Abstract
Background and Aims
Heterostyly is a floral polymorphism characterized by the reciprocal position of stamens and stigmas in different flower morphs in a population. This reciprocal herkogamy is usually associated with an incompatibility system that prevents selfing and intra-morph fertilization, termed a heteromorphic incompatibility system. In different evolutionary models explaining heterostyly, it has been alternately argued that heteromorphic incompatibility either preceded or followed the evolution of reciprocal herkogamy. In some models, reciprocal herkogamy and incompatibility have been hypothesized to be linked together during the evolution of the heterostylous system.
Methods
We examine the incompatibility systems in species with different stylar polymorphisms from the genera Lithodora and Glandora (Boraginaceae). We then test whether evolution towards reciprocal herkogamy is associated with the acquisition of incompatibility. To this end, a phylogeny of these genera and related species is reconstructed and the morphological and reproductive changes that occurred during the course of evolution are assessed.
Key Results
Both self-compatibility and self-incompatibility are found within the studied genera, along with different degrees of intra-morph compatibility. We report for the first time extensive variability among members of the genus Glandora and related species in terms of the presence or absence of intraspecies polymorphism and heteromorphic incompatibility. Overall, our results do not support a tight link between floral polymorphism and incompatibility systems.
Conclusions
The independent evolution of stylar polymorphism and incompatibility appears to have occurred in this group of plants. This refutes the canonical view that there is strong linkage between these reproductive traits.
Keywords: Character transitions, Lithodora, Glandora, Boragineae, evolution, heterostyly, incompatibility system, ITS, Lithospermeae, phylogenetic reconstruction, trnLUAA intron
INTRODUCTION
Despite a century and a half of studies on the evolutionary significance of heterostyly following the extensive treatment by Darwin (1877), there are still many open questions, and there is a lack of general agreement about the significance of and steps necessary to build this fascinating reproductive system in plants. Heterostyly is a morphological and reproductive polymorphism in which plant populations are composed of two (distyly) or three (tristyly) floral morphs that differ reciprocally in the height of their stigmas and anthers (Ganders, 1979). Additionally, heterostylous species usually possess a sporophytic diallelic incompatibility system, which prevents self- and intra-morph fertilization; this is termed a heteromorphic incompatibility system (Mather and de Winton, 1941). There is a set of ancillary traits that often accompany such a system (e.g. stigmatic papillae and pollen grain polymorphisms), although a number of deviations from the complete set of traits have been observed (for a review, see Ganders, 1979).
Several hypotheses about the evolution of heterostyly have been proposed, but two of them, representing quite different alternatives, are the most noteworthy: those proposed by Charlesworth and Charlesworth (1979) and by Lloyd and Webb (1992a, b). In these models, the incompatibility system has been interpreted differently. Darwin considered the evolution of incompatibility in heterostylous plants to be an ‘incidental and purposeless’ result of dissimilarities in pollen growth patterns in styles of different length (Darwin, 1877, p. 265). Later, incompatibility was considered of primary importance in the evolution of heterostyly. In particular, Charlesworth and Charlesworth (1979), following Baker (1966), proposed that the acquisition of a very simple, diallelic incompatibility system is the first necessary step in the evolutionary process, serving as a mechanism to avoid inbreeding effects in selfing plants. This system is considered highly inefficient because the plants are separated into two isolated mating groups within a population, as determined by the possession of different incompatibility alleles. The appearance of reciprocal herkogamy would be the consequence of two mutations tightly linked to the incompatibility locus that are selected to guarantee mating between plants of opposite groups. In contrast, and following Darwin (1877), Lloyd and Webb (1992a) proposed that heterostyly is mostly a morphological device that promotes precise, legitimate pollinations between morphs. Inbreeding is not invoked as the key selective factor, but an increase in the precision of cross-pollination and avoidance of interference between sex organs within the flower are instead considered to be the selected features (Barrett, 2002). Incompatibility reactions would evolve after the emergence of reciprocal herkogamy as a result of pollen specialization for legitimate pollination (Lloyd and Webb, 1992b). Heteromorphic incompatibility operates through the failure of each class of pollen (legitimate or illegitimate) to grow in a particular type of style, allowing growth only if legitimate cross-pollination is achieved. In this context, it is at least possible for the morphological changes to occur without any accompanying heteromorphic incompatibility. Recent studies using character reconstruction in Narcissus species have provided evidence for the Darwinian Lloyd and Webb (1992a) hypothesis (Pérez-Barrales et al., 2006). However, aside from the wide occurrence of heterostyly across families of angiosperms, the phenomenon has not been well documented in taxonomic groups outside the Amaryllidaceae.
The Boraginaceae is known to be one of the lineages in which heterostyly evolved (e.g. Ganders, 1979; Schoen et al., 1997; Ferrero et al., 2009; Cohen, 2011), and this family displays a wide variety of reproductive systems involving stylar polymorphism (Ray and Chisaki, 1957; Opler et al., 1975; Philipp and Schou, 1981; Schou and Philipp, 1983, 1984; Casper, 1985; De Jong and Klinkamer, 1989; Weller and Ornduff, 1991; Schoen et al., 1997; Ferrero et al., 2009, 2011a). Within the heterostylous taxa of the Boraginaceae, full self-compatibility appears to be common, as it has been observed in the distylous Cryptantha flava (Casper, 1985) and in some species of Pulmonaria (although with different strengths, Olesen, 1979; Brys et al., 2008). In most of these cases, however, self-compatibility seems to be a derived condition selected by reproductive assurance (see, for example, Schoen et al., 1997), produced through recombination within the heterostyly incompatibility supergene (Mather and de Winton, 1941).
Recent studies show extensive variation in style polymorphism within the tribe Lithospermeae, specifically in the genera Glandora and Lithodora (Thompson, 2005; Thomas et al., 2008; Ferrero et al., 2009; Cohen, 2011), suggesting that this group is well suited as a model system to test hypotheses about the evolution of heterostyly. In all species within the genera Lithodora and Glandora, two morphs with different style lengths are observed, called long (L) and short (S) morphs, although anther position varies widely. Thus, in stylar dimorphic species, the anthers remain at approximately the same height in both morphs, and in distylous species the anther height tends to be reciprocal to that of the stigma of the opposite morph (Ferrero et al., 2009, 2011a). Facing such diversity, we analyse the incompatibility systems found in six species of Glandora and Lithodora exhibiting different degrees of stylar polymorphism, which allows us to examine whether the incompatibility reaction is linked to style polymorphism, as predicted by the Charlesworth and Charlesworth (1979) model, or whether the two traits are not necessarily associated, as predicted by Lloyd and Webb (1992a). To that end, we have conducted hand pollination experiments using style dimorphic and distylous species, and we have investigated the reproductive and morphological shifts that occurred during the course of evolution using phylogenetic analysis of sequences from the nuclear internal transcribed spacer (ITS) region and the plastid trnLUAA intron.
MATERIALS AND METHODS
Study species
Traditionally, Lithodora (Griseb.) has formerly consisted of seven species and five subspecies distributed around the Mediterranean Basin. Recently, based on molecular data, this genus has been split into Glandora D.C. Thomas, Weigend and Hilger and Lithodora (Griseb.) D.C. Thomas, Weigend and Hilger (Thomas et al., 2008). All species in these two genera are perennial shrubs with style dimorphic flowers showing different degrees of reciprocity (Ferrero et al., 2009, 2011a). The plants occur mainly on rocky limestone habitats and cliffs, except for the widespread Glandora prostrata, which grows on acid soils. Their flowering period extends from February to July depending on the species and location. Like many heterostylous plants, these species present actinomorphic, tubular flowers that are pollinated by long-tongued pollinators (mainly solitary bees, bee-flies and butterflies; Ferrero et al., 2011b). Single flower life span is 3–5 d. Pollen is released from the first day onward, and nectar is secreted abundantly at the base of the ovaries at a rate of 1·2–3·6 µL per flower per day, depending on the species (Ferrero, 2011b). We carried out our study using six populations located in Spain, Portugal and Morocco, where most of the floral diversity occurs. We selected one population of each species from the genus Glandora (except G. rosmarinifolia, endemic to Italy) and one species from the genus Lithodora (L. fruticosa), thus accounting for much of the polymorphism present in the genus Lithodora as it was formerly defined (Ferrero et al., 2009, 2011a) (see Table 1 for details). Other species from tribe Lithospermeae and the tribe Anchuseae, for which information on morphological and reproductive traits as well as DNA sequence data were available, were also included in the study to serve as outgroups (see Table 1 and the Molecular analysis section for details).
Table 1.
Taxa included in the study with details on the studied populations of Glandora and Lithodora species: type of stylar polymorphism, location and co-ordinates of the studied localities where the breeding systems were carried out and GenBank accession numbers are provided
| Species | Stylar polymorphism | Locality (reference) | Co-ordinates | GenBank accession no. ITS* | GenBank accession no. trnL intron* |
|---|---|---|---|---|---|
| Anchusa officinalis | Stylar dimorphism | Denmark (Philipp and Schou, 1981) | – | AY045710 | AY045703 |
| Borago officinalis | Non-herkogamous monomorphism | Spain (Montaner et al., 2000) | – | AY383283 | AY383245 |
| Echium vulgare | Approach herkogamy | UK (Corbet, 1978) | – | FJ789862 | FJ789844 |
| E. wildpretii | Approach herkogamy | Spain (Dupont et al. 2004) | – | L43314 | L43316 |
| Glandora diffusa | Distyly | Spain: Suances (Ferrero et al., 2009) | 43°26′30″N 04°02′50′′W | FJ789863 | FJ789845 |
| G. moroccana | Distyly | Morocco: Akchour (Ferrero et al., 2009) | 35°14′12′′N 05°10′19′′W | FJ789867 | FJ789849 |
| G. nitida | Distyly | Spain: S. Mágina (Ferrero et al., 2009) | 37°42′06′′N 03°27′53′′W | FJ789868 | FJ789850 |
| G. oleifolia | Distyly | Spain: St. Aniol (Ferrero et al., 2009) | 42°19′01′′N 02°35′13′′W | FJ789869 | FJ789851 |
| G. prostrata | Relaxed style dimorphism | Spain: Corrubedo (Ferrero et al., 2009) | 42°34′35′′N 09°05′20′′W | FJ789869 | FJ789853 |
| Lithodora fruticosa | Stylar dimorphism | Spain: Hueva (Ferrero et al., 2009) | 40°28′9′′N 02°057′2′′W | FJ789864 | FJ789846 |
| Lithospermum caroliniense | Distyly | USA (Levin, 1968) | – | EU044876 | EU044914 |
| Macromeria viridiflora | Approach herkogamy | USA (Boyd, 2004) | – | EU044870 | EU044908 |
*Reported in Böhle et al. (1996); Hilger et al. (2004); Thomas et al. (2008); Ferrero et al. (2009).
Floral polymorphism and reciprocity
In order to describe the types of stylar polymorphisms observed in the studied species, we calculate the degree of reciprocity between sexual whorls for each population, following Sánchez et al. (2008). This index is based on a comparison of the positions of every anther of each flower with the stigmas of all plants of the opposite morph in a population, thus accounting for both the reciprocal positions of organs and their variability within the flower and within the population. Lithodora and Glandora show a variety of stylar polymorphisms and degrees of reciprocity, distyly being the most common polymorphism within both genera (Ferrero et al., 2009, 2011a). However, L. fruticosa is style dimorphic, i.e. its flowers have two different lengths for the style but only one for the anthers. In addition, G. prostrata shows relaxed style dimorphism, a recently described polymorphism in which there are also two lengths for the style and two similar average lengths for the stamens in each morph, but the stamens are spread along the flower tube and display a distribution of different heights (Ferrero et al., 2009, 2011a).
Hand pollination experiments
To determine the types of incompatibility systems present in the studied species, controlled pollination experiments were conducted in the field during the spring of 2007 and 2008. On each plant, we marked and bagged four flower buds that were randomly assigned to one of the following treatments: (1) obligatory self-pollination; (2) intra-morph cross-pollination (L × L or S × S); (3) inter-morph cross-pollination (L × S or S × L); or (4) spontaneous self-pollination. For treatments (2) and (3), flower buds were emasculated. To study the extent to which the amount of pollen deposited on stigmas may limit fruit and seed production, on each plant, we marked two additional flowers that were not bagged (natural pollination was allowed), to which we applied supplementary manual inter-morph cross-pollination (treatment 5). Unmanipulated flowers (treatment 6) were also studied as controls (natural pollination was allowed). The six treatments were carried out on each plant, with at least 11 plants treated for each morph (sample sizes are summarized in Table 2). For inter- and intra-morph cross-pollinations, we used a mixture of pollen from the anthers of the recently opened flowers of several plants located 15–30 m away from the experimental plants.
Table 2.
Number of treated flowers [n (total number of L plants treated, total number of S plants treated)], fruit set and seed to ovule ratio (S/O) for Glandora and Lithodora studied species under different hand pollination treatments
|
G. diffusa |
G. moroccana |
G. nitida |
G. oleifolia |
G. prostrata |
L. fruticosa |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pollination treatment | Morph | n (15, 16) | Fruit set | S/O | n (16, 18) | Fruit set | S/O | n (20, 21) | Fruit set | S/O | n (12, 11) | Fruit set | S/O | n (29, 29) | Fruit set | S/O | n (16, 17) | Fruit set | S/O |
| Self-pollination | L | 13 | 0·23 | 0·08 | 16 | 0·47 | 0·12 | 14 | 0·07 | 0·02 | 9 | 0·22 | 0·06 | 28 | 0·18 | 0·08 | 10 | 0·1 | 0·03 |
| S | 15 | 0·40 | 0·15 | 18 | 0·22 | 0·06 | 13 | 0·23 | 0·06 | 10 | 0·10 | 0·03 | 27 | 0·22 | 0·09 | 15 | 0 | 0 | |
| Total | 28 | 0·32 | 0·12 | 35 | 0·34 | 0·09 | 27 | 0·14 | 0·04 | 19 | 0·16 | 0·04 | 55 | 0·20 | 0·09 | 25 | 0·04 | 0·01 | |
| Intra-morph (L × L, S × S) | L | 14 | 0·36 | 0·13 | 15 | 0·53 | 0·13 | 18 | 0·22 | 0·06 | 8 | 0·38 | 0·09 | 26 | 0·46 | 0·23 | 11 | 0·45 | 0·11 |
| S | 15 | 0·47 | 0·17 | 18 | 0·56 | 0·14 | 17 | 0·06 | 0·01 | 9 | 0·11 | 0·03 | 26 | 0·46 | 0·29 | 13 | 0·31 | 0·12 | |
| Total | 29 | 0·41 | 0·14 | 33 | 0·55 | 0·14 | 35 | 0·14 | 0·04 | 17 | 0·24 | 0·06 | 52 | 0·46 | 0·26 | 24 | 0·38 | 0·11 | |
| Inter-morph (L × S, S × L) | L | 15 | 0·47 | 0·15 | 16 | 0·5 | 0·13 | 18 | 0·50 | 0·14 | 8 | 0·25 | 0·06 | 27 | 0·41 | 0·24 | 14 | 0·57 | 0·18 |
| S | 14 | 0·36 | 0·21 | 15 | 0·2 | 0·06 | 19 | 0·42 | 0·11 | 9 | 0·33 | 0·08 | 25 | 0·52 | 0·24 | 14 | 0·21 | 0·09 | |
| Total | 29 | 0·41 | 0·18 | 31 | 0·35 | 0·10 | 37 | 0·46 | 0·12 | 17 | 0·29 | 0·07 | 52 | 0·46 | 0·24 | 28 | 0·39 | 0·13 | |
| Spontaneous self-pollination | L | 15 | 0 | 0 | 16 | 0 | 0 | 17 | 0 | 0 | 11 | 0 | 0 | 27 | 0 | 0 | 14 | 0 | 0 |
| S | 14 | 0 | 0 | 18 | 0 | 0 | 17 | 0 | 0 | 11 | 0 | 0 | 24 | 0 | 0 | 13 | 0 | 0 | |
| Total | 30 | 0 | 0 | 35 | 0 | 0 | 34 | 0 | 0 | 22 | 0 | 0 | 51 | 0 | 0 | 27 | 0 | 0 | |
| Supplementary pollination | L | 9 | 0·89 | 0·44 | 16 | 0·65 | 0·19 | 15 | 0·60 | 0·15 | 11 | 0·55 | 0·14 | 11 | 0·73 | 0·23 | 8 | 0·63 | 0·14 |
| S | 13 | 0·46 | 0·29 | 17 | 0·59 | 0·15 | 14 | 0·50 | 0·12 | 10 | 0·40 | 0·10 | 16 | 0·44 | 0·17 | 9 | 0·56 | 0·17 | |
| Total | 22 | 0·64 | 0·35 | 34 | 0·62 | 0·15 | 29 | 0·55 | 0·14 | 21 | 0·48 | 0·12 | 27 | 0·56 | 0·19 | 17 | 0·59 | 0·16 | |
| Control | L | 12 | 0·58 | 0·25 | 15 | 0·47 | 0·12 | 13 | 0·23 | 0·06 | 11 | 0·27 | 0·07 | 9 | 0·67 | 0·30 | 10 | 0·50 | 0·16 |
| S | 12 | 0·67 | 0·29 | 17 | 0·76 | 0·19 | 17 | 0·24 | 0·06 | 10 | 0·30 | 0·08 | 9 | 0·44 | 0·19 | 8 | 0·13 | 0·06 | |
| Total | 24 | 0·63 | 0·27 | 32 | 0·63 | 0·16 | 30 | 0·23 | 0·06 | 21 | 0·29 | 0·07 | 18 | 0·55 | 0·26 | 18 | 0·33 | 0·13 | |
Floral morphs: L, long styled; S, short styled. Results are presented for each floral morph and the total for each species.
Statistical comparisons between treatments were based on the seed to ovule ratio (S/O). We conducted two independent analyses, one for the incompatibility system and another for the pollen limitation experiment. In both, the effects of pollination treatment, morph and species were considered fixed factors and explored by Generalized Estimating Equation (GEE) models and an ‘independent’ correlation matrix structure. We chose this matrix because it was the one best tuned for our data. The S/O ratio was modelled as a binomial distribution, with logit used as the link function. A backward stepwise elimination procedure was used to determine the minimal adequate model.
Reproductive indices
In order to characterize the breeding system of each species quantitatively, we calculated the following indices, using the S/O values obtained in the manual pollination experiments.
Self- and morph compatibility and self-fertilization indices
The self-compatibility index (SCI) (Lloyd and Schoen, 1992) was calculated as the ratio of S/O values from self-pollination and inter-morph crosses. We extended this index for calculating the degree of within-morph compatibility (MCI) in style polymorphic species, which was calculated as the ratio of S/O values from intra-morph crosses and inter-morph crosses. The same criteria used for SCI were used for MCI to differentiate the types of morph compatibility. An SCI or MCI ratio of 0·75 was used as the threshold value (Lloyd and Schoen, 1992). A value <0·75 indicates that the species are self-incompatible (SCI) or incompatible with others of the same morph (MCI). A value >0·75 for the SCI or MCI indicates self-compatibility or intra-morph compatibility, respectively.
Percentage of pollen limitation
Limitation of reproductive success due to low pollen delivery was measured as the percentage of pollen limitation (PPL), calculated as follows:
In this equation, PS represents the S/O value of pollen-supplemented flowers, and C represents the S/O value of control flowers (Jules and Rathcke, 1999).
Molecular analysis
A matrix of two DNA regions (ITS; trnLUAA intron) was constructed using sequences from GenBank for Glandora, Lithodora and other members of Lithospermeae, as well as from some members of the Anchuseae for which incompatibility system information is available. We chose Anchusa officinalis and Borago officinalis as outgroups, following Thomas et al. (2008). The sequences from the Glandora and Lithodora species correspond to the same populations used in the experiments described above, and they have been phylogenetically analysed by others (Ferrero et al., 2009). Information about taxa and accession numbers is summarized in Table 1. Alignment of the two concatenated DNA regions was carried out with ClustalW (Thompson et al., 1994) as implemented in MEGA 4 (Tamura et al., 2007). Maximum parsimony (MP) and Bayesian inference reconstructions were carried out. Parsimony analyses were run using Fitch parsimony with PAUP* (Swofford, 1999), operated in a Windows environment using the PAUPUp graphical interface (Calendini and Martin, 2005). Equal weighting of all characters and of transitions/transversions was used. Heuristic searches were replicated 100 times under the random taxon addition sequences option. Support for monophyly of groups was assessed by ‘full’ bootstrapping (1000 replicates) using the heuristic search strategy mentioned above. The Hierarchical Likelihood Ratio Test (hLRT) and Akaike Information Criterion (AIC) were implemented for each of the two partitions independently using MrModeltest 3·7 (Posada and Crandall, 1998; Nylander, 2002) to determine the simplest model of sequence evolution that best fits the sequence data. A Bayesian inference of phylogeny with Markov chain Monte Carlo sampling was conducted using MrBayes 3·1·2 (Ronquist and Huelsenbeck, 2003). One cold chain and three heated chains were run simultaneously for 10 million generations, and one tree per 100 generations was sampled (four MCMC, chain temperature = 0·2; sample frequency = 100; and burn-in = 10000). A general time-reversible model of DNA substitution and a shape parameter of the gamma distribution model (GTR + G) were used, with different parameter settings for the two genes. We estimated the 50 % majority-rule consensus of the remaining trees and used posterior probability (PP) as an alternative estimate of robustness.
Ancestral character reconstruction
The evolution of heterostyly and incompatibility systems assuming MP was reconstructed using the 50 % majority-rule consensus tree recovered from the Bayesian analysis of the combined nuclear and plastid data sets. Three states of sexual polymorphism were considered, which are the three present in Lithodora and Glandora (Ferrero et al., 2009, 2011a), and two types of monomorphism were considered: 0, non-herkogamous monomorphism (one morph with equal heights of stigma and anthers); 1, approach herkogamy (one morph with the stigma above the anthers); 2, stylar dimorphism (two morphs differing in stigma height but with a similar anther position among morphs); 3, distyly (two morphs with a reciprocal position of anthers and stigmas); 4, relaxed style dimorphism (similar to distyly, but with anthers spread along the corolla tube at different heights; see Ferrero et al. 2011a for further detail); and three states for the incompatibility system characterization on the basis of the results obtained from the experimental manipulations: 0, self- and intra-morph compatibility (self-compatibility in monomorphism); 1, self-incompatibility and intra-morph compatibility; and 2, self- and intra-morph incompatibility. For the characterization of the taxa added from GenBank, we consulted Ferrero et al. (2009, 2011a) for descriptions of their polymorphism, and Levin (1968), Philipp and Schou (1981), Corbet (1978), Montaner et al. (2000), Dupont et al. (2004) and Boyd (2004) for information about their incompatibility systems.
Stylar polymorphisms and incompatibility systems were reconstructed by MP using the ‘Unordered’ model in Mesquite 2·01 (Maddison and Maddison, 2007). According to this model, the cost of changing state is 1, and all changes among polymorphism states are equally probable. We also used a likelihood-based reconstruction method, which maximize the probability that the states would evolve under a stochastic model of evolution (Schluter et al., 1997; Pagel, 1999). Maximum likelihood (ML) methods find the state that maximizes the probability of arriving at the observed states in the terminal taxa, given a particular model of evolution for each node. Maximum likelihood reconstruction was carried out based on an Mk1 model in which any particular change is equally probable (‘Markov k-state 1 parameter model’).
Tests for correlated trait evolution were conducted using Bayesian mutation mapping (Nielsen and Huelsenbeck, 2002), as implemented in SIMMAP Version 1.5 (Bollback, 2006), on the final 101 sampled post-burn-in trees of the Bayesian reconstruction. The method accounts not only for phylogenetic uncertainty, but also for mapping uncertainty by allowing the rate of evolution of the character to vary. SIMMAP uses a Gamma prior on the overall substitution rate of a character, in which both parameters of the Γ (Gamma) distribution can be defined. To calculate the priors, an MCMC analysis of 100 000 cycles was first performed on the Bayesian consensus tree to sample the overall rate values (Gamma prior). The samples from the posterior distribution of these parameters were then used to find the best-fitting Gamma distribution using the MASS and TeachingDemos packages (Venables and Ripley, 2002; Snow, 2005) for the R statistical computing platform (version 2·8·1, R Development Core Team, 2008).
The overall evolutionary rate prior had the parameters α = 4·5, β = 0·11 and κ = 60 for the stylar polymorphism and α = 5·079, β = 0·012 and κ = 60 for the incompatibility system.
The D statistic in SIMMAP was used to calculate the correlation between the states of each character (stylar polymorphism and incompatibility system), and the M statistic was used to evaluate the correlations between character histories along the phylogeny for the two characters, where f is the fraction of time one state is associated with another in a character history. We let the program take ten stochastic draws from the prior distribution, and ten realizations were sampled for each tree.
RESULTS
Floral polymorphism and reciprocity
The reciprocity index ranged from 0·018 in G. diffusa to 0·094 in G. prostrata (Table 4), with low index values indicating high levels of reciprocity, and vice versa.
Table 4.
Type of polymorphism, reciprocity index (following Sánchez et al., 2008), self-compatibility index (SCI, following Lloyd and Schoen, 1992), morph compatibility index (MCI, see details in the Materials and methods) and percentage of pollination limitation (PPL, following Jules and Rathcke, 1999) for the studied species
| Species | Stylar polymorphism | Reciprocity index | SCI | MCI | PPL |
|---|---|---|---|---|---|
| Glandora diffusa | Distyly | 0·0181 | 0·64 | 0·81 | 23·12 |
| G. moroccana | Distyly | 0·0254 | 0·91 | 1·13 | 9·09 |
| G. nitida | Distyly | 0·0302 | 0·29 | 0·29 | 57·71 |
| G. oleifolia | Distyly | 0·0225 | 0·54 | 0·80 | 40·0 |
| G. prostrata subsp. prostrata | Relaxed stylar dimorphism | 0·0945 | 0·36 | 1·08 | –35·71 |
| Lithodora fruticosa | Stylar dimorphism | 0·0762 | 0·07 | 0·86 | 22·73 |
Hand pollination experiments and reproductive indices
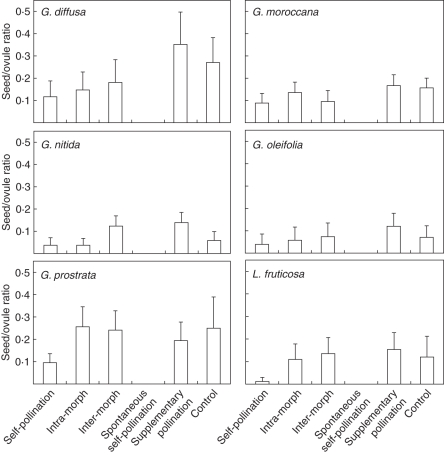
The results of hand pollination experiments in terms of the S/O are presented in Fig. 1 and Table 2. There were no progeny after spontaneous selfing, as no unpollinated bagged flower produced fruit, indicating that all of the studied species depend on pollinators to achieve fertilization. For this reason, the spontaneous self-pollination treatment was excluded in the statistical analysis. For the hand pollination treatment effect, we found significant differences in S/O between species and between treatments, as well as interactive effects in the simplest model (Table 3, Fig. 1). There were no significant differences between morphs in any case (P > 0·280). In G. diffusa, G. moroccana and G. oleifolia, there were no significant differences in the S/O between any treatments (Fig. 1). Moreover, S/O values in both self-pollination and intra-morph treatments were greater than zero, indicating that these species are equally self- and intra-morph compatible (Fig. 1). For these species, the values for SCI ranged between 0·54 and 0·91, and MCI values were >0·80 (Table 4). Significant differences in S/O ratio between treatments were found for G. nitida, G. prostrata and L. fruticosa (Fig. 1). However, whereas G. prostrata and L. fruticosa presented similar S/O values in intra- and inter-morph crosses, thus indicating that they are morph-compatible species (SCI < 0·36 and MCI > 0·86), G. nitida showed similar low levels of the S/O between both intra-morph and self-pollinations (SCI = 0·29 and MCI = 0·29), clearly displaying a heteromorphic incompatibility system (Table 4).
Fig. 1.
Mean (with 95 % confidence interval) of the seed-to-ovule ratio in the studied species of Lithodora and Glandora following hand-pollination treatments. Treatments included self-pollination, intra-morph cross-pollination (L × L, S × S), inter-morph cross-pollination (L × S, S × L), spontaneous self-pollination, natural pollination plus supplementary pollination and control (natural) pollination. Because there were no statistical differences between floral morphs within species, the results for both morphs are plotted together for each taxon.
Table 3.
Results of the Generalized Estimating Equations analysis for the comparisons of the seed/ovule ratios after different pollination treatments
| Self-pollination, intra-morph, inter-morph crosses |
Control supplementary pollination |
|||||
|---|---|---|---|---|---|---|
| Source | Wald χ2 | d.f. | P | Wald χ2 | d.f. | P |
| Species | 34·35 | 5 | 0·000 | 34·81 | 5 | 0·000 |
| Treatment | 17·90 | 2 | 0·000 | 4·06 | 1 | 0·044 |
| Species × treatment | 18·64 | 10 | 0·045 | 7·46 | 5 | 0·188 |
Analyses were carried out independently for the self-pollination, intra-morph crosses and inter-morph crosses on one hand and the control supplementary pollination on the other. Spontaneous self-pollination was not included in the analysis as it was nearly zero in most cases.
For the pollen limitation experiment, significant differences were found between species (P < 0·001) and treatments (P < 0·044) in the simplest model (Table 3, Fig. 1). Significant differences between treatments were only found for G. nitida (Fig. 1). The PPL was revealed to be relatively high for G. nitida (57·7 %) and G. oleifolia (40·0 % Table 4).
Molecular analysis
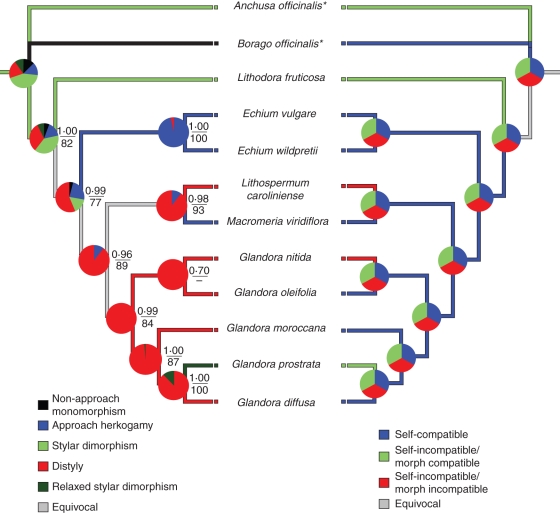
For the ITS and trnLUAA intron regions, the aligned sequences were 237 and 465 bp in length, respectively. In the MP analysis, 557 characters are constant, 82 are parsimony uninformative and 63 are parsimony informative. In the phylogenetic reconstruction, one polytomy is depicted in the outgroup, and the relationship between G. nitida and G. oleifolia is not well supported (0·70 PP). Bootstrap values and PP are quite high for the remaining reconstruction (77 % and 0·96 PP as minimum values; Fig. 2).
Fig. 2.
Evolution of sexual polymorphisms and incompatibility systems within some genera of Boraginaceae, based on the majority-rule consensus tree recovered from the Bayesian analysis of the combined ITS and trnLUAA intron data set. Numbers above branches on the left tree indicate posterior probabilities from the Bayesian analysis under the GTR + G model. Numbers below branches are bootstrap values from the MP analysis. The most-parsimonious states are shown under the unordered model (coloured lines of the tree). For the ML character reconstruction, probabilities at each node are reported as proportional likelihoods for each character (sectors of the pie charts).
Ancestral character reconstruction
Reconstruction of stylar polymorphism using MP methods indicates that distyly is the most likely ancestral condition in Glandora and that relaxed style dimorphism is a derived condition (Fig. 2). According to the MP reconstruction, self- and morph compatibility is the ancestral condition in the genus Glandora, while heteromorphic incompatibility and self-incompatibility are derived conditions. However, following this method, the condition ancestral to the ingroup (i.e. including also Lithodora, Echium, Lithospermum and Macromeria) cannot be resolved (Fig. 2). The ML reconstruction method does not clarify any of the more ancestral stages for style polymorphism and does not resolve any ancestral state for the incompatibility systems. M and D statistics (Huelsenbeck et al., 2003) for measuring the correlation of stylar polymorphism and incompatibility system were not significant (M = 0·014, P = 0·326; D = 0·111, P = 0·285).
DISCUSSION
A wide variety of style polymorphisms has been recently reported for Lithodora, Glandora and other members of the tribe Lithospermeae. This level of variation is one of the highest described thus far at the family level (Ferrero et al., 2009). Here we report that there is also extensive variation in incompatibility systems, mostly within Glandora. Both style polymorphism and incompatibility are critical features for testing the alternative hypotheses regarding the evolution of heterostyly (Charlesworth and Charlesworth, 1979; Lloyd and Webb, 1992a). This taxonomic group presents an opportunity to determine which of these two hypotheses is best supported by phylogenetic evidence. Our results indicate that stylar polymorphism is not necessarily coupled with the incompatibility system.
Implications for the evolution of distyly
The great variety of incompatibility systems in the study group and the relatively small number of species precluded any sound ML reconstruction of evolutionary transitions of the relevant traits. Nevertheless, the parsimony analysis indicates that self-incompatibility evolved independently in Lithospermum caroliniense, G. nitida and G. prostrata, although the latter is of a contrasted type (morph compatible). This is similar to what was previously found in other related Boraginaceae (L. fruticosa, this study; Anchusa officinalis, Phillip and Schou, 1981). At least within Glandora, incompatibility systems both linked and unlinked to style polymorphism have evolved in different species. Such a contrasting pattern within a single genus is reported here for the first time. In this respect, it is noteworthy that within Boraginaceae, some Anchusa species show an incompatibility system that is also unlinked to style length variation (Philipp and Schou, 1981; Schou and Philipp, 1983, 1984), whereas other style polymorphic species of the Lithospermum or Amsinckia genera typically show self-incompatibility that is linked to style morphs (Levin, 1968; Ganders, 1979). Thus, Boraginaceae is a very good candidate for further work exploring the genetic, physiological and evolutionary relationships between homomorphic and heteromorphic incompatibility systems (Lloyd and Webb, 1992a; Barrett and Shore, 2008).
In a previous study of character reconstruction in Lithospermeae (Ferrero et al., 2009), we found two striking results: that style dimorphism is the ancestral condition to distyly in the genus Lithodora sensu lato (including Glandora), supporting current models for the evolution of distyly (Charlesworth and Charlesworth, 1979; Lloyd and Webb's 1992a), and that a newly described stylar polymorphism, termed relaxed stylar dimorphism, is derived from distyly in the genus Glandora (G. prostrata, with variable anther height within a single flower). The results presented here for the reconstruction of incompatibility system evolution can be considered additional support for the Darwinian hypothesis of Lloyd and Webb (1992a). We observed strong variation within the group and an apparent lack of association between incompatibility system and style length variation, and the Darwinian hypothesis predicts no such association between these two reproductive traits.
The sequence of evolutionary events that occurred in the studied taxonomic group is best represented by a model of heterostyly evolution that is partially in keeping with some of the premises of Lloyd and Webb (1992a, b). In particular, the acquisition of a full heteromorphic self-incompatible distylous condition as a final step (G. nitida) of evolution from a self-compatible distylous ancestor fits well with the Lloyd and Webb model. Thus, Glandora represents a challenging system to study the physiological, molecular, genetic and ecological (pollen transfer dynamics) basis for the acquisition of the supergene coding for reciprocal herkogamy and heteromorphic incompatibility. It is also worth noting that even ancillary traits, such as the pollen size dimorphism or pollen production traits thought to be associated with the same supergene (Dulberger, 1992), are only found in G. nitida. Glandora nitida is the only studied species that displays all of the traits usually associated with heterostyly (Ferrero et al., 2011a; this study).
Incompatibility system variation
Incompatibility systems are diverse in their genetic basis and physiological expression, although they are all thought to be mechanisms for avoiding self-crossing (Barrett, 2002). Whereas homomorphic (without morphological variation within the population) incompatibility systems are variable in their genetic control and site of reaction (Allen and Hiscock, 2008), heteromorphic incompatibility systems are frequently sporophytic and diallelic. The latter usually present two or three crossing groups and a linkage between the incompatibility locus and loci involved in discrete, reciprocal morph variation in stamen and stigma heights (Gibbs, 1986). It is commonly thought that homomorphic and heteromorphic incompatibility systems evolved independently and have distinct modes of action (Gibbs, 1986; Barrett and Shore, 2008). There is no available information on the genetic basis and the physiological mechanisms of the incompatibility system operating in our studied species, but our crossing experiments provide some information about the possible mechanisms involved.
In general, we observed low S/O values in all experimental pollination treatments; the values were all <0·44. Lower values of seed set have been reported previously for the Boraginaceae (Casper and Wiens, 1981; Goulson et al., 1998), which might be related to intrinsic limitations on the development of the four ovules in the flower. In light of our results, we are able to report full self-compatibility, self-incompatibility with intra-morph compatibility and self-incompatibility with intra-morph incompatibility. To our knowledge, this is the first report of such a wide-ranging set of incompatibility systems found within such a small group of species, and we believe that this observation casts doubt on their supposedly independent evolutionary origins. Full self-compatibility is a widespread feature in the studied group (G. diffusa, G. moroccana and G. oleifolia), and it is even found in some related species, such as Echium vulgare, E. wildpretii and Macromeria viridiflora (Corbet et al., 1978; Boyd, 2004; Dupont et al., 2004). Despite the lack of strong support for self-compatibility as a derived state in our reconstruction (see parsimony analysis in Fig. 2), it seems reasonable to assume that self-compatibility in the three species in our group was selected for reproductive assurance, as has been shown in other heterostylous groups (Schoen et al., 1997; Pannell and Barrett, 1998). It is worth noting that two of the three self-compatible species (G. oleifolia and G. moroccana) are narrow endemic species with very small and isolated populations, such that reproductive assurance is more likely to apply due to the scarcity of mates. However, these species still rely on insects for pollination (see results of supplemental and autonomous hand pollination experiments), which probably indicates a mixture of self-geitonogamous and cross-pollination that would result in a typical mixed-mating system for these species.
The three narrow endemic species, comprising a few small and sparse populations, show the lowest values of S/O in all the hand cross-pollination treatments (<10 %), irrespective of their being self-compatible (G. moroccana in N Morocco and G. oleifolia in the E Pyrenees, NE Spain) or self-incompatible (G. nitida in S Spain). There is no information on their population genetic structure, although the restricted range and small population size may have led to low genetic variation and high biparental inbreeding levels in their populations (Allee effect; Courchamp et al., 1999; Stephens et al., 1999). Moreover, the two narrowest and most threatened endemics (G. nitida and G. oleifolia; Bañares et al., 2003) suffer to some extent from the effects of pollen limitation (high PPL), an additional cause of low reproductive output in small populations and narrow distribution ranges (Knight et al., 2005).
In this study, we found that the three cases of style polymorphism in which there is a lack of perfect reciprocity share a particular incompatibility system: self-incompatibility with intra-morph compatibility. This association has been reported in other species, such as Mirabilis froebelii (Bateman, 1968), Quinchamalium santalense (Riveros et al., 1987), several species of Narcissus (Dulberger, 1964; Barrett et al., 1997; Sage et al., 1999; Baker et al., 2000; Arroyo et al., 2002; Pérez-Barrales et al., 2006) and Anchusa hybrida (Dulberger, 1970). Perhaps the presence of this incompatibility system, which is unlinked to style polymorphism (probably gametophytic and multiallelic; Dulberger, 1964), is only a by-product of the historical (phylogenetic) effects in these particular plant groups, but this claim requires a wider sampling of taxa to be conclusive. In this respect, it is worth noting that a recent paper by Cohen (2011) reports a variability of floral morphology in the phylogeny of Lithospermum, with several independent gains of heterostyly. It is desirable to have data on the breeding systems of these species in order to have a larger sample size for estimating phylogenetic correlations between these critical traits.
ACKNOWLEDGEMENTS
The authors thank I. Stanescu, R. Ajbilou, M. Ater, G. Carballo and J. M. Sánchez for their help with field work; A. Velando for his comments and help with the statistical analysis; S. Rocha for logistical support for the phylogenetic reconstructions; and F. E. Narbona, S. D. Johnson and J. R. Pannell for their helpful comments. The work of D. Levin and three anonymous reviewers contributed substantial improvements to the final version. This research was supported by the Spanish Dirección General de Investigación, Ciencia y Tecnología (DGICYT) through the grants CGL2006-13847-CO2-01 + 02, CGL2009-10466 and CGL2009-12565, FEDER funds from the European Union, the Agencia Española de Cooperación Internacional (AECI) through the Projects A/6962/06, A/017570/08, A/016832/08 and A/023775/09, CYTED COCAMGLOINBIEMO project (409AC0369), the Xunta de Galicia through the grant PGIDT04-PXIC31003PN, and Plan Andaluz de Investigación through excellence grants P07-RNM-02869 and P09-RNM-5280. The Ministerio de Educación y Ciencia (MEC) financed the work of V.F. through a PhD scholarship (AP-2004-6394), together with the Fundación Ramón Areces. The Portuguese Foundation for Science and Technology financed the work of S.C. (BD/10901/2002 and BPD/41200/2007).
LITERATURE CITED
- Allen AM, Hiscock SJ. Evolution and phylogeny of self-incompatibility systems in angiosperms. In: Franklin-Tong VE, editor. Self-incompatibility in flowering plants: evolution, diversity and mechanisms. Berlin: Springer-Verlag; 2008. pp. 73–101. [Google Scholar]
- Arroyo J, Barrett SCH, Hidalgo R, Cole WW. Evolutionary maintenance of stigma-height dimorphism in Narcissus papyraceus (Amaryllidaceae) American Journal of Botany. 2002;89:1242–1249. doi: 10.3732/ajb.89.8.1242. [DOI] [PubMed] [Google Scholar]
- Baker HG. The evolution, functioning and breakdown of heteromorphic incompatibility systems. I. The Plumbaginaceae. Evolution. 1966;20:349–368. doi: 10.1111/j.1558-5646.1966.tb03371.x. [DOI] [PubMed] [Google Scholar]
- Baker AM, Thompson JD, Barrett SCH. Evolution and maintenance of stigma-height dimorphism in Narcissus. II. Fitness comparisons between style morphs. Heredity. 2000;84:514–524. doi: 10.1046/j.1365-2540.2000.00686.x. [DOI] [PubMed] [Google Scholar]
- Bañares Á, Blanca G, Güemes J, Moreno JC, Ortiz S, editors. Atlas y libro rojo de la flora vascular amenazada de España. Madrid: Dirección General de Conservación de la Naturaleza; 2003. [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;4:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. Variation and evolution of breeding systems in the Turnera ulmifolia L. complex (Turneraceae) Evolution. 1987;41:340–354. doi: 10.1111/j.1558-5646.1987.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. New insights on heterostyly: comparative biology, ecology and genetics. In: Franklin-Tong VE, editor. Self-incompatibility in flowering plants: evolution, diversity and mechanisms. Berlin: Springer-Verlag; 2008. pp. 3–32. [Google Scholar]
- Barrett SCH, Cole WW, Arroyo J, Cruzan MB, Lloyd DG. Sexual polymorphisms in Narcissus triandrus (Amaryllidaceae): is this species tristylous? Heredity. 1997;78:135–145. [Google Scholar]
- Bateman AJ. The role of heterostyly in Narcissus and Mirabilis. Evolution. 1968;22:645–646. doi: 10.1111/j.1558-5646.1968.tb03999.x. [DOI] [PubMed] [Google Scholar]
- Böhle U-R, Hilger HH, Martin W. Island colonization and evolution of the insular woody habit in Echium L. (Boraginaceae) Proceedings of the National Academy of Sciences, USA. 1996;93:11740–11745. doi: 10.1073/pnas.93.21.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollback JP. SIMMAP: Stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics. 2006;7:88. doi: 10.1186/1471-2105-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AE. Breeding system of Macromeria viridiflora (Boraginaceae) and geographic variation in pollinator assemblages. American Journal of Botany. 2004;11:1809–1813. doi: 10.3732/ajb.91.11.1809. [DOI] [PubMed] [Google Scholar]
- Brys R, Jacquemyn H, Beeckman T. Morph-ratio variation, population size and female reproductive success in distylous Pulmonaria officinalis (Boraginaceae) Journal of Evolutionary Biology. 2008;21:1281–1289. doi: 10.1111/j.1420-9101.2008.01569.x. [DOI] [PubMed] [Google Scholar]
- Calendini F, Martin JF. PaupUP version, 1·0·3·1. A free graphical frontend for PAUP* Dos software. 2005 http://www.agro-montpellier.fr/sppe/Recherche/JFM/PaupUp/main.htm . [Google Scholar]
- Casper BB. Self-compatibility in distylous Cryptantha flava (Boraginaceae) New Phytologist. 1985;99:149–154. [Google Scholar]
- Casper BB, Wiens D. Fixed rates of random ovule abortion in Cryptantha flava (Boraginaceae) and its possible relation to seed dispersal. Ecology. 1981;62:866–869. [Google Scholar]
- Charlesworth D, Charlesworth B. A model for the evolution of distyly. American Naturalist. 1979;114:467–498. [Google Scholar]
- Cohen JI. A phylogenetic analysis of morphological and molecular characters of Lithospermum L. (Boraginaceae) and related taxa: evolutionary relationships and character evolution. Cladistics. 2011;27:1–22. doi: 10.1111/j.1096-0031.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- Corbet SA. Bee visits and the nectar of Echium vulgare L. and Sinapis alba L. Ecological Entomology. 1978;3:25–37. [Google Scholar]
- Courchamp F, Clutton-Brock T, Grenfell BT. Inverse density dependence and the Allee effect. Trends in Ecology and Evolution. 1999;14:405–410. doi: 10.1016/s0169-5347(99)01683-3. [DOI] [PubMed] [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- De Jong TJ, Klinkhamer GL. Limiting factors for seed production in Cynoglossum officinale. Oecologia. 1989;80:167–172. doi: 10.1007/BF00380146. [DOI] [PubMed] [Google Scholar]
- Dulberger R. Flower dimorphism and self-compatibility in Narcissus tazetta L. Evolution. 1964;18:361–363. [Google Scholar]
- Dulberger R. Floral dimorphism in Anchusa hybrida Ten. Israel Journal of Botany. 1970;19:37–41. [Google Scholar]
- Dulberger R. Floral polymorphisms and their functional significance in the heterostylous syndrome. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. pp. 41–84. [Google Scholar]
- Dupont YL, Hansen DM, Valido A, Olesen JM. Impact of introduced honey bees on native pollination interactions of the endemic Echium wildpretii (Boraginaceae) on Tenerife, Canary Islands. Biological Conservation. 2004;118:301–311. [Google Scholar]
- Ferrero V, Arroyo J, Vargas P, Thompson JD, Navarro L. Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae) Perspectives in Plant Ecology, Evolution and Systematics. 2009;1(1):111–125. [Google Scholar]
- Ferrero V, Chapela I, Arroyo J, Navarro L. Reciprocal style polymorphisms are not so discrete: the case of heterostyly in Lithodora and Glandora (Boraginaceae) Plant Biology. 2011a;13(Suppl. 1):7–18. doi: 10.1111/j.1438-8677.2009.00307.x. [DOI] [PubMed] [Google Scholar]
- Ferrero V, Castro S, Sánchez JM, Navarro L. Stigma–anther reciprocity, pollinators and pollen transfer efficiency in populations of heterostylous species of Lithodora and Glandora (Boraginaceae) Plant Systematics and Evolution. 2011b;291:267–276. [Google Scholar]
- Ganders FR. The biology of heterostyly. New Zealand Journal of Botany. 1979;17:607–635. [Google Scholar]
- Gibbs PE. Do homomorphic and heteromorphic self-incompatibility systems have the same sporophytic mechanism? Plant Systematics and Evolution. 1986;154:285–323. [Google Scholar]
- Goulson D, Stout JC, Hawson SA, Allen JA. Floral display size in comfrey, Symphytum oficinale L. (Boraginaceae): relationships with visitation by three bumblebee species and subsequent seed set. Oecologia. 1998;113:502–508. doi: 10.1007/s004420050402. [DOI] [PubMed] [Google Scholar]
- Hilger HH, Selvi F, Papini A, Bigazzi M. Molecular systematic of Boraginaceae tribe Boragineae based on ITS1 and trnL sequences, with special reference to Anchusa sensu lato. Annals of Botany. 2004;94:201–212. doi: 10.1093/aob/mch132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jules ES, Rathcke BJ. Mechanisms of reduced Trillium recruitment along edges of old-growth forest fragments. Conservation Biology. 1999;13:784–793. [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology, Evolution and Systematics. 2005;36:467–497. [Google Scholar]
- Levin DA. Breeding system of Lithospermum caroliniense: adaptation and counteradaptation. American Naturalist. 1968;102:427–441. [Google Scholar]
- Lloyd DG, Schoen DJ. Self- and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences. 1992;153:358–369. [Google Scholar]
- Lloyd DG, Webb CJ. The evolution of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992a. pp. 151–178. [Google Scholar]
- Lloyd DG, Webb CJ. The selection of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992b. pp. 179–207. [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis, version 2·01 for Windows. 2007 Computer program and documentation distributed by the authors at website: http://mesquiteproject.org . [Google Scholar]
- Mather K, de Winton D. Adaptation and counter-adaptation of the breeding system in Primula: the nature of breeding systems. Annals of Botany. 1941;5:297–311. [Google Scholar]
- Montaner C, Floris E, Alvarez JM. Is self-compatibility the main breeding system in borage (Borago officinalis L.)? Theoretical and Applied Genetics. 2000;101:185–189. [Google Scholar]
- Nielsen RH, Huelsenbeck JP. Detecting positively selected amino acid sites using posterior predictive p-values. Pacific Symposium on Biocomputing. 2002;7:576–588. doi: 10.1142/9789812799623_0054. [DOI] [PubMed] [Google Scholar]
- Nylander JAA. Testing models of evolution – MrModeltest version 1·1b. 2002 Computer program and documentation distributed by author, website: http://www.ebc.uu.se/systzoo/staff/nylander.html . [Google Scholar]
- Opler PA, Baker HG, Frankie GW. Reproductive biology of some Costa Rica Cordia species (Boraginaceae) Biotropica. 1975;7:234–247. [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral states on phylogenies. Systematic Biology. 1999;48:612–622. [Google Scholar]
- Pannel JR, Barrett SCH. Baker's Law revisited: reproductive assurance in a metapopulation. Evolution. 1998;52:657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Barrales R, Vargas P, Arroyo J. New evidence for the Darwinian hypothesis of heterostyly: breeding systems and pollinators in Narcissus sect. Apodanthi. New Phytologist. 2006;171:553–567. doi: 10.1111/j.1469-8137.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- Philipp M, Schou O. An unusual heteromorphic incompatibility system: distyly, self incompatibility, pollen load and fecundity in Anchusa officinalis (Boraginaceae) New Phytologist. 1981;89:693–703. [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA sustitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. 2008 R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org . [Google Scholar]
- Ray PM, Chisaki HF. Studies on Amsinckia. American Journal of Botany. 1957;44:529–544. [Google Scholar]
- Riveros M, Arroyo MTK, Humana AM. An unusual kind of distyly in Quinchamalium chilense (Santalaceae) on Volcan Casablanca, Southern Chile. American Journal of Botany. 1987;74:313–320. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sage TL, Strumas F, Cole WW, Barrett SCH. Differential ovule development following self- and cross pollination: the basis of self-sterility in Narcissus triandrus (Amaryllidaceae) American Journal of Botany. 1999;86:855–870. [PubMed] [Google Scholar]
- Sánchez JM, Ferrero V, Navarro L. A new approach to the quantification of degree of reciprocity in distylous (sensu lato) plant populations. Annals of Botany. 2008;102:463–472. doi: 10.1093/aob/mcn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D, Price T, Mooers AØ, Ludwig D. Likelihood of ancestor states in adaptive radiation. Evolution. 1997;51:1699–1711. doi: 10.1111/j.1558-5646.1997.tb05095.x. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Johnston MO, L'Heureux AM, Marsolais JV. Evolutionary history of the mating system in Amsinckia (Boraginaceae) Evolution. 1997;51:1090–1099. doi: 10.1111/j.1558-5646.1997.tb03956.x. [DOI] [PubMed] [Google Scholar]
- Schou O, Philipp M. An unusual heteromorphic incompatibility system II. Pollen tube growth and seed sets following compatible and incompatible crossing within Anchusa officinalis L. (Boraginaceae) In: Mulcahy DL, Ottaviano E, editors. Pollen: biology and implications for plant breeding. New York: Elsevier; 1983. pp. 219–227. [Google Scholar]
- Schou O, Philipp M. An unusual heteromorphic incompatibility system. 3. Genetic control of distyly and self incompatibility in Anchusa officinalis (Boraginaceae) Theoretical and Applied Genetics. 1984;68:139–144. doi: 10.1007/BF00252330. [DOI] [PubMed] [Google Scholar]
- Snow G. TeachingDemos: demonstrations for teaching and learning. 2005 R package version 1·5. http://cran.r-project.org/web/packages/TeachingDemos/TeachingDemos.pdf . [Google Scholar]
- Stephens PA, Sutherland WJ, Freckleton RP. What is the Allee effect? Oikos. 1999;87:185–190. [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4·07b. Sunderland, MA: Sinauer Associates; 1999. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software, version 4·0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Weigend M, Hilger HH. Phylogeny and systematic of Lithodora (Boraginaceae-Lithospermeae) and its affinities to the monotypic genera Mairetis, Halacksya and Paramoltkia based on ITS1 and trnLUAA – sequence data and morphology. Taxon. 2008;57:79–97. [Google Scholar]
- Thompson JD. Plant evolution in the Mediterranean. Oxford: Oxford University Press; 2005. [Google Scholar]
- Thompson JD, Higgins DG, Gibon TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4th edn. New York: Springer; 2002. [Google Scholar]
- Weller SG, Ornduff R. Pollen tube growth and inbreeding depression in Amsinckia grandiflora (Boraginaceae) American Journal of Botany. 1991;78:801–804. [Google Scholar]