Abstract
Background and Aims
The number of flowers blooming simultaneously on a plant may have profound consequences for reproductive success. Large floral displays often attract more pollinator visits, increasing outcross pollen receipt. However, pollinators frequently probe more flowers in sequence on large displays, potentially increasing self-pollination and reducing pollen export per flower. To better understand how floral display size influences male and female fitness, we manipulated display phenotypes and then used paternity analysis to quantify siring success and selfing rates.
Methods
To facilitate unambiguous assignment of paternity, we established four replicate (cloned) arrays of Mimulus ringens, each consisting of genets with unique combinations of homozygous marker genotypes. In each array, we trimmed displays to two, four, eight or 16 flowers. When fruits ripened, we counted the number of seeds per fruit and assigned paternity to 1935 progeny.
Key Results
Siring success per flower declined sharply with increasing display size, while female success per flower did not vary with display. The rate of self-fertilization increased for large floral displays, but siring losses due to geitonogamous pollen discounting were much greater than siring gains through increased self-fertilization. As display size increased, each additional seed sired through geitonogamous self-pollination was associated with a loss of 9·7 seeds sired through outcrossing.
Conclusions
Although total fitness increased with floral display size, the marginal return on each additional flower declined steadily as display size increased. Therefore, a plant could maximize fitness by producing small displays over a long flowering period, rather than large displays over a brief flowering period.
Keywords: Bumble-bee, floral display size, functional gender, geitonogamy, male selfing rate, mating system, Mimulus ringens, paternity analysis, pollen discounting, pollination, self-fertilization, siring success
INTRODUCTION
In many natural plant populations the number of flowers blooming simultaneously varies greatly among individuals. As the abundance of floral resources can strongly influence patterns of pollinator behaviour, this variation in floral display size may have profound consequences for reproductive success. Plants with many open flowers often attract more pollinator visits than plants with few flowers, potentially increasing outcross pollen receipt and resulting seed-set (Schmid-Hempel and Speiser, 1988; Mitchell, 1994; Galloway et al., 2002). Frequent pollinator visits to large floral displays might also enhance pollen export and siring success (Willson and Price, 1977; Broyles and Wyatt, 1990). However, pollinators usually probe more flowers in sequence on large displays than on small displays (Darwin, 1876; Dudash, 1991; Robertson, 1992; Mitchell et al., 2004). These within-plant pollinator movements are likely to be costly both for male and for female reproductive success, especially when pollen carryover is limited (Harder and Barrett, 1995; Barrett and Harder, 1996; Lau et al., 2008). For example, self-fertilization resulting from geitonogamous (among-flower, within-plant) self-pollination may lower fitness due to inbreeding depression (Snow et al., 1996; Eckert, 2000). In addition, geitonogamous self-pollination may reduce the pool of gametes that can be exported to other plants (‘pollen discounting’; Holsinger et al., 1984; Harder and Barrett, 1995; Barrett, 2003; Lau et al., 2008), offsetting siring advantages associated with an increased rate of pollinator visitation. Thus, although larger floral displays may enhance outcross siring success (e.g. Broyles and Wyatt, 1990), losses of pollen due to within-plant pollinator movements may be so severe that the gain in number of outcross seeds sired may diminish with increases in display size (Lau et al., 2008).
Most empirical studies of the effects of floral display size focus on just one or two components of reproductive success, typically female fecundity or female selfing rate (e.g. Crawford, 1984; Snow et al., 1996; Eckert, 2000; Karron et al., 2004). By contrast, the effects of floral display on siring success have only rarely been quantified (Harder and Barrett, 1995; Lau et al., 2008) and the quality of sired offspring, incorporating the male selfing rate and inbreeding depression, have not previously been reported. Here we present the first study to quantify the effects of floral display on both male and female fitness, combining data on the number and quality of offspring through the two sexual functions.
A novel feature of our research is that we manipulated floral display size in arrays where we could quantify self-siring and outcross-siring of individual plants using unambiguous paternity assignment. This allowed us to examine the relationship between these parameters without the estimation errors typically associated with statistical inference of selfing rates and paternity (Ritland, 1990, 2002; Morgan, 1998; Lau et al., 2008). Another unique feature of our research is that we incorporate data on inbreeding depression and both male and female selfing rates into our calculations of total reproductive success.
We address the following questions: (1) Do male and female reproductive success respond differently to an increase in floral display size? (2) As display size increases, are outcross siring losses due to geitonogamous pollen discounting greater than siring gains through increased self-fertilization? (3) What is the overall effect of floral display size on fitness?
MATERIALS AND METHODS
Study species
Mimulus ringens L. (Phrymaceae) is a wetland perennial herb native to central and eastern North America (Grant, 1924). Daily floral display varies widely within M. ringens populations, often ranging from one to more than 20 open flowers. The anthers dehisce before dawn, and individual flowers typically receive 1–4 probes by bumble-bee workers between 0530 and 1100 h (Mitchell et al., 2004, 2005; Karron et al., 2006). By 1100 h most stigmas have closed, and corollas are shed by late afternoon, approximately 12 h following anthesis. In the year of this study (2000) Bombus fervidus workers accounted for more than 80 % of all floral visits, with four other species of Bombus accounting for the remainder (Mitchell et al., 2004).
Mimulus ringens is self-compatible, and nearly all flowers produce capsules containing 700–5500 seeds (Karron et al., 2006). Female selfing rates vary widely within and among populations, largely because of the effects of plant spacing and floral display size on the frequency of geitonogamous pollination (Karron et al., 1995a, 2004, 2009), but also due to other factors such as the extent of heterospecific pollen loss when competitors for pollination are present (Bell et al., 2005; Flanagan et al., 2009). In our main study population seeds resulting from controlled self and outcross hand-pollinations do not differ in seed-set per fruit, seed mass, germination rate or seedling survival (J. D. Karron et al., unpubl. res.). However, self progeny have lower fitness than outcross progeny in flower production, pollen fertility and seed production. Overall the cumulative fitness of self progeny was 21·1 % lower than the fitness of outcross progeny (J. D. Karron and R. J. Mitchell, unpubl. res.).
Use of experimental arrays to facilitate paternity analysis
To facilitate paternity exclusion, we bred a set of 16 Mimulus genets with unique combinations of homozygous genotypes at four unlinked allozyme loci (Karron et al., 1995a, 2004). Once genets with these 16 multilocus combinations of homozygous genotypes had been identified, we clonally propagated the genets so that we could explore the effects of floral display size manipulations on a common genetic background (Karron et al., 1995a).
On 7 June 2000 we planted replicate arrays into each of four isolated experimental gardens at the UW-Milwaukee Field Station (Saukville, WI, USA). To minimize pollen dispersal between arrays, gardens were separated by a minimum of 75 m of vegetation containing a high abundance of several unrelated bumble-bee-pollinated plants. Gene flow from natural populations was unlikely as the nearest natural population was >15 km away. In each garden we planted a single array of 36 Mimulus plants in a square grid with 0·8-m spacing. In the centre of each array we planted single ramets of each of 15 different genets. We arranged these ‘central’ genets in a different random order in each array. To minimize edge effects on patterns of pollinator visitation, we surrounded the 15 central genets in each array with a ‘border’ composed of 21 ramets of a 16th genet (genet ‘D’; Fig. 1). We fertilized and weeded our experimental arrays to ensure that all plants produced sufficient flowers for each display size treatment.
Fig. 1.
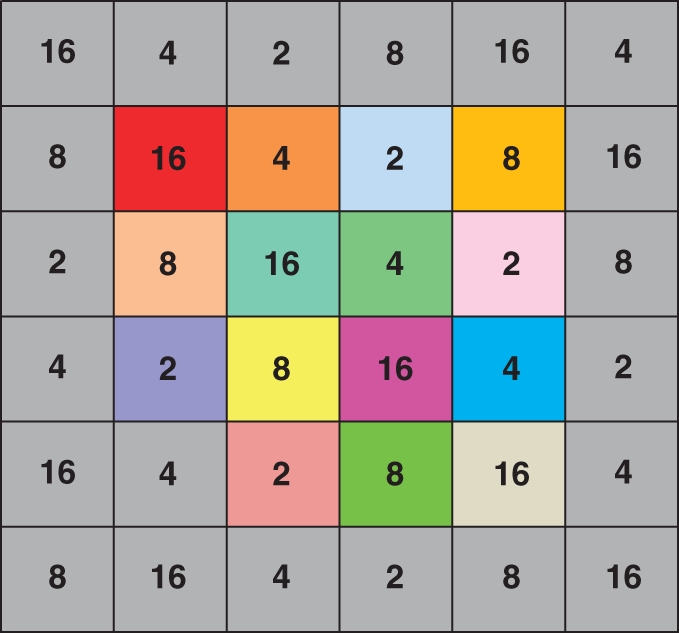
Arrangement of genets and floral display size treatments in one of the four experimental arrays. Numbers indicate the floral display size treatment for each spatial position. Colour indicates the genetic identity of individual plants. The 15 brightly coloured locations in the centre of the array represent single ramets of 15 genetically distinct individuals. The grey locations on the border, and at the lower left corner, of the central 4 × 4 square indicate multiple ramets of border genet ‘D’. Note that each central plant is surrounded by two plants with each of the floral display treatments. Therefore, each central plant has an equivalent neighbourhood in terms of display size.
Floral display size treatments
On 10 and 11 August 2000, during the peak period of flowering, we experimentally manipulated floral display size by trimming displays on all 36 plants in each array to one of four display sizes (two, four, eight or 16 flowers) that span much of the range in display observed in natural M. ringens populations. Using scissors we removed excess flowers in the early morning hours, before pollinators became active. As shown in Fig. 1, we used a regular spatial arrangement of display sizes so that each ‘central genet’ was surrounded by two plants with each display size. We rotated floral display size classes among arrays such that each ‘central genet’ experienced all four display sizes.
We assigned the same floral display size treatment to individual plants on both 10 and 11 August. The manipulation on 10 August served to allow pollinators to acclimate to display sizes, as traplining bees may exhibit behaviours related to a previous day's floral display (Thomson, 1999). Therefore, we did not tag the fruits of 10 August for paternity analysis. On 11 August, we recorded patterns of pollinator visitation during several 20-min observation periods in all four experimental arrays from 0620 until 1100 h. To do this, two teams of three observers rotated among the gardens, recording a total of 1310 bumble-bee visits (72 foraging bouts). Here we report the frequency of geitonogamous (within-plant) moves; more complete examinations of pollinator responses are reported elsewhere (Karron et al., 2004; Mitchell et al., 2004). At 1400 h, 3 h after all stigmas closed, we tied labelled plastic tags to pedicels of all open flowers.
Determining seed-set, selfing rates and patterns of paternity
We harvested tagged fruits on 14 September 2000 and stored them individually in centrifuge tubes at 4 °C. To quantify female reproductive success on the 15 unique genets in the centre of each array, we used a dissecting microscope to count the number of seeds in each of two fruits from each of these 60 plants. Total seeds mothered by a daily floral display was then calculated as [number of open flowers × mean seeds/flower].
To genotype seedlings for paternity assignment, we established progeny arrays for all 60 ‘central’ genets (15 plants with unique genotypes per array × four arrays). Using a separate pot for each fruit, we germinated seeds from all tagged fruits on two- and four-flower displays, and from four randomly chosen fruits on eight- and 16-flower displays. Germination rates were uniformly high (>85 %) in all four display size classes. Two-week-old seedlings were transplanted into 5-cm cells in plastic flats, and then grown for three additional weeks until large enough for genotyping.
We genotyped up to ten progeny (mean = 9·9 seedlings) from each fruit following the methods described in Karron et al. (2004). Paternity was assigned unambiguously to a total of 1935 seedlings by simple exclusion (Karron et al., 1995a, 1997). We classified each sampled seedling as self or outcross, and identified the sire of all outcross seeds.
To score overall male function success we estimated the total number of seeds sired by each plant. To do this we first evaluated the number of seeds sired by each donor on each recipient as [(proportion of seeds on plantj that were sired by plantk) × (total seeds mothered on plantj)]. We then calculated male reproductive success for each donor by summing that donor's siring success across all 15 of the ‘central genets’ in an array. In our analysis we do not include seeds mothered by ramets of genet ‘D’ (including both border plants and the central ramet with genotype ‘D’) because selfed seeds on these plants cannot be distinguished from seeds sired by another ‘D’ ramet. We estimated functional gender following Lloyd (1980) by assessing the proportion of reproductive success that was achieved through female function [seeds mothered/(seeds mothered + seeds sired)]. We calculated selfing rate through female function as the proportion of all seeds mothered by that plant that were selfed, and calculated selfing rate through male function as the proportion of all seeds sired by that plant that were selfed.
To calculate total fitness we first weighted the contribution of selfed offspring by 0·789, the mean relative fitness of self to outcross progeny in this population. For female function fitness, we then summed the number of outcross seeds and the weighted number of selfed seeds for each maternal plant, and divided each mother's total across the array by the total weighted number of seeds mothered by central genets in that array (Devlin et al., 1992). Likewise, for male function fitness we used an analogous procedure, summing the outcross seeds and the weighted number of selfed seeds for each sire, then divided that total by the weighted number of seeds sired by central genets in that array. Finally, to calculate total fitness we averaged the proportional success through male and through female function for each genet. For most of the response variables described above we calculated success per flower by dividing the total by the number of flowers in the display.
Data analysis
We tested the effects of Floral Display Size Treatment, Array and Interaction on measures of reproductive success, using a fixed-effects ANOVA (Proc GLM in SAS version 9·2), treating Display Size as a categorical factor. Display size was the only significant term in all but one ANOVA. Therefore, in most cases we present only the test for effects of Display Size, using a pooled error term. However, in one ANOVA the Interaction and Array terms were significant, so we report the full ANOVA for that case (self seeds sired per plant). Exploratory analyses indicated no strong differences among genets in any response variables, so we do not consider that source of variation further. In one of our analyses (seeds sired per flower) ln-transformation was necessary to meet assumptions of ANOVA. To test for proportionality of increase in response variables with Display Size we used ln–ln regression and tested for a slope of one, following Klinkhamer and de Jong (2005), using the ‘test’ option of SAS Proc REG.
RESULTS
Floral display size affected male and female reproductive success in very different ways. On a per-flower basis, the number of seeds sired decreased significantly for larger floral displays (F3,56 = 23·46, P < 0·0001). Individual flowers on two-flower displays sired an average of 7406 seeds (back-transformed from LS-mean of ln values), nearly three times as many progeny as were sired by individual flowers on 16-flower displays (Fig. 2A). In contrast, the number of seeds mothered per flower did not vary significantly across display size treatments (F3,56 = 0·13, P = 0·95). Individual fruits averaged approx. 4100 seeds in all four floral display size treatments (Fig. 2A), and all flowers produced seeds. On a per-plant basis (multiplying the per-flower values by floral display size), larger floral displays strongly increased both the number of seeds sired (F3,56 = 18·22, P < 0·0001) and the number of seeds mothered (F3,56 = 119·8, P < 0·0001). Although number of seeds mothered and sired per plant both rose with floral display, siring success per plant increased much more gradually (Fig. 2B). Sixteen-flower displays mothered 7·8 times as many seeds as two-flower displays, but only sired 2·6 times as many seeds. Indeed, the increase in seeds sired showed decelerating gains (a negative first derivative; see Fig. 2A) with increases in floral display (ln–ln regression rejects the hypothesis that the slope is 1·0; slope = 0·46, F1,58 = 70, P > 0·0001). And unlike the male response, the first derivative for seeds mothered was constant, so that seeds mothered increased in direct proportion to the increase in display size (ln–ln regression testing for slope = 1·0, slope = 0·98, F1,58 =0·10, P > 0·75). As a result of these sexual differences in response to display, functional gender varied significantly among floral display treatments (Fig. 2C; F3,56 = 20·68, P < 0·0001), with proportionately more male function success for small displays, and more female function success for large displays.
Fig. 2.
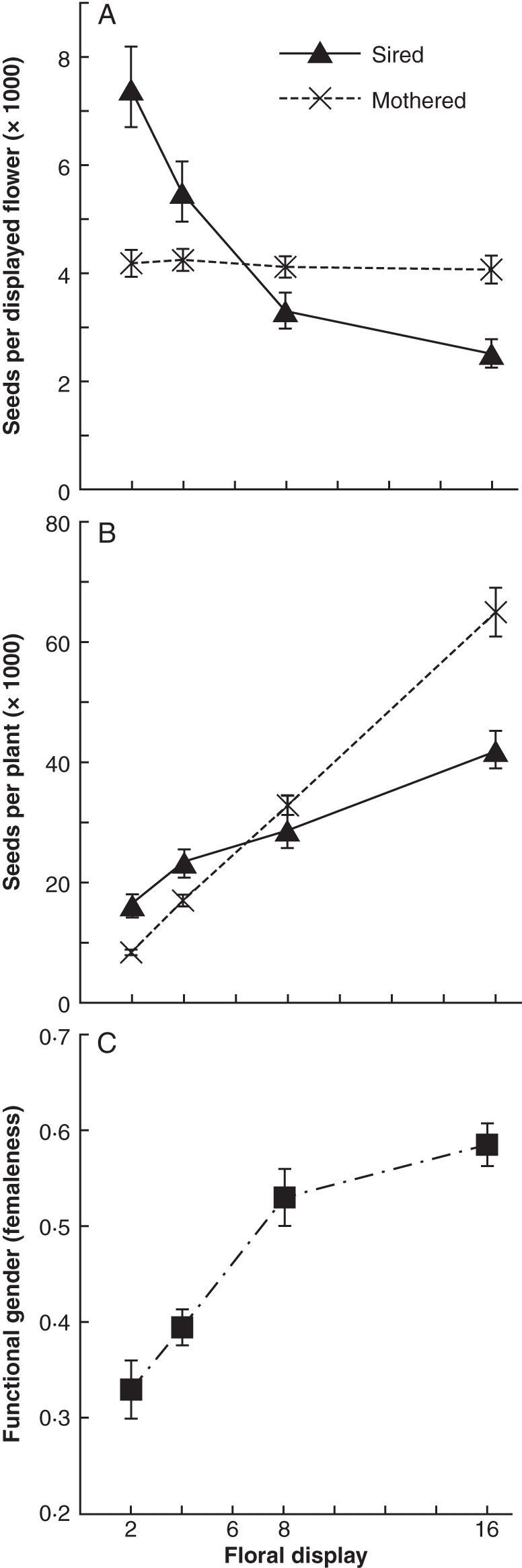
(A) Effects of floral display size on the mean number of seeds sired or mothered by each open flower (mean ± s.e.); n = 15 plants per data point. (B) Effects of floral display size on the mean number of seeds sired or mothered by all displayed flowers on a plant (mean ± s.e.); n = 15 plants per data point. Values for seeds sired per displayed flower are back-transformed from ln-transformed LS mean and s.e., and statistical tests in the text were based on ln-transformed values to meet assumptions of the analysis. (C) Effect of floral display size on the functional gender of individual plants (mean ± s.e.). Functional genders below 0·5 indicate higher reproductive success through male function than through female function; n = 15 plants per data point.
Siring success per flower through outcrossing and selfing responded very differently to changes in floral display size (Fig. 3A). The number of outcross seeds sired per flower varied significantly across display sizes (F3,56 = 22·78, P < 0·00001), declining from 7167 ± 904 outcross seeds sired per flower in two-flower displays to just 1062 ± 97 outcross seeds sired per flower for 16-flower displays. By contrast, the number of self seeds sired per flower increased slightly with display size (F3,56 = 2·80, P < 0·05). Note that the increased self siring per flower in 16-flower displays was not sufficient to offset the marked decline in outcross siring success in these large displays. The strikingly different effects of floral display size on numbers of self and outcross seeds sired per flower were also apparent on a per-plant basis (Fig. 3B). The number of outcross seeds sired per display did not vary significantly among floral display size treatments (F3,56 = 0·78, P = 0·5), with a mean value of 16 910 ± 1017 seeds. By contrast, the number of self seeds sired per plant was strongly and significantly affected by floral display size (F3,44 = 55·19, P < 0·00001), increasing from 1819 ± 318 in two-flower displays to 25045 ± 2638 in 16-flower displays. In this one analysis (self seeds sired per flower) the array and interaction terms were significant (F3,44 = 3·64, P < 0·02; F9,44 = 2·81, P < 0·02, respectively); this reflects lower than expected self seed production for 16-flowered plants in one of the arrays (mean = 11379 vs. a mean of 28461 for the other arrays). All other responses were consistent across treatment–array combinations.
Fig. 3.
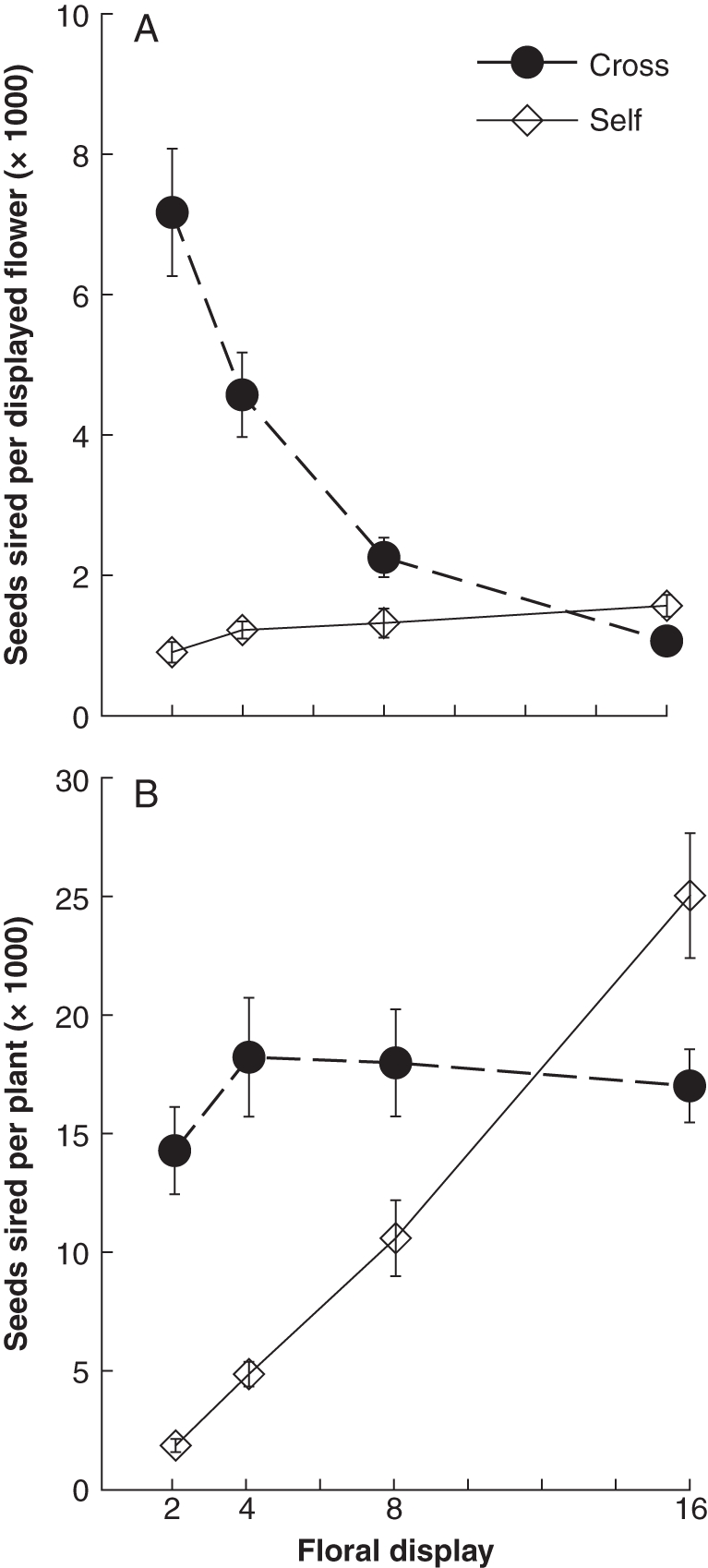
(A) Effects of floral display size on the mean number of outcross and self seeds sired by each open flower (mean ± s.e.); n = 15 plants per data point. (B) Effects of floral display size on the mean number of outcross or self seeds sired by all displayed flowers on a plant (mean ± s.e.); n = 15 plants per data point.
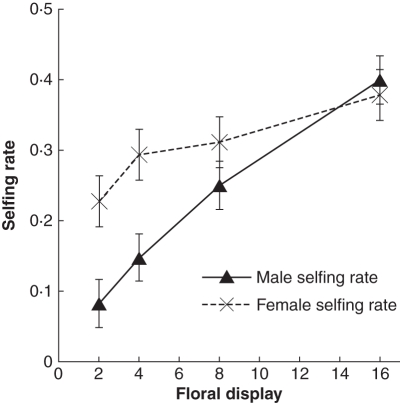
The proportion of pollinator moves that were within-plant (geitonogamous) increased significantly with floral display size (Fig. 4; F3,503= 338, P < 0·001), and this was associated with a reduction in the number of outcross seeds sired per flower (Fig. 4; correlation of treatment means; r = –0·983, P < 0·02, n = 4). Concurrently, selfing rates increased significantly with floral display size (Fig. 5), both from the female perspective (F3,56 = 3·33, P < 0·026) and from the male perspective (F3,56 = 16·49, P < 0·0001). The changes in male selfing rate were especially pronounced, increasing from 0·083 ± 0·016 on two-flower displays to 0·400 ± 0·051 on 16-flower displays.
Fig. 4.
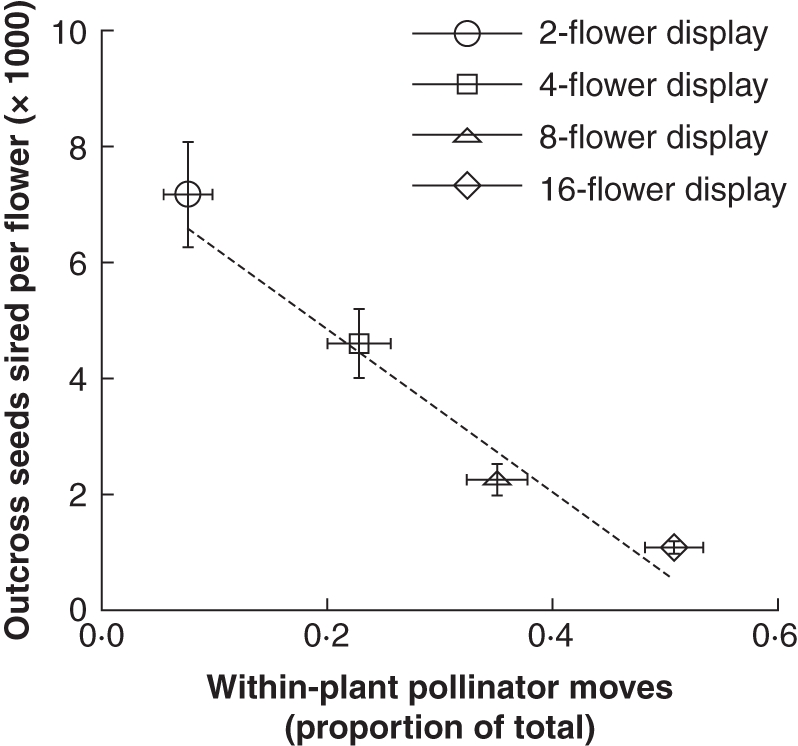
Effect of geitonogamous pollinator movements on outcross siring success per flower (mean ± s.e.) for plants in each floral display size treatment; n = 66–189 plant visits per data point for the x-axis, n = 15 plants per data point for the y-axis. Dashed line represents a linear regression fitted to the means.
Fig. 5.
Effect of floral display size on mean selfing rates of individual plants (mean ± s.e.). The male selfing rate is the proportion of sired seeds resulting from self-fertilization. The female selfing rate is the proportion of mothered seeds resulting from self-fertilization. n = 15 plants per data point.
Total fitness per flower, accounting for selfing rates and inbreeding depression, decreased significantly with floral display size (F3,56 = 18·0, P < 0·0001). Each flower in the two-flower displays had more than twice the fitness of each flower in the 16-flower displays (Fig. 6A). When looked at on a per-plant basis, however, total fitness increased with display size (Fig. 6B; F3,56 = 85, P < 0·0001), although the gains in fitness were less than proportional to the increase in display size (ln–ln regression rejects the hypothesis of a 1·0 slope; slope = 0·65, F1,58 = 70, P > 0·0001).
Fig. 6.
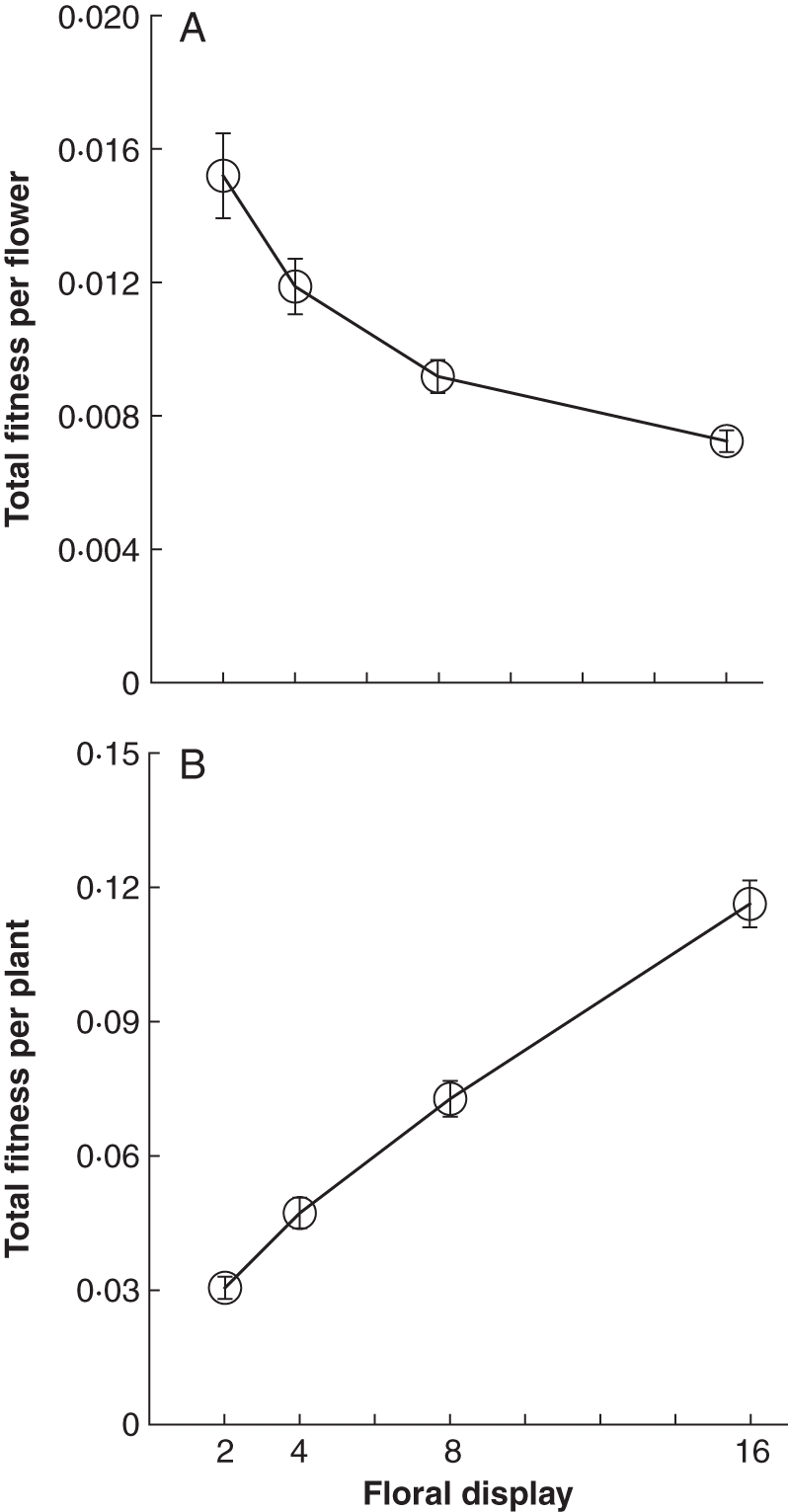
(A) Effect of floral display size on mean total fitness per flower (mean ± s.e.). Total fitness per flower was calculated as the fractional contribution to population seed yield through both male and female function. Fitnesses were adjusted to account for inbreeding depression in selfed seeds. (B) Effect of floral display size on mean total fitness of all displayed flowers on a plant (mean ± s.e.). n = 15 plants per data point.
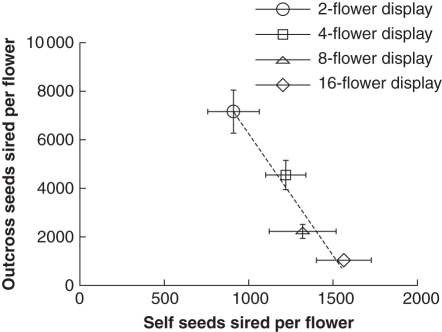
Increased selfing for large floral displays was associated with a decrease in the number of outcross seeds sired, demonstrating strong pollen discounting (Fig. 7). Linear regression indicates that each additional seed sired through geitonogamous self-pollination was associated with a loss of 9·7 seeds sired through outcrossing.
Fig. 7.
Relationship between self seeds sired per flower and outcross seeds sired per flower for each floral display size treatment (mean ± s.e.); n = 15 plants per data point. The dashed line represents a linear regression for these means; regression equation: Outcross Seeds Sired per Flower = 15922 – 9·7 × (Self Seeds Sired per Flower); r2 = 0·95, P < 0·025).
DISCUSSION
Experimental manipulation of floral display size affected male and female reproductive success in very different ways. Siring per flower declined sharply on larger displays, while seeds mothered per flower did not vary with display size (Fig. 2A). The decline in siring success was even more striking when siring was partitioned into outcross-siring and self-siring (Fig. 3A). On average, each flower on two-flower displays sired 6·7 times as many outcross seeds as did individual flowers on 16-flower displays. This reduction in outcross-siring per flower was much greater than the increase in self-siring in large displays (Fig. 3A), demonstrating that geitonogamous pollen discounting can strongly influence male reproductive success (Harder and Barrett, 1995; Barrett and Harder, 1996; Lau et al., 2008). We first explore why pollen discounting is so strong in Mimulus ringens, and then consider the implications of our findings for the evolution of floral display size.
Geitonogamous pollen discounting
With an increase in Mimulus display size, each additional seed sired through geitonogamous self-pollination was associated with a loss of 9·7 seeds sired through outcrossing (Fig. 7). This strong per-flower decline in outcross siring, coupled with a modest increase in selfing, is consistent with the results from a study of Ipomoea purpurea, in which each additional self seed was associated with an outcross siring loss of 3·99 seeds (Lau et al., 2008). Our finding suggests that when pollinators probe several Mimulus flowers in sequence on many-flowered displays, pollen that could potentially play a role in siring seeds on other plants is instead deposited onto self stigmas, lost to other floral structures or lost to grooming (Fig. 4; Harder and Wilson, 1998). Such costs would be especially likely in a species with very restricted pollen carryover. In fact, pollen-mediated gene dispersal is highly restricted in M. ringens (Karron et al., 1995b). Nearly 60 % of the pollen from a donor flower is dispersed to the next flower probed by a pollinator, and nearly 90 % of the pollen from a donor flower is dispersed to the first two recipient flowers (Holmquist et al., 2011). Therefore, when pollinators probe three or more flowers consecutively on a display, pollen from the first flowers probed will have little opportunity to contribute to outcross siring success.
Pollen discounting may also explain another intriguing pattern in our results: on a per-plant basis, an 800 % increase in Mimulus display size caused just a 19 % increase (non-significant) in outcross siring success. It is as if the last one or two flowers probed by a pollinator are the only ones that contribute appreciably to outcross siring. This finding differs markedly from several other studies, which found that outcross siring success per plant increases strongly with increasing floral display size, or at least exhibits saturating gains (Broyles and Wyatt, 1990; Lau et al., 2008). The diminished outcross siring success in large Mimulus displays causes a dramatic shift in functional gender from predominantly male in small displays to predominantly female in large displays (Fig. 3). A similar shift in functional gender has also been shown in other species, including Ipomopsis (Campbell, 1989), Asclepias (Broyles and Wyatt, 1990) and Solanum (Elle and Meagher, 2000).
The shift in functional gender explains why male and female selfing rates respond differently to floral display size (Fig. 5). These two parameters have the same numerator (self seeds sired by a plant equals self seeds mothered) but different denominators (total seeds sired by a plant is not equivalent to total seeds mothered), and therefore may vary considerably when functional gender is skewed. Male selfing rates are a fundamental parameter for modelling pollen discounting and mating system evolution (Harder and Wilson, 1998), yet to our knowledge the effect of floral display size on male selfing rates has not previously been quantified empirically.
Evolution of floral display size
Knowledge of how male and female reproductive success are each affected by floral display size provides a rare opportunity to evaluate sex-specific responses to a floral trait. Indeed, we found that the sexual functions differed greatly in their response to increased floral display size, so that plants with large floral displays gain most of their reproductive fitness through female function, while those with small displays do better through male function. This is the opposite of what is predicted by the ‘male function hypothesis’ (Sutherland and Delph, 1984; Campbell, 1989; Burd and Callahan, 2000), and contributes to a growing body of research indicating that selection on floral display size occurs through both sexual functions (Ashman and Morgan, 2004). One possible implication of this pattern of response to floral display size is that there might be selection for size-specific changes in allocation to each function, with many-flowered plants allocating relatively more resources to ovules than to pollen.
Selection in hermaphrodites operates on the level of the individual (Broyles and Wyatt, 1990; Conner, 2006), so although knowledge of sex-specific responses is valuable, total fitness responses across male and female function are most directly relevant for understanding the evolutionary effects of floral display size. Ideally, total fitness estimates should not only combine measures of reproductive success for the two sexual functions, but should also consider both the quantity and quality of offspring mothered and sired. Such calculations for M. ringens reveal that total fitness per plant increases strongly with daily floral display size (Fig. 6B). Thus, the plants with the most flowers had the highest fitness. However, each additional flower provides diminishing returns because of the costs of geitonogamy and inbreeding depression, so that per-flower total fitness is highest on small displays (Fig. 6A).
The finding that total fitness per flower is greatest on small displays implies that selection should favour the strategy of presenting a few flowers each day, while flowering over a prolonged period (Crawford, 1984; de Jong et al., 1992; Harder and Barrett, 1995; Lau et al., 2008). However M. ringens plants exhibit considerable plasticity in size; plants in natural populations often display 1–3 flowers each day over 2–3 weeks, but clones of such plants can be grown in pots or experimental arrays to produce daily floral displays with 15 or more open flowers for up to 6 weeks (R. J. Mitchell and J. D. Karron, unpubl. res.). With such great plasticity, plants that change floral display strategy with size (to display proportionally fewer flowers per day and extend the flowering season when large) would reduce the costs of geitonogamy and gain an advantage over alternative strategies. There is some evidence suggesting that this may occur in M. ringens: daily floral display size increased less than proportionally with total flower production (slope of ln–ln regression = 0·53, R. J. Mitchell and J. D. Karron, unpubl. res.). Thus, plastic responses in floral display strategy with increases in plant size may represent evolved responses to avoid the costs of geitonogamous selfing and pollen discounting.
The magnitude of the effect of floral display size on reproduction through male and female function is likely to depend on the ecological context (Harder and Barrett, 1995). In particular, the effects of floral display should be strongest when there is substantial variation in display size among individuals, as frequently occurs in natural populations of M. ringens. By contrast, if populations had little or no variation in display, the effects of floral display size would probably be much less pronounced.
Conclusions
The number of open flowers on Mimulus ringens floral displays strongly influenced pollinator behaviour and resulting patterns of reproductive success. Although seed-set per flower did not vary with display size treatment, siring success per flower declined markedly on large displays. As a result, a plant could in principle maximize fitness by producing small displays over a long flowering window, rather than large displays over a brief window.
The observed reduction in siring success for large display sizes is largely attributable to geitonogamous pollen discounting. As display size increased, each additional seed sired through geitonogamous self-pollination was associated with a loss of nearly ten seeds sired through outcrossing. Such strong responses suggest that pollen discounting may play a critical role in the evolution of floral display strategies, especially in species with limited pollen carryover.
ACKNOWLEDGEMENTS
We thank Tom Schuck for assistance propagating M. ringens and Karsten Holmquist, John Bell, Jim Reinartz, Gretchen Meyer, Forrest Meekins, Lori Artiomow, Nichole Poirier and Todd Egan for help and encouragement in the field. This work was supported by the National Science Foundation (grant DEB 9816712 to J.D.K. and DEB 9903308 to R.J.M.).
LITERATURE CITED
- Ashman T-L, Morgan MT. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proceedings of the Royal Society of London B. 2004;271:553–559. doi: 10.1098/rspb.2003.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH. Mating strategies in flowering plants: the outcrossing-selfing paradigm and beyond. Philosophical Transactions of the Royal Society of London B. 2003;358:991–1004. doi: 10.1098/rstb.2003.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD. Ecology and evolution of plant mating. Trends in Ecology and Evolution. 1996;11:73–79. doi: 10.1016/0169-5347(96)81046-9. [DOI] [PubMed] [Google Scholar]
- Bell JM, Karron JD, Mitchell RJ. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology. 2005;86:776–785. [Google Scholar]
- Broyles SB, Wyatt R. Paternity analysis in a natural population of Asclepias exaltata: multiple paternity, functional gender, and the ‘pollen-donation’ hypothesis. Evolution. 1990;44:1454–1468. doi: 10.1111/j.1558-5646.1990.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Burd M, Callahan H. What does the male function hypothesis claim? Journal of Evolutionary Biology. 2000;13:735–742. [Google Scholar]
- Campbell DR. Inflorescence size: test of the male function hypothesis. American Journal of Botany. 1989;76:730–738. [Google Scholar]
- Conner JK. Ecological genetics of floral evolution. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 260–277. [Google Scholar]
- Crawford TJ. What is a population? In. In: Shorrocks B, editor. Evolutionary ecology. Oxford: Blackwell Scientific Publications; 1984. pp. 135–173. [Google Scholar]
- Darwin C. The effects of cross and self fertilisation in the vegetable kingdom. London: Murray; 1876. [Google Scholar]
- Devlin B, Clegg J, Ellstrand NC. The effect of flower production on male reproductive success in wild radish populations. Evolution. 1992;46:1030–1042. doi: 10.1111/j.1558-5646.1992.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Dudash MR. Plant size effects on female and male function in hermaphroditic Sabatia angularis (Gentianaceae) Ecology. 1991;72:1004–1012. [Google Scholar]
- Eckert CG. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology. 2000;81:532–542. [Google Scholar]
- Elle E, Meagher TR. Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). II. Paternity and functional gender. American Naturalist. 2000;156:622–636. doi: 10.1086/316997. [DOI] [PubMed] [Google Scholar]
- Flanagan RJ, Mitchell RJ, Knutowski D, Karron JD. Interspecific pollinator movements reduce pollen deposition and seed production in Mimulus ringens (Phrymaceae) American Journal of Botany. 2009;96:809–815. doi: 10.3732/ajb.0800317. [DOI] [PubMed] [Google Scholar]
- Galloway LF, Crigliano T, Gremski K. The contribution of display size and dichogamy to potential geitonogamy in Campanula americana. International Journal of Plant Sciences. 2002;163:133–139. [Google Scholar]
- Grant AL. A monograph of the genus Mimulus. Annals of the Missouri Botanical Garden. 1924;11:99–388. [Google Scholar]
- Harder LD, Barrett SCH. Mating cost of large floral displays in hermaphrodite plants. Nature. 1995;373:512–515. [Google Scholar]
- Harder LD, Wilson WG. A clarification of pollen discounting and its joint effects with inbreeding depression on mating system evolution. American Naturalist. 1998;152:684–695. doi: 10.1086/286199. [DOI] [PubMed] [Google Scholar]
- Holmquist KG, Mitchell RJ, Karron JD. Influence of pollinator grooming on pollen mediated gene dispersal in Mimulus ringens (Phrymaceae) Plant Species Biology. 2011 in press. doi: 10.1111/j.1442-1984.2011.00329.x. [Google Scholar]
- Holsinger KE, Feldman MW, Christiansen FB. The evolution of self-fertilization in plants: a population genetic model. American Naturalist. 1984;124:446–453. [Google Scholar]
- de Jong TJ, Klinkhamer PGL, Van Staalduinen MJ. The consequences of pollination biology for selection of mass or extended blooming. Functional Ecology. 1992;6:606–615. [Google Scholar]
- Karron JD, Thumser NN, Tucker R, Hessenauer AJ. The influence of population density on outcrossing rates in Mimulus ringens. Heredity. 1995a;75:175–180. [Google Scholar]
- Karron JD, Tucker R, Thumser NN, Reinartz JA. Comparison of pollinator flight movements and gene dispersal patterns in Mimulus ringens. Heredity. 1995b;75:612–617. [Google Scholar]
- Karron JD, Jackson RT, Thumser NN, Schlicht SL. Outcrossing rates of individual Mimulus ringens genets are correlated with anther-stigma separation. Heredity. 1997;79:365–370. [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. The influence of floral display size on selfing rates in Mimulus ringens. Heredity. 2004;92:242–248. doi: 10.1038/sj.hdy.6800402. [DOI] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ, Bell JM. Multiple pollinator visits to Mimulus ringens (Phrymaceae) flowers increase mate number and seed set within fruits. American Journal of Botany. 2006;93:1306–1312. doi: 10.3732/ajb.93.9.1306. [DOI] [PubMed] [Google Scholar]
- Karron JD, Holmquist KG, Flanagan RJ, Mitchell RJ. Pollinator visitation patterns strongly influence among-flower variation in selfing rate. Annals of Botany. 2009;103:1379–1383. doi: 10.1093/aob/mcp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkhamer PGL, de Jong T. Evolutionary ecology of plant reproductive strategies. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Lau JA, Miller RE, Rausher MD. Selection through male function favors smaller floral display size in the common morning glory Ipomoea purpurea (Convolvulaceae) American Naturalist. 2008;172:63–74. doi: 10.1086/588080. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. Sexual strategies in plants III. A quantitative method for describing the gender of plants. New Zealand Journal of Botany. 1980;18:103–108. [Google Scholar]
- Mitchell RJ. Effects of floral traits, pollinator visitation, and plant size on Ipomopsis aggregata fruit production. American Naturalist. 1994;143:870–889. [Google Scholar]
- Mitchell RJ, Karron JD, Holmquist KG, Bell JM. The influence of Mimulus ringens floral display size on pollinator visitation patterns. Functional Ecology. 2004;18:116–124. [Google Scholar]
- Mitchell RJ, Karron JD, Holmquist KG, Bell JM. Patterns of multiple paternity in fruits of Mimulus ringens (Phrymaceae) American Journal of Botany. 2005;92:885–890. doi: 10.3732/ajb.92.5.885. [DOI] [PubMed] [Google Scholar]
- Morgan MT. Properties of maximum likelihood male fertility estimation in plant populations. Genetics. 1998;149:1099–1103. doi: 10.1093/genetics/149.2.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritland K. A series of FORTRAN computer programs for estimating plant mating systems. Journal of Heredity. 1990;81:235–237. [Google Scholar]
- Ritland K. Extensions of models for the estimation of mating systems using n independent loci. Heredity. 2002;88:221–228. doi: 10.1038/sj.hdy.6800029. [DOI] [PubMed] [Google Scholar]
- Robertson AW. The relationship between floral display size, pollen carryover and geitonogamy in Myosotis colensoi (Kirk) Macbride (Boraginaceae) Biological Journal of the Linnean Society. 1992;46:333–349. [Google Scholar]
- Schmid-Hempel P, Speiser B. Effects of inflorescence size on pollination in Epilobium angustifolium. Oikos. 1988;53:98–104. [Google Scholar]
- Snow AA, Spira TP, Simpson R, Klips RA. The ecology of geitonogamous pollination. In: Lloyd DG, Barrett SCH, editors. Floral biology. New York: Chapman and Hall; 1996. pp. 191–216. [Google Scholar]
- Sutherland S, Delph LF. On the importance of male fitness in plants: patterns of fruit-set. Ecology. 1984;65:1093–1104. [Google Scholar]
- Thomson JD. Trapline foraging by bumblebees: I. Persistence of flight-path geometry. Behavioral Ecology. 1999;7:158–164. [Google Scholar]
- Willson MF, Price PW. The evolution of inflorescence size in Asclepias (Asclepiacaecae) Evolution. 1977;31:495–511. doi: 10.1111/j.1558-5646.1977.tb01040.x. [DOI] [PubMed] [Google Scholar]