub-dioecy, plasticity costs would render sub-dioecy unstable, favouring canalized males over hermaphrodites. This study highlights the importance of interactions between environmental conditions and hermaphrodite sex expression for the stability of dimorphic sexual systems.
Keywords: Dioecy, gender plasticity, gynodioecy, plant sexual systems, sex-allocation plasticity, sex-differential plasticity, sub-dioecy, Wurmbea biglandulosa, Wurmbea dioica
INTRODUCTION
The transition to separate sexes (dioecy) from cosexual ancestors has occurred repeatedly in flowering plants and is often associated with harsh or resource-limited conditions (Darwin, 1877; Renner and Ricklefs, 1995; Sakai and Weller, 1999; Weiblen et al., 2000). Several evolutionary pathways are possible, but a particularly common route begins with the invasion and spread of a female (male-sterile) mutant, and the subsequent co-occurrence of hermaphrodites and females (gynodioecy). Because females produce ovules but not pollen, the evolution of gynodioecy requires that females have a seed fitness advantage relative to hermaphrodites (Lloyd, 1976; Charlesworth and Charlesworth, 1978). Ecological context plays a key role in modifying the relative seed fertility of the sex morphs and providing a female advantage. For example, previous studies have revealed how variation in pollinator service, pollen limitation, herbivores and pathogens may affect relative seed fertility of females and allow them to establish and be maintained in populations (McCauley and Brock, 1998; Ashman, 2002; Lopez-Villavicencio et al., 2005; Alsono et al., 2007).
An alternative mechanism that could explain why females are often more common in resource-limited environments is the ability of hermaphrodites to respond to changes in environment by altering allocation to male and female functions (gender plasticity; Delph, 2003). Specifically, if hermaphrodites respond to low resource conditions by reducing investment in seeds compared with pollen, then females may achieve the seed fertility advantage required to establish and then spread in populations. For this advantage to be realized, however, females should express less plasticity in response to resource variation than hermaphrodites [i.e. sex-differential plasticity (SDP); Delph, 1990a, 2003; Delph and Wolfe, 2005]. Consistent with the SDP hypothesis, studies across environmental gradients have generally shown associations between site quality, or indicators of quality such as plant vigour and the relative seed fitness of females and hermaphrodites. Most of these studies have also reported higher frequencies of females in more resource-limited sites (e.g. Darwin, 1877; Delph, 1990a; further examples in table 1 of Delph, 2003 and table 11·2 of Ashman, 2006; but see Van Etten and Chang, 2009). Further, studies that have manipulated resources in the field and glasshouse have shown that SDP in fruit production can be substantial enough to more than double the seed fertility of females relative to that of hermaphrodites under low compared with high resource conditions (Delph, 1990b; Barr, 2004; Dorken and Mitchard, 2008; Spigler and Ashman, 2011). Collectively, these studies underscore the importance of gender plasticity mediated by the environment in explaining establishment and spread of females in populations.
Once females have established, selection favours hermaphrodites that allocate more resources to male function than female function because they accrue greater fitness through pollen than through ovules. Such selection is expected eventually to culminate in the replacement of hermaphrodites by males in populations and the evolution of dioecy (Charlesworth and Charlesworth, 1978; Charlesworth, 1989). However, as outlined by several authors, gender plasticity may hinder the evolution of dioecy by lowering the costs of producing seeds (Delph and Lloyd, 1991; Barrett et al., 1999; Delph and Wolfe, 2005). In particular, if gender plasticity allows hermaphrodites to vary female function depending on environmental conditions, then more seeds could be produced during periods of high resource availability when seed production costs are lower. This could allow hermaphrodites to be retained in populations, stabilizing gynodioecy or sub-dioecy (Delph and Wolfe, 2005). On the other hand, if gender plasticity imposes costs, expressed as reduced male function when female function is increased, then canalized individuals that produce fewer seeds or no seeds may be favoured over gender-plastic hermaphrodites. Because costs of plasticity are likely to be more pronounced in low resource environments, plasticity might be expected to vary positively with resource availability (Ashman, 2006). This could affect sexual system evolution, such that dioecy is most likely to evolve in low resource environments, while gynodioecy and sub-dioecy are maintained in environments that are less severe (Case and Ashman, 2007).
Australian Wurmbea species are diminutive, insect-pollinated geophytes that vary in sexual system from cosexual through gynodioecy and sub-dioecy to dioecy (reviewed by Barrett and Case, 2006). Sexual system variation within and between closely related W. biglandulosa (cosexual and gynodioecious populations) and W. dioica (sub-dioecious and putatively dioecious populations) provides unique opportunities to study the evolution of separate sexes. Previous work on these species has revealed both a greater incidence of sexually dimorphic populations and lower female investment by hermaphrodites in resource-limited, particularly arid, habitats (Barrett, 1992; Case and Barrett, 2004a; Vaughton and Ramsey, 2002, 2004). In part, these patterns can be attributed to divergent selection imposed by shifts in pollination biology and flower size (Case and Barrett, 2004b), and their consequent effects on plant mating and expression of inbreeding depression (Ramsey et al., 2006a, b). However, one feature of these species in particular indicates that gender plasticity might also play a role. Hermaphrodites can produce both staminate and perfect flowers, but vary the number of the latter, indicating that female function is controlled primarily at the flower rather than at the fruit level, whereas male function is largely dictated by total flower production (Barrett et al., 1999; Ramsey and Vaughton, 2001; Vaughton and Ramsey, 2002). This should enable these plants to curtail investment in female function without altering male function. Indeed, Barrett et al. (1999) reported that the production of perfect flowers was size dependent in sub-dioecious W. dioica, and concluded that female allocation was plastic and adjusted depending on resource status (see also Ramsey and Vaughton, 2001). Here we use the term phenotypic males to describe plants in the field with only staminate flowers because we cannot distinguish whether such plants are constant (canalized) or inconstant (gender plastic). We refer to phenotypic males that subsequently produce at least one perfect flower under experimental conditions as hermaphrodites, whereas those that do not are canalized males. Hermaphrodites are clearly recognized because they produce at least one flower with a well-developed pistil. Flowers on all polleniferous plants produce six stamens.
In this study, we use a combination of field and glasshouse studies to characterize gender plasticity in hermaphrodites from cosexual and gynodioecious W. biglandulosa populations and sub-dioecious and putatively dioecious W. dioica populations. Our overall aim is to assess how gender plasticity might influence sexual system evolution and stability. To achieve this, three approaches are used. (1) Resource conditions are manipulated to assess the proportion of phenotypic males that are gender plastic, and to test for SDP in female function by comparing hermaphrodites and females and in male function by comparing hermaphrodites and canalized males. (2) Pollen and flower production, and plant mass of phenotypic males and hermaphrodites are compared to assess size effects on male function and potential size thresholds that allow investment in female function. (3) Costs of gender plasticity are assessed by examining responses of male traits (pollen production and flower diameter) to increasing female function (perfect flowers) in hermaphrodites and by comparing responses of pollen production to plant size in hermaphrodites and phenotypic males.
MATERIALS AND METHODS
Study species and sites
Wurmbea dioica (R.Br.) F.Muell. ssp. dioica and W. biglandulosa (R.Br.) T.D.Macfarl. ssp. biglandulosa (Colchicaceae) are winter-growing geophytes found in grassland or on granite outcrops in south-eastern Australia. Plants have a corm, an annual shoot with three leaves and bloom in spring, producing an erect cymose inflorescence with 1–10 flowers. Major pollinators are flies and bees. Tepals are white with a conspicuous purple nectary near the base. Fruit capsules produce up to 50 seeds (Macfarlane, 1987). These Wurmbea species are closely related, being placed in the same clade in a recent phylogenetic study of the genus (Case et al., 2008).
Populations of W. dioica are either sub-dioecious (phenotypic males, hermaphrodites and females coexist) or putatively dioecious (phenotypic males and females). Populations of W. biglandulosa are either cosexual (hermaphrodites only) or gynodioecious (hermaphrodites and females), but in all populations some phenotypic males are present (Barrett, 1992; Vaughton and Ramsey, 2002). In both species, the genetic mechanism of male sterility is unknown. Hermaphroditic plants produce varying proportions of perfect and staminate flowers and phenotypic males produce staminate flowers only. Females produce pistillate flowers only (Barrett, 1992; Ramsey and Vaughton, 2001; Vaughton and Ramsey, 2002).
Sub-dioecious W. dioica populations are found in eastern Victoria and New South Wales, whereas putatively dioecious populations are found in western areas of these states that experience lower rainfall. Cosexual W. biglandulosa populations are restricted to tableland sites in northern New South Wales and southern Queensland, whereas gynodioecious populations occur in drier areas in northern, central and southern New South Wales and eastern Victoria. Four populations were studied, one of each of the four sexual systems based on the frequencies of females, phenotypic males and hermaphrodites from field surveys. Populations were from Wyperfeld, Victoria [putatively dioecious W. dioica (hereafter, dioecious; 0·43 females, 0·57 phenotypic males, 0·0 hermaphrodites); 35°30'S, 142°00'E]; Wallan, Victoria [sub-dioecious W. dioica (0·45, 0·48, 0·07); 37°42'S, 144°59'E]; Goonoowigal, New South Wales [gynodioecious W. biglandulosa (0·19, 0·11, 0·70); 29°59'S, 150°52'E]; and Rocky River, New South Wales [cosexual W. biglandulosa (0·0, 0·05, 0·95); 30°36'S, 151°29'E]. With regard to habitat and average sex frequencies, these populations are representative of others with the same sexual systems that we have studied (e.g. Ramsey and Vaughton, 2001; Vaughton and Ramsey, 2002).
Gender plasticity of phenotypic males
We examined whether phenotypic males could produce perfect flowers (i.e. become hermaphrodites) when placed under two resource conditions. Methods follow those of Ramsey and Vaughton (2001), except here levels of water and mineral nutrients are manipulated. Phenotypic males were excavated from the dioecious (n = 66) and sub-dioecious (n = 100) W. dioica populations and the gynodioecious and cosexual W. biglandulosa populations (both n = 50) during flowering (July–September), they were planted in 350 cm3 pots containing a low nutrient soil mixture and the pots were placed in a glasshouse. The soil was kept moist and, when plants senesced in early summer (October–November), watering was reduced until April (autumn) the following year. Leaf and flower primordia are formed in autumn/early winter and expand in spring; treatments were started to coincide with this initiation.
For each population, plants were assigned haphazardly to either a high resource [100 mL of water week−1 + 10 g of slow-release, low phosphorus nutrient fertilizer pellets (Osmocote, Scotts, Maryville, OH, USA)] or a low resource (40 mL + 2 g) treatment; sample sizes were equal in each treatment. Treatments were repeated for 2 years, and as each plant bloomed it was scored as gender plastic if at least one perfect flower was produced, as evidenced by the presence of a pistil. All plants survived and bloomed at least once. All perfect flowers were hand cross-pollinated with pollen donors from the relevant population. This ensured that previously phenotypic males could produce seeds. In the three sexually dimorphic populations, 20 female plants were also excavated, planted, cultivated and monitored in the same fashion as the phenotypic males (n = 10 plants/treatment/population).
Phenotypic males vs. hermaphrodites: pollen production, flower number and plant mass
Here and elsewhere, the dioecious W. dioica population is not included because no hermaphrodites were found despite examining several thousand plants over large areas over a 3-year period. In the other populations (sub-dioecious W. dioica, gynodioecious and cosexual W. biglandulosa), pollen production of phenotypic males and hermaphrodites (n = 20 plants/sex morph/population) was assessed. Plants were selected haphazardly while walking transects through populations and were separated by at least 2 m. From the first flower of each plant, one undehisced anther was collected. Each anther was placed into a 1·5 mL Eppendorf tube, 200 µL of lactophenol–aniline blue was added and, after thorough mixing, the number of pollen grains in four replicate haemocytometer grids was counted (e.g. Ramsey and Vaughton, 2001). The mean of these replicates was used and multiplied by six (anthers/flower) to obtain pollen production per flower. For each population, pollen production of phenotypic males and hermaphrodites was compared, using one-way analysis of variance (ANOVAs). Data were ln-transformed to ensure homogeneity of variances and normality of residuals. JMP was used to analyse data (v. 5·01a; SAS Institute, 2002). Here and elsewhere, means (± s.e.) are presented.
The size of phenotypic males and hermaphrodites was assessed in the three populations (sub-dioecious W. dioica: n = 25 males, n = 37 hermaphrodites; gynodioecious and cosexual W. biglandulosa: both n = 30 males, n = 30 hermaphrodites). The total flower number per plant was counted, and the plants were excavated and each placed into a paper bag. Later, roots and corms were washed and dried on paper towels. Plants (roots, corms and leaves) were individually placed in paper bags, dried at 80 °C for about 72 h and then weighed. In all populations, corms accounted for approx. 80 % of plant mass. For each population, we assessed whether sex morph (male vs. hermaphrodite; fixed factor) influenced the number of flowers produced with generalized linear models (GLMs) using the glm function in R (R v. 2·12·2; R Development Core Team, 2011). We specified Poisson error structures with log-link functions; deviances were not overdispersed and χ2 values are given. Plant dry mass of the sex morphs was compared using one-way ANOVAs; data transformation was not required.
Effects of resources on production of perfect and total flowers
The effect of high and low resource treatments on flower production of the sex morphs from sub-dioecious W. dioica and gynodioecious and cosexual W. biglandulosa populations was examined. We were interested in whether (1) total flower production and perfect flower production by hermaphrodites from the three populations exhibited similar plasticity responses to varying resource conditions; and (2) SDP exists in female function between hermaphrodites and females (sub-dioecious W. dioica and gynodioecious W. biglandulosa) and in male function between hermaphrodites and phenotypic males (sub-dioecious W. dioica). In hermaphrodites, total flowers estimate investment in male function because all flowers produce pollen but not necessarily ovules, whereas perfect flowers estimate investment in female function because they produce ovules.
Plants were excavated from the three populations in August and they were potted and maintained on glasshouse benches as described above. As plants started to senesce, watering was reduced until May the following year when treatments were initiated. Plants were then assigned haphazardly to either high resources (100 mL of water week−1 + 10 g of Osmocote) or low resources (40 mL + 2 g). When plants bloomed about 3 months later, the total number of flowers produced on all plants and also the number of perfect flowers on hermaphrodites were counted.
To ensure males from the sub-dioecious W. dioica population were not gender plastic, 40 phenotypic males were assigned to each resource treatment. As plants flowered, phenotype was assessed as either male or hermaphrodite, and the latter were excluded; however, some males in the low resource treatment could have been gender plastic, but failed to produce perfect flowers (final n = 18 and 30 males in high and low treatments, respectively). In addition to these males, 20 females were assigned to each treatment, and 35 and 25 hermaphrodites were assigned to the high and low treatments, respectively (final n = 34 and 23, respectively). For the gynodioecious W. biglandulosa population, 20 hermaphrodites and 20 females were assigned to each resource treatment (n = 20 plants/sex morph/treatment). For the cosexual W. biglandulosa population, 40 hermaphrodites were assigned to each treatment (low resources, final n = 39).
For all analyses, GLMs were used with the glm function in R (R v. 2·12·2; R Development Core Team, 2011). We specified quasi-Poisson errors with log-link functions to correct for overdispersion and present F-tests as suggested by Crawley (2007). In two analyses, perfect flower production (female function) and total flower production (male function) by hermaphrodites from the three populations were compared, with population, resource treatment and their interaction as fixed factors. A significant interaction would indicate that plastic responses to resource conditions differ among hermaphrodites from different populations. In three analyses, we tested for SDP in female and male functions. In the sub-dioecious W. dioica population, perfect flower production and total flower production of hermaphrodites were compared with flower production of females and phenotypic males, respectively. Sex morph, resource treatment and their interaction were fixed factors; a significant interaction would indicate SDP. Similarly, in the gynodioecious W. biglandulosa population, perfect flower production of hermaphrodites and flower production of females were compared.
Costs of gender plasticity
Hermaphrodites were examined in the field to assess whether the number of perfect flowers affected two traits associated with male fitness: pollen production and flower diameter; larger flowers attract more pollinators and hasten pollen removal (Vaughton and Ramsey, 1998). In the three populations, we marked plants as first flowers opened, ensuring that they were hermaphrodites. From the first flower of every plant, one undehisced anther was collected, pollen production was estimated as described above and flower diameter (mean of two orthogonal measurements) was measured. On each plant the number of perfect flowers and total flowers was recorded. Final sample sizes were 147, 50 and 79 plants for the sub-dioecious W. dioica and gynodioecious and cosexual W. biglandulosa populations, respectively.
For the two male traits, the association with the number of perfect flowers per plant was assessed using multiple linear regressions; traits were dependent variables and the numbers of perfect and total flowers were independent variables. This allowed us to examine effects of perfect flower production on pollen production and flower diameter while holding constant any effects of total flower production. For each trait in the three populations, partial correlation coefficients and t-values calculated from the multiple linear regressions (Greene, 2003) are presented. Least-square means of traits, adjusted for total flower number, were calculated and these means were plotted against the number of perfect flowers per plant.
For the sub-dioecious W. dioica population, the relationship between the number of total flowers per plant and pollen production in the first flower of phenotypic males and hermaphrodites was also examined to assess how plant size affected male function. Total flowers reflect plant size as well as the investment in male function (correlation between flowers and vegetative mass: males, r2 = 0·49, P <0·001, n = 24; hermaphrodites, r2 = 0·45, P <0·001, n = 36). Pollen from phenotypic males (n = 120) was counted as described above and the hermaphrodites from the previous analyses were used (n = 147). To test for differences between regression coefficients of the relationships, an analysis of covariance (ANCOVA) was calculated, with sex morph (males vs. hermaphrodites), total flowers (covariate) and their interaction as factors. A significant interaction would demonstrate that the regression coefficients differ and that increasing flower production (plant size) affects pollen production differently in the sex morphs. Simple linear regressions were then computed for each sex morph to obtain linear equations. Untransformed data met statistical assumptions.
RESULTS
Gender plasticity of phenotypic males
Gender plasticity varied among the four populations. In the dioecious W. dioica population, no phenotypic males in the low and high resource treatments produced perfect flowers and thus did not express plasticity. In the sub-dioecious W. dioica population, some phenotypic males expressed plasticity under both resource treatments, but fewer produced perfect flowers under low than high resources [42 % vs. 66 %; binomial test to compare two proportions (Crawley, 2007), χ2 = 4·87, d.f. = 1, P1-tailed = 0·014]. In contrast, in both W. biglandulosa populations all phenotypic males were plastic and produced perfect flowers under both resource conditions. Following cross-pollinations, all plants with perfect flowers produced fruits with seeds. Female plants in the dimorphic populations remained male sterile.
Phenotypic males vs. hermaphrodites: pollen production, flower number and plant mass
In the sub-dioecious W. dioica population, phenotypic males produced twice as much pollen per flower as hermaphrodites (Fig. 1A; F1,38 = 9·70, P = 0·003), but plants were similar in size (Fig. 1B, flower number: χ2 = 0·27, d.f. = 1, P = 0·601; Fig. 1C, mass: F1,60 = 0·02, P = 0·883). In the W. biglandulosa populations phenotypic males produced 40 % less pollen than hermaphrodites (Fig. 1A; gynodioecious: F1,38 = 11·81, P = 0·001; cosexual: F1,38 = 18·85, P < 0·001). Phenotypic males were smaller than hermaphrodites (Fig. 1B, flower number: gynodioecious: χ2 = 4·50, d.f. = 1, P = 0·034; cosexual: χ2 = 12·04, d.f. = 1, P < 0·001; Fig. 1C, mass: gynodioecious: F1,58 = 19·36, P < 0·001; cosexual: F1,58 = 22·16, P < 0·001).
Fig. 1.
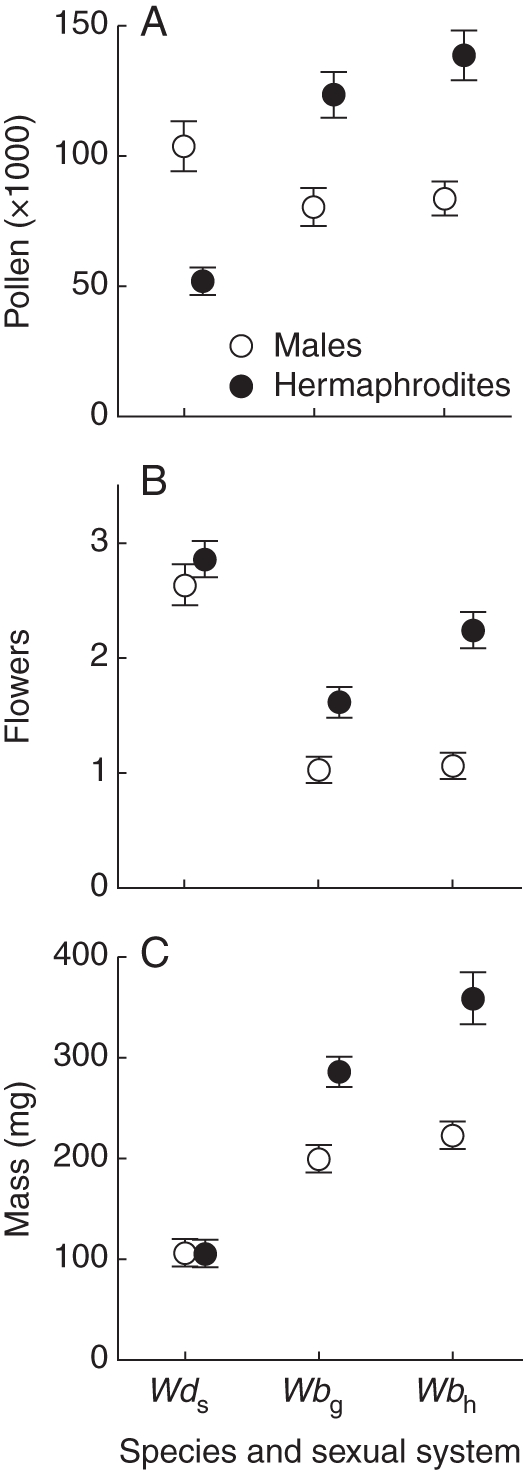
Pollen grain production (A), numbers of total flowers (B) and plant mass (C) for phenotypic males and hermaphrodites, as indicated, of sub-dioecious Wurmbea dioica (Wds), and gynodioecious (Wbg) and cosexual (Wbc) W. biglandulosa. Data are means (± s.e.).
Effects of resources on production of perfect and total flowers
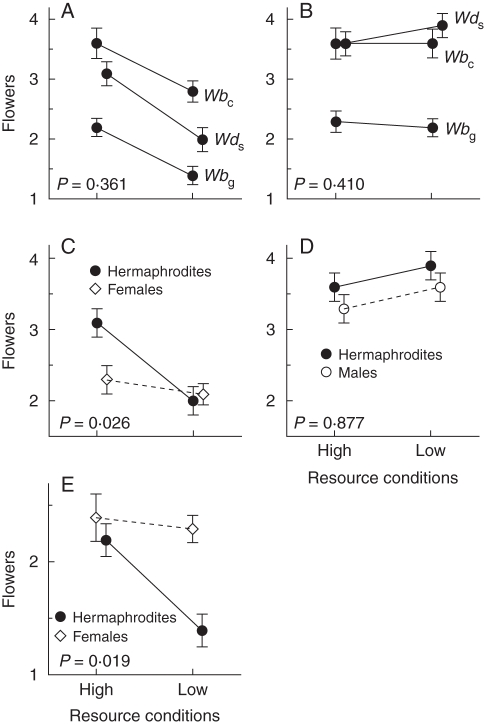
Flower production by hermaphrodites from the three populations responded similarly to changes in resources as indicated by non-significant population × resource treatment interactions (Table 1, Fig. 2A, B). Resource treatment and population both significantly affected perfect flower production. Fewer flowers were produced under low compared with high resources. Hermaphrodites from the cosexual W. biglandulosa population produced more flowers than those from the sub-dioecious W. dioica population, which in turn produced more than those from the gynodioecious W. biglandulosa population (Table 1, Fig. 2A). Population, but not resources, significantly affected total flower production. Hermaphrodites from the cosexual W. biglandulosa and sub-dioecious W. dioica populations produced more flowers than those from the gynodioecious W. biglandulosa population (Table 1, Fig. 2B).
Table 1.
F-ratios for generalized linear models comparing the number of perfect flowers or total flowers produced by plants grown under high and low resource conditions for sub-dioecious Wurmbea dioica (Wds), and gynodioecious (Wbg) and cosexual (Wbc) W. biglandulosa.
| Factors | A | B | Factors | C | D | E |
|---|---|---|---|---|---|---|
| Population | 21·62*** (2,170) | 23·71*** (2,170) | Sex morph | 13·83*** (1,93) | 2·21 (1,101) | 10·34** (1,76) |
| Resources | 21·66*** (1,170) | 0·10 (1,170) | Resources | 1·87 (1,93) | 2·41 (1,101) | 12·52*** (1,76) |
| Population × resorces | 1·02 (2,170) | 0·90 (2,170) | Sex × resources | 5·13* (1,93) | 0·10 (1,101) | 5·74* (1,76) |
A and B are two-factor analyses with population, resource treatment and their interaction as fixed factors. A and B assess perfect flower (female function) and total flower (male function) production, respectively, of hermaphrodites from the three populations in response to resources. C, D and E are two-factor analyses with sex morph, resource treatment and their interaction as fixed factors. C and D assess female function of hermaphrodites and females, and male function of hermaphrodites and phenotypic males from Wds, respectively. E assesses female function of hermaphrodites and females from Wbg. Numerator and denominator degrees of freedom are given in parentheses. ***P < 0·001, **P < 0·01, *P ≤ 0·026. Analyses correspond to data presented in Fig. 2.
Fig. 2.
Numbers of flowers under high and low resource conditions for sub-dioecious Wurmbea dioica (Wds), and gynodioecious (Wbg) and cosexual (Wbc) W. biglandulosa. (A) and (B) compare perfect flower and total flower production, respectively, by hermaphrodites for Wds, Wbg and Wbc. For (A) and (B), P-values are for population × resource interactions, testing whether hermaphrodites respond similarly to resource conditions. For Wds, (C) compares production of flowers with female function by hermaphrodites and females, as indicated, and (D) compares production of flowers with male function by hermaphrodites and phenotypic males, as indicated. For Wbg, (E) compares perfect flower production by hermaphrodites and females. For (C–E), P-values are for sex morph × resource interactions, testing sex-differential plasticity of hermaphrodites and unisexuals. Data are means (± s.e.). Analyses for each figure are given in Table 1.
SDP for female function was detected in the sub-dioecious W. dioica and gynodioecious W. biglandulosa populations, as indicated by significant sex morph × resource treatment interactions (Table 1, Fig. 2C, E). Production of perfect flowers (female function) by hermaphrodites decreased under low resources, but flower production by females was unaffected. In contrast, SDP for male function was not detected in the sub-dioecious W. dioica population, and total flower production by hermaphrodites and phenotypic males was similar under both high and low resources (Table 1, Fig. 2D).
Cost of gender plasticity
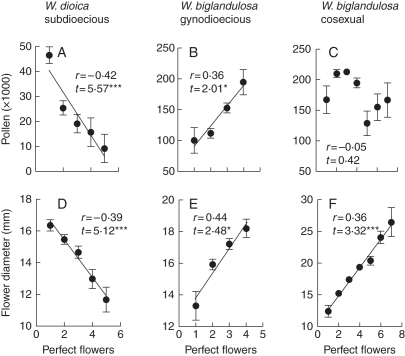
The relationships between the number of perfect flowers per plant and the male traits in hermaphrodites varied among the sexual systems (Fig. 3). For sub-dioecious W. dioica, pollen production and flower diameter were negatively correlated with perfect flowers (Fig. 3A, D). In contrast, for gynodioecious W. biglandulosa, both traits were positively correlated with perfect flowers (Fig. 3B, E). For cosexual W. biglandulosa, flower diameter was positively correlated, but pollen production was not correlated, with perfect flowers (Fig. 3C, F).
Fig. 3.
Number of perfect flowers vs. pollen production (A–C) and flower diameter (D–F) for hermaphrodites of sub-dioecious Wurmbea dioica (Wds), and gynodioecious (Wbg) and cosexual (Wbc) W. biglandulosa. Data are means (± s.e.), adjusted for total flower number per plant. Partial correlation coefficients (r) and t-values were calculated from multiple linear regressions with perfect and total flowers per plant as independent variables (Wds, Wbg and Wbc d.f. = 147, 47 and 76, respectively). ***P < 0·001, *P < 0·05.
For the sub-dioecious W. dioica population, the sex morph × covariate (total flowers) interaction of the ANCOVA comparing pollen production by phenotypic males and hermaphrodites in the first flower was significant (F1,263 = 12·19, P <0·001). This indicates that regression coefficients for the relationship between pollen production and total flower production differed between the sex morphs (Fig. 4). For males, the relationship was significant (F 1,118 = 23·71, P < 0·001), and for every additional flower produced per plant, the first flower produced about 12 000 additional pollen grains. In contrast, pollen production in hermaphrodites did not respond to increasing flower number (F1,145 = 0·52, P = 0·471; Fig. 4).
Fig. 4.
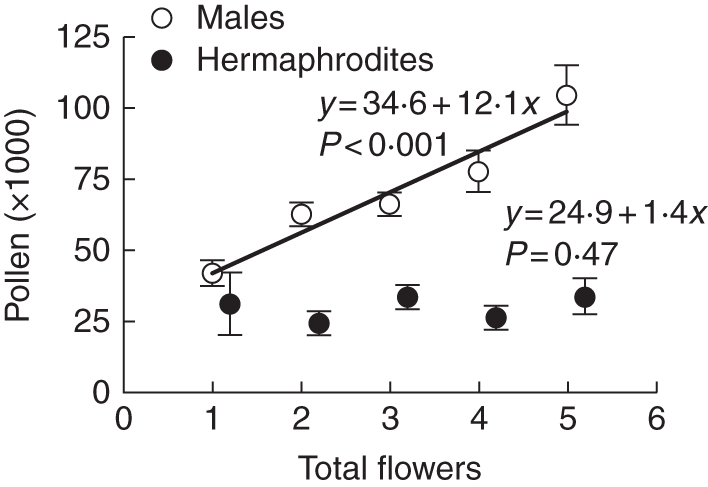
Linear regressions of the number of total flowers vs. pollen production for males (n = 120) and hermaphrodites (n = 147), as indicated, of sub-dioecious Wurmbea dioica. Means (± s.e.) and equations of regression lines are given. Coefficients for the intercept and slope have been changed to be consistent with the scale of the y-axis and require multiplication by 1000.
DISCUSSION
Our results highlight the influence of gender plasticity mediated by the environment on sex expression of hermaphrodites in W. dioica and W. biglandulosa. In response to shifts in resources, hermaphrodites adjusted numbers of perfect flowers (female function), but not total flowers (male function). Consistent with the SDP hypothesis, perfect flower production by hermaphrodites was more variable than flower production by females. In contrast, total flower production by hermaphrodites was the same as that of canalized males. Costs of plasticity, expressed as negative correlations between perfect flower production and pollen production per flower and flower size, were not universal, indicating that their role in selection for canalized males would vary among the sexual systems. Below we discuss each of these findings and their implications for sexual system evolution and stability in these Wurmbea species.
Phenotypic male and hermaphrodite plasticity
Relocation of phenotypic males from the field to the glasshouse and manipulation of resource conditions demonstrated the importance of resource availability on female investment, but also showed that gender plasticity varied among the populations with contrasting sexual systems. At one extreme, all phenotypic males from the cosexual and gynodioecious W. biglandulosa populations were gender plastic and produced perfect flowers in the glasshouse, regardless of resource conditions. At the other extreme, in the dioecious W. dioica population phenotypic males exhibited no evidence of gender plasticity. This and other putatively dioecious populations that we have surveyed are found in areas of much lower rainfall than sub-dioecious W. dioica populations (see also Barrett, 1992). Phenotypic males in dioecious populations are probably canalized males, reflecting the effect of differentiated sex chromosomes (Charlesworth and Guttman, 1999).
The sub-dioecious W. dioica population showed a mixed response, with 66 % of phenotypic males producing perfect flowers under high resource conditions and only 42 % under low resource conditions. The remaining plants were invariant and were either canalized males or gender-plastic males with a very high resource threshold for the initiation of female function (see also Barrett et al., 1999; Ramsey and Vaughton, 2001). In sub-dioecious Fragaria virginiana, populations also comprise males that are either plastic or canalized in their sex expression (Spigler and Ashman, 2011). In this species the mechanism of genetic control involves ‘proto-sex’ chromosomes with occasional recombination occurring between two linked loci that carry either male or female sterility mutations (Spigler et al., 2008, 2010). Whether sex determination in sub-dioecious W. dioica involves ‘proto-sex’ chromosomes remains to be established.
In both cosexual and gynodioecious W. biglandulosa populations, the expression of male and female function was size dependent. Phenotypic males weighed less and produced fewer flowers and less pollen per flower than hermaphrodites, indicating a size or resource threshold that must be reached for plants to express both sexual functions (Lloyd and Bawa, 1984). Under natural conditions, most plants readily exceed this threshold because phenotypic males occur at low frequencies in populations (<13 %; Vaughton and Ramsey, 2002). In contrast, in the sub-dioecious W. dioica population, plant size and flower production did not differ between phenotypic males and hermaphrodites, indicating a higher threshold to initiate female function than W. biglandulosa. Interestingly, phenotypic males in the sub-dioecious W. dioica population produced almost twice as much pollen per flower as hermaphrodites, consistent with a resource trade-off between male and female functions. These results could be explained if pistil reduction genetic modifiers occur in sub-dioecious W. dioica and function to increase a resource threshold required to initiate perfect flower production (Delph and Lloyd, 1991). Plants with a high threshold should have more available resources to invest in pollen. The operation of genetic modifiers is consistent with our observation of variable levels of plasticity under high and low resource conditions in the sub-dioecious W. dioica population compared with the cosexual and gynodioecious W. biglandulosa populations. Further, genetic modifiers and differences in resource availability could account for the substantially lower frequency of hermaphrodites in natural populations (approx. 7 %) than we obtained in the glasshouse (Barrett et al., 1999; Ramsey and Vaughton, 2001). Overall, differences in the frequencies of gender-plastic phenotypic males among the W. dioica and W. biglandulosa populations provide evidence for selection against plasticity in favour of male-biased allocation and male canalization as female frequencies increase in populations (Charlesworth, 1989).
Despite the observed variation in the frequency of gender-plastic phenotypic males, changes in resource availability elicited similar responses in hermaphrodites from the sub-dioecious W. dioica and gynodioecious and cosexual W. biglandulosa populations. Hermaphrodites in these populations reduced their production of perfect flowers (female function), but not total flower production (male function), as resources decreased. Whether this plasticity in female function represents a direct response to resource variation or is mediated indirectly through changes in plant size with resource availability in unknown (e.g. Bishop et al., 2010). Nevertheless, our results are consistent with sex allocation theory, which predicts that individuals should invest more in female function than in male function as resource availability increases. These predictions arise because resource costs of producing seeds are greater than those of producing pollen, and male fitness, but not female fitness, is expected to decelerate with increasing plant size (Klinkhamer et al., 1997; Zhang and Jiang, 2002).
Sex-differential plasticity
Consistent with the SDP hypothesis, production of perfect flowers by hermaphrodites was reduced more than flower production by females under low compared with high resource conditions in both the gynodioecious W. biglandulosa and sub-diocecious W. dioica populations. Our finding of SDP in female function concurs with other studies that have experimentally investigated sex-based differences in plasticity (Delph, 1990b; Barr, 2004; Dorken and Mitchard, 2008; Spigler and Ashman, 2011; but see Case and Ashman, 2007). These studies have also shown that SDP can translate into a >2-fold seed fertility advantage to females under low resource conditions, whereas under high resource conditions sex-based differences in fertility are minimal (Barr, 2004; Dorken and Mitchard, 2008; Spigler and Ashman, 2011). We did not examine seed production in our study. However, the SDP that we observed could provide a mechanism accounting, at least in part, for the variation in relative seed fertility of hermaphrodites observed among natural populations in both sub-dioecious W. dioica and gynodioecious W. biglandulosa (Case and Barrett, 2004a; Vaughton and Ramsey, 2004). Gender plasticity, therefore, could play a role in maintaining females in sexually dimorphic populations of Wurmbea, along with inbreeding avoidance, which has also been shown to be important (Ramsey et al., 2006a, b). In contrast to SDP in female function, we found no evidence of SDP in male function in the sub-dioecious W. dioica population, at least as evidenced by the production of total flowers by hermaphrodites and canalized males. Thus, gender plasticity does not appear to be a factor affecting relative male function of canalized males and hermaphrodites, although the effect of resource availability on pollen production per flower remains to be examined.
The SDP hypothesis does not make predictions concerning the relative investment in female and male function by hermaphrodites in cosexual populations. Nevertheless, in W. biglandulosa low resources elicited a response in hermaphrodites from the cosexual population similar to that in the two sexually dimorphic populations. Further, plasticity of hermaphrodites in the cosexual population was greater than that of females in the gynodioecious population. Assuming that females in the gynodioecious population are a reasonable proxy for a newly arisen male-sterile mutant, this SDP could indicate the potential of gender plasticity to mediate the invasion of females into cosexual populations. However, whether gender plasticity exhibited by hermaphrodites in the cosexual population represents an ancestral condition is unknown. Possibly this cosexual population represents a reversion from gynodioecy (e.g. Case et al., 2008). If ancestral gynodioecious populations experienced more favourable resource conditions over prolonged periods, then females might not achieve the required fertility advantage to be maintained.
In the cosexual population of W. biglandulosa, hermaphrodite plasticity combined with the tendency of small plants to produce only staminate flowers creates a male-biased floral sex ratio that low resource conditions exacerbate. This would provide an advantage to newly arisen females because they accrue fitness by contributing through the under-represented sex role in populations, thereby increasing their likelihood of establishing (Sarkissian et al., 2001). Given current climatic conditions, however, cosexual populations of W. biglandulosa appear to be resistant to female invasion. Our previous work has shown that hermaphrodites in cosexual populations are highly fecund and outcrossed (outcrossing rate, t = 0·97). Also experimentally emasculated cosexuals and intact cosexuals did not differ in seed production, indicating that newly arisen females are unlikely to achieve the necessary seed fertility advantage to establish (Ramsey et al., 2006a). However, if climatic conditions became drier, then gender plasticity, coupled with changes in pollination and plant mating, could cause a decline in the seed fertility of hermaphrodites, increasing the likelihood that females would establish.
Costs of plasticity and sexual system stability
Costs of plasticity in hermaphrodites were assessed indirectly by examining responses of pollen production per flower and flower diameter to increasing perfect flower production. It was found that costs of plasticity varied across sexual systems, but we recognize that our data are based on single populations of each sexual system and multiple populations would be required to test this proposal rigorously. Nevertheless, in the gynodioecious W. biglandulosa population costs of plasticity on male investment were not apparent. In fact, pollen production per flower and flower diameter were both positively correlated with the number of perfect flowers per plant. This result may be explained if plants with more perfect flowers were better resourced, allowing investment in both sexual functions, while avoiding male and female trade-offs (van Noordwijk and de Jong, 1986). In the absence of plasticity costs, we could expect the retention of female function by hermaphrodites to be favoured. Further, if increasing plant size diminishes the ability to sire seeds, then hermaphrodites with a capacity for female function could be maintained over more canalized types with increased pollen or flower production (Klinkhamer et al., 1997). Advantages of female function, however, may be minimal in gynodioecious W. biglanduosa. Unlike cosexual populations, hermaphrodites in gynodioecious populations experience low outcrossing (t = 0·68) and severe inbreeding depression, which eliminates selfed progeny (δ approx. 0·93; Ramsey et al., 2006a).
In the sub-dioecious W. dioica population, plasticity was costly, as evidenced by negative relationships between the number of perfect flowers and both pollen production per flower and flower diameter. Further, it was found that hermaphrodites produced less pollen per flower than phenotypic males in response to total flower production, an indicator of plant size as well as overall male function. Phenotypic males, therefore, could have a pollen production advantage compared with hermaphrodites, and this advantage would be expected to increase in high resource environments when hermaphrodites produce more perfect flowers. Studies that manipulate resources under controlled conditions could provide support for these ideas, particularly if relative pollen fertility of the sex morphs is environment dependent. Costs of plasticity on pollen production have been reported in sub-dioecious F. virginiana. Hermaphrodites with high fruit set (i.e. more plastic) produced less pollen than less plastic individuals (Ashman, 2006). Our study cannot address whether costs to male function are also manifested when hermaphrodites do not initiate female function. However, if such intrinsic costs of plasticity occur, then hermaphrodites would be further disadvantaged relative to more canalized male genotypes.
For gender plasticity to be adaptive in the presence of plasticity costs, fitness gains through seeds must outweigh losses through pollen. Recent modelling has identified scenarios whereby plastic hermaphrodites are maintained in sub-dioecious populations (Ehlers and Bataillon, 2007). For example, female fitness of hermaphrodites may be increased through reproductive assurance in metapopulations, if seed production allows unoccupied habitat to be colonized (Pannell et al., 2008). In W. dioica, however, fitness gains by hermaphrodites through seeds are minimal because low outcrossing rates (t = 0·36) coupled with severe inbreeding depression (δ approx. 0·95) greatly reduce hermaphrodite seed fitness (Ramsey et al., 2006b). Fitness gains through seeds, therefore, are unlikely to outweigh losses through pollen caused by trade-offs between female and male functions. Based on our previous and present studies, we suggest that sub-dioecy is unstable and that hermaphrodites are likely to be eliminated in favour of canalized males in sub-dioecious W. dioica populations.
Conclusions: implications of SDP for sexual system stability
Our findings indicate that gender plasticity mediated by environmental conditions plays a role in sexual system evolution and stability in Wurmbea. In W. biglandulosa, SDP, through its influence on the relative female function of hermaphrodites and females, contributes to stabilizing gynodioecy in low resource environments. In contrast, because SDP is minimized in high resource environments the cosexual system is stable. Further, in such environments gynodioecy could revert to a cosexual system, providing that the pollination environment ensures outcrossing. In sub-dioecious W. diocia, the presence of SDP in female function would maintain high female frequencies. Despite the absence of SDP in male function, sub-dioecy is unlikely to be stable because costs of plasticity coupled with low outcrossing rates would favour less plastic hermaphrodites or canalized males, leading to the transition to dioecy. Surprisingly, our findings indicate that high resource environments could exacerbate costs. As hermaphrodites increase female function, they decrease male function, but the increased female function fails to compensate for the reduced male function because most seeds are selfed and do not survive. Under prolonged low resource conditions, female function would be continuously suppressed and loss-of-function mutations could eventually result in female sterility. This would explain the lack of gender plasticity in the dioecious W. dioica population.
ACKNOWLEGEMENTS
We thank Jeff Karron for the opportunity to contribute to this issue. Three anonymous reviewers made welcome comments on earlier drafts of the manuscript. We thank Richard Willis for glasshouse assistance, and Stuart Cairns for statistical advice.
LITERATURE CITED
- Alonso C, Mutikainen P, Herrera CM. Ecological context of breeding system variation: sex, size and pollination in a (predominantly) gynodioecious shrub. Annals of Botany. 2007;100:1547–1556. doi: 10.1093/aob/mcm254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman T-L. The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology. 2002;83:1175–1184. [Google Scholar]
- Ashman T-L. The evolution of separate sexes: a focus on the ecological context. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 204–222. [Google Scholar]
- Barr CM. Soil moisture and sex ratio in a plant with nuclear–cytoplasmic sex inheritance. Proceedings of the Royal Society B: Biological Sciences. 2004;271:1935–1939. doi: 10.1098/rspb.2004.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH. Gender variation and the evolution of dioecy in Wurmbea dioica (Liliaceae) Journal of Evolutionary Biology. 1992;5:423–444. [Google Scholar]
- Barrett SCH, Case AL. The ecology and evolution of gender strategies in plants: the example of Australian Wurmbea (Colchicaceae) Australian Journal of Botany. 2006;54:417–433. [Google Scholar]
- Barrett SCH, Case AL, Peters GB. Gender modification and resource allocation in subdioecious Wurmbea dioica (Colchicaceae) Journal of Ecology. 1999;87:123–137. [Google Scholar]
- Bishop EJ, Spigler RB, Ashman T-L. Sex-allocation plasticity in hermaphrodites of sexually dimorphic Fragaria virginiana (Rosaceae) Botany. 2010;88:231–240. [Google Scholar]
- Case A, Barrett SCH. Environmental stress and the evolution of dioecy: Wurmbea dioica (Colchicaceae)) in Western Australia. Evolutionary Ecology. 2004a;18:145–164. [Google Scholar]
- Case A, Barrett SCH. Floral biology of gender monomorphism and dimorphism in Wurmbea dioica (Colchicaceae) in Western Australia. International Journal of Plant Sciences. 2004b;165:289–301. [Google Scholar]
- Case AL, Ashman T-L. An experimental test of the effects of resources and sex ratio on maternal fitness and phenotypic selection in gynodioecious Fragaria virginiana. Evolution. 2007;61:1900–1911. doi: 10.1111/j.1558-5646.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- Case AL, Graham SW, Macfarlane TD, Barrett SCH. A phylogenetic study of evolutionary transitions in sexual systems in Australasian Wurmbea (Colchicaceae) International Journal of Plant Sciences. 2008;169:141–156. [Google Scholar]
- Charlesworth B, Charlesworth D. A model for the evolution of dioecy and gynodioecy. American Naturalist. 1978;112:975–997. [Google Scholar]
- Charlesworth D. Allocation to male and female function in hermaphrodites, in sexually polymorphic populations. Journal of Theoretical Biology. 1989;139:327–342. [Google Scholar]
- Charlesworth D, Guttman DS. The evolution of dioecy and plant sex chromosome systems. In: Ainsworth CC, editor. Sex determination in plants. Oxford: BIOS Scientific Publishers; 1999. pp. 25–49. [Google Scholar]
- Crawley MJ. The R book. Chichester, UK: John Wiley and Sons; 2007. [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- Delph LF. Sex-ratio variation in the gynodioecious shrub Hebe strictissima (Scrophulariaceae) Evolution. 1990a;44:134–142. doi: 10.1111/j.1558-5646.1990.tb04284.x. [DOI] [PubMed] [Google Scholar]
- Delph LF. Sex-differential resource allocation patterns in the subdioecious shrub Hebe subalpina. Ecology. 1990b;71:1342–1351. [Google Scholar]
- Delph LF. Sexual dimorphism in gender plasticity and its consequences for breeding system evolution. Evolution and Development. 2003;5:31–39. doi: 10.1046/j.1525-142x.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- Delph LF, Lloyd DG. Environmental and genetic control of gender in the dimorphic shrub Hebe subalpina. Evolution. 1991;45:1957–1964. doi: 10.1111/j.1558-5646.1991.tb02701.x. [DOI] [PubMed] [Google Scholar]
- Delph LF, Wolfe DE. Evolutionary consequences of gender plasticity in genetically dimorphic breeding systems. New Phytologist. 2005;166:119–128. doi: 10.1111/j.1469-8137.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- Dorken ME, Mitchard ETA. Phenotypic plasticity of hermaphrodite sex allocation promotes the evolution of separate sexes: an experimental test of the sex-differential plasticity hypothesis using Sagittaria latifolia (Alismataceae) Evolution. 2008;62:971–978. doi: 10.1111/j.1558-5646.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- Ehlers BK, Bataillon T. ‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New Phytologist. 2007;174:194–211. doi: 10.1111/j.1469-8137.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- Greene WH. Econometric analysis. 5th edn. Upper Saddle River, NJ: Prentice Hall; 2003. [Google Scholar]
- Klinkhamer PGL, de Jong TJ, Metz H. Sex and size in cosexual plants. Trends in Ecology and Evolution. 1997;12:260–265. doi: 10.1016/s0169-5347(97)01078-1. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. The transmission of genes via pollen and ovules in gynodioecious angiosperms. Theoretical Population Biology. 1976;9:299–316. doi: 10.1016/0040-5809(76)90050-2. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Bawa KS. Modification of the gender of seed plants in varying conditions. Evolutionary Biology. 1984;17:255–338. [Google Scholar]
- Lopez-Villavicencio M, Branca A, Giraud T, Shyoff JA. Sex-specific effect of Microbotryum violaceum (Uredinales) spores on healthy plants of the gynodioecious Gypsophila repens (Caryophyllaceae) American Journal of Botany. 2005;92:896–900. doi: 10.3732/ajb.92.5.896. [DOI] [PubMed] [Google Scholar]
- Macfarlane TD. Wurmbea (Liliaceae) Flora of Australia. 1987;45:387–405. [Google Scholar]
- McCauley DE, Brock MT. Frequency-dependent fitness in Silene vulgaris, a gynodioecious plant. Evolution. 1998;52:30–36. doi: 10.1111/j.1558-5646.1998.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Pannell JR, Dorken ME, Pujol B, Berjano R. Gender variation and transitions between sexual systems in Mercurialis annua (Euphorbiaceae) International Journal of Plant Sciences. 2008;169:129–139. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Ramsey M, Vaughton G. Sex expression and sexual dimorphism in subdioecious Wurmbea dioica (Colchicaceae) International Journal of Plant Sciences. 2001;162:589–597. [Google Scholar]
- Ramsey M, Vaughton G, Peakall R. Inbreeding avoidance and the evolution of gender dimorphism in Wurmbea biglandulosa (Colchicaceae) Evolution. 2006a;60:529–537. [PubMed] [Google Scholar]
- Ramsey M, Vaughton G, Peakall R. Does inbreeding avoidance maintain gender dimorphism in Wurmbea dioica (Colchicaceae)? Journal of Evolutionary Biology. 2006b;19:1497–1506. doi: 10.1111/j.1420-9101.2006.01129.x. [DOI] [PubMed] [Google Scholar]
- Renner SS, Ricklefs RE. Dioecy and its correlates in the flowering plants. American Journal of Botany. 1995;82:596–606. [Google Scholar]
- Sakai AK, Weller SG. Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer; 1999. pp. 1–31. [Google Scholar]
- SAS Institute. JMP. Ver. 5·01a. Cary, NC: SAS Institute; 2002. [Google Scholar]
- Sarkissian TS, Barrett SCH, Harder LH. Gender variation in Sagittaria latifolia (Alismataceae): is size all that matters? Ecology. 2001;82:360–373. [Google Scholar]
- Spigler RB, Ashman T-L. Sex ratio and subdioecy in Fragaria virginiana: the roles of plasticity and gene flow examined. New Phytologist. 2011;190:1058–1068. doi: 10.1111/j.1469-8137.2011.03657.x. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Lewers KS, Main DS, Ashman T-L. Genetic mapping of sex determination in a wild strawberry, Fragaria virginiana, reveals earliest form of sex chromosome. Heredity. 2008;101:507–517. doi: 10.1038/hdy.2008.100. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Lewers K, Johnson A, Ashman T-L. Comparative mapping reveals autosomal origin of sex chromosome in octoploid Fragaria virginiana. Journal of Heredity. 2010;101 (Suppl. 1):S107–S117. doi: 10.1093/jhered/esq001. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Chang S-M. Effects of environmental heterogeneity on the distribution of sexes within and among populations in a gynodioecious species. Geranium maculatum. New Phytologist. 2009;183:649–660. doi: 10.1111/j.1469-8137.2009.02940.x. [DOI] [PubMed] [Google Scholar]
- Van Noordwijk AJ, de Jong G. Acquisition and allocation of resources: their influence on variation in life history tactics. American Naturalist. 1986;128:137–142. [Google Scholar]
- Vaughton G, Ramsey M. Floral display, pollinator visitation and reproductive success in the dioecious perennial herb Wurmbea dioica (Liliaceae) Oecologia. 1998;115:93–101. doi: 10.1007/s004420050495. [DOI] [PubMed] [Google Scholar]
- Vaughton G, Ramsey M. Evidence of gynodioecy and sex ratio variation in Wurmbea biglandulosa (Colchicaceae) Plant Systematics and Evolution. 2002;232:167–179. [Google Scholar]
- Vaughton G, Ramsey M. Dry environments promote the establishment of females in monomorphic populations of Wurmbea biglandulosa (Colchicaceae) Evolutionary Ecology. 2004;18:323–341. [Google Scholar]
- Weiblen GD, Oyama RK, Donoghue MJ. Phylogenetic analysis of dioecy in monocotyledons. American Naturalist. 2000;155:46–58. doi: 10.1086/303303. [DOI] [PubMed] [Google Scholar]
- Zhang D-Y, Jiang X-H. Size-dependent resource and sex allocation in herbaceous perennial plants. Journal of Evolutionary Biology. 2002;15:74–83. [Google Scholar]