Abstract
Background
The ‘gynodioecy–dioecy pathway’ is considered to be one of the most important evolutionary routes from hermaphroditism to separate sexes (dioecy). Despite a large accumulation of evidence for female seed fertility advantages in gynodioecious species (females and hermaphrodites coexist) in support of the first step in the gynodioecy–dioecy pathway, we still have very little evidence for the second step, i.e. the transition from gynodioecy to dioecy.
Scope
We review the literature to evaluate whether basic predictions by theory are supported. To establish whether females' seed fertility advantage and frequencies are sufficient to favour the invasion of males, we review these for species along the gynodioecy–dioecy pathway published in the last 5 years. We then review the empirical evidence for predictions deriving from the second step, i.e. hermaphrodites' male fertility increases with female frequency, selection favours greater male fertility in hermaphrodites in gynodioecious species, and, where males and hermaphrodites coexist with females (subdioecy), males have greater male fertility than hermaphrodites. We review how genetic control and certain ecological features (pollen limitation, selfing, plasticity in sex expression and antagonists) influence the trajectory of a population along the gynodioecy–dioecy pathway.
Conclusions
Females tend to have greater seed fertility advantages over hermaphrodites where the two coexist, and this advantage is positively correlated with female frequency across species, as predicted by theory. A limited number of studies in subdioecious species have demonstrated that males have an advantage over hermaphrodites, as also predicted by theory. However, less evidence exists for phenotypic selection to increase male traits of hermaphrodites or for increasing male function of hermaphrodites in populations with high female frequency. A few key case studies underline the importance of examining multiple components of male fertility and the roles of pollen limitation, selfing and plasticity, when evaluating advantages. We conclude that we do not yet have a full understanding of the transition from gynodioecy to dioecy.
Keywords: Breeding system, dioecy, gynodioecy, male fertility, pollen limitation, plasticity, sex ratio, sexual systems, subdioecy, trioecy
INTRODUCTION
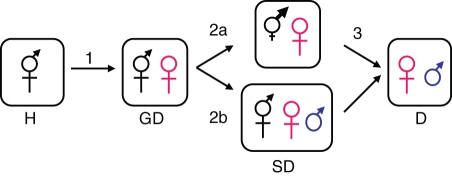
While separate sexes are thought of as the rule in the animal world, they are the exception in flowering plants. Only 6 % of angiosperm species have completely separate sexes (males and females, i.e. dioecy), but dioecy exists in approximately 38 % of flowering families where it has arisen independently many times, underlining this evolutionary transition as a fundamental one in flowering plants (Renner and Ricklefs, 1995). There are five recognized pathways by which dioecy can arise from hermaphroditism: (1) directly, (2) via gynodioecy, (3) via androdioecy, (4) via monoecy and (5) via heterostyly (Bawa, 1980; Webb, 1999; Torices et al., 2011). An important pathway that has received perhaps the most attention, and is the focus of this review, is the ‘gynodioecy–dioecy’ pathway (hereafter, ‘G-D pathway’) (e.g. Charlesworth and Charlesworth, 1978; Ross, 1978; Barrett, 1992; Webb, 1999; Weiblen et al., 2000; Ehlers and Bataillon, 2007; but see Renner and Rickleffs, 1995, for a greater role of monoecy). Stated briefly and in general terms (based on Charlesworth and Charlesworth, 1978), the G-D pathway can be broken down into three transitional phases (Fig. 1). In the first phase, a male sterility mutation arises in a hermaphroditic population. In order for male-sterile individuals or ‘females’ to successfully establish in the population, they must achieve a seed-fertility advantage over the resident hermaphrodites, the magnitude of which will depend, in part, on the underlying genetic control of male sterility (Lewis, 1941; Lloyd, 1974, 1975; Charlesworth and Charlesworth, 1978). Once females are maintained alongside hermaphrodites (the population is considered gynodioecious), their very presence leads to hermaphrodites gaining more of their fitness through male function than female function (Lloyd, 1976; Charlesworth, 1989). Because of the feedback between the relative fertility of the sexes and sex ratio in the population, female frequency is related to their seed advantage, and hermaphrodites experience greater selective pressure to invest more in male function where females contribute the most to population seed production. This situation sets the stage for the second phase of the G-D pathway, wherein a full (or several partial) female-sterility mutation(s) invades the gynodioecious population. If this mutation also increases male function sufficiently to compensate for loss of female function then these new ‘males’ will successfully establish and the population is considered subdioecious. We note that the distinction between ‘male’ and a ‘hermaphrodite’ may be blurry at first as these pollen-bearing individuals will probably express variability in residual female function, and in cases with partial female-sterility alleles, hermaphrodites may gradually become male-biased (Fig. 1, steps 2a vs. 2b) (e.g. Ross, 1977, 1982). For the purposes of this review, we define a ‘male’ as a pollen-bearing individual that has a genetic reduction in female function as a result of a single full- or multiple partial-female sterility mutations such that it is entirely or almost entirely female-sterile. The latter case may occur if selection does not act to completely eliminate infrequent fruit production (Charlesworth, 1989). In the final transition from subdioecy to dioecy (Fig. 1, step 3), hermaphrodites are lost from the population as they are supplanted by males.
Fig. 1.
Diagram illustrating the basic stages of the gynodioecy–dioecy (G-D) pathway. The first transition involves the invasion of a male-sterile individual (female) into an hermaphroditic (‘H’) population. If females successfully establish, the population can be said to be gynodioecious (‘GD’) (step 1). The presence of females places selective pressure on hermaphrodites to invest in male function at the cost of female function, and males invade the population (step 2). Males may invade either via gradual reductions in female fertility and increases in male fertility (2a) or via major mutations influencing male and female fertility (2b). These populations are subdioecious (‘SD’). In the final transition, pure males supplant hermaphrodites such that the system is completely dioecious (‘D’) (step 3) (based on Charlesworth and Charlesworth, 1978; Charlesworth & Gutman, 1999).
Whereas the first step in the G-D pathway may be discrete – individuals are either female, i.e. completely male-sterile, or hermaphroditic, i.e. male- and female-fertile – the later steps are less clear (Lloyd, 1976; Ross, 1982). Perhaps it is for this reason that we have much empirical evidence of the seed fertility of females relative to hermaphrodites in gynodioecious populations and the genetic and ecological factors influencing it (e.g. reviewed in Webb, 1999; Jacobs and Wade, 2003; Shykoff et al., 2003; Bailey and Delph, 2007; Delph et al., 2007; McCauley and Olson, 2008; McCauley and Bailey, 2009; Dufay and Billard 2011), but have comparatively little evidence documenting latter stages in the G-D pathway. Thus, rather than provide a comprehensive review on the entire G-D pathway, here we review the literature to identify whether fundamental predictions are met for the later stages of the G-D pathway. Specifically, we ask the following questions. (1) Are conditions for selection for males met? That is, are females found in populations at high frequencies and with high seed fertility advantages? (2) Do hermaphrodites have greater male fertility where females are more common (gynodioecious populations) or do males have greater male fertility than hermaphrodites (subdioecious populations)? (3) Is there evidence of selection for increased male function in hermaphrodites or selection favouring males over hermaphrodites? In addition, we consider the factors that may influence the final transition to dioecy, or conversely, the conditions under which hermaphrodites are likely to be maintained in subdioecious populations. To do so we first touch on the role of genetics of sex determination, and then focus on pollen limitation, selfing, plasticity and plant antagonists (e.g. herbivores) using theoretical models and empirical studies as our foundation. Together, this work highlights existing evidence and sheds light on the complexities that may retard the transition to dioecy. We identify approaches to study aspects that remain unknown and recommend that efforts shift emphasis from what maintains and regulates females in gynodioecious populations to understanding the selective pressures that favour increased maleness and the factors that regulate male frequencies in subdioecious populations in order to understand the complete G-D pathway.
FEMALE FERTILITY AND FREQUENCY: SETTING THE STAGE FOR THE EVOLUTION OF MALES
The presence of females in hermaphroditic populations is expected to place selective pressure on hermaphrodites to gain more of their fitness through male function and less through female function. The strength of this selection is predicted to increase with female frequency and the magnitude of their seed fertility advantage over hermaphrodites (Charlesworth, 1989). Thus before reviewing the evidence for latter stages of the G-D pathway, it is worthwhile to examine the distribution of these features in species at intermediate stages of this pathway. To accomplish this, we performed a literature search. We searched ISI Web of Science using the keywords ‘gyndioec*’, ‘subdioec*’ and ‘trioec*’ for studies published between 2005 and 2010. We chose to focus the number of years examined for the question of female frequency and fertility advantage because of the large number of studies with these keywords (666 between 1945 and the present listed in ISI); earlier work on females' seed fertility advantage has been reviewed in the context of the transition from hermaphroditism to gynodioecy (Couvet et al., 1990; Shykoff et al., 2003). We included studies in which the species was denoted as ‘trioecious’ (males, females and hermaphrodites) because these systems may also reflect a point along a continuum (Lloyd, 1976; Webb, 1976; Ross, 1982; Fig. 1), but note that the terms ‘subdioecy’ and ‘trioecy’ have been defined differently or used to indicate distinct systems (Ross, 1982; Gregorius et al., 1983; Sakai and Weller, 1991; Maurice et al., 1994, 1998; Maurice and Fleming, 1995; Seger and Eckhart, 1996; Sakai and Weller, 1999; Ehlers and Bataillon, 2007). For example, Gregorius et al. (1983), Maurice et al. (1998) and Sakai and Weller (1999) all distinguished trioecy from subdioecy in that the former describes cases in which there are morphologically distinct females with pistillate flowers, males with staminate flowers and bisexuals with perfect flowers, whereas the latter indicates that some unisexuals may be ‘imperfectly differentiated’ (Gregorius et al., 1983). These terms have also been used to invoke different evolutionary processes, with trioecy denoting a stable evolutionary system and subdioecy as transitional one (e.g. Maurice et al., 1994, 1998; Maurice and Flemming, 1995). Thus, we also recorded for each study how the sexual system was identified by the authors. To ensure that we captured the full range of relative fertilities in species that have been studied, we also examined species included in a recent review by Ehlers and Bataillon (2007, table 1) that were near the dioecy end of the spectrum but that had evidence of ‘fruiting males’ or ‘inconstant males’. We used the ratio of seed fertility of females to hermaphrodites (‘F : H’) (or, where applicable, of ‘fruiting’ or ‘inconstant’ male morphs) reported by the authors or calculated it from the data presented. Where multiple components of female reproduction were reported, we selectively chose one in the following order of preference: seed number per plant, fruit number per plant, proportion fruit-set, proportion seed-set. We also recorded sex ratio (proportion of females) when reported for the study population. When multiple populations (or treatments) were included, we calculated the average fertility or sex ratio. Ultimately, we compiled F : H seed fertility data from 58 studies on 43 species and sex ratio from 51 studies on 38 species (Supplementary Data, available online). We used a Spearman's rank correlation to assess the relationship between F : H seed fertility and sex ratio.
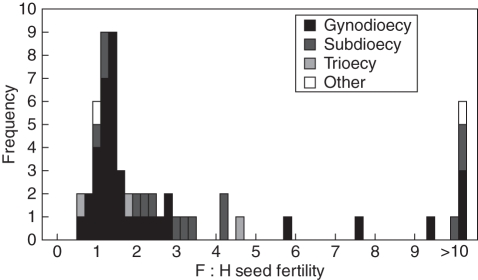
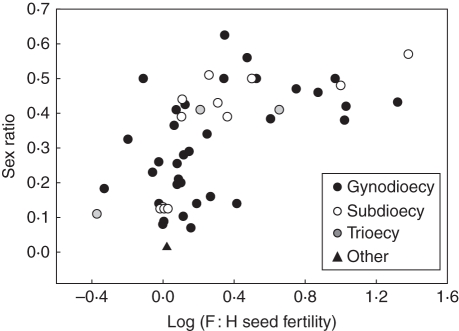
The results of our search revealed a nearly continuous distribution of relative seed fertilities across species with a modal bin at 1·4 (Fig. 2). Approximately 40 % (23) of the studies demonstrated that females have at least a two-fold advantage over hermaphrodites, great enough for females to persist assuming nuclear control, and even greater than needed under cytoplasmic-nuclear control (Lewis, 1941; Lloyd, 1974, 1975; Charlesworth and Charlesworth, 1978). These results are consistent with a meta-analysis of earlier published data that demonstrated higher seed fertility of females than hermaphrodites (Shykoff et al., 2003). Across studies, mean F : H seed (±s.d.) fertility was 3·2 ± 4·5 (adjusted by removing two influential data points over a order magnitude greater than the next highest value; 22·1 ± 118·5 including those values). These fertility estimates do not include potential offspring quality differences associated with differences in selfing by the sex morphs and thus potential for inbreeding depression in the progeny; therefore, the prevalence and degree of female advantage is likely to be even greater than presented in Fig. 2. The proportion of females ranged from 0·014 to 0·63 (mean 0·32 ± 0·16) across the species reviewed. Consistent with theory on the evolution of gynodioecy (Lloyd, 1976), female frequency was strongly positively correlated with F : H seed fertility across studies (rs = 0·62, P < 0·0001; Fig. 3). A similar positive slope is found in a review of earlier published data (r = 0·37, P = 0·02; Couvet et al., 1990: data in appendix with one outlier removed). Taken together, these data demonstrate that, for many species, females are frequent and have a pronounced seed fertility advantage over hermaphrodites and thus ought to exert selection for increased investment in male function in hermaphrodites and/or the invasion of pure males. These species provide prime opportunities to investigate the second phase of the G-D pathway.
Fig. 2.
Histograms showing the distribution of the relative seed fertility of females to hermaphrodites (F : H seed fertility) in the literature reviewed. Different filled bars indicate when species represented in those bins were defined using terms other than ‘gynodioecy’. The category defined as ‘other’ indicates species described as having ‘leaky dioecy’, ‘almost dioecy’ or ‘polygamous’. Note that species described as ‘subdioecious’ and ‘trioecious’ are found across the entire range of the distribution.
Fig. 3.
Scatter plot showing the correlation (rs = 0·62, P < 0·0001) between sex ratio (proportion females) and the relative fertility of females to hermaphrodites (F : H seed fertility, log-transformed) across studies reviewed. Different symbols indicate how the original authors defined the species according to sexual system. ‘Other’ indicates species described as having ‘leaky dioecy’, ‘almost dioecy’ or ‘polygamous’.
It is worth noting that there does not appear to be a clear pattern as to where species classified as ‘subdioecious’ or ‘trioecious’ fall along this continuum (Fig. 2). In fact, the extremities of the range of F : H fertility values are represented by a ‘trioecious’ species (Opuntia robusta; Del Castillo and Argueta, 2009) and one classified as ‘subdioecious’ [Silene acaulis subsp. exscapa, which has females, males and males that set fruit (‘H’); Maurice et al., 1998]. This underlines that the use of sexual system labels may obscure where along the G-D pathway a species resides and probably does not inform on the direction of the evolutionary trajectory. Instead, we argue that it is more informative to characterize the relative fertilities and the conditions that influence them.
EVIDENCE FOR INCREASED ‘MALENESS’: OUTCOMES OF SELECTION
Whereas the mechanisms that influence the relative fertilities of females and hermaphrodites in the initial stages of the G-D pathway are well studied (see citations in Introduction), we have much less empirical evidence for the dynamics of the latter stage of the pathway. A first approach to filling this gap is to examine the outcome of selection by focusing on whether male function of hermaphrodites varies with sex ratio in gynodioecious populations or, where males have been identified (in subdioecious populations), assessing their fertility relative to hermaphrodites. Before we evaluate these outcomes, however, we briefly review the ways in which ‘maleness’ has been quantified, i.e. the metrics of male fertility.
Components of ‘maleness’
There are multitudes of ways to estimate ‘maleness’, but what is the best metric for our purposes? Most researchers focus on allocation to male function and measure components of male fertility. For example, flower number is a common and perhaps the most tractable component of male function (e.g. Bell, 1985; Schoen and Stewart, 1986; Sutherland, 1987; Queller, 1997; Ashman and Penet, 2007). Flower number alone, however, can be potentially misleading because it can also be selected through female fertility (Broyles and Wyatt, 1990; Conner et al., 1996; Ashman and Penet, 2007; Van Etten, 2009). In fact, some studies have found that females have more flowers than either hermaphrodites or males (reviewed in Shykoff et al., 2003) or that ‘pure’ males have fewer flowers than ‘inconstant’ males (i.e. hermaphrodites; Wolfe and Shmida, 1997). A less sexually ambiguous component of male fertility is pollen production, estimated either as anther number per flower or per plant, or, preferably, as pollen production per flower or plant. Unfortunately, just as ovule number is not necessarily a good predictor of fruit or seed production, pollen production can also be an imprecise surrogate for male fertility. For example, males and hermaphrodites may differ with respect to pollen viability, or incompatibility haplotypes (i.e. alleles may be distributed differentially between sex morphs; Saumitou-Laprade et al., 2010) and pollen production may not correlate with pollen export (Ashman, 1998). Consequently, predictions based on pollen production alone could potentially lead to premature conclusions about realized male function. Others have also warned that assumptions about relationships between a single component of male function and male fitness should be viewed cautiously (e.g. Snow and Lewis, 1993; Ashman, 1998; Campbell, 2000), underlining the importance of measuring multiple components. Ideally, studies should measure these multiple components and directly estimate siring success in the field using genetic markers to assign paternity. Relatively few studies, even in hermaphroditic species, however, have examined siring success in experimental or natural populations, as achieving high exclusion probabilities for paternity assignment is difficult and expensive in wild populations (reviewed in Ashley, 2010). Yet this information is key not only for estimating the relative male fertility of males and hermaphrodites, but also for estimating total (i.e. female and male) fitness of hermaphrodites under different contexts and thus conditions for their maintenance. Ultimately, realized male fertility is desired; such estimates would not only account for siring success but also the viability of subsequent offspring (Campbell, 2000).
Does the male fertility of hermaphrodites vary as predicted across populations in gynodioecious species?
In gynodioecious species, where pollen-bearing individuals show a continuum of allocation to female function, simple comparisons between males and hermaphrodites are often not possible. However, even in such species we can take advantage of the continuous distribution of fruiting abilities to examine relationships between selective context and components of maleness. For example, we know from previous work that hermaphrodites are generally ‘less female’ relative to females in gynodioecious populations (i.e. F : H seed fertility > 1·0, Fig. 2) and, as predicted by theory, this fertility difference often becomes greater where females are more common, as seen in this study across species (Fig. 3) and in other studies within species (reviewed in Ashman, 2006). However, whether this pattern reflects decreased female function of hermaphrodites in response to selection by high female frequencies or increased female seed production that enables them to achieve higher frequencies is unknown. Moreover, such patterns do not inform as to whether hermaphrodites are ‘more male’ along this gradient of female frequencies relative to hermaphrodites in populations where females are rare or have only recently invaded.
In one study, McCauley and Brock (1998) manipulated the sex ratio of experimental arrays of gynodioecious Silene vulgaris. They found that, as expected, hermaphrodites were functionally more male, i.e. they fertilized more seeds per capita, when they were rare compared with when they were more common. These results illustrate the frequency-dependent nature of gender in gynodioecious environments and that female frequency creates the conditions for selection.
However, when we examine whether increased allocation to components of male fertility (an outcome of such selection) is observed in the few studies that have examined it, we do not see strong support for it. Ashman (2006) reviewed studies in gynodioecious species and found that, although there was evidence of increased allocation to male function with increased female frequency across species with different sexual systems (Manicacci et al., 1998), of the three studies that assessed male function and female frequency within species, none had found supporting evidence. All of these studies used pollen per flower as the main metric of ‘maleness’. Our own recent work also did not find support for this prediction among populations of Fragaria virginiana in Ohio and Pennsylvania (correlation between female frequency and population mean pollen per anther: r = 0·06, P = 0·82) (R. B. Spigler and T.-L. Ashman, unpubl. res.). But there is a pattern across species: the number of anthers per flower is higher in the dioecious species of Fragaria relative to the gynodioecious or subdioecious species (Ashman et al., 2011). Ehlers and Thompson (2004) found that hermaphrodite total pollen production was positively correlated with the number of female flowers open in the gynodioecious perennial Thymus vulgaris, and although their study examined variation across the flowering season within populations, it lends support for the prediction that hermaphrodite reproductive allocation is male-biased where female frequencies are high. We did not find in our searches additional published studies that have tested this hypothesis within species, indicating that more studies are needed. Preferably, such studies could be done in the greenhouse to examine whether hermaphrodites from populations of varying female frequencies are genetically differentiated for multiple components of male fertility, as described above. Experimental arrays of hermaphrodites from these populations along with females could be used to examine whether hermaphrodites from high-female-frequency populations have greater siring success or whether a greater proportion of their total fitness comes through male versus female function (see below).
Do males have higher male fertility than hermaphrodites in subdioecious species?
Males are able to invade gynodioecious populations when they have a male fertility advantage over hermaphrodites that more than compensates for their reduction in female fertility, although the size of this advantage will depend upon the selfing rate, inbreeding depression and equilibrium frequency of females (Charlesworth and Charlesworth, 1978). Thus, in subdioecious populations en route to dioecy, we would predict that males have higher male fertility than hermaphrodites. To evaluate this prediction, we reviewed studies where males and hermaphrodites (the latter alternatively referred to as ‘inconstant males’ or ‘fruiting males’) were identified and components of their male fertilities compared. In addition to those studies identified through the search criteria above, we searched ISI Web of Science for studies using the following combination of terms from 1991 to 2010: males and hermaphrodit* and (pollen or flower*). We compiled relative fertility of males to hermaphrodites (‘M : H’), either as reported by the author or calculated from reported M and H fertility values (averaged when presented for more than one population or treatment), and noted the metrics used.
We found 14 studies on 13 species (Table 1), with male frequencies ranging from 0·09 to 0·71. Of these, most (11 of 14) revealed that males had greater male fertility than hermaphrodites (M : H pollen fertility >1·0) for at least one component. In five of 14 studies, the males had more that twice the male fertility of hermaphrodites. An emergent property of these studies, however, is the importance of examining several components of male fertility because the magnitude and/or the direction of the difference (M > H vs. M < H) varies with the component studied. For example, in the desert shrub Ochradenus baccatus, males make significantly more pollen per flower (based on stamen biomass), but produce significantly fewer inflorescences (Table 1; Wolfe and Shmida, 1997). However, in general, a greater male advantage (mean M : H pollen fertility, ± s.d.) was seen for traits related to pollen production (per anther, per flower, or per plant: 2·4 ± 1·80) rather than flower number (1·3 ± 0·84) or pollen viability (1·2 ± 0·25), and for the three cases where hermaphrodites had greater fertility than males it was in terms of flower number. Of the two studies that examined siring success based on hand pollinations and assessment of paternity with genetic markers, one found that males and hermaphrodites did not differ significantly whereas the other found that males sired ten times the number of offspring as hermaphrodites, based on single donor crosses, and that males almost completely excluded hermaphrodites from siring offspring when in competition (Table 1). In contrast to the pattern seen above for F : H seed fertility, however, we did not find evidence for a significant positive correlation between mean estimate of M : H pollen fertility (based on species in Table 1) and male frequency (rs = –0·42, P = 0·15).
Table 1.
Comparison of relative fertility of males to hermaphrodites across studies and components of male fertility
| Species | Proportion males | Mating system1 | Flower number | Anther number | Pollen production2 | Pollen viability | Siring success | Study |
|---|---|---|---|---|---|---|---|---|
| Astilbe biternata | 0·52 | – | 1·06 | – | – | – | – | Olson (2001) |
| Circium arvense3 | 0·71 | – | 1·11 | – | 1·12–1·81 | 1·03 | – | Kay (1985) |
| Clusia nemorosa | – | SC | 3·30 | 1·33–5·33 | – | 1·00 | – | Lopes and Machado (1998) |
| Coccoloba cereifera3 | 0·16 | – | 1·67–2·18 | – | 1·50–4·21 | 1·07–1·19 | – | Silva et al. (2008) |
| Fraxinus excelsior | 0·33 | SI | – | – | – | – | ≥ 10 | Morand-Prieur et al. (2003) |
| Jacaratia mexicana | 0·38 | – | – | 1·72 | – | – | – | Aguirre et al. (2009) |
| Ochradenus baccatus | 0·53 | SC | 0·25 | 1·01 | 1·954 | – | – | Wolfe and Shmida (1997) |
| Opuntia robusta | 0·09 | SC | M > H5 | – | 6·50 | – | – | Del Castillo and Argueta (2009) |
| Pachycereus pringlei | 0·23 | SC | 1·56 | – | 1·52 | – | – | Fleming et al. (1994) |
| Schiedea globosa | 0·46 | SC | 0·586 | 0·97 | 1·03 | – | – | Sakai and Weller (1991) |
| Silene acaulis subsp. acaulis | 0·31 | SC | 1·09 | – | 1·21 | 1·63 | 0·94 | Phillip et al. (2009) |
| Silene acaulis subsp. cenisia | – | – | 0·70 | – | M = H | M = H | – | Maurice et al. (1998) |
| Wurmbea dioica | 0·50 | SC | 1·09 | – | 1·12/2·117 | 0·948 | – | Jones and Burd (2001) |
| Wurmbea dioica | 0·50 | SC | 1·03 | – | 1·2–1·4 | 1·04–1·11 | – | Ramsey and Vaughton (2001) |
Values in bold indicate where male and hermaphrodite fertilities were found to be significantly different by the authors.
1 Mating system of hermaphrodites (SC, self-compabtile; SI, self-incompatible).
2 Pollen production per plant, flower or anther.
3 Estimates depend on hermaphrodite morph (n = 2) examined.
4 Stamen biomass per flower.
5 Where fertilities were not directly reported or difficult to estimate from figures, we present the direction of the relationship as reported by the authors.
6 Flowers per infloresence*infloresence per genet.
7 Larger ratio based on estimate adjusted for differences in perianth size.
8 Pollen grain size.
One particularly comprehensive comparison found that males of subdioecious Silene acaulis produced 1·2 times the number of pollen grains and 1·6 times the number of viable pollen grains as hermaphrodites, but the two pollen-bearing morphs did not differ in their expected siring success when examined using single and mixed loads of pollen on females in the field (Philipp et al., 2009). Such results would lead us to conclude nevertheless that males have higher fertility because of their greater pollen production, but estimates of plant size in natural populations revealed that hermaphrodites are 2·7 times larger than and produce 2·5 times the number of flowers as males, potentially negating males' per-flower pollen advantage. In fact, when Phillip et al. (2009) extrapolated these differences in a model accounting for the current frequency of all three sexes (females, hermaphrodites and males), and several components of male success, they concluded that hermaphrodites would increase in the population, maintaining subdioecy or leading to a reversion to gynodioecy. This model allowed the authors to make predictions about the direction of evolution on the G-D pathway.
EVIDENCE FOR INCREASED ‘MALENESS’: SELECTION FOR MALES?
Although the studies reviewed above speak to the outcome of selection, they do not inform on whether such selection is occurring or can occur. According to theory, when females are the major contributors of the next generation of seed as a result of their high frequencies and F : H seed ratios, selection should favour hermaphrodites with high allocation to male function. However, direct tests of this prediction are rare: to our knowledge a phenotypic selection approach (sensu Lande and Arnold, 1983) to test this fundamental prediction has been taken in only two gynodioecious/subdioecious species.
Van Etten (2009) created experimental gardens of gynodioecious Geranium maculatum with varying frequencies of females (13, 26 and 42 %) and assigned paternity using six microsatellite markers. She found that although direction and strength of selection on pollen production increased with female frequency (from –0·08 to 0·07 at highest female frequencies), this pattern was not statistically significant (P = 0·11), and the opposite pattern (stronger positive selection at low frequencies than high) was observed for flower number. T-L. Ashman (unpubl. res.) also created experimental gardens but with Fragaria virginiana and a constant sex ratio (50 % females) and scored paternity with four microsatellites. Pooled across two replicates, she found significant selection through siring success to increase pollen production per flower (β′ = 0·08, P < 0·05), flower number per plant (β′ = 0·30, P < 0·05) and to reduce fruit-set (β′ = –0·09, P < 0·05) of hermaphrodites. In an array experiment analogous to these two, but with an androdioecious species (males and hermaphrodites), Dorken and Pannell (2009) demonstrated a selection response for hermaphrodites that were female-biased in their floral sex allocation in Mercurialis annua when males were absent but no such selection response when males were present at high (50 %) frequencies.
Taken together, these studies suggest that the presence of females, at least at high frequencies, can lead to selection to increase male traits and reduce female traits. However, whether these result in a selection response is only known from the study of an androdioecious species. Combining selection gradient analyses with knowledge of genetic (co)variance matrices will be necessary to predict evolutionary response (see, for example, Ashman, 2005), and ideally confirmation with empirical study of realized response to selection.
FACTORS INFLUENCING THE LATER STAGES OF THE G-D PATHWAY
Inarguably, one reason for the diverse data reviewed above is that the G-D evolutionary trajectory is influenced by a suite of interacting genetic and ecological factors. In Table 2 we summarize several key theoretical treatments of the G-D pathway to illustrate the diversity of evolutionary stable endpoints predicted – after females invade – when the entire range of genetic and ecological factors are invoked. We note, however, that because of the vast body of theoretical work on the subject of evolutionary pathways to dioecy, an exhaustive review of theoretical models is beyond the scope of this paper. Instead, in this section, we briefly review different types of genetic control of sex determination that can be involved and then focus our discussion on the roles of ecological factors that are likely to be universally important.
Table 2.
Summary of key theoretical treatments of the G-D pathway
| Genetic control |
Ecological parameters |
Stable sexual system outcomes† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model citation | Loci* | Linkage | Dominance | H : F | H : M | S | PL | IBD | G | SD | D |
| Ho and Ross (1974) | 2(2)N | L, U | Recessive male sterility; dominant female sterility; heterosis | √ | √ | 0 | √ | √ | √ | ||
| Ross and Weir (1976) (building on Ho and Ross, 1974; Lewis, 1942) | 1(2)N; 2(2)N | L, U | Recessive male sterility; dominant female sterility; heterosis | √ | √ | √ | 0 | √ | √ | √ | |
| Charlesworth and Charlesworth (1978) | 2(2)N | L, U | All possibile combinations | √ | √ | √ | 0 | √ | √ | √ | √ |
| Ross (1978) | 2(2)N | Recessive male sterility; dominant female sterility; heterosis | √ | √ | √ | 0 | √ | √ | √ | ||
| Gregorius et al. (1983) | 1(2)N | n/a | Three types considered: (1) Aa females, (2) Aa hermaphrodites, (3) Aa males; other sexes homozygous | √ | √ | √ | √ | √ | √ | ||
| Maurice et al. (1994) | 1C + 2(3)N or 3(2)N | U | Dominant restorer alleles (M > H) | √ | √ | 0 | √ | √ | √ | ||
| Shultz, 1994 | various: C-N or N | L | dominant restorer and female-sterility alleles | √ | √ | √ | √ | √ | √ | √ | √ |
| Maurice and Flemming (1995) | n/a | n/a | n/a | √ | √ | √ | √ | √ | √ | √ | √ |
| Seger and Eckart (1996) (extension of classic resource-allocation models, e.g. Charnov et al., 1976; Charnov, 1982) | N | n/a | n/a | √ | √ | 0 | √ | √ | √ | ||
| Ehlers and Bataillon (2007) | 1(3)N or 2(2) | U | Male heterogamety: AA females, Aa males; aa males exist in ‘recent’ dioecy | √ | √ | √ | √ | √ | √ | √ | √ |
* Loci = no. of loci (no. of alleles) considered; where applicable ‘N’ indicates nuclear, ‘C’ indicates cytoplasmic and ‘C-N’ represents nuclear-cytoplasmic. Linkage refers to nuclear loci only; L = linked, U = unlinked. Dominance refers to dominance of alleles determining male and female function; genotypes are given where dominance does not apply. H : F, relative female function of hermaphrodites (H) to females (F). H : M, relative male function of hermaphrodites (H) to males (M). S, selfing rate of hermaphrodites. PL, pollen limitation. IBD, inbreeding depression.
† GD, gynodioecy; SD, subdioecy (or trioecy); D, dioecy.
Genetic control of male and female function
One important genetic consideration is whether sex is determined by nuclear loci only or a combination of cytoplasmic and nuclear genes. When sex is determined by nuclear genes only, the number of loci and their linkage relationships are important as well as the dominance of alleles at those loci (Table 2). For instance, one influential model posits that females are created by an initial male sterility mutation that is followed by a female sterility mutation at a second locus (facilitating the transition of gynodieocy to subdioecy). Once these loci are linked, have complementary dominance and recombination between them is suppressed, full dioecy and sex chromosomes can evolve (Charlesworth and Charlesworth, 1978). Recombination suppression leads to a female-determining chromosome (carrying the male sterility and female fertility alleles) and a male-determining one (carrying the male fertility and female sterility alleles). Consistent with these predictions, researchers have revealed two-locus control with recombination in subdioecious Fragaria virginiana (Spigler et al., 2008) but a single region without apparent recombination in dioecious Fragaria chiloensis (Goldberg et al., 2010). One should note, however, that although chromosomes carrying both sterility alleles will go extinct, those carrying both fertility alleles can persist in subdioecious populations (e.g. three morphs exist: females, males and hermaphrodites), and sex is determined by a single region or ‘locus’ with three ‘alleles’ (see Vitus, Marguerit et al., 2009). In other models (e.g. Ho and Ross, 1974; Ross and Weir, 1976; Table 2), heterosis is a key component to the evolution of dioecy, although as in Charlesworth and Charlesworth (1978), complete dioecy will require suppression of recombination between the two sex-determining loci.
Females can also be created by cytoplasmic male sterility (CMS), and this maternally inherited mutation commonly leads to gynodioecy (Lewis, 1941, 1942; Bailey and Delph, 2007; for extensive review of the evolutionary dynamics of gynodioecy with CMS see: Couvet et al., 1990; Jacobs and Wade, 2003; Bailey and Delph, 2007; Delph et al., 2007; McCauley and Bailey, 2009). And, although the evolution of dioecy from gynodioecy may be less likely when CMS is involved than when under strict nuclear control (Lloyd, 1974; Ross, 1978; Richards, 1986; reviewed in Schultz, 1994), at least two theoretical models (Table 2) have demonstrated that gynodioecious species with CMS can evolve to dioecy, even at an accelerated pace relative to nuclear-only control (Maurice et al., 1994; Schultz, 1994).
While multiple genetic systems can lead to dioecy or subdioecy (Table 2), for most species studied, we simply do not have complete knowledge of the genetic control of sex determination (see reviews in Charlesworth and Mank, 2010; Dufay and Billard, 2011). Initial assessments of the class of genetic control (e.g. involvement of cytoplasmic or nuclear factors) can be made via reciprocal crosses and field observations of sex ratios (Bailey and Delph, 2007). In addition, genetic mapping and analysis of quantitative trait loci can identify the number of loci housing sterility alleles (Wang et al., 2004; Spigler et al., 2011), or nuclear restorers of CMS (e.g. Fishman and Willis, 2006; Barr and Fishman, 2010).
Ecological features
Importantly, the factors controlling the invasion of males in gynodioecious populations may be independent of the genetic underpinnings of male sterility; instead, ecological conditions may largely dictate male absence or presence (Table 2; across-species relationship Fig. 3; Schultz, 1994). Thus in the remainder of this paper, we do not differentiate between systems based on genetic control, and instead focus on three main ecological parameters (in addition to fertility ratios discussed previously) considered in the majority of models (hermaphrodite selfing rate, pollen limitation and inbreeding depression; Table 2) and two additional ecological factors that have been either recently incorporated (plasticity in sex allocation; Ehlers and Bataillon, 2007) or not yet explicitly incorporated (plant–enemy interactions) in models but that are predicted to impact sexual system evolution (Table 3).
Table 3.
Ecological factors that affect the second step in the evolution of dioecy. Predicted effect on sexual system, mechanisms of action, and example citations are given for each
| Ecological factor | Expected consequence for sexual system* | Mechanism† | Example citation |
|---|---|---|---|
| Pollen limitation | Selects for SD | Reproductive assurance maintains H | Maurice and Fleming (1995), Ehlers and Bataillon (2007) |
| Selfing by hermaphrodites | Selects for SD | In combination with pollen limitation favours H | Ehlers and Bataillon (2007), Del Castillo and Argueta (2009) |
| Retards D | Geitonogamous selfing and pollen discounting disfavours M | de Jong et al. (1999) | |
| Selects for D | Prior selfing with negligible pollen discounting in combination with high inbreeding depression favours M | de Jong et al. (1999) | |
| Inbreeding depression | Selects for D | Reduces advantage of selfing by H | Charlesworth and Charlesworth (1978), Maurice and Fleming (1995) |
| Sex allocation plasticity | Selects for SD | Plasticity of H favoured in heterogeneous environments | Delph and Wolf (2005), Ashman (2006), Ehlers and Bataillon (2007) |
| Antagonists | Retards D | Male-biased damage reduces pollen fitness gain; male-biased damage increases pollen limitation of F | Ashman (2002) |
| Selects for D | Damage to H seed reduces their fitness relative to M | Ashman (2002) |
* SD, subdioecy; D, dioecy.
† H, hermaphrodites; F, female; M, male.
Pollen limitation, selfing and inbreeding depression
Pollen limitation can arise either because of limited mate availability (low pollen to ovule ratios) or because of limited pollination services (Wilcock and Neiland, 2002; Knight et al., 2005). In general, as females become more common within populations along the G-D pathway, the pollen to ovule ratio in the population decreases, limiting the availability of male gametes and thus potentially females' seed fertility (Lewis, 1941; Lloyd, 1974; Maurice and Fleming, 1995; McCauley and Brock, 1998; Case and Ashman, 2009). This effect can be exacerbated when pollinators preferentially visit pollen-bearing morphs (e.g. Bell, 1985; Ashman and Stanton, 1991; Eckhart, 1992; Ashman, 2000; Case and Ashman, 2009), such that pollen is distributed disproportionately among hermaphrodite ovules, increasing their relative seed fertility. Males, however, might actually have an advantage relative to hermaphrodites under a scenario of low pollen to ovule ratios if greater pollen quantity or quality results in a greater total number of ovules sired. On the other hand, one could argue that low pollen to ovule ratios results in a less competitive pollen environment where even lower quality pollen from hermaphrodites may successfully sire offspring (e.g. Morand-Prieur et al., 2003).
Where pollen limitation is due to limited pollinator services, however, males as well as females will be at a disadvantage relative to hermaphrodites if hermaphrodites are capable of autonomous self-pollination, a mechanism of reproductive assurance. Consistent with this prediction, Ehlers and Bataillon (2007) reviewed studies on subdioecious species, and of those species where pollen limitation was found, all but one were self-compatible. They suggested that selfing ability contributes to the maintenance of ‘inconstant males’ in these species. Although pollen limitation is often not explicitly included or is set to zero in most theoretical models (Table 2), at least two demonstrate that, under pollen-limited conditions, increased selfing promotes the maintenance of hermaphrodites and stable subdioecy (Maurice and Flemming, 1995; Ehlers and Bataillon, 2007; Table 2).
These models also demonstrated that the net outcome of pollen limitation on F : H and M : H fertilities will depend not only on selfing ability but also the level of inbreeding depression. High levels of inbreeding depression can potentially negate the benefits of reproductive assurance by reducing realized reproductive success in hermaphrodites, thus equalizing the sexes or favouring males and females. The role of inbreeding depression has been shown to influence F : H seed fertility in several species in the context of earlier stages of the G-D pathway (e.g. Sakai et al., 1997; Thompson and Tarayre, 2000; Chang, 2007; Dufay et al., 2010; Dufay and Billard, 2011; but see Miyake and Olson, 2009). Studies of this role in the latter stages of the G-D pathway are still needed, as inbreeding depression may also influence components of male function, increasing M : H fertility. For example, inbred individuals might have reduced pollen production and/or viability (e.g. Carr and Dudash, 1997; Glaettli and Goudet, 2006).
In a particularly comprehensive study, Del Castillo and Argueta (2009) studied the combined effects of pollen limitation, selfing and inbreeding depression on the relative fertilities of the sexes in ‘trioecious’ cactus Opuntia robusta. They showed that pollen to ovule ratios in the population were sufficient such that pollen limitation was more likely due to low pollinator visitation. Greater pollen limitation of females than hermaphrodites led to a fertility disadvantage for females (F : H seed fertility = 0·42) and significantly greater variation in seed production by females. Because this limitation was due to limited pollinator services, this also meant that even though males produce 6·5 times as much pollen as hermaphrodites per flower (Table 1), they will nevertheless be at a disadvantage relative to hermaphrodites when pollinators are scarce because males' pollen export depends on pollinators, whereas hermaphrodites can autonomously self. Compounding this fitness differential, Del Castillo and Argueta (2009) found that selfing occurs prior to opportunities for outcross pollination (‘prior selfing’, sensu Lloyd, 1975). Thus, even when pollinators are not limiting, males will still have reduced fitness if prior self-fertilization by hermaphrodites limits males' access to hermaphrodite ovules, which comprise the majority in the population. The combination of pollen limitation and prior selfing in hermaphrodites, in conjunction with limited to no pollen discounting and no inbreeding depression (Del Castillo and Argueta, 2009), can potentially explain why males are rare relative to hermaphrodites in the population. These results raise questions about whether trioecy is stable in this system or whether it may revert to hermaphroditism.
The work by Del Castillo and Arugueta (2009) raises an important distinction about the role of selfing in the evolution of separate sexes. Specifically, the timing of selfing is important. Timing and modes of selfing have previously been shown to influence the evolution of separate sexes (e.g. Lloyd, 1975; de Jong et al., 1999; de Jong and Geritz, 2001), although via different processes from ‘ovule preemption’, as suggested in Opuntia robusta. For example, Lloyd (1975) concluded that prior or competing selfing (i.e. self pollen arrives prior to or during outcross pollen deposition, respectively) should maintain gynodioecy because inbreeding depression of hermaphrodite offspring provides females with an outcrossing advantage, whereas delayed selfing (i.e. self pollen is deposited after opportunities for outcrossing have passed) should maintain hermaphrodites because it can provide reproductive assurance. On the other hand, de Jong et al. (1999) suggested that prior selfing coupled with negligible pollen discounting should actually favour dioecy, but that selfing via geitonogamy (transfer of self pollen among flowers within a plant) with high levels of pollen discounting will maintain (or lead to) hermaphroditism, provided inbreeding depression is low. One should note that de Jong et al.'s (1999) models do not depend on a gynodioecy intermediate in the evolution of dioecy from hermaphroditism, but rather serve as an example of how mode of selfing can influence the dynamics.
Future modelling efforts on the G-D pathway should explore how timing and mode of selfing can influence the stability of subdioecy once gynodioecy is established and empirical efforts in subdioecious species should take steps to characterize the mode of selfing as well as pollen discounting. Moreover, studies in subdioecious systems are needed to test whether pollinator limitation reduces males' siring success more than hermaphrodites' and the generality of the role of prior selfing in precluding a significant proportion of males from siring seeds of hermaphrodites. Researchers could take advantage of known, documented population variation in the degree of pollen limitation and timing of selfing, or create experimental arrays where these conditions could be manipulated (e.g. Ashman and Diefenderfer, 2001) and paternity analysis more easily be assigned to estimate male fitness.
Sex allocation plasticity
Resource-dependent plasticity in sex expression or allocation (hereafter ‘sex-allocation plasticity) of hermaphrodites is another factor that can influence a population's location along the G-D pathway (Table 2). In the earliest stages of the G-D pathway, sex-allocation plasticity is thought to play a major role in the initial invasion and spread of females (Delph, 1990, 2003; Delph and Wolf, 2005). Stated briefly, if hermaphrodites reduce allocation to female function in favour of male function in resource-poor habitats, because of the cost of producing seeds, then females can more easily gain the seed-fertility advantage needed to invade hermaphrodite populations in these habitats. However, if hermaphrodites can respond to increased resources by increasing their female fertility, e.g. in resource-rich habitats, then females’ advantage, and thus their invasion or spread, will be limited. Several studies have shown patterns of negative relationships between female frequency and resource availability in the field consistent with this hypothesis (reviewed in Delph, 2003; Delph and Wolf, 2005) and provided experimental evidence for sex-differential plasticity (Delph, 1990, 2003; Barr, 2004; Dorken and Mitchard, 2008; Spigler and Ashman, 2011).
As we move further along the G-D pathway, however, it has been suggested that plasticity ought to retard the transition to complete dioecy, promoting stable subdioecy (Barrett et al., 1999; reviewed in Delph and Wolf, 2005; Ashman, 2006). This can occur because, as stated above, hermaphrodites may be maintained and continue to produce seeds in high-resource habitats, or because, even where females are common, plasticity enables hermaphrodites to escape female-mediated selection against seed production in low-resource habitats where they appear phenotypically male. In our recent work with F. virginiana, for example, plasticity underlies the absence of population-level genetic differentiation in fruit production of pollen-bearing morphs (hermaphrodites and males), despite strong variation in female frequency, consistent with the hypothesis that plasticity maintains hermaphrodites (Spigler and Ashman, 2011). Population variation in plasticity itself, however, may be critical in these dynamics because those without plastic hermaphrodites could provide an arena for male invasion.
Whether sex-allocation plasticity can maintain hermaphrodites with canalized (i.e. non-plastic) males, thereby stabilizing subdioecy, or whether plasticity will be lost from populations as they evolve towards dioecy will depend largely in part on whether this plasticity is adaptive; yet there exists only very limited evidence for an adaptive role. Sex-allocation theory provides at least one rationale for why sex-allocation plasticity may be adaptive: at small plant sizes (and presumably low resources), the cost of producing fruits makes it more advantageous to produce pollen. But, as plant size (or resources) increases, fitness-returns from continual investment in pollen diminish whereas fitness increases linearly for seed production (de Jong and Klinkhamer, 1989; Pickering and Ash, 1993; Klinkhamer et al., 1997; Paquin and Aarssen, 2004). Accordingly, individuals that are plastic in their sex allocation might have the greatest fitness – particularly in a heterogeneous environment – compared with individuals that are canalized in their sex expression. Although evidence for different pollen and seed fitness functions exists (reviewed in Ashman, 2006), we still do not know whether they are adaptive. Recently, we demonstrated that the degree of plasticity in fruit production of hermaphrodites of F. virginiana is positively correlated with in situ heterogeneity in soil resources, suggesting a potential adaptive basis (Spigler and Ashman, 2011). Results from another study in the same species, however, point toward a significant cost of plasticity; individuals that were more plastic in fruit had reduced pollen production (Ashman, 2006), but to our knowledge costs of sex-allocation plasticity have not been examined in other species. If the costs outweigh the benefits, then in certain contexts canalized males can out-perform hermaphrodites, leading to the transition to complete dioecy. Experiments that examine total fitness (seed production and siring success) in the field or experimental arrays under different resource conditions can test whether plastic hermaphrodites garner a fitness advantage over males.
Ultimately, a theoretical framework is needed that accounts for the dynamics influencing the relative fitness of these types of pollen-bearing individuals across resource gradients, including the frequency of resource patches and gene flow among them, and that can make predictions about the stability of subdioecy versus transition to dioecy. In this regard, Ehlers and Bataillon (2007) made a significant contribution toward building a theoretical framework for the role of plastic hermaphrodites (‘inconstant males’) in the evolution of dioecy in the context of the G-D pathway (Table 1). Interestingly, although their models did not attach any adaptive value to plasticity of sex expression, they demonstrate that given the right ecological conditions (pollen limitation and selfing) and genetic underpinnings (‘ancient’ rather than ‘recent’ dioecy; unlinked rather than linked loci), stable subdioecy can exist with hermaphrodites as inconstant males.
Recent work points toward additional factors that need to be considered when building upon the work of Ehlers and Bataillon (2007). First, in light of the evidence demonstrating resource-mediated plasticity (see above), plasticity under a heterogeneous environment should be incorporated, such that plasticity is not expressed as a constant variable. Second, the degree of sex-allocation plasticity can vary across genotypes (Dorken and Barrett, 2004; Bishop et al., 2010) and populations (Dorken and Barrett, 2003; Spigler and Ashman, 2011). This variation suggests that sex-allocation plasticity has the potential to evolve, although studies on its heritability are needed. In particular, it has been suggested that plasticity may evolve with sex ratios: high frequencies of females may select for males over plastic hermaphrodites if there is a cost to plasticity (Ashman, 2006), although in the only study to examine the relationship between sex-allocation plasticity and sex ratio no relationship was found (Spigler and Ashman, 2011). Finally, the potential genetic mechanisms that underlie sex allocation plasticity need to be explored, including whether epigenetic modification of sex determination is involved. Such trans-generational inheritance of this type of variation (Boyko and Kovulchuk, 2008; Chinnusamy and Zhu, 2009; Angers et al., 2010) may provide a means by which males could arise as descendants of plastic hermaphrodites in low-resource environments or even potentially explain an alternative origin of males from hermaphrodites (Gorelick, 2003).
Antagonistic interactions
Interactions between plants and enemies (e.g. herbivores, pathogens, nectar robbers) may also affect the G-D transition (reviewed in Ashman 2002, 2006). Male-biased antagonism, in particular, is seen consistently in dioecious species (males suffer more herbivore damage than females; Ågren et al., 1999; Cornelissen and Stiling, 2005), and in most gynodioecious species, hermaphrodites suffer more than females (Ashman, 2002; Zhang et al., 2009; but see Alonso, 2003; Collin and Shykoff, 2010). There is even evidence that damage can be greater to phenotypically more male individuals where males and hermaphrodites coexist or among differentially male-biased hermaphrodites (Verdú et al., 2004; Ashman and Penet, 2007). Male-biased antagonism may occur if males are a better resource for antagonists or if females are better defended than males or hermaphrodites (Cornelissen and Stiling, 2005; Cepeda-Cornejo and Dirzo, 2010; Tsuji and Sota, 2010). In addition to patterns of sex-differential susceptibility, it is worth noting that response to herbivory may vary among the sexes (i.e. tolerance) (Ågren et al., 1999; Ashman et al., 2004; Cole and Ashman, 2005; Narbona and Dirzo, 2010). For these reasons, the role of male-biased antagonistic interactions on influencing the transition from subdioecy to dioecy merits greater attention.
The effect of antagonists may be to retard the evolution of full dioecy by opposing selection for males and male-biased hermaphrodites (Table 2). Ashman (2002) described several ways that male-biased attack by antagonists could influence the evolution of dioecy from subdioecy. For example, if herbivory shifts the relationship between fitness gains and pollen investment from accelerating to saturating, the benefits from increased investment in pollen will diminish. Under this scenario, female-biased hermaphrodites would be favoured. Such diminishing returns may occur because certain herbivores, in particular florivores, are attracted to nitrogen-rich pollen. Moreover, male-biased florivore damage might also translate into reduced pollinator visitation to males and reduced pollen export and siring success; the latter may be compromised even further if herbivory influences pollen quality as well (reviewed in McCall and Irwin, 2006). For example, using path analysis Ashman and Penet (2007) demonstrated that weevil florivores preferentially attacked hermaphrodites with more flowers and more pollen per flower and thus reduced the siring success of these individuals. Interestingly, in this same species there is a positive association between damage to hermaphrodites and deviation from predicted sex ratio (based on Lloyd's 1976 model) suggesting that weevil damage may represent a selective force influencing sex ratio in the wild (Ashman, 2006).
Alternatively, male-biased antagonism might promote dioecy (Table 2). Even if males have higher rates of herbivory, hermaphrodites could still suffer greater fitness losses when herbivory increases their selfing rate and so lowers the quality of subsequent offspring. Penet et al. (2009), for example, showed that herbivory led to increased selfing, probably via autonomous autogamy, in F. virginiana. Similar increases in selfing as a consequence of herbivory have been shown in other species (Elle and Hare, 2002; Steets and Ashman, 2004; Steets et al., 2007). This suggests that where selfing increases and inbreeding depression is significant, hermaphrodites may be at a greater disadvantage than males.
The studies reviewed here suggest that antagonists could represent an important, yet largely unstudied, aspect of the ecological context influencing location of a population along the G-D continuum (Ashman, 2006). Future studies on the relative fitness of males and hermaphrodites or selection for male and/or male-biased hermaphrodites should incorporate the role of antagonists. Specifically, we need to evaluate whether the negative effects of male-biased antagonism are strong enough to outweigh any male fertility advantage males (or male-biased hermaphrodites) have over hermaphrodites in the absence of antagonists. Studies using path analyses to identify the components that influence fitness and selection studies such as those by Ashman and Penet (2007) and Wise and Hébert (2010) represent excellent examples of work that should be applied to subdioecious systems.
CONCLUSIONS
A large body of empirical evidence has convincingly demonstrated that females often have a seed fertility advantage over hermaphrodites and that this correlates with their frequency in wild populations, as predicted by the theory. Although this evidence also suggests that hermaphrodites have reduced female fertility where females are common in gynodioecious species, the few studies that have examined whether these hermaphrodites are also ‘more male’ have not found this to be the case. However, in species where males and hermaphrodites can be distinguished, males tend to have greater male fertility than hermaphrodites, and the two studies of selection via siring success in gynodioecious species show that selection for increased male traits can occur. Given the paucity of the evidence and the multitude of ecological factors that can influence M : H fertility, we need to concentrate future efforts on advancing our understanding of what dictates the frequency of males. Such work will require a combination of genetic and experimental approaches to estimate siring success, selection studies, and field studies and experiments examining the role of various ecological factors in subdioecious species or among hermaphrodites in gynodioecious species from populations varying greatly in their sex ratio.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank J. Karron for inviting us to contribute to this special issue and A. Jantzi for help with the literature review. We thank current and past members of the Ashman laboratory for assistance. This manuscript was improved by comments from two anonymous reviewers and D. Levin. This work was supported by funds from the University of Pittsburgh and NSF (DEB 0449488 and 1020523) to T.L.A.
LITERATURE CITED
- Ågren J, Danell K, Elmqvist T, Ericson L, Hjältén J. Sexual dimorphism and biotic interactions. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer; 1999. pp. 217–246. [Google Scholar]
- Aguirre A, Vallejo-Marin M, Piedra-Malagon EM, Cruz-Ortega R, Dirzo R. Morphological variation in the flowers of Jacaratia mexicana A. DC. (Caricaceae), a subdioecious tree. Plant Biology. 2009;11:417–424. doi: 10.1111/j.1438-8677.2008.00154.x. [DOI] [PubMed] [Google Scholar]
- Alonso C. Herbivores do not discriminate between leaves of female and hermaphrodite individuals of gynodioecious Daphne laureola (Thymelaeaceae) Oikos. 2003;101:505–510. [Google Scholar]
- Angers B, Castonguay E, Massicotte R. Environmentally induced phenotypes and DNA methylation: how to deal with unpredictable conditions until the next generation and after. Molecular Ecology. 2010;19:1283–1295. doi: 10.1111/j.1365-294X.2010.04580.x. [DOI] [PubMed] [Google Scholar]
- Ashley MV. Plant parentage, pollination, and dispersal: how DNA microsatellites have altered the landscape. Critical Reviews in Plant Sciences. 2010;29:148–161. [Google Scholar]
- Ashman TL. Is relative pollen production or removal a good predictor of relative male fitness? An experimental exploration with a wild strawberry (Fragaria virginiana, Rosaceae) American Journal of Botany. 1998;85:1166–1171. [PubMed] [Google Scholar]
- Ashman TL. Pollinator selectivity and its implications for the evolution of dioecy and sexual dimorphism. Ecology. 2000;81:2577–2591. [Google Scholar]
- Ashman TL. The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology. 2002;83:1175–1184. [Google Scholar]
- Ashman TL. The limits on sexual dimorphism in vegetative traits in a gynodioecious plant. American Naturalist. 2005;166:S5–S16. doi: 10.1086/444598. [DOI] [PubMed] [Google Scholar]
- Ashman TL. The evolution of separate sexes: a focus on the ecological context. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. New York: Oxford University Press; 2006. pp. 204–222. [Google Scholar]
- Ashman T-L, Diefenderfer C. Sex ratio represents a unique context for selection on attractive traits: consequences for the evolution of sexual dimorphism. The American Naturalist. 2001;157:334–347. doi: 10.1086/319192. [DOI] [PubMed] [Google Scholar]
- Ashman TL, Penet L. Direct and indirect effects of a sex-biased antagonist on male and female fertility: consequences for reproductive trait evolution in a gender-dimorphic plant. American Naturalist. 2007;169:595–608. doi: 10.1086/513150. [DOI] [PubMed] [Google Scholar]
- Ashman T, Stanton M. Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana ssp. spicata (Malvaceae) Ecology. 1991;72:993–1003. [Google Scholar]
- Ashman TL, Cole DH, Bradburn M. Sex-differential resistance and tolerance to herbivory in a gynodioecious wild strawberry. Ecology. 2004;85:2550–2559. [Google Scholar]
- Ashman T, Spigler RB, Goldberg M, Govindarajulu R. Fragaria: a polyploid lineage for understanding sex chromosome evolution. In: Navajas-Pérez R, editor. New insights on plant sex chromosomes. Chap. VI. Hauppauge, NY: Nova Science Publishers, in press; 2011. [Google Scholar]
- Bailey MF, Delph LF. A field guide to models of sex-ratio evolution in gynodioecious species. Oikos. 2007;116:1609–1617. [Google Scholar]
- Barr CM. Soil moisture and sex ratio in a plant with nuclear–cytoplasmic sex inheritance. Proceedings of the Royal Society of London Biological Sciences. 2004;271:1935–1939. doi: 10.1098/rspb.2004.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CM, Fishman L. The nuclear component of a cytonuclear hybrid incompatibility in Mimulus maps to a cluster of pentatricopeptide repeat genes. Genetics. 2011;184:455–465. doi: 10.1534/genetics.109.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH. Gender variation and the evolution of dioecy in Wurmbea dioica (Liliaceae) Journal of Evolutionary Biology. 1992;5:423–444. [Google Scholar]
- Barrett SCH, Case AL, Peters GB. Gender modification and resource allocation in subdioecious (Colchicaceae) Journal of Ecology. 1999;87:123–137. [Google Scholar]
- Bawa KS. Evolution of dioecy in flowering plants. Annual Review of Ecology and Systematics. 1980;11:15–39. [Google Scholar]
- Bell G. On the function of flowers. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1985;224:223–265. [Google Scholar]
- Bishop E, Spigler R, Ashman T. Sex-allocation plasticity in hermaphrodites of sexually dimorphic Fragaria virginiana (Rosaceae) Botany. 2010;88:231–240. [Google Scholar]
- Boyko A, Kovalchuk I. Epigenetic control of plant stress response. Environmental and Molecular Mutagenesis. 2008;49:61–72. doi: 10.1002/em.20347. [DOI] [PubMed] [Google Scholar]
- Broyles SB, Wyatt R. Paternity analysis in a natural population of asclepias exaltata: multiple paternity, functional gender,, the ‘pollen-donation hypothesis’. Evolution. 1990;44:1454–1468. doi: 10.1111/j.1558-5646.1990.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Campbell D. Experimental tests of sex-allocation theory in plants. Trends in Ecology and Evolution. 2000;15:227–231. doi: 10.1016/s0169-5347(00)01872-3. [DOI] [PubMed] [Google Scholar]
- Carr DE, Dudash MR. The effects of five generations of enforced selfing on potential male and female function in Mimulus guttatus. Evolution. 1997;51:1797–1807. doi: 10.1111/j.1558-5646.1997.tb05103.x. [DOI] [PubMed] [Google Scholar]
- Case AL, Ashman TL. Resources and pollinators contribute to population sex-ratio bias and pollen limitation in Fragaria virginiana (Rosaceae) Oikos. 2009;118:1250–1260. [Google Scholar]
- Cepeda-Cornejo V, Dirzo R. Sex-related differences in reproductive allocation, growth, defense and herbivory in three dioecious neotropical palms. PLoS ONE. 2010;5:e9824. doi: 10.1371/journal.pone.0009824. doi:10.1371/journal.pone.0009824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SM. Gender-specific inbreeding depression in a gynodioecious plant, Geranium maculatum (Geraniaceae) American Journal of Botany. 2007;94:1193. doi: 10.3732/ajb.94.7.1193. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. A model for the evolution of dioecy and gynodioecy. American Naturalist. 1978;112:975–997. [Google Scholar]
- Charlesworth D. Allocation to male and female function in hermaphrodites, in sexually polymorphic populations. Journal of Theoretical Biology. 1989;139:327–342. [Google Scholar]
- Charlesworth D, Mank JE. The birds and the bees and the flowers and the trees: lessons from genetic mapping of sex determination in plants and animals. Genetics. 2010;186:9–31. doi: 10.1534/genetics.110.117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov EL. The theory of sex allocation. Princeton, NJ: Princeton University Press; 1982. [PubMed] [Google Scholar]
- Charnov EL, Maynard Smith J, Bull JJ. Why be a hermaphrodite? Nature. 1976;263:125–126. [Google Scholar]
- Chinnusamy V, Zhu J-K. Epigenetic regulation of stress responses in plants. Current Biology. 2009;12:1–9. doi: 10.1016/j.pbi.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DH, Ashman TL. Sexes show differential tolerance to Spittlebug damage and consequences of damage for multi-species interactions. American Journal of Botany. 2005;92:1708–1713. doi: 10.3732/ajb.92.10.1708. [DOI] [PubMed] [Google Scholar]
- Collin CL, Shykoff JA. Flowering phenology and female fitness: impact of a pre-dispersal seed predator on a sexually polymorphic species. Plant Ecology. 2010;206:1–13. [Google Scholar]
- Conner JK, Rush S, Kercher S, Jennetten P. Measurements of natural selection on floral traits in wild radish (Raphanus raphanistrum). 2. Selection through lifetime male and total fitness. Evolution. 1996;50:1137–1146. doi: 10.1111/j.1558-5646.1996.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Cornelissen T, Stiling P. Sex-biased herbivory: a meta-analysis of the effects of gender on plant-herbivore interactions. Oikos. 2005;111:488–500. [Google Scholar]
- Couvet D, Atlan A, Belhassen E, Gliddon C, Gouyon PH, Kjellberg F. Coevolution between two symbionts: the case of cytoplasmic male-sterility in higher plants. In: Futuyma D, Antonovics J, editors. Oxford surveys in evolutionary biology. Vol. 7. Oxford: Oxford University Press; 1990. pp. 225–249. [Google Scholar]
- Del Castillo RF, Argueta ST. Reproductive implications of combined and separate sexes in a trioecious population of Opuntia robusta (Cactaceae) American Journal of Botany. 2009;96:1148–1158. doi: 10.3732/ajb.0800301. [DOI] [PubMed] [Google Scholar]
- Delph LF. Sex-differential resource allocation patterns in the subdioecious shrub Hebe subalpina. Ecology. 1990;71:1342–1351. [Google Scholar]
- Delph LF. Sexual dimorphism in gender plasticity and its consequences for breeding system evolution. Evolution & Development. 2003;5:34–39. doi: 10.1046/j.1525-142x.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- Delph LF, Wolf DE. Evolutionary consequences of gender plasticity in genetically dimorphic breeding systems. New Phytologist. 2005;166:119–128. doi: 10.1111/j.1469-8137.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- Delph LF, Touzet P, Bailey MF. Merging theory and mechanism in studies of gynodioecy. Trends in Ecology & Evolution. 2007;22:17–24. doi: 10.1016/j.tree.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Dorken ME, Barrett SCH. Gender plasticity in Sagittaria sagittifolia (Alismataceae), a monoecious aquatic species. Plant Systematics and Evolution. 2003;237:99–106. [Google Scholar]
- Dorken ME, Barrett SCH. Phenotypic plasticity of vegetative and reproductive traits in monoecious and dioecious populations of Sagittaria latifolia (Alismataceae): a clonal aquatic plant. Journal of Ecology. 2004;92:32–44. [Google Scholar]
- Dorken ME, Mitchard ETA. Phenotypic plasticity of hermaphrodite sex allocation promotes the evolution of separate sexes: an experimental test of the sex-differential plasticity hypothesis using Sagittaria latifolia (Alismataceae) Evolution. 2008;62:971–978. doi: 10.1111/j.1558-5646.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- Dorken ME, Pannell JR. Hermaphroditic sex allocation evolves when mating opportunities change. Current Biology. 2009;19:514–517. doi: 10.1016/j.cub.2009.01.067. [DOI] [PubMed] [Google Scholar]
- Dufay M, Billard E. How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Annals of Botany. 2011;109:505–519. doi: 10.1093/aob/mcr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufay M, Pannell JR. The effect of pollen versus seed flow on the maintenance of nuclear-cytoplasmic gynodioecy. Evolution. 2010;64:772–784. doi: 10.1111/j.1558-5646.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- Dufay M, Lahiani E, Brachi B. Gender variation and inbreeding depression in gynodioecious-gynomonoecious Silene nutans (Caryophyllaceae) International Journal of Plant Sciences. 2010;171:53–62. [Google Scholar]
- Eckhart VM. Spatio-temporal variation in abundance and variation in foraging behavior of the pollinators of gynodioecious Phacelia linearis (Hydrophyllaceae) Oikos. 1992;64:573–586. [Google Scholar]
- Ehlers BK, Bataillon T. ‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New Phytologist. 2007;174:194–211. doi: 10.1111/j.1469-8137.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- Ehlers BK, Thompson JD. Temporal variation in sex allocation in hermaphrodites of gynodioecious Thymus vulgaris L. Journal of Ecology. 2004;92:15–23. [Google Scholar]
- Elle E, Hare JD. Environmentally induced variation in floral traits affects the mating system in Datura wrightii. Functional Ecology. 2002;16:79–88. [Google Scholar]
- Fishman L, Willis JH. A cytonuclear incompatibility causes anther sterility in Mimulus hybrids. Evolution. 2006;60:1372–1381. doi: 10.1554/05-708.1. [DOI] [PubMed] [Google Scholar]
- Fleming TH, Maurice S, Buchmann SL, Tuttle MD. Reproductive biology and relative male and female fitness in a trioecious cactus, Pachycereus pringlei (Cactaceae) American Journal of Botany. 1994;81:858–867. [Google Scholar]
- Glaettli M, Goudet J. Inbreeding effects on progeny sex ratio and gender variation in the gynodioecious Silene vulgaris (Caryophyllaceae) New Phytologist. 2006;172:763–773. doi: 10.1111/j.1469-8137.2006.01866.x. [DOI] [PubMed] [Google Scholar]
- Goldberg MT, Spigler RB, Ashman T-L. Comparative genetic mapping points to different sex chromosomes in sibling species of wild strawberry (Fragaria) Genetics. 2010;186:1425–1433. doi: 10.1534/genetics.110.122911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick R. Evolution of dioecy and sex chromosomes via methylation driving Muller's ratchet. Biological Journal of the Linnean Society. 2003;80:353–368. [Google Scholar]
- Gregorius H, Ross M, Gillet E. Selection in plant populations of effectively infinite size. V. Biallelic models of trioecy. Genetics. 1983;103:529. doi: 10.1093/genetics/103.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TY, Ross M. Maintenance of males and females in hermaphrodite populations. Heredity. 1974;32:113–118. doi: 10.1111/j.1558-5646.1976.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Jacobs MS, Wade MJ. A synthetic review of the theory of gynodioecy. American Naturalist. 2003;161:837–851. doi: 10.1086/375174. [DOI] [PubMed] [Google Scholar]
- de Jong TJ, Geritz SAH. Gradual evolution towards dioecy in cosexual plants. Selection. 2001;2:133–146. [Google Scholar]
- de Jong TJ, Klinkhamer PGL. Size-dependency of sex-allocation in hermaphroditic, monocarpic plants. Functional Ecology. 1989;3:201–206. [Google Scholar]
- de Jong TJ, Klinkhamer PGL, Rademaker MCJ. How geitonogamous selfing affects sex allocation in hermaphrodite plants. Journal of Evolutionary Biology. 1999;12:166–176. [Google Scholar]
- Jones A, Burd M. Vegetative and reproductive variation among unisexual and hermaphroditic individuals of Wurmbea dioica (Colchicaceae) Australian Journal of Botany. 2001;49:603–609. [Google Scholar]
- Kay Q. Hermaphrodites and subhermaphrodites in a reputedly dioecious plant, Cirsium arvense (L.) Scop. New Phytologist. 1985;100:457–472. [Google Scholar]
- Klinkhamer PGL, de Jong TJ, Metz H. Sex and size in cosexual plants. TREE. 1997;12:260–265. doi: 10.1016/s0169-5347(97)01078-1. [DOI] [PubMed] [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology, Evolution & Systematics. 2005;36:467–497. [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. American Naturalist. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. Male sterility in natural populations of hermaphrodite plants the equilibrium between females and hermaphrodites to be expected with different types of inheritance. New Phytologist. 1941;40:56–63. [Google Scholar]
- Lewis D. The evolution of sex in flowering plants. Biological Reviews. 1942;17:46–67. [Google Scholar]
- Lloyd DG. Theoretical sex ratios of dioecious and gynodioecious angiosperms. Heredity. 1974;32:11–34. [Google Scholar]
- Lloyd DG. Maintenance of gynodioecy and andriodioecy in angiosperms. Genetica. 1975;45:325–339. [Google Scholar]
- Lloyd DG. Transmission of genes via pollen and ovules in gynodioecious angiosperms. Theoretical Population Biology. 1976;9:299–316. doi: 10.1016/0040-5809(76)90050-2. [DOI] [PubMed] [Google Scholar]
- Lopes AV, Machado IC. Floral biology and reproductive ecology of Clusia nemorosa (Clusiaceae) in northeastern Brazil. Plant Systematics and Evolution. 1998;213:71–90. [Google Scholar]
- Manicacci D, Atlan A, Rossello JAE, Couvet D. Gynodioecy and reproductive trait variation in three Thymus species (Lamiaceae) International Journal of Plant Sciences. 1998;159:948–957. [Google Scholar]
- Marguerit E, Boury C, Minicki A, et al. Genetic dissection of sex determinism, inflorescence morphology and downy mildew resistance in grapevine. Theoretical and Applied Genetics. 2009;118:1261–1278. doi: 10.1007/s00122-009-0979-4. [DOI] [PubMed] [Google Scholar]
- Maurice S, Fleming TH. The effect of pollen limitation on plant reproductive systems and the maintenance of sexual polymorphisms. Oikos. 1995;74:55–60. [Google Scholar]
- Maurice S, Belhassen E, Couvet D, Gouyon PH. Evolution of dioecy: can nuclear-cytoplasmic interactions select for maleness? Heredity. 1994;73:346–354. doi: 10.1038/hdy.1994.181. [DOI] [PubMed] [Google Scholar]
- Maurice S, Desfeux C, Mignot A, Henry J. Is Silene acaulis (Caryophyllaceae) a trioecious species? Reproductive biology of two subspecies. Botany. 1998;76:478–485. [Google Scholar]
- McCall AC, Irwin RE. Florivory: the intersection of pollination and herbivory. Ecology Letters. 2006;9:1351–1365. doi: 10.1111/j.1461-0248.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- McCauley DE, Bailey MF. Recent advances in the study of gynodioecy: the interface of theory and empiricism. Annals of Botany. 2009;104:611–620. doi: 10.1093/aob/mcp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley D, Brock M. Frequency-dependent fitness in Silene vulgaris, a gynodioecious plant. Evolution. 1998;52:30–36. doi: 10.1111/j.1558-5646.1998.tb05135.x. [DOI] [PubMed] [Google Scholar]
- McCauley DE, Olson MS. Do recent findings in plant mitochondrial molecular and population genetics have implications for the study of gynodioecy and cytonuclear conflict? Evolution. 2008;62:1013–1025. doi: 10.1111/j.1558-5646.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- Miyake K, Olson MS. Experimental evidence for frequency dependent self-fertilization in the gynodioecious plant. Silene vulgaris. Evolution. 2009;63:1644–1652. doi: 10.1111/j.1558-5646.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- Morand-Prieur ME, Raquin C, Shykoff JA, Frascaria-Lacoste N. Males outcompete hermaphrodites for seed siring success in controlled crosses in the polygamous Fraxinus excelsior (Oleaceae) American Journal of Botany. 2003;90:949–953. doi: 10.3732/ajb.90.6.949. [DOI] [PubMed] [Google Scholar]
- Narbona E, Dirzo R. Experimental defoliation affects male but not female reproductive performance of the tropical monoecious plant Croton suberosus (Euphorbiaceae) Annals of Botany. 2010;106:359–369. doi: 10.1093/aob/mcq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MS. Patterns of fruit production in the subdioecious plant Astilbe biternata (Saxifragaceae) Ecology. 2001;89:600–607. [Google Scholar]
- Paquin V, Aarssen LW. Allometric gender allocation in Ambrosia artemisiifolia (Asteraceae) has adaptive plasticity. American Journal of Botany. 2004;91:430–438. doi: 10.3732/ajb.91.3.430. [DOI] [PubMed] [Google Scholar]
- Penet L, Collin CL, Ashman TL. Florivory increases selfing: an experimental study in the wild strawberry, Fragaria virginiana. Plant Biology. 2009;11:38–45. doi: 10.1111/j.1438-8677.2008.00141.x. [DOI] [PubMed] [Google Scholar]
- Philipp M, Jakobsen RB, Nachman G. A comparison of pollen-siring ability and life history between males and hermaphrodites of subdioecious Silene acaulis. Evolutionary Ecology Research. 2009;11:787–801. [Google Scholar]
- Pickering C, Ash J. Gender variation in hermaphrodite plants: evidence from five species of alpine Ranunculus. Oikos. 1993;68:539–548. [Google Scholar]
- Queller D. Pollen removal, paternity, and the male function of flowers. American Naturalist. 1997;149:585–594. [Google Scholar]
- Ramsey M, Vaughton G. Sex expression and sexual dimorphism in subdioecious Wurmbea dioica (Colchicaceae) International Journal of Plant Sciences. 2001;162:589–597. [Google Scholar]
- Renner S, Ricklefs R. Dioecy and its correlates in the flowering plants. American Journal of Botany. 1995;82:596–606. [Google Scholar]
- Richards AJ. Plant breeding systems. London: George Allen & Unwin; 1986. [Google Scholar]
- Ross MD. Behavior of a sex-differential fertility gene in hermaphrodite populations. Heredity. 1977;38:279–290. [Google Scholar]
- Ross MD. The evolution of gynodioecy and subdioecy. Evolution. 1978;32:147–188. doi: 10.1111/j.1558-5646.1978.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Ross MD. Five evolutionary pathways to subdioecy. American Naturalist. 1982;119:297–318. [Google Scholar]
- Ross M, Weir B. Maintenance of males and females in hermaphrodite populations and the evolution of dioecy. Evolution. 1976;30:425–441. doi: 10.1111/j.1558-5646.1976.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Sakai AK, Weller SG. Ecological aspects of sex expression in subdioecious Schiedea globosa (Caryophyllaceae) American Journal of Botany. 1991;78:1280–1288. [Google Scholar]
- Sakai AK, Weller SG. Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer; 1999. [Google Scholar]
- Sakai AK, Weller SG, Chen ML, Chou SY, Tasanont C. Evolution of gynodioecy and maintenance of females: the role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea adamantis (Caryophyllaceae) Evolution. 1997;51:724–736. doi: 10.1111/j.1558-5646.1997.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Saumitou-Laprade P, Vernet P, Vassiliadis C, et al. A self-incompatibility system explains high male frequencies in an androdioecious plant. Science. 2010;327:1648–1650. doi: 10.1126/science.1186687. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Stewart SC. Variation in male reproductive investment and male reproductive success in white spruce. Evolution. 1986;40:1109–1120. doi: 10.1111/j.1558-5646.1986.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Schultz ST. Nucleo-cytoplasmic male sterility and alternative routes to dioecy. Evolution. 1994;48:1933–1945. doi: 10.1111/j.1558-5646.1994.tb02224.x. [DOI] [PubMed] [Google Scholar]
- Seger J, Eckhart VM. Evolution of sexual systems and sex allocation in plants when growth and reproduction overlap. Proceedings of the Royal Society B, Biological Sciences. 1996;263:833–841. [Google Scholar]
- Shykoff JA, Kolokotronis S-O, Collin CL, López-Villavicencio M. Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia. 2003;135:1–9. doi: 10.1007/s00442-002-1133-z. [DOI] [PubMed] [Google Scholar]
- Silva CA, Oliva MA, Vieira MF, Fernandes GW. Trioecy in Coccoloba cereifera Schwacke (Polygonaceae), a narrow endemic and threatened tropical species. Brazilian Archives of Biology and Technology. 2008;51:1003–1010. [Google Scholar]
- Snow AA, Lewis PO. Reproductive traits and male fertility in plants: empirical approaches. Annual Review of Ecology and Systematics. 1993;24:331–351. [Google Scholar]
- Spigler RB, Ashman TL. Sex ratio and subdioecy in Fragaria virginiana: the roles of plasticity and gene flow examined. New Phytologist. 2011;190:1058–1068. doi: 10.1111/j.1469-8137.2011.03657.x. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Lewers KS, Main DS, Ashman TL. Genetic mapping of sex determination in a wild strawberry, Fragaria virginiana, reveals earliest form of sex chromosome. Heredity. 2008;101:507–517. doi: 10.1038/hdy.2008.100. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Lewers K, Ashman T-L. Genetic architecture of sexual dimorphism in a subdioecious plant with a proto-sex chromosome. Evolution. 2011;65:1114–1126. doi: 10.1111/j.1558-5646.2010.01189.x. [DOI] [PubMed] [Google Scholar]
- Steets JA, Ashman T-L. Herbivory alters the expression of a mixed-mating system. American Journal of Botany. 2004;91:1046–1051. doi: 10.3732/ajb.91.7.1046. [DOI] [PubMed] [Google Scholar]
- Steets JA, Knight TM, Ashman TL. The interactive effects of herbivory and mixed mating for the population dynamics of Impatiens capensis. American Naturalist. 2007;170:113–127. doi: 10.1086/518178. [DOI] [PubMed] [Google Scholar]
- Sutherland S. Why hermaphroditic plants produce many more flowers than fruits: experimental tests with Agave mckelveyana. Evolution. 1987;41:750–759. doi: 10.1111/j.1558-5646.1987.tb05850.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Tarayre M. Exploring the genetic basis and proximate causes of female fertility advantage in gynodioecious Thymus vulgaris. Evolution. 2000;54:1510–1520. doi: 10.1111/j.0014-3820.2000.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Torices R, Méndez M, Gómez JM. Where do monomorphic sexual systems fit in the evolution of dioecy? Insights from the largest family of angiosperms. New Phytologist. 2001;190:234–248. doi: 10.1111/j.1469-8137.2010.03609.x. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Sota T. Sexual differences in flower defense and correlated male-biased florivory in a plant–florivore system. Oikos. 2010;119:1848–1853. [Google Scholar]
- Van Etten ML. The evolution and maintenance of gynodioecy in Geranium maculatum. University of Georgia, Athens: GA; 2009. PhD thesis. [Google Scholar]
- Verdú M, García-Fayos P, Gleiser G. Mites attack males of the sexually polymorphic tree Acer opalus more harmfully and more often. Functional Ecology. 2004;18:592–597. [Google Scholar]
- Wang LH, Zhang BX, Lefebvre V, Huang SW, Daubèze AM, Palloix A. QTL analysis of fertility restoration in cytoplasmic male sterile pepper. Theoretical and Applied Genetics. 2004;109:1058–1063. doi: 10.1007/s00122-004-1715-8. [DOI] [PubMed] [Google Scholar]
- Webb C. Flowering periods in the gynodioecious species Gingidia decipiens (Umbelliferae) New Zealand Journal of Botany. 1976;14:207–210. [Google Scholar]
- Webb CJ. Empirical studies: evolution and maintenance of dimorphic breeding systems. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer; 1999. pp. 61–95. [Google Scholar]
- Weiblen GD, Oyama RK, Donoghue MJ. Phylogenetic analysis of dioecy in monocotyledons. American Naturalist. 2000;155:46–58. doi: 10.1086/303303. [DOI] [PubMed] [Google Scholar]
- Wilcock C, Neiland R. Pollination failure in plants: why it happens and when it matters. Trends in Plant Science. 2002;7:270–277. doi: 10.1016/s1360-1385(02)02258-6. [DOI] [PubMed] [Google Scholar]
- Wise MJ, Hébert JB. Herbivores affect natural selection for floral-sex ratio in a field population of horsenettle, Solanum carolinense. Ecology. 2010;91:937–943. doi: 10.1890/09-1373.1. [DOI] [PubMed] [Google Scholar]
- Wolfe LM, Shmida A. The ecology of sex expression in a gynodioecious Israeli desert shrub (Ochradenus baccatus) Ecology. 1997;78:101–110. [Google Scholar]
- Zhang YW, Yang CF, Zhao JM, Guo YH. Selective nectar robbing in a gynodioecious plant (Glechoma longituba) enhances female advantage. Journal of Evolutionary Biology. 2009;22:527–535. doi: 10.1111/j.1420-9101.2008.01669.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.