Abstract
Background and Aims
How and why plants evolve to become selfing is a long-standing evolutionary puzzle. The transition from outcrossing to highly selfing is less well understood in self-compatible (SC) mixed-mating (MM) species where potentially subtle interactions between floral phenotypes and the environment are at play. We examined floral morphological and developmental traits across an entire SC MM genus, Collinsia, to determine which, if any, predict potential autonomous selfing ability when pollinators are absent (AS) and actual selfing rates in the wild, sm, and to best define the selfing syndrome for this clade.
Methods
Using polymorphic microsatellite markers, we obtained 30 population-level estimates of sm across 19 Collinsia taxa. Species grand means for the timing of herkogamy (stigma–anther contact) and dichogamy (stigmatic receptivity, SR), AS, floral size, longevity and their genetic correlations were quantified for 22 taxa.
Key Results
Species fell into discrete selfing and outcrossing groups based on floral traits. Loss of dichogamy defines Collinsia's selfing syndrome. Floral size, longevity and herkogamy also differ significantly between these groups. Most taxa have high AS rates (>80 %), but AS is uncorrelated with any measured trait. In contrast, sm is significantly correlated only with SR. High variance in sm was observed in the two groups.
Conclusions
Collinsia species exhibit clear morphological and developmental traits diagnostic of ‘selfing’ or ‘outcrossing’ groups. However, many species in both the ‘selfing’ and the ‘outcrossing’ groups were MM, pointing to the critical influence of the pollination environment, the timing of AS and outcross pollen prepotency on sm. Flower size is a poor predictor of Collinsia species' field selfing rates and this result may apply to many SC species. Assessment of the variation in the pollination environment, which can increase selfing rates in more ‘outcrossing’ species but can also decrease selfing rates in more ‘selfing’ species, is critical to understanding mating system evolution of SC MM taxa.
Keywords: Mating system evolution, selfing rate, outcrossing rate, floral traits, floral development, flower size, reproductive assurance, Collinsia, genetic correlations, selfing syndrome, mixed mating
INTRODUCTION
Angiosperm species display an impressive array of reproductive phenotypes associated with mating systems. Phenotypes range from large, showy, strictly outcrossing flowers to inconspicuous ones with reduced attractive traits associated with a highly selfing mating system termed the ‘selfing syndrome’ (Ritland and Ritland, 1989; Sicard and Lenhard, 2011). The tremendous diversity of floral traits in angiosperms and their effects on plant mating systems have been the focus of both theoretical and empirical studies for over a century, starting with Darwin's monographs on plant sexual systems (Darwin, 1876, 1877). The evolutionary shift toward selfing is associated with changes not only in floral phenotypes but also with shifts in habitat and life history (Ornduff, 1969; Barrett et al., 1996). Because mating system is the primary determinant of the distribution of genetic variation within and among contemporary populations of a species it plays a central role in the capacity of species for local adaptation, persistence and evolution (Stebbins, 1957; Grant, 1981; Hamrick and Godt, 1996; Takebayashi and Morrell, 2001).
Our understanding of the transition from pollen-vectored outcross fertilization to autonomous self-fertilization (e.g. Stebbins, 1957; Grant, 1981) remains incomplete, despite a long history of research (reviewed by Jain, 1976; Goodwillie et al., 2005; Igic and Kohn, 2006). A set of phenotypic changes in floral traits are known to accompany this shift to selfing (Ornduff, 1969), including: diminished individual flower or display size (reviewed by Goodwillie et al., 2010), reduced allocation to male and female function (Cruden, 1977; Delesalle et al., 2008; Mazer et al., 2009), shorter floral life spans (e.g. Wyatt, 1984; Primack, 1985; Runions and Geber, 2000; Mazer et al., 2010), smaller physical distance between male and female structures (hereafter herkogamy) (e.g. Webb and Lloyd, 1986; Herlihy and Eckert, 2007), and increased temporal overlap of male and female phases within a flower (hereafter dichogamy; e.g. Bertin and Newman, 1993; Totland and Schulte-Herbrüggen, 2003). Recent insight into the causes of the evolution of selfing comes from genetic and genomic studies of selfing taxa derived from highly outcrossing taxa that focus on the formation of self-compatible (SC) taxa by the breakdown of self-incompatibility (SI) (e.g. Solbrig and Rollins, 1977; Goodwillie, 1999; Busch, 2005; Foxe et al., 2009; Guo et al., 2009; Busch et al., 2010) or the transition to homostyly from heterostylous taxa (e.g. Vallejo-Marín and Barrett, 2009; Ness et al., 2010). In these studies, the evolution of SC highly selfing taxa from highly outcrossing taxa was attributed to strong to moderate population bottlenecks driven by reproductive assurance (Foxe et al., 2009; Guo et al., 2009; Ness et al., 2010; Busch et al., 2011) although some support for the automatic selection of selfing is also emerging (Busch et al., 2011).
The morphological and developmental changes that occur during the shift to high rates of selfing in SC taxa are less well understood and potentially subtler than those described above, but nonetheless are known to occur (e.g. Ritland and Ritland, 1989; Geber and Moeller, 2006; Baldwin et al., 2011). Because SC species already have the potential to self, individuals can produce both selfed and outcrossed progeny termed mixed mating and species often display wide and continuous variation in their actual selfing rates in nature (reviewed in Goodwillie et al., 2005; Eckert et al., 2010). For SC species in general, herkogamy and dichogamy have long been considered key determinants of mating patterns (Darwin, 1862; Müller, 1883; Lloyd and Webb, 1986; Webb and Lloyd, 1986; Bertin and Newman, 1993). But because they present an incomplete barrier to self-pollen compared with SI or heteromorphic flowers, even small changes in herkogamy or dichogamy in SC species can exert large functional changes in selfing ability.
Within species, instances of reduced herkogamy or dichogamy within and among populations are associated with higher rates of fruit-set in the absence of pollinators (e.g. Ennos, 1981; Schoen, 1982; Ritland and Ritland, 1989; Carr and Fenster, 1994; Elle and Hare, 2002) and generally higher average selfing rates (Barrett and Shore, 1987; Holtsford and Ellstrand, 1992; Motten and Antonovics, 1992; Karron et al., 1997; Brunet and Eckert, 1998; Takebayashi et al., 2006). Furthermore, differences in herkogamy among populations can affect the variance in selfing rate. For example, in populations of the SC perennial Aquiligia canadense, high levels of herkogamy are associated with increased variance in selfing rate relative to those with low herkogamy (Eckert et al., 2009). As herkogamy and dichogamy control the potential for autonomous selfing in SC species (reviewed by Wyatt, 1988; Runions and Geber, 2000), and populations and species differ phenotypically and genetically for these traits, herkogamy and dichogamy should be central in defining ‘selfing syndromes’ in SC mixed-mating genera.
Herkogamy and dichogamy also control the timing of autonomous selfing within a flower's lifespan, which is an additional dimension of the mating system syndrome important for mixed-mating taxa. The timing of selfing determines the likelihood of outcross pollen receipt and the evolutionary transition toward selfing (Lloyd and Schoen, 1992; Eckert et al., 2010). Lloyd (1992, table 1, and references therein) defines prior, competing and delayed selfing as distinguishable by their degree of overlap with the timing of potential outcross pollen receipt. Prior selfing occurs before (e.g. Fishman and Wyatt, 1999), competing selfing occurs during (e.g. Leclerc-Potvin and Ritland, 1994) and delayed selfing occurs after (e.g. Kalisz et al., 1999) the temporal opportunity for outcross pollination. Despite strong inbreeding depression, selfing is always favoured if ovules would otherwise be unfertilized (Lloyd, 1979, 1992; Schoen and Brown, 1991; Jarne and Charlesworth, 1993). Furthermore, pollen competition may diminish the realized effects of inbreeding depression, especially for mixed-mating taxa (reviewed by Armbruster and Rogers, 2004). Ecological conditions determine the fitness consequences of the timing of selfing. Conditions of chronically scarce pollinators or mates should favour prior selfing. In these environments prior selfers benefit little from outcrossing, particularly if deleterious alleles that cause inbreeding depression have been purged (Charlesworth and Charlesworth, 1987; Lloyd, 1987; Jarne and Charlesworth, 1993). Diminished herkogamy and dichogamy promote prior selfing, and diminished floral-attractive traits are expected to evolve subsequently (Charlesworth and Charlesworth, 1987; Lloyd, 1987; Takebayashi and Morrell, 2001). In contrast, delayed selfing is favoured where mates are abundant but pollinators are unreliable (e.g. Kalisz and Vogler, 2003; Kalisz et al., 2004; Eckert et al., 2010). Delayed selfers typically have high levels of herkogamy and/or dichogamy and will not experience selection for reduced flower size, floral display or floral longevity, because the retention of pollinator-attractive traits allow delayed selfing species to function primarily as out-crossers when pollinators are available (e.g. Armbruster et al., 2002).
In addition, the pollination environment can radically change the actual outcrossing rate of an SC species, regardless of the timing of selfing. For example, if pollinators visit early selfing species, then outcrossing rates will increase, especially if outcross pollen also arrives early or achieves higher fertilization success via prepotency (Lloyd, 1992; Lloyd and Schoen, 1992). The temporal lag between a species' ability to self due to changes in herkogamy or dichogamy and its attractive traits is one potential explanation for the maintenance of outcrossing in populations of prior selfers. Conversely, while delayed selfing species are expected to be highly outcrossing, variable pollinator visitation will increase their actual selfing rate through reproductive assurance (Herlihy and Eckert, 2002; Kalisz et al., 2004). Finally, regardless of the timing of autonomous selfing, the absence of pollinators has the potential to drive high selfing rates for any SC taxa. So, variation in the pollination environment has the potential to dampen the strength of the correlation between indirect indicators of mating system (e.g. floral size) and actual outcrossing rates of SC mixed-mating taxa.
Whereas the selfing syndrome is well defined for SC species derived from SI or heteromorphic taxa, how well mixed-mating SC taxa will fit the traditional description of selfing or outcrossing syndromes is an open question. To understand the general patterns of mating-system traits for mixed-mating genera, we need to determine the distributions and correlations among species' mean floral characters and assess how these characters functionally influence both actual selfing rates in the field and potential selfing rates in the absence of pollinators. Most studies do not quantify actual selfing rates, but rather rely on indirect metrics such as flower size as mating system surrogates. The lack of actual selfing rate data has the potential to make the dichotomy between the selfing and outcrossing syndromes more distinct than it is in reality. Although numerous studies of floral trait evolution related to mating system have been conducted in single species (e.g. Schoen, 1982; Holtsford and Ellstrand, 1992; Eckert et al., 2009) or have compared traits of large- vs. small-flowered species within a genus (e.g. Fenster et al., 1995; Fishman and Wyatt, 1999; Goodwillie, 1999; Delesalle et al., 2008; Mazer et al., 2009), we know of no comprehensive analysis of the functional relationships between floral traits and the actual selfing rate and autonomous selfing potential across an entire genus.
Taxa in the mixed-mating SC hermaphroditic genus Collinsia (Plantaginaceae) exhibit an impressive range of floral morphological and developmental traits associated with variation in mating system and pollinator environments (detailed below), making it a model clade for mating system research. Here we quantify both the autonomous selfing potential in the absence of pollinators and actual field selfing rates and the correlation of these two traits with flower size, flower shape, flower longevity, herkogamy and dichogamy across 22 taxa of Collinsia to address three sets of questions. (1) Can we detect groups of highly selfing taxa (selfing syndrome) vs. more outcrossing taxa in the mixed-mating genus Collinsia? If so, what traits define these mating system syndromes? (2) Can we identify floral traits that correlate with autonomous selfing potential in the absence of pollinators? Are these same traits also strong predictors of actual selfing rates in the wild? (3) If distinct mating-system groups are found, do the more outcrossing taxa in this genus exhibit higher variance in actual selfing rates than the more selfing taxa, as might be expected in a variable pollination environment?
MATERIALS AND METHODS
Plant material
The genus Collinsia comprises 26 minimum rank taxa including 22 species plus four additional varieties (Baldwin et al., 2011). All members of the genus are spring-flowering annuals found exclusively in North America. All Collinsia flowers are SC, hermaphroditic and generally protandrous, with the male phase preceding the female phase (Kalisz et al., 1999; Armbruster et al., 2002). The genus exhibits a wide range of flower sizes (Fig. 1; Armbruster et al., 2002; Randle et al., 2009), and selfing rates range from highly selfing, to mixed mating, to highly outcrossing (e.g. Weil and Allard, 1964; Charlesworth and Mayer, 1995; Kalisz et al., 2004; and data presented below). The presence of multiple pairs of sister taxa, where each pair is represented by a small- flowered and a large-flowered species that differ in floral developmental traits and autonomous selfing rates (Randle et al., 2009), suggests that mating system transitions may be common in this genus.
Fig. 1.
Range of flower sizes in the genus Collinsia. Scale bar = 1 mm.
The bilaterally symmetrical flowers of Collinsia are unusual in having a folded ventral petal that encloses the reproductive parts and traps pollen not collected by pollinators (Fig. 2). Similar flowers with keel petals are found elsewhere only in Papilionid legumes and this trait has undoubtedly influenced mating system evolution of Collinsia species through its effects on outcrossing success and self–pollination ability. Like members of the snapdragon family, Collinsia taxa have persistent styles that remain attached to the ovary after corolla drop. Bees are the primary floral visitors of all Collinsia species (Rust and Clement, 1977; Kalisz and Vogler, 2003; Randle et al., 2009), and visitation rates are known to affect the extent to which autonomous selfing occurs (Kalisz et al., 2004). Reproductive assurance through autonomous selfing is context-dependent in Collinsia. Field experiments have shown that flower size (Elle and Carney, 2003), local population size (Kennedy and Elle, 2008a) and the local pollinator environment (Kalisz and Vogler, 2003; Kalisz et al., 2004) affect the extent to which selection favours autonomous selfing and mating-system syndrome traits in Collinsia species.
Fig. 2.
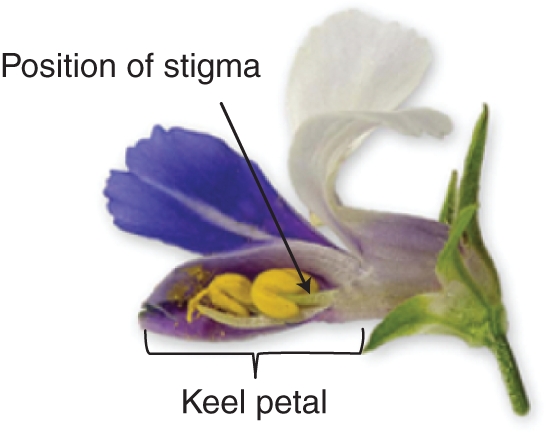
Flower of Collinsia verna with one lateral petal and half of keel petal removed to reveal stamen development and pollen placement at the front of the keel petal. This flower is in stage 1 = one mature stamen.
Autonomous self-pollination (AS) is achieved in Collinsia flowers via a developmental process we term dynamic herkogamy. In general, AS is achieved as follows: the four stamens mature in sequence during the male phase of a flower (e.g. Kalisz et al., 1999). Each filament elongates in turn, placing its dehiscing anther sac at the front of the keel (Fig. 2). In the subsequent female phase, the stigma becomes receptive and style elongation moves the stigma forward in the keel, closing the stigma–anther gap. AS can occur when the stigma contacts self–pollen, in a mature anther or the keel petal. For the subset of species measured to date, Collinsia species vary in their timing of stigma–anther contact and stigmatic receptivity (Kalisz et al., 1999; Armbruster et al., 2002). Although all Collinsia species are SC, proficiency at AS and fruit-set in the absence of pollinators is known to vary among populations and between individuals within populations of C. heterophylla (Charlesworth and Mayer, 1995) and C. verna (Kalisz and Vogler, 2003) as well as among species (Randle et al., 2009). The timing of AS is documented only for C. verna and was determined to be primarily delayed with the potential for some competing selfing (Kalisz and Vogler, 2003). Outcross pollen can adhere to the stigma of Collinsia flowers before they are receptive (Kalisz and Vogler, 2003; Lankinen et al., 2007) and may pre-empt later arriving self–pollen. However, early stigma–anther contact coupled with early stigma receptivity could give self-pollen priority. In general we expect that across Collinsia taxa, these component traits of early selfing will correlate with high levels of autonomous fruit-set in the absence of pollinators and with low outcrossing rates in the wild.
Seed collection and cultivation of plants
Naturally produced seeds were collected by maternal family from wild populations of each of 22 taxa between 2000 and 2006 and stored in the lab. In 2006 and 2007, we planted and germinated seeds from the collection and grew individuals in the University of Pittsburgh plant growth facilities. For each of the 22 taxa, we grew one to six populations and 15–35 families/population. The majority of these individuals were harvested as seedlings to estimate outcrossing rates (described below) and the remainder were grown to flowering and used to quantify floral morphological and developmental traits. Distinct maternal families were used for each morphological or developmental trait. To standardize for floral position effects, all floral morphological and developmental traits were measured only on flowers from whorls 3–5. Because the number of flowers per whorl is low for some species and because some of our floral measurements were destructive, a single floral trait was measured per individual plant.
Data collection and analyses
Collinsia flower size
One mature flower from each of 3–6 individuals/1–3 populations/22 species was collected. Flowers were digitized and analysed as described by Randle et al. (2009). In brief, our template consisted of 76 points (eight primary points and 68 secondary points) that were placed onto the scaled digital image of each flower at predefined intervals and landmark locations to capture shape and size information. To capture floral size variation among species, a principal components analysis (PCA) using the AAMToolbox was conducted (Hanna; http://fizz.cmp.uea.ac.uk/wiki/AAMToolbox/index.php/AAMToolbox).
Floral longevity
For each of the 22 taxa, we marked the calyxes or subtending leaves of six unopened flowers on each of six plants from different seed families using Duncan™ non-toxic fabric paint. We checked all marked flowers daily between 0800 and 0900 h and again between 1500 and 1800 h and recorded the current stage of floral development (stage 1 = one mature stamen, stage 4 = four mature stamens) until the corolla abscised. We define floral longevity as the cumulative duration of stages 1–4, as these stages define the primary period of pollen export and receipt (Armbruster et al., 2002; Kalisz and Vogler, 2003). We calculated the species mean floral longevity and its standard error by calculating the mean floral lifespan for each individual plant/population and averaged the individual means to calculate the population mean. Where applicable, population means were averaged to calculate a grand mean for the species or taxon.
Stage of stigma–anther contact
In Collinsia, stigma–anther contact (SAC) is achieved by dynamic herkogamy, as described above. The timing of SAC for each species quantifies the extent to which herkogamy functions to promote outcrossing across Collinsia. For each of the 22 taxa, two flowers per stage × 4 stages from 5–12 individuals each from a different maternal family/population and from one to six populations were scored for SAC. For each flower, we determined its SAC value by noting both its stage (stage 0 = no mature stamen, stage 4 = all four stamens mature) and if its style was sufficiently elongated to place its stigma in contact with any of the anthers. If the style had not elongated and the stigma was not in contact with any anthers by the time the corolla was abscising, that flower was given an SAC score of 5. The earliest stage of SAC across all flowers within a plant was used as that individual's SAC value. Because of the large number of concurrent measurements, flowers for some species were collected and preserved in vials containing 70 % alcohol for later assessment of stage of SAC. We calculated the species mean SAC and its standard error by calculating the mean stage SAC across all individual plants/population. Where applicable, the population means were averaged to calculate a grand mean for the species or taxon.
Stigmatic receptivity
The timing of stigmatic receptivity for each species quantifies the extent to which dichogamy functions in Collinsia. We tested for stigmatic receptivity using the stigmatic peroxidase activity method of Kearns and Inouye (1993), which is positively correlated with the presence of pollen tubes in the styles of Collinsia species (Armbruster et al., 2002). False positive results can occur if pollen grains adhere to the stigma before it is receptive (Kearns and Inouye, 1993). As pollen was shown to adhere to unreceptive stigmas of C. verna and C. heterophylla, we eliminated the possibility of false positives as follows. We excised styles from fresh flowers across all six floral developmental stages: (stage 0 = no mature stamens, stage 5 = corolla abscission). Excised styles were checked under a 20× light microscope for the presence of pollen grains; any stigma with pollen was excluded from the sample. The remaining styles were placed on a glass slide in a drop of 3 % hydrogen peroxide covered with a cover slip and examined under the microscope. Bubbling of the stigmatic surface indicates peroxidase activity of a receptive stigma. Two flowers per stage ×6 stages from each of 5–10 individuals each from a different maternal family/population were used for each species. We calculated the species mean stage of stigmatic receptivity and its standard error by calculating the mean stage of receptivity for each individual plant/population and averaged the individual means to calculate the population mean. Where applicable, the population means were averaged to calculate a grand mean for the species or taxon.
Ability to autonomously self-fertilize (AS)
Following Charlesworth and Mayer (1995), we used the ability of an unmanipulated flower to make a fruit as our estimate of autonomous selfing proficiency. We marked the calyx or subtending leaf of flowers on each plant using Duncan™ non-toxic fabric paint (n = 5–10 flowers per plant from 5–25 plants/population per species). Marked flowers were monitored after corolla abscission to determine if a successful fruit was produced. We had sufficient data to estimate the autonomous selfing for 20 of our 22 species. Individual AS ability was calculated as Σ(number of fruits)/Σ(number of sampled flowers) per plant. We calculated the species mean AS ability and its standard error by calculating the AS ability for each individual plant/population and averaged the individual means to calculate the population mean. Where applicable, the population means were averaged to calculate a grand mean for the species or taxon.
Selfing rate (sm)
We used polymorphic microsatellite markers developed for C. verna (Dunn et al., 2006) and C. sparsiflora (J. W. Wright et al., USDA, Forest Service Pacific Southwest Research Station, unpubl. res.) to assess selfing rates of the Collinsia taxa. Twelve to 20 maternal sibships, each with a minimum of seven seedlings, were used for each population/species. DNA from seedling tissue was extracted, and four to seven microsatellite markers were amplified and then scored using Genemapper™ software as described by Dunn et al. (2006). For each population the sibship microsatellite genotypic data were analysed using the MLTR 2·2 software program (Ritland, 1990; http://genetics.forestry.ubC.ca/ritland/programs.html). This maximum-likelihood estimation method was performed with 500 bootstraps with family re-sampling to provide estimates of the mean and standard error of the multilocus outcrossing rate, tm, for each population/species. We were unable to estimate tm for Collinsia wrightii and C. torreyi because none of the markers would amplify in these species. Also, we could not include C. callosa because low germination rates/maternal sibship precluded adequate sample sizes for outcrossing rate estimation. We obtained 30 estimates of tm, representing 19 of the 22 taxa. Where applicable (seven species of 19 species), the multiple estimates of tm among populations within a species were averaged to calculate a grand mean for the species or taxon. Finally, because published estimates of tm for two species, C. verna and C. heterophylla (Kalisz et al., 2004 and Kalisz lab unpubl. res.; Charlesworth and Mayer, 1995, respectively), were consistent with the estimates from the current study, we calculated a grand mean across all estimates for these two species. Values of tm were used to calculate the multi-locus selfing rate, sm, where sm = 1 – tm.
Distribution of floral traits
Because highly selfing SC species and their highly outcrossing SI or heteromorphic ancestors are known to exhibit distinct phenotypic classes, we looked for discrete phenotypic groupings across the measured floral traits in Collinsia. To do so we tested for normality using the Shapiro–Wilkes test statistic, W, plotted each floral trait's distribution and visually inspected each for bimodality.
Genetic correlations
Our experimental design includes measurements on multiple replicates (individuals and populations) of each species in a common environment. These data were used to calculate a species- or taxon-level grand mean for each floral trait. With this approach, the non-genetic effects are averaged within and among species, and the correlation coefficients provide estimates of genetic correlations of floral traits across the genus (Lynch and Walsh, 1998). We calculated the Spearman rank correlation (r) among the following floral morphological and developmental traits: floral size (PC1), stage of SAC, stage of stigmatic receptivity, floral longevity, actual selfing rate, sm, and the potential to autonomously self in the absence of pollinators across the entire data set.
Except for the mating system estimations, all analyses for this paper were generated using SAS software, version 9·2 (SAS Institute Inc.).
RESULTS
Flower size
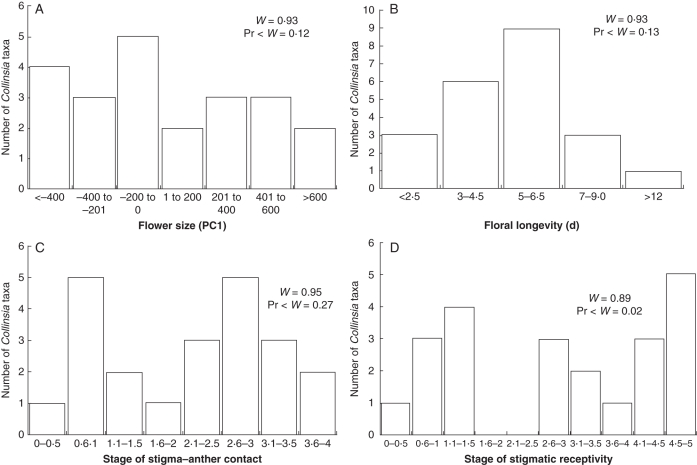
The first three PCs from the PCA describe 89 % of the floral morphological variation observed. PC1 corresponds to overall floral size and accounts for 76 % of the variation. Small flowers have positive values whereas large flowers have negative PC1 values. PC1 is used in all further analyses as our estimate of flower size. (Note: For our analyses, we multiplied PC1 by –1·0, giving large flowers positive PC1 values and small flowers negative PC1 values. These PC1 values are reported from here on.) Collinsia flower size exhibits a continuous distribution with PC1 scores from –532 in C. parviflora to +687 in C. corymbosa (Fig. 3A). This is a five-fold difference in corolla height from the smallest to the largest flowers (<5 to >25 mm).
Fig. 3.
Distribution of floral morphological and developmental traits across the genus Collinsia. (A) Flower size (PC1). (B) Floral longevity (in days). (C) Stage of stigma–anther contact. (D) Stage of stigmatic receptivity. See text for description of stages. Shapiro–Wilkes test for normality (W) indicates normal distributions for all traits except stage of stigmatic receptivity.
Floral developmental traits
Floral developmental traits and floral size trait distributions and grand means for all traits measured for the 22 taxa are presented in Fig. 3 and the Appendix, respectively. All floral trait distributions quantified in this study met the Shapiro–Wilkes criteria for normality except the timing of stigmatic receptivity (Fig. 3D).
Average individual floral lifespan (stages 1–4) of Collinsia taxa is 5·2 d. Floral longevity ranges from short-lived flowers in C. rattanii that are open for 2·5 d (60 h) to long-lived flowers of C. bartsiifolia that are open for nearly 10 d (232 h). Collinsia taxa also exhibited a similar wide array in the herkogamy (SAC) and dichogamy. For example, in C. wrightii, mean SAC is stage 0·14, indicating that the stigma contacts the anther before the anthers have dehisced, defining this species as a prior selfer. In contrast, C. tinctoria and C. heterophylla express late mean SAC. Stigmatic receptivity appears to be strongly bimodal (Fig. 3D). Collinsia taxa express either early stigmatic receptivity (> stage 1·5) and are probably early selfers (E) or express late receptivity (> stage 2·5) and are later selfers (L), with no taxa falling between these values. Stigmatic receptivity occurred at or before stage 1 in C. wrightii, C. sparsiflora var. collina, C. rattanni, C. callosa and C. parviflora, suggesting that these species are prior selfers. Average stigmatic receptivity occurred after the corolla had abscised (i.e. > stage 4) in C. bartisifolia, C. heterophylla, C. corymbosa, C. tinctoria and C. greenii. These species are probably delayed selfers, and any seed produced via autonomous selfing must be the result of self-pollen adhering to unreceptive stigmas at the time of SAC.
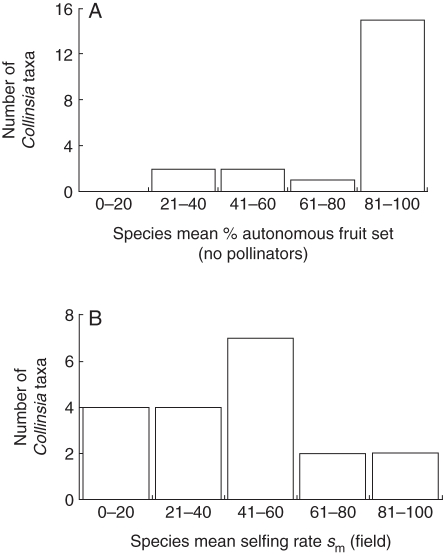
Most species of Collinsia are proficient autonomous selfers (Fig. 4A). Averaged across all species, 81 % of flowers produce fruits in the absence of pollinators. Species with autonomous selfing rates lower than this average include the larger flowered C. grandiflora, C. verna and C. sparsiflora var. arvensis and the smaller flowered C. sparsiflora var. collina and C. parviflora. In contrast to the generally high potential selfing rates across the genus, of the 19 taxa for which we have estimated sm (Fig. 4B), only two species are functionally highly selfing (sm > 0·8) and four species are functionally highly outcrossing (sm < 0·2). The remaining 13 taxa (68 %) fit within the 20–80 % range, the standard criteria defining mixed mating (Goodwillie et al., 2005). The average field selfing rate sm = 0·52 for the genus overall.
Fig. 4.
(A) Distribution of average autonomous selfing ability in the absence of pollinators for 19 taxa in the genus Collinsia. ΣFruit production/Σflower production gives an estimate of autogamy. (B) Distribution of actual species mean selfing rates (sm) estimated for 19 taxa within the genus Collinsia using polymorphic microsatellite markers.
Genetic correlations among traits
We found consistently positive significant genetic correlations among floral size, floral longevity, SAC and stage of stigmatic receptivity (Table 1). The strongest correlations were seen between floral size and SAC (r = 0·73, P < 0·0001), flower size and floral longevity (r = 0·72, P < 0·0001), and floral longevity and SAC (r = 0·65, P < 0·001). No floral traits were correlated with the potential to self in the absence of pollinators. In contrast, the multi-locus selfing rate, sm, was significantly negatively correlated only with stage of stigmatic receptivity, a measure of dichogamy in Collinsia (r = –0·57, P < 0·01; Table 1). Although we expected a significant negative correlation between flower size and sm, this correlation was neither strong nor significant (r = –0·44, P < 0·06; Table 1).
Table 1.
Genetic correlations between the average species-level floral traits calculated for taxa in the genus Collinsia
| Floral trait | Flower size (PC1) | Floral longevity | Stage of stigma–anther contact | Stage of stigmatic receptivity | Selfing rate (field) | Autonomous selfing rate (no pollinators) |
|---|---|---|---|---|---|---|
| Flower size (PC1) | 1 | 0·72 | 0·73 | 0·63 | –0·44 | 0·31 |
| 0·0001 | 0·0001 | 0·002 | 0·06 | 0·19 | ||
| 22 | 22 | 22 | 19 | 20 | ||
| Floral longevity | 1 | 0·65 | 0·52 | –0·21 | 0·20 | |
| 0·001 | 0·01 | 0·38 | 0·42 | |||
| 22 | 22 | 19 | 20 | |||
| Stage of stigma–anther contact | 1 | 0·63 | –0·31 | 0·22 | ||
| 0·001 | 0·19 | 0·34 | ||||
| 22 | 19 | 20 | ||||
| Stage of stigmatic receptivity | 1 | –0·57 | 0·16 | |||
| 0·01 | 0·49 | |||||
| 19 | 20 | |||||
| Selfing rate (field) | 1 | –0·14 | ||||
| 0·58 | ||||||
| 20 | ||||||
| Autonomous selfing rate (no pollinators) | 1 |
Within each trait, the first row= Spearman rank correlations (r); the second row = P value; and third row = sample size, number of species. Significant correlations are in bold type.
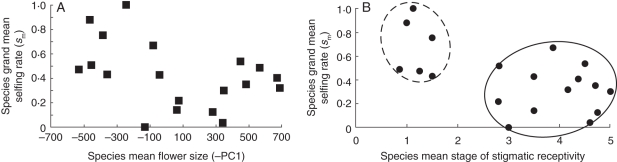
To identify possible selfing and outcrossing groups, we plotted sm against the species mean flower size and the species mean stage of receptivity (Fig. 5). The relationship between flower size and sm does not reveal clear groupings expected for mating system syndromes. Rather, we see a continuous bivariate distribution of both traits (Fig. 5A). In clear contrast, mating system groups are obvious based on the timing of stigmatic receptivity (Fig. 5B), suggesting a diagnostic character for selfing vs. outcrossing syndromes in Collinsia. We tested for differences between these two groups for all the measured floral traits to characterize mating system syndromes for Collinsia. To do so, the 22 taxa were assigned to one of two groups (early selfing = E or later selfing = L; Appendix) based on species mean stage of receptivity (early stigmatic receptivity < stage 1·5, late receptivity > stage 2·5, respectively). We used the GLM procedure in SAS with a Bonferroni t-test to determine if the group means were significantly different. All floral traits measured differed significantly between the two groups except for the potential to self in the absence of pollinators (Table 2). Furthermore, actual selfing rates in the field for the early selfing group (E) ranged from sm = 0·43 to 0·999 with a mean of 0·67. The later selfing group (L) ranged from sm = 0 to 0·67, with a mean of 0·30 (Table 2; Appendix).
Fig. 5.
(A) Relationship between species mean flower size (–PC1) vs. species mean selfing rates (sm) in Collinsia (y = –0·0003x – 0·44; R2 = 0·18). (B) Relationship between species mean stage of stigmatic receptivity vs. species mean selfing rates (sm) in Collinsia (y = –0·11x – 0·25; R2 = 0·34). Species fall into two distinct groups based on stage of stigmatic receptivity <1·5 or stage of stigmatic receptivity >2·8. See text for details.
Table 2.
Mean trait values for early selfing (selfing syndrome) and later selfing (outcrossing syndrome) groups in the genus Collinsia
| Early selfing group (E) | Late selfing group (L) | F value (Pr > F) | |
|---|---|---|---|
| Flower size (–PC1) | –298·0 ± 125·7a (n = 8) | 204·7 ± 80·4b (n = 14) | 9·57 (0·006) |
| Flower longevity (h) | 87·53 ± 12·5a (n = 8) | 154·84 ± 14·4b (n = 14) | 9·96 (0·005) |
| Stage of stigma–anther contact | 1·45 ± 0·35a (n = 8) | 2·82 ± 0·21b (n = 14) | 13·02 (0·002) |
| Stage of stigmatic receptivity | 1·07 ± 0·13a (n = 8) | 4·04 ± 0·21b (n = 14) | 99·70 (<0·0001) |
| Actual selfing rate, sm | 0·67 ± 0·10a (n = 7) | 0·30 ± 0·06b (n = 12) | 11·69 (0·0033) |
| Potential selfing rate AS (no pollinators) | 0·802 ± 0·09 (n = 7) | 0·818 ± 0·10 (n = 14) | 0·02 (0·8871) |
Values shown are means±s.e. (n = number of species in the group). Means with different letters are significantly different.
DISCUSSION
Examination of the distribution of selfing syndrome traits in this mixed mating clade allowed us to identify a critical trait that may be diagnostic and foster the maintenance of outcrossing or promote selfing: dichogamy. When stigmatic receptivity occurs early in a Collinsia flower's lifespan, the separation of male and female phases is functionally cancelled. Collinsia species in this early selfing group express a mean stigmatic receptivity of Stage 1·07, indicating that receptivity is coincident with dehiscence of the first anther. Early selfing is highly correlated with smaller flower size, shorter floral lifespan and early SAC (Table 1). Thus, Collinsia taxa within the early selfing group fit the general pattern described for other early selfing species (Wyatt, 1984; Primack, 1985; Guerrant, 1989; Sato, 2002; Snell and Aarssen, 2005; Weber and Goodwillie, 2007). Indeed, when we grouped species based their dichogamy phenotypes [later selfing species (putatively highly outcrossing) vs. early selfing species (putatively high selfing)] the two groups differed significantly for all floral morphological and developmental traits measured (Table 2). Average floral trait constellations of the two Collinsia groups align with those of highly outcrossing (SI and heteromorphic) taxa and their derived highly selfing SC taxa (Sicard and Lenhard, 2011). However, we found four instances where flower size does not match the expectation based on these dichogamy groups. This mismatch was seen in the placement of one larger flowered species in the early selfing group (C. concolor) and the placement of three smaller flowered species in the late selfing group (C. parryi, C. sparsiflora var. sparsiflora and C. violacea). These data underline the potential for high lability of dichogamy in Collinsia species and may provide support for the rapid evolution of traits affecting mating system traits and a lag in florally attractive traits (Takebayashi and Morrell, 2001).
Autonomous selfing potential in the absence of pollinators is uncorrelated with any of the floral morphological or developmental traits measured in this study (Table 1). This result was unexpected given that both C. heterophylla and C. verna have been to shown to exhibit significant genetic variance in autonomous selfing ability among individuals within populations (Charlesworth and Mayer, 1995; Kalisz and Vogler, 2003). Low variation in autonomous selfing potential among species observed here undoubtedly constrains our ability to detect a correlation, if one was present. The majority (17 of 22) of Collinsia taxa are proficient autonomous selfers, with approx. 80–100 % of the flowers of these species producing a fruit in the absence of pollinators (Fig. 5A). Only five species have lower than average rates of autonomous selfing and these are members of both the early selfing group (C. parviflora, C. sparsiflora var. collina) and the late selfing group (C. grandiflora, C. sparsiflora var. arvensis and C. verna) (Appendix). The dynamic components of herkogamy and dichogamy are the likely explanation for the high functional efficiency of autonomous selfing across the genus. This genus-wide high autonomous selfing proficiency has the potential to partially decouple the relationship between indirect estimates of mating system (e.g. flower size, herkogamy) and the actual selfing rate in the wild. In general, pronounced increases in the selfing rates of species expected to be highly outcrossing (i.e. those in the later selfing group for Collinsia) will occur when pollinators are absent.
Although we expected a significant correlation between flower size and sm, these two traits were not significantly correlated. Field selfing rate is significantly negatively correlated only with dichogamy (timing of stigmatic receptivity). While dichogamy clearly delimits early and later selfing groups in Collinsia, the correlation of dichogamy with sm (r = –0·57) provides only modest predictive power of the actual selfing rates for individual Collinsia species (Fig. 5B). The lack of a strong correlation highlights the role of the variable pollination environment, and that the generally high autonomous selfing ability across the genus is shaping the actual selfing rates in any year for any population.
We expected both low mean values of sm and low variation in sm among species in the early selfing group because their low values of herkogamy and dichogamy were expected to drive high rates of selfing overall. However, the variance in selfing rate among the early group was similar to that of the later selfing group (σ2 in sm = 0·06 vs. 0·04, respectively) and the two groups expressed a wide range in sm (0·43–0·99 vs. 0–0·67, respectively). Our results contrast with those from Aquilegia canadensis, where populations with higher values of herkogamy exhibit higher variance in selfing rates across years or sites than populations with low values of herkogamy (Eckert et al., 2009).
Our results point to the importance of the pollination environment, the timing of outcross pollen receipt and the prepotency of outcross pollen in determining actual outcrossing rates. For example, the value sm = 0·22 in one population of C. parviflora, a species with small, short-lived flowers and early selfing, can only be explained by pollinators delivering outcross pollen immediately after flowers open combined with prepotency. The fact that pollinators were observed visiting even the earliest stage of flowers of C. verna with no anthers presenting pollen (Kalisz and Vogler, 2003) suggests that pollinators could deliver pollen to stigmas of prior selfing species such as C. parviflora and drive higher than expected outcrossing rates. Ecological conditions and prepotency can combine to push early selfers to resemble highly outcrossing species.
Likewise, Collinsia species with large, long-lived flowers and later selfing can exhibit surprisingly high selfing rates, as, for example, with C. tinctoria (sm = 0·55) and C. heterophylla (sm = 0·68; M population from Charlesworth and Mayer, 1995). In addition to the costs of floral maintenance (Schoen and Ashman, 1995), species with large, long-lived flowers and large flower displays bear additional costs of lost outcrossing opportunities through pollinator-mediated within-plant selfing (geitonogamy; e.g. de Jong et al., 1993) even when pollinators are abundant. Thus, it is likely that the outcrossing rate of Collinsia in the later selfing group is decreased by either geitonogamy or, during times of low pollinator service, through high autonomous selfing potential that provides reproductive assurance (e.g. Herlihy and Eckert, 2002; Kalisz et al., 2004). Although a significant negative correlation between flower size/display size and selfing rate was found across angiosperms (Goodwillie et al., 2010), our data suggest that variable pollinator environments and prepotency of outcross pollen could weaken the correlation between the marker-based estimates of selfing and indirect metrics of mating system for Collinsia and probably other SC mixed mating species.
This study has demonstrated that the actual mating system of most Collinsia species is mixed, with a substantial proportion of selfed progeny. Theory predicts that when an outcrossing population begins to self-pollinate, a rapid evolutionary transition to a highly selfing state will occur due to fast purging of genetic load and the loss of inbreeding depression (Lande and Schemske, 1985; Charlesworth and Charlesworth, 1987; Charlesworth et al., 1990). However, in four species of Collinsia measured to date, inbreeding depression estimates are consistently low to modest across multiple populations (C. heterophylla, Mayer et al., 1996; C. verna, Kalisz, 1989; Kalisz et al., 2004; C. parviflora, Kennedy and Elle, 2008b; C. corymbosa, S. Kalisz unpubl. res.), suggesting that inbreeding depression is not high enough to oppose the evolution of complete selfing. If we assume that these values of inbreeding are representative of the genus, then the low sm values of the early selfing group are unexpected. Low values of inbreeding depression in the later selfing group are similarly surprising, but for different reasons. Theoretically, if deleterious mutations combine to eliminate all selfed progeny from a population prior to their reproduction, then there is no opportunity for selection to remove homozygous deleterious or even lethal mutations from that population. This ‘selective interference’ of purging (Lande et al., 1994) can completely negate the effects of purging below a threshold selfing rate (Porcher and Lande, 2005), and species will be stably mixed mating. However, species that experience selective interference are expected to express high inbreeding depression levels, similar to those of highly outcrossing species (Husband and Schemske, 1996) and this was recently shown to be true in a broad survey across mixed-mating taxa (Winn et al., 2011). The lack of high levels of inbreeding depression in the later selfing group of Collinsia suggests that selective interference is not a viable explanatory mechanism. Explanations for the generally low inbreeding depression combined with mixed mating remain future empirical and theoretical challenges in this clade.
Finally, a recent review of the selfing syndrome (Sicard and Lenhard, 2011) highlights the need for understanding the molecular genetic basis of traits that define mating systems shifts. Although some progress has been made in understanding the genetics of floral size and herkogamy (reviewed by Sicard and Lenhard, 2011), changes in dichogamy, as seen in Collinsia, were not discussed. If changes in dichogamy evolve faster or more easily than morphological traits, as suggested for four of the species measured, then dichogamy may contribute to the frequent and rapid shifts in mating system observed in mixed-mating taxa such as Collinsia. And if a mating system is labile, such that changes in the timing of male and female phases rapidly respond to selection, then species in the selfing syndrome may not be trapped in a dead-end scenario (reviewed by Takebayashi and Morrell, 2001), but rather may be able to respond to selection for increased outcrossing when the environment changes. Indeed, variable selection by pollinators has been proposed to maintain both floral traits that promote outcrossing and those that ensure delayed autonomous selfing in specialized flowers (reviewed by Fenster and Martens-Rodriguez, 2007). The large positive genetic correlations among floral traits across Collinsia species (Table 1) suggest that if biotic and abiotic selective pressures act on mating system traits within this genus, then there is potential for their rapid evolution. High levels of heritabilty for floral traits are common (reviewed by Ashman and Majetic, 2006; Caruso, 2006; Sicard and Lenhard, 2011). Likewise, within species of Collinsia, high values of genetic variance for mating system traits reported for both C. heterophylla (Charlesworth and Mayer, 1995; Lankinen et al., 2007) and C. verna (Spigler et al., ms.) support the idea that populations within this genus maintain the evolutionary potential for shifts in mating system. Furthermore, patterns of divergence in flower size and mating system among sister taxa of Collinsia suggest a likely role for mating system in speciation events in this clade (Randle et al., 2009; Baldwin et al., 2011). Understanding the selective factors that drive the evolution of selfing (e.g. selection for rapid flowering time, reproductive assurance, transmission advantage) may be critical for understanding the origin of new species of Collinsia and other SC mixed maters.
ACKNOWLEDGEMENTS
We thank Ellen York and Jessica Dunn for expert technical assistance in the lab and the University of Pittsburgh Plant Growth Facility, B. Baldwin, M. Park, K. Hanley, W. S. Armbruster, E. Elle, C. Ivey, C. Heckel, T. Holtsford, J. McGraw, J. Paul and S. Rauth for seeds of various Collinsia taxa, and past and present members of the Kalisz lab, especially R. Spigler, and three reviewers for insightful comments. This work was supported by a grant from the National Science Foundation (NSF DEB-0324764) to S.K. and REU supplements to this award supported research by D.C., A.B., M.F. and C.B. and NSF DEB 0709638 to A.R. and S.K.
APPENDIX
Species of Collinsia; dichogamy group; and grand means of floral traits (s.e.), number of populations, number of plant families sampled. Outcrossing rate in the wild, (range of tm), number of population-level estimates of tm (Note: selfing rate sm = 1 – tm). SAC, stigma–anther contact
| Species | Dichogamy group: early selfing, E; later selfing, L | Flower size (–PC1) | Flower longevity (h) | Herkogamy: stage of SAC | Dichogamy: stage of stigmatic receptivity | Autonomous selfing rate AS (no pollinators) | Outcrossing rate in the wild (Ritland's tm) |
|---|---|---|---|---|---|---|---|
| C. antonina | E | –385·6 (54·0), 2,7 | 85·6 (1·9), 1,6 | 2·50 (0·50), 1,5 | 1·50 (0·92), 1,5 | 0·83 (0·17), 1,7 | 0·25, ( ),1 |
| C. bartisifolia | L | 280·0 (20·5), 2,9 | 287·4 (58·4), 3,15 | 3·17 (0·33), 2,5 | 4·75 (0·25), 2,8 | 1·00 (0·00), 1,6 | 0·88, (0·82–0·93), 2 |
| C. callosa | E | –296·0 (13·8), 1,6 | 121·6 (16·2), 1,6 | 1·83 (0·60), 1,5 | 0·88 (0·43), 1,5 | 0·93 (0·07), 1,5 | na |
| C. childii | E | –359·0 (22·9), 1,3 | 59·2 (0·9), 2,13 | 1·00 (0·00), 1,5 | 1·50 (0·45), 1,5 | 0·89 (0·07), 1,7 | 0·57, ( ), 1 |
| C. concolor | E | 563·2 (23·6), 1,3 | 157·3 (1·6), 1,6 | 0·86 (0·26), 1,7 | 0·86 (0·26), 1,7 | 1·00 (0·00), 1,5 | 0·51, ( ), 1 |
| C. corymbosa | L | 687·2 (35·9), 1,6 | 186·3 (11·4), 1,7 | 2·25 (0·25), 1,5 | 4·17 (0·08), 1,8 | 0·83 (0·07), 1,7 | 0·6, (0·60–0·77), 2 |
| C. grandiflora | L | 348·8 (34·6), 2,6 | 150·2 (26·5), 2,13 | 2·25 (0·14), 1,5 | 5·00 (0·00), 1,5 | 0·65 (0·01), 1,5 | 0·7, (0·45–0·87), 4 |
| C. greenii | L | 341·3 (39·7), 2,6 | 151·8 (35·5), 1,5 | 2·83 (0·67), 1,5 | 4·60 (0·24), 1,5 | 1·00 (0·00), 1,5 | 0·96, ( ), 1 |
| C. heterophylla | L | 446·7 (100·5), 3,9 | 212·3 (37·9), 3,9 | 3·63 (0·38), 2,5 | 4·50 (0·50), 2,5 | 0·82 (0·18), 2,13 | 0·46, (0·32–0·64), 5 |
| C. linearis | L | –42·6 (20·8), 2,6 | 96·2 (5·4), 3,18 | 2·83 (0·60), 1,5 | 3·50 (0·16), 1,5 | 0·85 (0·07), 1,5 | 0·57, (0·53–0·62), 2 |
| C. multicolor | L | 481·4 (46·8), 2,7 | 101·3 (3·7), 1,12 | 3·50 (0·50), 1,5 | 4·71 (0·04), 1,6 | 1·00 (0·00), 1,6 | 0·65, (0·54–0·77), 2 |
| C. parryii | L | –131·4 (16·8), 1,3 | 80·8 (1·7), 1,6 | 2·63 (0·38), 1,7 | 3·00 (0·52), 1,5 | 0·83 (0·17), 1,6 | 1·00, ( ), 1 |
| C. parviflora | E | –532·2 (24·2), 1,3 | 60·4 (5·0), 1,6 | 1·00 (0·00), 1,5 | 1·25 (0·14), 1,5 | 0·58 (0·16), 1,5 | 0·53, (0·27–0·78), 2 |
| C. rattanii | E | –465·0 (13·5) 3,10 | 55·3 (4·7), 2,16 | 1·00 (0·00), 1,5 | 1·00 (0·00), 1,5 | 1·00 (0·00), 2,5 | 0·12, ( ), 1 |
| C. sparisiflora var. arvensis | L | 73·5 (81·1), 1,3 | 143·7 (9·7), 1,5 | 1·50 (0·50), 1,5 | 2·80 (0·37), 1,5 | 0·39 (0·01), 1,5 | 0·78, ( ), 1 |
| C. sparsiflora var. collina | E | –456·0 (43·0), 1,9 | 83·0 (7·1), 1,5 | 1·00 (0·00), 1,5 | 1·13 (0·13), 1,5 | 0·38 (0·15), 1,7 | 0·001, ( ), 1 |
| C. sparsiflora var. sparsiflora | L | –245·8 (41·3), 1,5 | 155·7 (32·4), 1,5 | 1·33 (0·17), 1,5 | 2·83 (0·44), 1,5 | 0·92 (0·08), 1,5 | 0·49, ( ), 1 |
| C. tinctoria | L | 669·6 (38·8), 2,6 | 198·7 (21·7), 1,5 | 4·00 (0·20), 1,5 | 4·38 (0·47), 1,5 | 1·00, (0·00), 1,5 | 0·60, (0·45–0·74), 2 |
| C. torreyi | L | –23·7 (31·9), 1,3 | 120·2 (8·3), 1,6 | 3·00 (0·00), 1,5 | 5·00 (0·00), 1,5 | na | na |
| C. verna | L | 61·0 (11·09), 3,9 | 129·3 (3·1), 1,6 | 3·75 (0·31), 1,6 | 3·61 (0·16), 1,12 | 0·37, (0·17), 1,5 | 0·91, (0·72–0·97), 7 |
| C. violacea | L | –80·5 (38·4), 1,3 | 154·0 (2·6), 1,11 | 3·00 (0·00), 1,5 | 3·88 (0·13), 1,5 | 0·81, (0·12), 1,6 | 0·33, ( ), 1 |
| C. wrightii | E | –453·6 (23·2), 1,6 | 78·0 (12·7), 1,5 | 0·14 (0·07), 1,9 | 0·41 (0·20), 1,17 | na | na |
LITERATURE CITED
- Armbruster WS, Rogers DG. Does pollen competition reduce the cost of inbreeding? American Journal of Botany. 2004;91:1939–1943. doi: 10.3732/ajb.91.11.1939. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Mulder CPH, Baldwin BG, et al. Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae s.l.) American Journal of Botany. 2002;89:37–49. doi: 10.3732/ajb.89.1.37. [DOI] [PubMed] [Google Scholar]
- Ashman T-L, Majetic C. Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity. 2006;96:343–352. doi: 10.1038/sj.hdy.6800815. [DOI] [PubMed] [Google Scholar]
- Baldwin BG, Kalisz S, Armbruster WS. Phylogenetic perspectives on diversification, diversity, and phytogeography of Collinsia and Tonella (Plantaginaceae) American Journal of Botany. 2011;98:731–753. doi: 10.3732/ajb.1000346. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Shore JS. Variation and evolution of breeding systems in the Turnera ulmifolia L complex (Turneraceae) Evolution. 1987;41:340–354. doi: 10.1111/j.1558-5646.1987.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD, Worley AC. The comparative biology of pollination and mating in flowering plants. Philosophical Transactions Royal Society B. 1996;351:1271–1280. [Google Scholar]
- Bertin RI, Newman CM. Dichogamy in Angiosperms. Botanical Review. 1993;59:112–152. [Google Scholar]
- Busch JW. The evolution of self-compatibility in geographically peripheral populations of Leavenworthia alabamica (Brassicaceae) American Journal of Botany. 2005;92:1503–1512. doi: 10.3732/ajb.92.9.1503. [DOI] [PubMed] [Google Scholar]
- Busch JW, Joly S, Schoen DJ. Does mate-limitation in self-incompatible species promote the evolution of selfing? The case of Leavenworthia alabamica. Evolution. 2010;64:1657–1670. doi: 10.1111/j.1558-5646.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Busch JW, Joly S, Schoen DJ. Demographic signatures accompanying the evolution of selfing in Leavenworthia alabamica. Molecular Biology and Evolution. 2011;28:1717–1729. doi: 10.1093/molbev/msq352. [DOI] [PubMed] [Google Scholar]
- Brunet J, Eckert CG. Effects of floral morphology and display on outcrossing in blue columbine, Aquilegia caerulea (Ranunculaceae) Functional Ecology. 1998;12:596–606. [Google Scholar]
- Carr DE, Fenster CB. Levels of genetic variation and covariation for Mimulus (Scrophulariaceae) floral traits. Heredity. 1994;72:606–618. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Charlesworth D, Mayer S. Genetic variability of plant characters in the partial inbreeder Collinsia heterophylla (Scrophulariaceae) American Journal of Botany. 1995;82:112–120. [Google Scholar]
- Charlesworth D, Morgan MT, Charlesworth B. Inbreeding depression, genetic load, and the evolution of outcrossing in a multilocus system with no linkage. Evolution. 1990;44:1469–1498. doi: 10.1111/j.1558-5646.1990.tb03839.x. [DOI] [PubMed] [Google Scholar]
- Caruso CM. Adaptive evolution: the ecological genetics of floral traits. Heredity. 2006;97:86–87. doi: 10.1038/sj.hdy.6800853. [DOI] [PubMed] [Google Scholar]
- Cruden RW. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the various contrivances by which British and foreign orchids are fertilised by insects and on the good effects of intercrossing. 1st edn. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The effects of cross and self fertilisation in the vegetable kingdom. London: John Murray; 1876. [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- Delesalle VA, Mazer SJ, Paz H. Temporal variation in the pollen:ovule ratios of Clarkia (Onagraceae) taxa with contrasting mating systems: field populations. Journal of Evolutionary Biology. 2008;21:310–323. doi: 10.1111/j.1420-9101.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- Dunn JL, Dierkes L, Pico FX, Kalisz S. Primer Note: Identification of microsatellite loci in Collinsia verna. Molecular Ecology Notes. 2006;6:1212–1215. [Google Scholar]
- Eckert CG, Ozimec B, Herlihy CR, Griffin CA, Routley MB. Floral morphology mediates temporal variation in the mating system of a self-compatible plant. Ecology. 2009;90:1540–1548. doi: 10.1890/08-1063.1. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Kalisz S, Geber MA, et al. Plant mating systems in a changing world. Trends in Ecology and Evolution. 2010;25:35–43. doi: 10.1016/j.tree.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Elle E, Carney R. Reproductive assurance varies with flower size in Collinsia parviflora (Scrophulariaceae) American Journal of Botany. 2003;90:888–896. doi: 10.3732/ajb.90.6.888. [DOI] [PubMed] [Google Scholar]
- Elle E, Hare JD. Environmentally induced variation in floral traits affects the mating system in Datura wrightii. Functional Ecology. 2002;16:79–88. [Google Scholar]
- Ennos RA. Quantitative studies of the mating system in two sympatric species of Ipomoea (Convolvulaceae) Genetica. 1981;57:93–98. [Google Scholar]
- Fenster CB, Martens-Rodriguez S. Reproductive assurance and the evolution of pollination specialization. International Journal of Plant Science. 2007;168:215–228. [Google Scholar]
- Fenster CB, Diggle PK, Barrett SCH, Ritland K. The genetics of floral development differentiating two species of Mimulus (Scrophulariaceae) Heredity. 1995;74:258–266. [Google Scholar]
- Foxe JP, Slotte T, Stahl E, et al. Recent speciation associated with the evolution of selfing in Capsella. Proceedings of the National Academy of Sciences USA. 2009;106:5241–5245. doi: 10.1073/pnas.0807679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman L, Wyatt R. Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae) Evolution. 1999;53:1723–1733. doi: 10.1111/j.1558-5646.1999.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Geber MA, Moeller DA. Pollinator responses to plant communities and implications for reproductive character evolution. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 102–119. [Google Scholar]
- Goodwillie C. Multiple origins of self-compatibility in Linanthus section Leptosiphon (Polemoniaceae) Evolution. 1999;40:1122–1131. doi: 10.1111/j.1558-5646.1999.tb05403.x. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations and empirical evidence. Annual Review of Ecology Evolution and Systematics. 2005;36:47–79. [Google Scholar]
- Goodwillie C, Sargent RD, Eckert CG, et al. Correlated evolution of mating system and floral display traits in flowering plants and its implications for the distribution of mating system variation. New Phytologist. 2010;185:311–321. doi: 10.1111/j.1469-8137.2009.03043.x. [DOI] [PubMed] [Google Scholar]
- Grant V. Plant speciation. New York: Columbia University Press; 1981. [Google Scholar]
- Guerrant EO. Early maturity, small flowers, and autogamy: a developmental connection? In. In: Bock JH, Linhart YB, editors. The evolutionary ecology of plants. Boulder, CO: Westview Press; 1989. pp. 61–84. [Google Scholar]
- Guo YL, Bechsbaard JS, Slotte T, et al. Recent speciation of Capsella rubella from Capsella grandiflora, associated with loss of self-incompatibility and an extreme bottleneck. Proceedings of the National Academy of Sciences USA. 2009;106:5245–5251. doi: 10.1073/pnas.0808012106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick JL, Godt MJ. Effects of life history traits on genetic diversity in plant species. Philosophical Transactions Royal Society B. 1996;351:1291–1298. [Google Scholar]
- Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. Evolutionary analysis of a key floral trait and its effect on the mating system in Aquilegia canadensis (Ranunculaceae) Evolution. 2007;61:1661–1674. doi: 10.1111/j.1558-5646.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- Holtsford TP, Ellstrand NC. Genetic and environmental variation in floral traits affecting outcrossing rate in Clarkia tembloriensis (Onagraceae) Evolution. 1992;46:216–225. doi: 10.1111/j.1558-5646.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- Igic B, Kohn JR. The distribution of plant mating systems: study bias against obligately outcrossing species. Evolution. 2006;60:1098–1103. [PubMed] [Google Scholar]
- Jain SK. The evolution of inbreeding in plants. Annual Review of Ecology and Systematics. 1976;7:469–495. [Google Scholar]
- Jarne P, Charlesworth D. The evolution of the selfing rate in functionally hermaphroditic plants and animals. Annual Review of Ecology and Systematics. 1993;24:441–466. [Google Scholar]
- de Jong TJ, Waser NM, Klinkhamer PGL. Geitonogamy: the neglected side of selfing. Trends in Ecology and Evolution. 1993;8:321–325. doi: 10.1016/0169-5347(93)90239-L. [DOI] [PubMed] [Google Scholar]
- Kalisz S. Fitness consequences of mating system, seed weight, and emergence date in winter annual. Collinsia verna. Evolution. 1989;43:1263–1272. doi: 10.1111/j.1558-5646.1989.tb02573.x. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Vogler DW. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology. 2003;84:2928–2942. [Google Scholar]
- Kalisz S, Vogler D, Fails B, Finer M, Shepard E, Herman T, Gonzales R. The mechanism of delayed selfing in Collinsia verna (Scrophulariaceae) American Journal of Botany. 1999;86:1239–1247. [PubMed] [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Karron JD, Jackson RT, Thumser NN, et al. Outcrossing rates of individual Mimulus ringens genets are correlated with anther–stigma separation. Heredity. 1997;79:365–370. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Boulder, CO: University Press of Colorado; 1993. [Google Scholar]
- Kennedy BF, Elle E. The reproductive assurance benefit of selfing: importance of flower size and population size. Oecologia. 2008a;155:469–477. doi: 10.1007/s00442-007-0924-7. [DOI] [PubMed] [Google Scholar]
- Kennedy BF, Elle E. The inbreeding depression cost of selfing: importance of flower size and population size in Collinsia parviflora (Veronicaceae) American Journal of Botany. 2008b;95:1596–1605. doi: 10.3732/ajb.0800322. [DOI] [PubMed] [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants. 1. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Lande R, Schemske DW, Schultz ST. High inbreeding depression, selective interference among loci, and the threshold selfing rate for purging recessive lethal mutations. Evolution. 1994;48:965–978. doi: 10.1111/j.1558-5646.1994.tb05286.x. [DOI] [PubMed] [Google Scholar]
- Lankinen Å, Armbruster WS, Antonsen L. Delayed stigma receptivity in Collinsia heterophylla (Plantaginaceae): genetic variation and possible adaptive significance in relation to pollen competition, delayed self-pollination, and mating-system evolution. American Journal of Botany. 2007;94:1183–1192. doi: 10.3732/ajb.94.7.1183. [DOI] [PubMed] [Google Scholar]
- Leclerc-Potvin C, Ritland K. Modes of self-fertilization in Mimulus guttatus (Scrophulariaceae): field experiment. American Journal of Botany. 1994;81:199–205. [Google Scholar]
- Lloyd DG. Some reproductive factors affecting the selection of self-fertilization in plants. American Naturalist. 1979;113:67–79. [Google Scholar]
- Lloyd DG. Allocation to pollen, seeds, and pollination mechanisms in self-fertilizing plants. Functional Ecology. 1987;1:83–89. [Google Scholar]
- Lloyd DG. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Science. 1992;153:370–380. [Google Scholar]
- Lloyd DG, Schoen DJ. Self and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Science. 1992;153:358–369. [Google Scholar]
- Lloyd DG, Webb CJ. The avoidance of interference between the presentation of pollen and stigmas in angiosperms I. Dichogamy. New Zealand Journal of Botany. 1986;24:135–162. [Google Scholar]
- Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- Mayer SS, Charlesworth D, Meyers B. Inbreeding depression in four populations of Collinsia heterophylla Nutt. (Scrophulariaceae) Evolution. 1996;50:879–891. doi: 10.1111/j.1558-5646.1996.tb03896.x. [DOI] [PubMed] [Google Scholar]
- Mazer SJ, Dudley LS, Delesalle VA, Paz H, Galusky P. Stability of pollen-ovule ratios in pollinator-dependent versus autogamous Clarkia sister taxa: testing evolutionary predictions. New Phytologist. 2009;183:630–648. doi: 10.1111/j.1469-8137.2009.02886.x. [DOI] [PubMed] [Google Scholar]
- Mazer SJ, Dudley LS, Hove AA, Emms SK, Verhoeven AS. Physiological performance in Clarkia sister taxa with contrasting mating systems: do early-flowering autogamous taxa aoid water stress relative to their pollinator-dependent counterparts? International Journal of Plant Sciences. 2010;171:1029–1047. [Google Scholar]
- Motten AF, Antonovics J. Determinants of outcrossing rate in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae) American Journal of Botany. 1992;79:419–427. [PubMed] [Google Scholar]
- Müller H. On the fertilization of flowers. London: MacMillan; 1883. [Google Scholar]
- Ness RW, Wright SI, Barrett SCH. Mating-system variation, demographic history and patterns of nucleotide diversity in the tristylous plant Eichhornia paniculata. Genetics. 2010;184:381–392. doi: 10.1534/genetics.109.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornduff R. Reproductive biology in relation to systematics. Taxon. 1969;18:121–133. [Google Scholar]
- Porcher E, Lande R. The evolution of self-fertilization and inbreeding depression under pollen discounting and pollen limitation. Journal of Evolutionary Biology. 2005;18:497–508. doi: 10.1111/j.1420-9101.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- Primack RB. Longevity of individual flowers. Annual Review of Ecology and Systematics. 1985;16:15–37. [Google Scholar]
- Randle AM, Slyder JB, Kalisz S. Can differences in autonomous selfing ability explain range size among sister-taxa pairs of Collinsia (Plantaginaceae)? An extension of Baker's Law. New Phytologist. 2009;183:618–629. doi: 10.1111/j.1469-8137.2009.02946.x. [DOI] [PubMed] [Google Scholar]
- Ritland K. A series of FORTRAN computer programs for estimating plant mating systems. Journal of Heredity. 1990;81:235–237. [Google Scholar]
- Ritland C, Ritland K. Variation of sex allocation among eight taxa of the Mimulus guttatus species complex (Scrophulariaceae) American Journal of Botany. 1989;76:1731–1739. [Google Scholar]
- Runions CJ, Geber MA. Evolution of the self-pollinating flower in Clarkia xantiana (Onagraceae). I. Size and development of floral organs. American Journal of Botany. 2000;87:1439–1451. [PubMed] [Google Scholar]
- Rust RW, Clement SL. Entomophilous pollination of the self-compatible species Collinsia sparsiflora Fisher and Meyer. Journal of the Kansas Entomological Society. 1977;50:37–48. [Google Scholar]
- Sato H. The role of autonomous self-pollination in floral longevity in varieties of Impatiens hypophylla (Balsaminaceae) American Journal of Botany. 2002;89:263–269. doi: 10.3732/ajb.89.2.263. [DOI] [PubMed] [Google Scholar]
- Schoen DJ. The breeding system of Gilia achillefolia – variation in the floral characteristics and outcrossing rate. Evolution. 1982;36:352–360. doi: 10.1111/j.1558-5646.1982.tb05051.x. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Brown AHD. Whole and part flower self-pollination in Glycine clandestina and G. argyrea and the evolution of autogamy. Evolution. 1991;45:1651–1664. doi: 10.1111/j.1558-5646.1991.tb02670.x. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Ashman T-L. The evolution of floral longevity: resource allocation to maintenance versus construction of repeated parts in modular organisms. Evolution. 1995;49:131–139. doi: 10.1111/j.1558-5646.1995.tb05965.x. [DOI] [PubMed] [Google Scholar]
- Sicard A, Lenhard M. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptations in plants. Annals of Botany. 2011;107:1433–1443. doi: 10.1093/aob/mcr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell R, Aarssen LW. Life history traits in selfing versus outcrossing annuals: exploring the ‘time-limitation’ hypothesis for the fitness benefit of self-pollination. BMC Ecology. 2005;5:2. doi: 10.1186/1472-6785-5-2. http://dx.doi.org/10.1186/1472-6785-5-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbrig OT, Rollins RC. The evolution of autogamy in species of the mustard genus Leavenworthia. Evolution. 1977;31:265–281. doi: 10.1111/j.1558-5646.1977.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Self fertilization and population variability in higher plants. American Naturalist. 1957;91:337–354. [Google Scholar]
- Takebayashi N, Morrell PL. Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. American Journal of Botany. 2001;88:1143–1150. [PubMed] [Google Scholar]
- Takebayashi N, Wolf DE, Delph LF. Effect of variation in herkogamy on outcrossing within a population of Gilia achilleifolia. Heredity. 2006;96:159–165. doi: 10.1038/sj.hdy.6800780. [DOI] [PubMed] [Google Scholar]
- Totland O, Schulte-Herbrüggen B. Breeding system, insect flower visitation, and floral traits of two alpine Cerastium species in Norway. Arctic, Antarctic, and Alpine Research. 2003;35:242–247. [Google Scholar]
- Vallejo-Marín M, Barrett SCH. Modification of flower architecture during early stages in the evolution of self-fertilization. Annals of Botany. 2009;103:951–962. doi: 10.1093/aob/mcp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CJ, Lloyd DG. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany. 1986;24:163–178. [Google Scholar]
- Weber JJ, Goodwillie C. Timing of self-compatibility, flower longevity, and potential for male outcross success in Leptosiphon jepsonii (Polemoniaceae) American Journal of Botany. 2007;94:1338–1343. doi: 10.3732/ajb.94.8.1338. [DOI] [PubMed] [Google Scholar]
- Weil J, Allard RW. The mating system and genetic variability in natural populations of Collinsia heterophylla. Evolution. 1964;18:515–525. [Google Scholar]
- Winn AA, Elle E, Kalisz S, et al. Analysis of inbreeding depression in mixed mating plants provides evidence for selective interference and stable mixed mating. Evolution. 2011 doi: 10.1111/j.1558-5646.2011.01462.x. (in press). [DOI] [PubMed] [Google Scholar]
- Wyatt R. The evolution of self-pollination in granite outcrop species of Arenaria (Caryophyllaceae). I. Morphological correlates. Evolution. 1984;38:804–816. doi: 10.1111/j.1558-5646.1984.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Wyatt R. Phylogenetic aspects of the evolution of self-pollination. In: Gottlieb LD, Jain SK, editors. Plant evolutionary biology. New York: Chapman and Hall; 1988. pp. 109–131. [Google Scholar]