Abstract
Background
The field of plant mating-system evolution has long been interested in understanding why selfing evolves from outcrossing. Many possible mechanisms drive this evolutionary trend, but most research has focused upon the transmission advantage of selfing and its ability to provide reproductive assurance when cross-pollination is uncertain. We discuss the shared conceptual framework of these ideas and their empirical support that is emerging from tests of their predictions over the last 25 years.
Scope
These two hypotheses are derived from the same strategic framework. The transmission advantage hypothesis involves purely gene-level selection, with reproductive assurance involving an added component of individual-level selection. Support for both of these ideas has been garnered from population-genetic tests of their predictions. Studies in natural populations often show that selfing increases seed production, but it is not clear if this benefit is sufficient to favour the evolution of selfing, and the ecological agents limiting outcross pollen are often not identified. Pollen discounting appears to be highly variable and important in systems where selfing involves multiple floral adaptations, yet seed discounting has rarely been investigated. Although reproductive assurance appears likely as a leading factor facilitating the evolution of selfing, studies must account for both seed and pollen discounting to adequately test this hypothesis.
Conclusions
The transmission advantage and reproductive assurance ideas describe components of gene transmission that favour selfing. Future work should move beyond their dichotomous presentation and focus upon understanding whether selection through pollen, seed or both explains the spread of selfing-rate modifiers in plant populations.
Keywords: Demography, inbreeding depression, mating systems, outcrossing, pollen discounting, pollination, seed discounting
INTRODUCTION
Even a casual study of floral diversity reveals a bewildering array of variation that has long been considered to be a paragon of the adaptive evolutionary process (Darwin, 1859). In comparison to other organismal groups, flowering plants are particularly variable with respect to the placement of male and female gametes within and among individuals (Darwin, 1876; Barrett, 2002). The vast majority of angiosperm species have perfect flowers, where the pollen and ovules of a single individual are held in close spatio-temporal proximity. Given this arrangement, plants are faced with a strategic decision, on whether to reproduce through outcrossing, selfing, or some mixture of these possibilities (Barrett and Eckert, 1990). This simple question has stimulated a rich body of theoretical and empirical work in the field of plant mating-system evolution (Lande and Schemske, 1985; Goodwillie et al., 2005). Addressing this idea is important because selfing appears to be driven by persistent natural selection in the wild, yet long periods of selfing have negative consequences on the genetic diversity, viability and diversification of plant lineages (Stebbins, 1957; Goldberg et al., 2010). If it is possible to understand the factors driving the recurrent evolution of selfing in nature, then this field of research will explain the ultimate mechanisms underlying a major evolutionary trend in flowering plants (Eckert et al., 2009a).
Theories on the evolution of self-fertilization have a deep history in plant evolutionary biology (Darwin, 1876; Fisher, 1941; Baker, 1955; Jain, 1976; Lloyd, 1979; Holsinger, 1996). In general, selfing is seen as a reproductive strategy that can replace outcrossing whenever the fitness of a selfing morph exceeds that of an outcrossing morph (Lloyd, 1979, 1992). Selection of selfing over outcrossing can theoretically occur for an extremely diverse array of reasons (Goodwillie et al., 2005), ranging from its ability to shield individuals and populations from maladaptive gene flow (Antonovics, 1968; Grossenbacher and Whittall, 2011), competitive interactions (Cheptou and Dieckmann, 2002), and antagonists (Koslow and DeAngelis, 2006) or its ability to increase seed production (Darwin, 1876; Lloyd, 1980) or allele transmission in natural populations (Fisher, 1941). Each of the potential benefits of selfing may be countered by inbreeding depression, which has received extensive theoretical and empirical attention in studies of mating-system evolution (Lande and Schemske, 1985; Porcher and Lande, 2005; Byers and Waller, 1999; Keller and Waller, 2002). In terms of the factors that favour selfing, most effort has focused on two specific hypotheses for the evolution of this reproductive strategy (Jain, 1976; Holsinger, 1996; Cheptou, 2004). The first and most long-standing hypothesis suggests that selfing evolves because it increases seed production when mates or pollinators are scarce, a phenomenon known as reproductive assurance (Darwin, 1876; Baker, 1955; Lloyd, 1965; Inouye et al., 1996). The second hypothesis was derived relatively recently from population-genetic models showing that a gene for selfing has a 3 : 2 transmission advantage over those causing outcrossing (Fisher, 1941; Holsinger, 1991). This potential gene-level advantage of selfing has been termed the automatic selection hypothesis.
The reason why these two hypotheses have received the greatest attention is because they concern the direct reproductive advantages of selfing, and do not invoke other potentially less general ecological agencies that favour selfing over outcrossing (Lloyd, 1979; Uyenoyama et al., 1993). In this paper, our goal is to discuss the current state of opinion on the relative importance of the reproductive assurance and transmission advantage hypotheses in explaining the repeated evolution of selfing in nature. We begin by revisiting theory concerning these components of natural selection so that the factors driving the evolution of selfing from outcrossing may be clearly understood (Lloyd, 1979, 1992). We follow this section by summarizing evidence gathered over the last 25 years toward addressing these hypotheses from observational, experimental and comparative approaches. Throughout this paper, we suggest that studies have too often treated these hypotheses as mutually exclusive alternatives, and have focused less often on their shared framework (Holsinger, 1996; Cheptou and Schoen, 2007). By discussing these hypotheses, their similarities, and their empirical support, we hope to focus future efforts toward best answering the question of why the evolution of selfing from outcrossing is the most commonly traversed axis in floral evolution in angiosperms.
THE BASIC MODEL FOR THE EVOLUTION OF SELFING
In considering these major hypotheses for the evolution of selfing, we revisit a phenotypic model developed by Lloyd (1992), as it describes gene transmission of plants in a stable population. In this example, one imagines the number of times gametes are passed on to offspring by competing plants, which are either entirely outcrossing or partially selfing. Outcrossing plants produce offspring through outcrossed ovules (xx) and also through fertilizing other ovules that are available to be outcrossed in the population. Success as an outcross pollen donor depends on pollen fitness, or the number of ovules fertilized with outcross pollen (px). The total fitness gained by an outcrossing morph is then:
In contrast, selfing plants pass on two copies of gametes through ovules that are self-fertilized (y), and the fitness of these seeds may be reduced by inbreeding depression (δ, i.e. fitness is scaled by a factor of 1 – δ). Remaining ovules (xs) are fertilized with outcross pollen, and this plant also achieves fitness through outcross pollen that fertilizes some number of ovules (ps):
Regardless of the actual shift in selfing rate, an increase in the amount of selfing will be selected whenever the gains from selfing exceed the losses, or when Ws > Wx:
The gains from selfing arise through increased allele transmission through selfed seeds which are discounted by inbreeding depression. The losses in numbers of offspring arise from the loss of outcrossed seeds (the absolute seed discount, xx – xs) and the loss of outcrossed pollen fitness (the absolute pollen discount, px – ps). This inequality is usually rearranged so that each of the discounts is expressed in terms of losses of outcrossed seeds and pollen fitness per selfed seed:
| (1) |
This entire inequality may be used in natural populations to determine whether selfing should evolve by natural selection. This perspective is useful because it identifies the components of fitness that are quantifiable and also potentially responsible for mating-system shifts. This perspective does not address the evolutionary stability of the selfing rate (Johnston, 1998; Goodwillie et al., 2010), but nevertheless can be used to illuminate why the direction and magnitude of selection on mating-system modifiers arises (Table 1).
Table 1.
Empirical quantities required to test the transmission advantage and reproductive assurance hypotheses in nature
| Term | Component | Parameters required to estimate component |
|---|---|---|
| δ = 1 – (Ws/Wx) | Inbreeding depression | Ws = lifetime fitness of selfed offspring |
| Wx = lifetime fitness of outcross offspring | ||
| (px – ps)/y | Pollen discounting rate | ps = number of outcrossed seeds sired by selfing morphs |
| px = number of outcrossed seeds sired by outcrossing morphs | ||
| (xx – xs)/y | Seed discounting rate | xs = number of outcrossed seeds made by selfing morphs |
| xx = number of outcrossed seeds made by outcrossing morphs | ||
| y = number of seeds produced by selfing |
Both hypotheses require estimates of inbreeding depression and pollen discounting. The transmission advantage hypothesis assumes that seed discounting is complete, so this quantity is relevant only to the reproductive assurance hypothesis.
TESTS OF HYPOTHESES BASED UPON THE MODEL
Transmission advantage hypothesis
Now that we understand the pathways whereby outcrossing and selfing plants compete, in a Darwinian sense, we can determine the necessary requirements for testing the transmission advantage hypothesis in nature. In a scenario where this mode of selection is operating, a mutation causing selfing spreads purely because it increases its transmission to offspring compared with a mutation that causes outcrossing, but there is no difference in the number of seeds produced by plants. In such a situation, selfed ovules (y) can be gained only by sacrificing ovules that are normally cross-fertilized in outcrossing plants (i.e. y = xx – xs). This hypothesis therefore describes selection operating at the level of genes causing outcrossing and selfing, since all else is equal at the individual level. Because of this perspective, selfing causes complete seed discounting, and the conditions for the evolution of selfing depend only upon the magnitude of inbreeding depression and the pollen-discounting rate:
| (2) |
If pollen discounting does not occur (px – ps = 0), then selfing evolves whenever δ < 1/2, as is expected under classic population-genetic theory (Fisher, 1941; Lande and Schemske, 1985). Pollen discounting is clearly the most important mechanism that can erase the transmission advantage of selfing, since selfing evolves under an increasingly narrower set of conditions as the magnitude of pollen discounting (px – ps) increases (Nagylaki, 1976; Holsinger, 1991). Pollen discounting may occur for a variety of reasons, such as when selfing causes a reduction in the floral attractiveness to pollinators, the number of pollen grains per flower, or the quality of pollen grains that compete with those made by outcrossers (Holsinger, 1996).
Given the potential importance of pollen discounting in counteracting selection of selfing, it is of great interest to know whether it is commonly observed in plant populations. The measurement of pollen discounting has been conducted in a number of scenarios where the outcross seed paternity of outcrossing and selfing plants has been measured in competition (Table 2). Over a broad number of studies in several years and populations of the species Ipomoea purpurea, pollen discounting has rarely been implicated for mutations that alter floral colour and increase selfing rates (Rausher and Fry, 1993; Fehr and Rausher, 2004; Fry and Rausher, 1997; Coberly and Rausher, 2008), although it has been observed in populations where selfing phenotypes are common (Chang and Rausher, 1998). In Eichhornia paniculata, a selfing morph experiences no pollen discounting in diverse populations, yet actually has an advantage (negative pollen discounting) over a similar outcrossing morph when morph diversity is reduced in populations (Kohn and Barrett, 1994). Pollen discounting in E. paniculata has also been shown to be more important for plants with large displays, as pollinators can transport pollen between flowers on the same plant, thereby reducing outcross siring success (Harder and Barrett, 1995; Eckert, 2000). These results collectively suggest that pollen discounting may not always operate in natural populations and is highly dependent upon plant life history, yet is critical for understanding the magnitude and direction of selection on the mating system.
Table 2.
Studies where direct competition of outcrossing and selfing morphs permitted estimates of pollen discounting
| Species | Trait(s) associated with selfing | Pollen discounting? (px– ps) | References |
|---|---|---|---|
| Ipomoea purpurea | Flower colour (A-locus) | ∼0 | Fehr and Rausher, 2004; Coberly and Rausher, 2008 |
| Ipomoea purpurea | Flower colour (W-locus) | ∼0 | Rausher and Fry, 1993; Fry and Rausher, 1997 |
| Ipomoea purpurea | Anther-stigma distance | ∼0: when selfing rare | Chang and Rausher, 1998 |
| >0: when selfing common | |||
| Eichhornia paniculata | Modified stamen in M morph | ∼0: trimorphic populations | Kohn and Barrett, 1994 |
| <0: mono-, di-morphic populations | |||
| Mimulus guttatus and M. micranthus | Multiple morphological traits | >0 | Ritland, 1991 |
| Senecio squalidus | Radiate vs. non-radiate morph | >0 | Holsinger, 1992 |
| Arenaria uniflora | Multiple morphological traits | >0 | Fishman, 2000 |
In each study, the average outcross paternity of outcrossing (px) and selfing (ps) plants was inferred with polymorphic molecular markers.
In species where selfing morphs harbour many floral adaptations associated with the selfing syndrome, there is a trend for higher levels of pollen discounting (Ritland, 1991; Holsinger, 1992; Fishman, 2000). Pollen discounting appears in these species because selfing morphs have reduced floral attractiveness (Holsinger, 1992) or reduced pollen quality (Ritland, 1991; Fishman, 2000). In two of these cases, experiments have utilized quite divergent outcrossing and selfing morphs, so some of the pollen discounting in these experiments may in fact be caused by pre-zygotic or early post-zygotic incompatibilities between lineages (Ritland, 1991; Fishman, 2000). Although there are relatively few estimates of pollen discounting in these types of systems, these results imply that pollen discounting can range from being negligible to important depending upon the context, and that morphological adaptations for selfing are likely to cause pollen discounting (Holsinger, 1996; Takebayashi and Delph, 2000). Since many of the floral traits that accompany selfing in these lineages may have arisen long after the initial transition from outcrossing to partial selfing, these cases may not adequately determine whether pollen discounting hindered the first stages of the transition to selfing.
Reproductive assurance hypothesis
Reproductive assurance is distinct from automatic selection because it invokes elevated seed production in selfing plants compared with outcrossers (i.e. xs + y > xx). This hypothesis therefore describes selection operating at the individual level, because it invokes variation in the number of offspring produced by plants. The idea that selfing is an efficient means of seed production has a long and storied history in plant evolutionary biology. This idea was originally cited in the works of Darwin (1859, 1876), and was reified by the observations of Baker (1955, 1965), Stebbins (1957) and Lloyd (1965, 1980), who examined bio-geographical and demographic correlates of selfing that arise in nature. Specifically, these important, early evolutionary botanists suggested that selfing evolves in isolated or marginal populations or those occurring on islands, as these habitats are often typified by chronically small populations or reductions in the efficiency of cross-pollination as a mode of reproduction. Currently, the specific agents limiting seed production through cross-pollination, such as the ephemeral habit, histories of invasion, and the reliance upon specialized pollinators, have been largely supported (Runions and Geber, 2000; Fenster et al., 2005; Fenster and Rodriguez, 2007; van Kleunen et al., 2008), although there are exceptions to this broad rule (Sutherland, 2004; Herlihy and Eckert, 2005; Cheptou and Massol, 2009). Given the broad interest in identifying the ecological factors that may cause the evolution of selfing because of its ability to provide reproductive assurance, we discuss important experimental issues and support for the hypothesis that have been identified in individual studies in recent history.
For the reproductive assurance hypothesis to explain the evolution of selfing, plants must be able to self-fertilize in the absence of a pollinator (Lloyd, 1992). Direct tests of the reproductive assurance hypothesis therefore require at minimum that the effect of the autonomous mode of selfing on seed production be quantified (Eckert and Schaefer, 1998). This component of selfing may be estimated by comparing the seed production of intact flowers, which have the capacity to autonomously self, with those that have been emasculated (Schoen and Lloyd, 1992). These manipulative experiments have been conducted in a wide diversity of species (>50 species), and there is broad support for autonomous selfing providing reproductive assurance (Eckert et al., 2006). That being said, the ability of selfing to provide reproductive assurance will also depend strongly on the timing and mode of self-pollen deposition, because it can lead to substantial seed discounting (Lloyd, 1992). Early self-pollen deposition (i.e. prior or competing selfing) should lead to large amounts of seed discounting, which can actually erase the reproductive assurance benefits of selfing (Herlihy and Eckert, 2002), while delayed selfing only boosts seed production once opportunities for outcrossing have passed. Seeing how seed discounting has been estimated much less often than pollen discounting (Vaughton and Ramsey, 2010; Vaughton et al., 2010), more work is needed to determine its magnitude in nature.
Even if emasculation experiments demonstrate that autonomous selfing provides reproductive assurance, elevated seed production in selfing plants is not enough to determine whether selfing should evolve by reproductive assurance. This problem arises because simple comparisons of seed production ignore seed discounting and the cost of meiosis in outcrossed seeds (Holsinger, 1996; Herlihy and Eckert, 2002; Cheptou and Schoen, 2007). If the cost of meiosis is accounted for, the evolution of selfing occurs when:
| (3) |
The numerator of this expression contains the number of gametes passed on to offspring by selfing plants minus the number of gametes passed on to offspring by outcrossing plants. As the number of ovules fertilized by self pollen (y) increases, conditions for the evolution of selfing become more favourable. In contrast, if either the seed discounts (xx – xs) or pollen discounts (px – ps) become increasingly large, selfing will be only be selected when values of inbreeding depression are increasingly small. This approach clarifies that both seed and pollen discounting contribute equally to cancel the advantage of selfing when it boosts seed production.
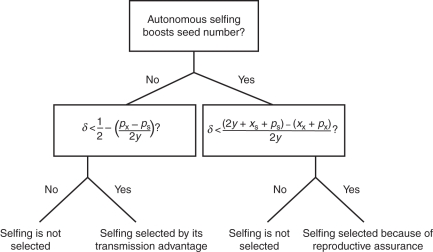
Outlining the logic behind the transmission advantage and reproductive assurance hypotheses is important because it clarifies the shared framework underlying these ideas (Fig. 1). Most importantly, both hypotheses describe selection of selfing because of the cost of meiosis incurred by outcross seeds. As such, these ideas are not independent of each other, even though they are often presented as such in the mating-system literature (Cheptou, 2007). The transmission advantage hypothesis is applicable only when the total seed production of plants must be equal, such that elevated selfing rates necessarily result in fewer outcross seeds (i.e. the seed discounting rate equals 1). If, however, autonomous selfing is associated with elevated seed production, it is still necessary to evaluate the magnitude of pollen and seed discounting, as each of these factors can counteract selection for selfing, even if it provides reproductive assurance (Fig. 1). Both of these discounting rates have rarely been measured in natural populations and, to our knowledge, never been estimated jointly in a single species to evaluate whether autonomous selfing should be selected (Herlihy and Eckert, 2002; Eckert et al., 2006).
Fig. 1.
The conceptual framework shared by two hypotheses for the evolution of selfing. Selfing will evolve by its gene-level transmission advantage if it is not erased by pollen discounting (px – ps) or inbreeding depression (δ). If selfing increases seed production, selfing will be selected if the number of alleles passed on by plants capable of selfing (2y + xs + ps) is greater than the number transmitted by outcrossers (xx + px), and exceeds a threshold inbreeding depression. Pollen and seed discounting (xx – xs) may both counter selection of selfing in this case.
ECOLOGICAL CORRELATION AND HISTORICAL APPROACHES
Ecological correlates of selfing
The approach outlined above requires a great deal of knowledge that is often specific to a single species and an environment. Since plants species differ widely in floral morphology, mode of selfing, pollination biology and environmental circumstances, a broader perspective may be more widely successful in addressing the relative importance of the transmission advantage and reproductive assurance hypotheses. Since the transmission advantage hypothesis assumes that outcrossing and selfing plants produce the same number of seeds, this idea cannot be addressed through large-scale ecological studies in natural populations. The reproductive assurance hypothesis, however, has long been thought to explain shifts to selfing, because this strategy is thought to be associated with pollinator-poor environments or scenarios where mates are uncommon (Wyatt, 1986; Husband and Barrett, 1991; Kalisz et al., 2004). Although there is interest in understanding the reasons why selfing evolves, most tests of the reproductive assurance hypothesis have focused on whether selfing boosts seed production [i.e. R = 1 – (seed setemasculated/seed setintact)]. As a consequence, reproductive assurance is operationally defined, as the seed benefit of selfing can arise in response to any number of a diverse suite of mechanisms in nature (Eckert and Schaefer, 1998; Kalisz and Vogler, 2003; Kalisz et al., 2004). Given this issue, more work is needed to test hypotheses about specific environmental or population-level characteristics that are responsible for selecting for selfing in nature.
Outcross pollen quantity may be constrained by any environmental or genetic factor that reduces the gross number of outcross pollen grains that are received at the stigma level by plants (Burd, 1994; Knight et al., 2005; Aizen and Harder, 2007). This concept of quantity was invoked by Baker (1955), Stebbins (1957) and Lloyd (1980), and has most often been interpreted to occur in small populations where mates are uncommon or when reduced pollinator availability or activity occurs. To shed light on the agents of selection that generate natural selection for selfing, we compiled data from studies that examined variables associated with increased selfing in plants. Studies that tested the reproductive assurance component of selfing (i.e. an increase in seed production through autonomous selfing) and a specific environmental correlate were included (Table 3). In general, reduced pollen quantity (i.e. low plant density or reduced pollinator activity) is often associated with a reproductive assurance benefit of selfing, although studies have also commonly failed to identify ecological agents associated with elevated seed production in self-fertile plants in comparison to their outcrossing relatives. Perhaps most surprising is the fact that very few studies have connected the reproductive assurance benefit of selfing specifically to how effective pollinators are in their foraging behaviour in maintaining outcrossing, even though these vectors are often invoked as causing reproductive assurance (Rick et al., 1979; Wyatt, 1986; Husband and Barrett, 1992; Karron et al., 2004; Moeller, 2006; Fishman and Willis, 2008; Fenster and Rodriguez, 2007; Bodbyl Roels and Kelly, 2011).
Table 3.
Studies examining associations between the reproductive assurance benefit of selfing and factors thought to limit outcross pollen in nature
| Species | Study* | Selfing = RA?† | Selfing variation§ | Potential correlate of ↑ selfing?¶ | References |
|---|---|---|---|---|---|
| Studies identifying an environmental correlate of RA | |||||
| Aquilegia canadensis | CP | Yes | s | ↓ Plant density | Herlihy and Eckert, 2004 |
| Arenaria uniflora | MP | Yes‡ | Flower size | ↑ Heterospecific pollen | Fishman and Wyatt, 1999 |
| Bulbine vagans | MP | Yes | R | Inclement weather | Vaughton and Ramsey, 2010 |
| Campanula spp. | CP | Yes | s | ↓ Pollinator visitation | Inouye et al., 1996 |
| Clarkia xantiana | CP, CG | Yes | Herkogamy | ↓ Pollinator abundance; ↓ plant density | Moeller and Geber, 2005; Moeller, 2006 |
| Collinsia parviflora | CP, CG | Yes | R, flower size | ↓ Pollinator visitation | Kennedy and Elle, 2008; Elle and Carney, 2003 |
| Eichhornia paniculata | CP | Yes‡ | s | ↓ Plant density | Barrett et al., 1989; Husband and Barrett, 1991 |
| Linanthus spp. | CP | Yes‡ | SC | ↑ Variation in pollen limitation | Goodwillie, 2001 |
| Phyllodoce aleutica | CP | Yes | s | ↓ Pollinator activity | Kameyama and Kudo, 2009 |
| Paris quadrifolia | CP | Yes | R | ↓ Plant density | Jacquemyn and Brys, 2008 |
| Primula vulgaris | CP, CG | Yes‡ | Heterostyly | ↓ Pollinator visitation | Piper et al., 1986 |
| Ranunculus reptans | CP | Unknown | SC | ↓ Mate availability | Willi, 2009 |
| Schizanthus spp. | MP | Yes | R | ↑ Pollinator specialization | Perez et al., 2009 |
| Studies not identifying an environmental correlate of RA | |||||
| Aquilegia canadensis | CP | Yes | s | ↓ Plant density range edges | Herlihy and Eckert, 2005; Eckert et al., 2009b |
| Arenaria uniflora | CP | Unknown | Flower size | ↓ Pollinator visitation | Wyatt, 1986 |
| Crepis sancta | CP | Unknown | s | Earlier successional stages | Cheptou et al., 2002 |
| Datura stramonium | CP | Yes | R | ↓ Plant density | van Kleunen et al., 2007 |
| Eichhornia paniculata | CP | Unknown | Heterostyly | ↓ Pollinator visitation | Husband and Barrett, 1992 |
| Eritrichium nanum | CP | Unknown | s | ↑ Altitude | Wirth et al., 2010 |
| Gesnerieae spp. | CP | Yes | R | ↑ Pollinator specialization | Marten-Rodriguez and Fenster, 2010 |
| Helleborus foetidus | CP | Yes | R | ↓ Pollinator visitation | Herrera et al., 2001 |
| Leavenworthia alabamica | CG | Yes‡ | SC | Range edges | Busch, 2005 |
| Nicotiana glauca | CP | Yes | R | ↓ Pollinator visitation | Schueller, 2004 |
* Studies involve either correlations among populations (CP), manipulations of pollination environment (MP) or common garden experiments (CG).
† ‘Yes’ denotes that a study used floral emasculation in natural populations to test if selfing provides reproductive assurance.
‡ In these cases, reproductive assurance is inferred because selfing plants produce more seed than closely related outcrossers
§ Studies were included if they measured the selfing rate inferred with molecular markers (s), variation in self-compatibility (SC), key indicators of selfing, or the reproductive assurance benefit of selfing R = 1 – (seed seetemasculated/seed setintact).
¶ Factors that were investigated to see if they correlate positively (↑) or negatively (↓) with an increase in selfing.
Reductions in pollen quantity, although dominant in the literature concerning reproductive assurance, may not be entirely sufficient to explain selection of selfing in natural populations. Outcross pollen quality may also generate selection for selfing when plants are unable to successfully use outcross pollen to produce viable offspring (Aizen and Harder, 2007). This constraint will be important when plants share S-alleles (Campbell and Husband, 2007), as these will result in self-incompatibility reactions that prevent pollen from fertilizing ovules (for a review, see Busch and Schoen, 2008). Mate-limitation of outcross seed production has commonly been observed to decline in the face of limited S-allele diversity (Young and Pickup, 2010; Campbell and Husband, 2007), although this is not always true for species with gametophytic pollen recognition (Holderegger et al., 2008). Incompatibilities arising because of pollen–pistil incompatibilities between species will also be important in limiting outcross pollen success in plant communities (Fishman and Wyatt, 1999), as pollen from other species should usurp ovules. Surprisingly, the heterospecific component of pollen quality has rarely been considered as a factor contributing to reproductive assurance (Fishman and Wyatt, 1999; Table 3), and this factor should be considered in the future, especially given recent broad support for overlapping species ranges in explaining shifts to selfing in Mimulus (Grossenbacher and Whittall, 2011).
Historical approaches to testing hypotheses for the evolution of selfing
Each of the approaches outlined so far in this paper involves the study of intra-specific variation in the mating system, but often devotes little consideration to the histories of these populations (Barrett et al., 1996). As is true for most major shifts in organismal structure and function, shifts in the mating system should produce population-genetic signatures that are readily discernible (Charlesworth, 2003). To understand why this may occur, we first consider the automatic selection hypothesis. For the innate transmission advantage of selfing to be realized, there must be sufficient vector-mediated transfer of pollen (Schoen et al., 1996). In such a scenario, the effective population size (Ne) of this population will decline in response to increased levels of selfing because this shift alters the time it takes for alleles to coalesce in a common ancestor. If the mating-system transition is to complete selfing, all plants will be homozygous and the two alleles found within individuals will be identical by descent; the reduced time to coalescence therefore results in a 50 % reduction in the effective population size. More generally, the shift to a selfing rate s causes a relatively rapid departure from random mating equalling F = s/(2 – s). This shift to greater amounts of selfing should therefore reduce the effective size of populations to a level equal to Ne = Ne/(1 + F) (Pollak, 1987).
If selfing evolves because of reproductive assurance, expectations for reductions in the effective population size may be much larger. In particular, with the reproductive assurance hypothesis, declines in Ne should be greater than expected under the transmission advantage hypothesis, because the loss of vector-mediated pollination service causes some individuals to produce fewer seeds. This inflated variation in fitness will depress Ne and thereby cause reductions in genetic diversity that are greater than expected under the transmission advantage hypothesis (Schoen et al., 1996). The transmission advantage hypothesis is therefore rejected whenever Ne falls below neutral expectations (i.e. Ne = Ne/(1 + F)), since variation in seed production among individuals will depress Ne. Comparisons of allozyme and nucleotide diversity in closely lineages have repeatedly shown large losses of genetic diversity upon the adoption of selfing (Hamrick and Godt, 1996; Charlesworth, 2003), or high variance in diversity among populations, as would be expected in the face of demographic instability (Schoen and Brown, 1991). Reproductive assurance again serves as an umbrella term that encapsulates many possible sources of selection that limit outcross pollen quantity and quality, such as the reliance upon an unreliable or currently depauperate pollinator fauna, ephemeral flowering periods where cross-pollination is unlikely, reductions in population size and stability, or heterospecific pollen interference. Rejecting the transmission advantage hypothesis is therefore useful, but alternative methods may be needed to determine the specific reason(s) why outcross pollen may have been limited during the transition to selfing.
Several recent studies have examined the population-genetic history of closely related outcrossing and selfing populations in three species (North American Arabidopsis lyrata, Eichhornia paniculata and Leavenworthia alabamica). These studies have largely failed to reject the transmission advantage hypothesis (Table 4). Interestingly, two of these comparisons (A. lyrata and L. alabamica) involve plant populations with little secondary adaptation to facilitate self-pollination, implying that these origins of selfing are recent, as is likely given recent glacial history and coalescent inferences (Foxe et al., 2010; Ness et al., 2010; Busch et al., 2011). In each of these studies, however, there is ongoing gene flow between populations, which complicates tests of the transmission advantage hypothesis. For example, the automatic selection hypothesis is not rejected in E. paniculata using a population-genetic approach (Ness et al., 2010), even though there are indications that reproductive assurance favours selfing from studies in natural populations (Barrett et al., 1989; Husband and Barrett, 1991; Kohn and Barrett, 1994). The body of work conducted in E. paniculata demonstrates that there may often be conflicting evidence for and against the automatic selection hypothesis. In this case, the most likely reason for the discrepancy is that migration events after the evolution of selfing have restored genetic diversity to a level that is consistent with the expectations of the transmission advantage of selfing.
Table 4.
Species where selfing is thought to have evolved recently from outcrossing, and where population-genetic data have been used to evaluate the mechanism of natural selection triggering the shift in mating system
| Selfing taxon | Timing of event(s) | Decline in Θ (4Neμ)* | Reject automatic selection? | Ecological correlation with selfing | References |
|---|---|---|---|---|---|
| Arabidopsis lyrata | <10 ka | <50 % | No | None known | Hoebe et al., 2009; Foxe et al., 2010 |
| Capsella rubella | <20 ka | >99 % | Yes | Floral adaptations, weedy habit | Foxe et al., 2009; Guo et al., 2009 |
| Clarkia xantiana ssp. parviflora | <61 ka | ∼80 % | Yes | Floral adaptations, loss of pollinators | J. B. Pettengill and D. A. Moeller, University of Minnesota, USA, unpubl. res. |
| Eichhornia paniculata | <125 ka | ∼50 % | No | Floral adaptations, selfing in smaller populations | Ness et al., 2010 |
| Leavenworthia alabamica | <48 ka | ∼10 % | No | No known floral adaptations | Busch et al., 2011 |
| <150 ka | 100 % | Yes | Floral adaptations, selfing in smaller populations | Busch et al., 2011 |
* Θ, scaled population parameter; μ, mutation rate per site per generation.
In contrast to cases where the shift to selfing did not involve a large loss of genetic diversity, there are three cases in which reproductive assurance has been implicated. Perhaps the strongest evidence comes from the mustard genus Capsella. In this genus, Capsella rubella is highly self-fertile and is thought to have evolved from a recent common ancestor with self-incompatible C. grandiflora. Interestingly, there has been a nearly complete loss of genetic diversity in C. rubella in comparison to its outcrossing sister species (Foxe et al., 2009; Guo et al., 2009). Selfing also appears to have evolved in response to reproductive assurance in a lineage of Leavenworthia alabamica and in Clarkia xantiana ssp. parviflora, as there have been large losses of genetic diversity in comparison to conspecific outcrossing populations (Table 4). Intriguingly, in each of these three recently derived selfing lineages (C. rubella, L. alabamica and C. xantiana ssp. parviflora) there are a large number of floral adaptations for self-pollination (e.g. shorter petals, alterations in anther position, and increased rates of spontaneous seed production). The joint observation of these derived floral syndromes, coupled with a genetic signature of a population bottleneck, provide perhaps the strongest support for the importance of reproductive assurance in driving shifts to self-fertilization.
SO WHICH MECHANISM IS MORE IMPORTANT IN EXPLAINING THE EVOLUTION OF SELFING?
The transmission advantage and reproductive assurance hypotheses have guided research in plant mating-system evolution for a very long time (Darwin, 1876; Fisher, 1941; Jain, 1976). It is important to remember that other hypotheses may explain shifts to selfing and have garnered empirical support (for a review, see Goodwillie et al., 2005). Further, the simple equations presented here ignore associations and evolutionary feedbacks that have been shown to be important in determining the eventual outcome of mating-system evolution (Uyenoyama et al., 1993; Cheptou and Schoen, 2007). Nevertheless, our simplified perspective is meant to focus upon the seed and pollen components of fitness that drive mating-system evolution. In this paper, we have endeavoured to show that the two major hypotheses for the evolution of selfing spring forth from the same framework, involving selection at the gene (transmission advantage) and both the gene and individual levels (reproductive assurance). The transmission advantage hypothesis is a special case, since it applies only when selfing does not boost seed production, whereas reproductive assurance applies more generally because it incorporates variation in seed production among outcrossing and selfing plants. In both of the conceptual approaches presented here (e.g. field experiments of competing plants or population genetic approaches), predictions of the reproductive assurance therefore include the cost of meiosis that forms the conceptual basis for the transmission advantage of selfing.
Since the transmission advantage hypothesis is a special case of the reproductive assurance hypothesis, it stands to reason that it will be applicable less often in natural populations. Interestingly, in two lineages where the evolution selfing does not involve floral adaptation for self-pollination, population-genetic analyses imply that this strategy may have evolved because of its transmission advantage (Table 4). It is more often the case, however, that selfing appears to boost seed production or to be associated with demographic instability (Eckert et al., 2006; Table 3), although this is not always true. More work must focus on recently derived selfing lineages to test accurately the predictions of the transmission advantage and reproductive assurance hypotheses with a population-genetic approach, since each of these mechanisms produces a unique historical signature on genetic diversity, but only over a relatively short time scale (Schoen et al., 1996). In direct studies of mating-system polymorphisms, observations of a seed benefit associated with selfing cannot be assumed to generate selection for selfing in nature, since the seed and pollen discounting rates may erase this selective advantage (Lloyd, 1992). Whether selfing evolves because it provides reproductive assurance must therefore be tested by explicitly measuring the pollen and seed discounting rates. Interestingly, no known study has jointly estimated these rates (Eckert et al., 2006). Both of these factors are sensitive to the timing of self-pollination and the pollination environment (Chang and Rausher, 1998; Vaughton et al., 2010), so future work must examine the spatial and temporal variability expected in the environment to understand fully the selection of mating-system modifiers. One very interesting conclusion taken from these studies is that the degree of pollen discounting is likely to be strongly dependent upon whether traits causing selfing have morphological impacts on flowers or pollen presentation, as predicted (Holsinger, 1996). No generality has yet emerged on the magnitude of seed discounting since it has rarely been estimated (Schoen and Lloyd, 1992), but this factor likely plays a major role in maintaining outcrossing, especially if selfing is not delayed (Lloyd and Schoen, 1992; Herlihy and Eckert, 2002).
The reproductive assurance hypothesis appears to have been supported by a diverse array of empirical tests, and is emerging as one of the most generally accepted reasons for the evolution of selfing in angiosperms. Indeed, direct studies have repeatedly shown that selfing boosts seed production, and population-genetic studies often find large losses of genetic diversity or high variance in diversity among populations, as would be expected in species where demographic instability triggers the evolution of selfing. Unfortunately, reproductive assurance is implicated by rejecting rather simplistic null hypotheses which may not adequately describe the expected complexity of mating-system evolution. In particular, it may often be the case that the process of mating-system evolution is aided by each of the direct advantages of selfing sequentially. This added layer of complexity has not yet been incorporated into population-genetic models, nor can it be addressed in field experiments lacking a historical component. Indeed, the initial spread of a mutation causing selfing might often be favoured because of reproductive assurance. Following the purging of some of the segregating mutational load, the equilibrium selfing rate might largely depend upon the tug-of-war between the transmission advantage of selfing and inbreeding depression. Making progress in understanding the relative importance of these mechanisms may be particularly difficult if this is often the case.
ACKNOWLEDGEMENTS
We thank each of the anonymous reviewers for their detailed and insightful comments on this paper, and J. Karron for inviting this review. We thank J. Pettengill and D. Moeller for sharing unpublished data concerning mating-system evolution in Clarkia.
LITERATURE CITED
- Aizen MA, Harder LD. Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology. 2007;88:271–281. doi: 10.1890/06-1017. [DOI] [PubMed] [Google Scholar]
- Antonovics J. Evolution in closely adjacent plant populations. V. Evolution of self fertility. Heredity. 1968;23:219–238. doi: 10.1038/sj.hdy.6800835. [DOI] [PubMed] [Google Scholar]
- Baker HG. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution. 1955;9:347–348. [Google Scholar]
- Baker HG. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The genetics of colonizing species. New York, NY: Academic Press; 1965. pp. 147–172. [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Eckert CG. Variation and evolution of mating systems in seed plants. In: Kawano S, editor. Biological approaches and evolutionary trends in plants. Tokyo: Academic Press; 1990. pp. 229–254. [Google Scholar]
- Barrett SCH, Morgan MT, Husband BC. The dissolution of a complex genetic polymorphism – the evolution of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae) Evolution. 1989;43:1398–1416. doi: 10.1111/j.1558-5646.1989.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Harder L, Worley AC. The comparative biology of pollination and mating in flowering plants. Philosophical Transactions of the Royal Society of London B. 1996;351:1271–1280. [Google Scholar]
- Bodbyl Roels SA, Kelly JK. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution. 2011 doi: 10.1111/j.1558-5646.2011.01326.x. in press. doi:10.1111/j.1558-5646.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd M. Bateman principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review. 1994;60:83–139. [Google Scholar]
- Busch JW. The evolution of self-compatibility in geographically peripheral populations of Leavenworthia alabamica (Brassicaceae) American Journal of Botany. 2005;92:1503–1512. doi: 10.3732/ajb.92.9.1503. [DOI] [PubMed] [Google Scholar]
- Busch JW, Joly S, Schoen DJ. Demographic signatures accompanying the evolution of selfing in Leavenworthia alabamica. Molecular Biology and Evolution. 2011;28:1717–1729. doi: 10.1093/molbev/msq352. [DOI] [PubMed] [Google Scholar]
- Busch JW, Schoen DJ. The evolution of self-incompatibility when mates are limiting. Trends in Plant Science. 2008;13:128–136. doi: 10.1016/j.tplants.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Byers DL, Waller DM. Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression. Annual Review of Ecology and Systematics. 1999;30:479–513. [Google Scholar]
- Campbell LG, Husband BC. Small populations are mate-poor but pollinator-rich in a rare, self-incompatible plant, Hymenoxys herbacea (Asteraceae) New Phytologist. 2007;174:915–925. doi: 10.1111/j.1469-8137.2007.02045.x. [DOI] [PubMed] [Google Scholar]
- Chang S-M, Rausher MD. Frequency-dependent pollen discouting contributes to maintenance of a mixed mating system in the common morning glory Ipomoea purpurea. American Naturalist. 1998;152:671–683. doi: 10.1086/286198. [DOI] [PubMed] [Google Scholar]
- Charlesworth D. Inbreeding effects on the genetic diversity of populations. Philosphical Transactions of the Royal Society of London B. 2003;358:1051–1070. doi: 10.1098/rstb.2003.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheptou P-O. Allee effect and self-fertilization in hermaphrodites: reproductive assurance in demographically stable populations. Evolution. 2004;58:2613–2621. doi: 10.1111/j.0014-3820.2004.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Cheptou P-O. Why should mating system biologists be demographers? Trends in Ecology and Evolution. 2007;22:562–563. doi: 10.1016/j.tree.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Cheptou P-O, Dieckmann U. The evolution of self-fertilization in density-regulated populations. Proceedings of the Royal Society of London B. 2002;269:1177–1186. doi: 10.1098/rspb.2002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheptou P-O, Massol F. Pollination fluctuations drive evolutionary syndromes linking dispersal and mating system. American Naturalist. 2009;174:46–55. doi: 10.1086/599303. [DOI] [PubMed] [Google Scholar]
- Cheptou P-O, Schoen DJ. Combining population genetics and demographical approaches in evolutionary studies of plant mating systems. Oikos. 2007;116:271–279. [Google Scholar]
- Cheptou P-O, Lepart J, Escarre J. Mating system variation along a successional gradient in the allogamous and colonizing plant Crepis sancta (Asteraceae) Journal of Evolutionary Biology. 2002;15:753–762. [Google Scholar]
- Coberly LC, Rausher MD. Pleiotropic effects of an allele producing white flowers in Ipomoea purpurea. Evolution. 2008;62:1076–1085. doi: 10.1111/j.1558-5646.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life. London: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- Darwin C. Effects of cross and self fertilization in the vegetable kingdom. London: John Murray; 1876. [Google Scholar]
- Eckert CG. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology. 2000;81:532–542. [Google Scholar]
- Eckert CG, Schaefer A. Does self-pollination provide reproductive assurance in Aquilegia canadensis? American Journal of Botany. 1998;85:919–924. [PubMed] [Google Scholar]
- Eckert CG, Kalisz S, Geber MA, et al. Plant mating systems in a changing world. Trends in Ecology and Evolution. 2009a;25:35–43. doi: 10.1016/j.tree.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Ozimec B, Herlihy CR, Griffin CA, Routley MB. Floral morphology mediates temporal variation in the mating system of a self-compatible plant. Ecology. 2009b;90:1540–1548. doi: 10.1890/08-1063.1. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Dart S. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder L, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 183–203. [Google Scholar]
- Elle E, Carney R. Reproductive assurance varies with flower size in Collinsia parviflora (Scrophulariaceae) American Journal of Botany. 2003;90:888–896. doi: 10.3732/ajb.90.6.888. [DOI] [PubMed] [Google Scholar]
- Fehr C, Rausher MD. Effects of variation at the flower-colour A locus on mating system parameters in Ipomoea purpurea. Molecular Ecology. 2004;13:1839–1847. doi: 10.1111/j.1365-294X.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- Fenster CB, Rodriguez SM. Pollination specialization and the evolution of reproductive assurance mechanisms through autonomous selfing. International Journal of Plant Sciences. 2007;93:1800–1807. [Google Scholar]
- Fenster CB, Diggle PK, Barrett SCH, Ritland K. The genetics of floral development differentiating two species of Mimulus (Scrophulariaceae) Heredity. 2005;74:258–266. [Google Scholar]
- Fisher RA. Average excess and average effect of a gene substitution. Annals of Eugenics. 1941;11:53–63. [Google Scholar]
- Fishman L. Pollen discounting and the evolution of selfing in Arenaria uniflora (Caryophyllaceae) Evolution. 2000;54:1558–1565. doi: 10.1111/j.0014-3820.2000.tb00701.x. [DOI] [PubMed] [Google Scholar]
- Fishman L, Willis JH. Pollen limitation and natural selection on floral characters in the yellow monkeyflower. Mimulus guttatus. New Phytologist. 2008;177:802–810. doi: 10.1111/j.1469-8137.2007.02265.x. [DOI] [PubMed] [Google Scholar]
- Fishman L, Wyatt R. Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae) Evolution. 1999;53:1723–1733. doi: 10.1111/j.1558-5646.1999.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Foxe JP, Slotte T, Stahl EA, Neuffer B, Hurka H, Wright SI. Recent speciation associated with the evolution of selfing in Capsella. Proceedings of the National Academy of Sciences of the USA. 2009;106:5241–5245. doi: 10.1073/pnas.0807679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JP, Stift M, Tedder A, Haudry A, Wright SI, Mable BK. Reconstructing origins of loss of self-incompatibility and selfing in North American Arabidopsis lyrata: a population genetic context. Evolution. 2010;64:3495–3510. doi: 10.1111/j.1558-5646.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- Fry JD, Rausher MD. Selection on a floral color polymorphism in the tall morning glory (Ipomoea purpurea): transmission success of the alleles through pollen. Evolution. 1997;51:66–78. doi: 10.1111/j.1558-5646.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Goldberg EE, Kohn JR, Lande R, Robertson KA, Smith SA, Igic B. Species selection maintains self-incompatibility. Science. 2010;330:459–460. doi: 10.1126/science.1194513. [DOI] [PubMed] [Google Scholar]
- Goodwillie C. Pollen limitation and the evolution of self-compatibility in Linanthus (Polemoniaceae) International Journal of Plant Sciences. 2001;162:1283–1292. [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics. 2005;11:15–39. [Google Scholar]
- Goodwillie C, Sargent RD, Eckert CG, et al. Correlated evolution of mating system and floral display traits in flowering plants and its implications for the distribution of mating system variation. New Phytologist. 2010;185:311–321. doi: 10.1111/j.1469-8137.2009.03043.x. [DOI] [PubMed] [Google Scholar]
- Grossenbacher DL, Whittall JB. Increased floral divergence in sympatric monkeyflowers. Evolution. 2011 doi: 10.1111/j.1558-5646.2011.01306.x. in press. doi:10.1111/j.1558-5646.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- Guo Y-L, Bechsgaard JS, Slotte T, et al. Recent speciation of Capsella rubella from Capsella grandiflora, associated with loss of self-incompatibility and an extreme bottleneck. Proceedings of the National Academy of Sciences of the USA. 2009;106:5246–5251. doi: 10.1073/pnas.0808012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick JL, Godt MJW. Effects of life history traits on genetic diversity in plant populations. Philosophical Transactions of the Royal Society of London B. 1996;351:1291–1298. [Google Scholar]
- Harder LD, Barrett SCH. Mating cost of large floral displays in hermaphroditic plants. Nature. 1995;373:512–515. [Google Scholar]
- Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. Experimental dissection of inbreeding and its adaptive significance in a flowering plant, Aquilegia canadensis (Ranunculaceae) Evolution. 2004;58:2693–2703. doi: 10.1111/j.0014-3820.2004.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. Evolution of self-fertilization at geographical range margins? A comparison of demographic, floral, and mating system variables in central vs. peripheral populations of Aquilegia canadensis (Ranunculaceae) American Journal of Botany. 2005;92:744–751. doi: 10.3732/ajb.92.4.744. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Sanchez-Lafuente AM, Medrano M, Guitian J, Cerda X, Rey P. Geographical variation in autonomous self-pollination levels unrelated to pollinator service in Helleborus foetidus (Ranunculaceae) American Journal of Botany. 2001;88:1025–1032. [PubMed] [Google Scholar]
- Hoebe PN, Stift M, Tedder A, Mable BK. Multiple losses of self-incompatibility in North-American Arabidopsis lyrata? Phylogeographic context and population genetic consequences. Molecular Ecology. 2009;23:4924–4939. doi: 10.1111/j.1365-294X.2009.04400.x. [DOI] [PubMed] [Google Scholar]
- Holderegger R, Haner R, Csencsics D, Angelone S, Hoebee SE. S-allele diversity suggests no mate limitation in small populations of a self-incompatible plant. Evolution. 2008;62:2922–2928. doi: 10.1111/j.1558-5646.2008.00498.x. [DOI] [PubMed] [Google Scholar]
- Holsinger KE. Mass-action models of plant mating systems: the evolutionary stability of mixed mating systems. American Naturalist. 1991;138:606–622. [Google Scholar]
- Holsinger KE. Ecological models of plant mating systems: the evolutionary stability of mixed mating systems. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman and Hall; 1992. pp. 169–191. [Google Scholar]
- Holsinger KE. Pollination biology and the evolution of mating systems in flowering plants. Evolutionary Biology. 1996;29:107–149. [Google Scholar]
- Husband BC, Barrett SCH. Colonization history and population genetic structure of Eichhornia paniculata in Jamaica. Heredity. 1991;66:287–296. [Google Scholar]
- Husband BC, Barrett SCH. Pollinator visitation in populations of tristylous Eichhornia paniculata in northeastern Brazil. Oecologia. 1992;89:365–371. doi: 10.1007/BF00317414. [DOI] [PubMed] [Google Scholar]
- Inouye K, Maki M, Masuda M. Evolution of Campanula flowers in relation to insect pollinators on islands. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman and Hall; 1996. pp. 377–301. [Google Scholar]
- Jacquemyn H, Brys R. Density-dependent mating and reproductive assurance in the temperate forest herb Paris quadrifolia (Trilliaceae) American Journal of Botany. 2008;95:294–298. doi: 10.3732/ajb.95.3.294. [DOI] [PubMed] [Google Scholar]
- Jain SK. The evolution of inbreeding in plants. Annual Review of Ecology, Evolution, and Systematics. 1976;7:469–495. [Google Scholar]
- Johnston MO. Evolution of intermediate selfing rates in plants: pollination ecology versus deleterious mutations. Genetica. 1998;102–3:267–278. [Google Scholar]
- Kalisz S, Vogler DW. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology. 2003;84:2928–2942. [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–886. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Kameyama Y, Kudo G. Flowering phenology influences seed production and outcrossing rate in populations of an alpine snowbed shrub, Phyllodoce aleutica: effects of pollinators and self-incompatibility. Annals of Botany. 2009;103:1385–1394. doi: 10.1093/aob/mcp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. The influence of floral display size on selfing rates in Mimulus ringens. Heredity. 2004;92:242–248. doi: 10.1038/sj.hdy.6800402. [DOI] [PubMed] [Google Scholar]
- Keller LF, Waller DM. Inbreeding effects in wild populations. Trends in Ecology and Evolution. 2002;17:230–241. [Google Scholar]
- Kennedy BF, Elle E. The reproductive assurance benefit of selfing: importance of flower size and population size. Oecologia. 2008;155:469–477. doi: 10.1007/s00442-007-0924-7. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Fischer M, Johnson SD. Reproductive assurance does not vary with population size in the alien invasive plant Datura stramonium. Oikos. 2007;116:1400–1412. [Google Scholar]
- van Kleunen M, Manning JC, Pasqualetto V, Johnson SD. Phylogenetically independent associations between autonomous self-fertilization and invasiveness. American Naturalist. 2008;171:195–201. doi: 10.1086/525057. [DOI] [PubMed] [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology, Evolution and Systematics. 2005;36:467–497. [Google Scholar]
- Kohn JR, Barrett SCH. Pollen discounting and the spread of a selfing variant in tristylous Eichhornia paniculata: evidence from experimental populations. Evolution. 1994;48:1576–1594. doi: 10.1111/j.1558-5646.1994.tb02197.x. [DOI] [PubMed] [Google Scholar]
- Koslow JM, DeAngelis D. Host mating system and the prevalence of disease in a plant population. Proceedings of the Royal Society of London B. 2006;273:1825–1831. doi: 10.1098/rspb.2006.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. The evolution of self-compatibility and racial differentiation in Leavenworthia (Cruciferae) Contributions from the Gray Herbarium of Harvard University. 1965;195:3–134. [Google Scholar]
- Lloyd DG. Some reproductive factors affecting the selection of self-fertilization in plants. American Naturalist. 1979;113:67–79. [Google Scholar]
- Lloyd DG. Demographic factors and mating patterns in angiosperms. In: Solbrig OT, editor. Demography and evolution in plant populations. Oxford: Blackwell Scientific; 1980. pp. 67–88. [Google Scholar]
- Lloyd DG. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences. 1992;153:370–380. [Google Scholar]
- Lloyd DG, Schoen DJ. Self- and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences. 1992;153:358–369. [Google Scholar]
- Marten-Rodriguez S, Fenster CB. Pollen limitation and reproductive assurance in Antillean Gesnerieae: a specialists vs. generalist comparison. Ecology. 2010;91:155–165. doi: 10.1890/08-2115.1. [DOI] [PubMed] [Google Scholar]
- Moeller DA. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology. 2006;87:1510–1522. doi: 10.1890/0012-9658(2006)87[1510:gsopcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Moeller DA, Geber MA. Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution. 2005;59:786–799. doi: 10.1554/04-656. [DOI] [PubMed] [Google Scholar]
- Nagylaki T. A model for the evolution of self-fertilization and vegetative reproduction. Journal of Theoretical Biology. 1976;58:55–58. doi: 10.1016/0022-5193(76)90138-7. [DOI] [PubMed] [Google Scholar]
- Ness RW, Wright SI, Barrett SCH. Mating-system variation, demographic history and patterns of nucleotide diversity in the tristylous plant Eichhornia paniculata. Genetics. 2010;184:381–392. doi: 10.1534/genetics.109.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Arroyo MT, Armesto JJ. Evolution of autonomous selfing accompanies increased specialization in the pollination system of Schizanthus (Solanaceae) American Journal of Botany. 2009;96:1168–1176. doi: 10.3732/ajb.0800306. [DOI] [PubMed] [Google Scholar]
- Piper JG, Charlesworth B, Charlesworth D. Breeding system evolution in Primula vulgaris and the role of reproductive assurance. Heredity. 1986;56:207–217. [Google Scholar]
- Pollak E. On the theory of partially inbreeding finite populations. 1. Partial selfing. Genetics. 1987;117:353–360. doi: 10.1093/genetics/117.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher E, Lande R. Loss of gametophytic self-incompatibility with evolution of inbreeding depression. Evolution. 2005;59:46–60. [PubMed] [Google Scholar]
- Rausher MD, Fry JD. Effects of a locus affecting floral pigmentation in Ipomoea-purpurea on female fitness components. Genetics. 1993;134:1237–1247. doi: 10.1093/genetics/134.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick CM, Forbes JF, Tanksley SD. Evolution of mating systems in Lycopersicon hirsutum as deduced from genetic variation in electrophoretic and morphological characters. Plant Systematics and Evolution. 1979;132:279–298. [Google Scholar]
- Ritland K. A genetic approach to measuring pollen discounting in natural plant populations. American Naturalist. 1991;138:1049–1057. [Google Scholar]
- Runions CJ, Geber MA. Evolution of the self-pollinating flower in Clarkia xantiana (Onagraceae). I. Size and development of floral organs. American Journal of Botany. 2000;87:1439–1451. [PubMed] [Google Scholar]
- Schoen DJ, Brown AHD. Interspecific variation in population gene diversity and effective population size correlates with the mating system in plants. Proceedings of the National Academy of Sciences of the USA. 1991;88:4494–4497. doi: 10.1073/pnas.88.10.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen DJ, Lloyd DG. Self- and cross-fertilization in plants. III. Methods of studying modes and functional aspects of fertilization. International Journal of Plant Sciences. 1992;153:381–393. [Google Scholar]
- Schoen DJ, Morgan MT, Bataillon T. How does self-pollination evolve? Inferences from floral ecology and molecular genetic variation. Philosophical Transactions of the Royal Society of London B. 1996;351:1281–1290. [Google Scholar]
- Schueller SK. Self-pollination in island and mainland populations of the introduced hummingbird-pollinated plant, Nicotiana glauca (Solanaceae) American Journal of Botany. 2004;91:672–681. doi: 10.3732/ajb.91.5.672. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Self-fertilization and population variability in flowering plants. American Naturalist. 1957;91:337–354. [Google Scholar]
- Sutherland S. What makes a weed a weed? Life history traits of native and exotic plants in the USA. Oecologia. 2004;141:24–39. doi: 10.1007/s00442-004-1628-x. [DOI] [PubMed] [Google Scholar]
- Takebayashi N, Delph LF. An association between a floral trait and inbreeding depression. Evolution. 2000;54:840–846. doi: 10.1111/j.0014-3820.2000.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Uyenoyama MK, Holsinger KE, Waller DM. Ecological and genetic factors determining the evolution of self-fertilization. Oxford Surveys in Evolutionary Biology. 1993;9:327–381. [Google Scholar]
- Vaughton G, Ramsey M. Pollinator-mediated selfing erodes the flexibility of the best-of-both-worlds mating strategy in Bulbine vagans. Functional Ecology. 2010;24:374–382. [Google Scholar]
- Vaughton G, Ramsey M, Johnson SD. Pollination and late-acting self-incompatibility in Cyrtanthus breviflorus (Amaryllidaceae): implications for seed production. Annals of Botany. 2010;106:547–555. doi: 10.1093/aob/mcq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi Y. Evolution towards self-compatibility when mates are limited. Journal of Evolutionary Biology. 2009;22:1967–1973. doi: 10.1111/j.1420-9101.2009.01806.x. [DOI] [PubMed] [Google Scholar]
- Wirth LR, Graf R, Gugerli F, Landergott U, Holderegger R. Lower selfing rate at higher altitudes in the alpine plant Eritrichium nanum (Boraginaceae) American Journal of Botany. 2010;97:899–901. doi: 10.3732/ajb.0900297. [DOI] [PubMed] [Google Scholar]
- Wyatt R. Ecology and evolution of self-pollination in Arenaria uniflora (Caryophyllaceae) Journal of Ecology. 1986;74:403–418. [Google Scholar]
- Young AG, Pickup M. Low S-allele numbers limit mate availability, reduce seed set and skew fitness in small populations of a self-incompatible plant. Journal of Applied Ecology. 2010;47:541–548. [Google Scholar]