Abstract
Background and Aims
Gynodioecy is a phylogenetically widespread and important sexual system where females coexist with hermaphrodites. Because dioecy can arise from gynodioecy, characterization of gynodioecy in close relatives of dioecious and sub-dioecious species can provide insight into this transition. Thus, we sought to determine whether Fragaria vesca ssp. bracteata, a close relative to F. chiloensis and F. virginiana, exhibits the functional and population genetic hallmarks of a gynodioecious species.
Methods
We compared reproductive allocation of females and hermaphrodites grown in the greenhouse and estimated genetic diversity (allelic diversity, heterozygosity) and inbreeding coefficients for field-collected adults of both sexes using simple sequence repeat (SSR) markers. We estimated mating system and early seed fitness from open-pollinated families of both sex morphs.
Key Results
Under greenhouse conditions, females and hermaphrodites allocated similarly to all reproductive traits except flower number, and, as a consequence, females produced 30 % fewer seeds per plant than hermaphrodites. Under natural conditions, hermaphrodites produce seeds by self-fertilization approx. 75 % of the time, and females produced outcrossed seeds with very little biparental inbreeding. Consistent with inbreeding depression, seeds from open-pollinated hermaphrodites were less likely to germinate than those from females, and family-level estimates of hermaphrodite selfing rates were negatively correlated with germination success and speed. Furthermore, estimates of inbreeding depression based on genetic markers and population genetic theory indicate that inbreeding depression in the field could be high.
Conclusions
The joint consideration of allocation and mating system suggests that compensation may be sufficient to maintain females given the current understanding of sex determination. Fragaria vesca ssp. bracteata exhibited similar sex morph-dependent patterns of mating system and genetic diversity, but less reproductive trait dimorphism, than its sub-dioecious and dioecious congeners.
Keywords: Dioecy, Fragaria chiloensis, Fragaria vesca ssp. bracteata, Fragaria virginiana, gynodioecy, inbreeding depression, selfing rate, SSR, strawberry
INTRODUCTION
Gynodioecy is a sexual system in flowering plants where females (i.e. male-sterile plants) coexist with hermaphrodites. It is common (approx. 7 % of species; Richards, 1997), phylogenetically widespread (50 families, Jacobs and Wade, 2003) and of importance both as an evolutionary stable sexual system and as the first step in the evolutionary pathway from hermaphroditism to dioecy (Charlesworth and Charlesworth, 1978; Jacobs and Wade, 2003; Bailey and Delph, 2007). Although cytoplasmic–nuclear sex determination is common (Dufay and Billard, 2011), nuclear sex determination has been found in several species and these can have closely related taxa that are dioecious (e.g. Schiedea, Weller and Sakai 1991; Fuschia, Berry et al., 2004; Fragaria, W. Njuguna et al., unpubl. res.; Carica, Wu et al., 2010). Thus, these gynodioecious species are key to gaining insight into how gynodioecy gives rise to dioecy (Charlesworth and Charlesworth, 1978; but see Maurice et al., 1994).
For gynodioecy to exist, females must compensate for their loss of male function, and the degree of compensation required is dictated by the genetic determination of male sterility. When sex determination is nuclear, females need a 2-fold advantage to be maintained (Lewis, 1941; Lloyd, 1975). This advantage is often achieved by females producing a greater quantity of seeds and/or better quality seeds than hermaphrodites (Darwin, 1877). Females are expected to be able to produce more seeds by reallocating resources not spent on pollen (e.g. Ashman, 1994), and higher seed production by females is often observed (reviewed in Shykoff et al., 2003; Dufay and Billard, 2011). One common avenue by which females produce higher quality seeds than hermaphrodites is through the avoidance of inbreeding depression. Since females must receive pollen from other individuals, they are obligate outcrossers, whereas self-compatible hermaphrodites are capable of self-pollination. These differences can lead to the progeny of females being more outcrossed than those of hermaphrodites (reviewed in Collin and Shykoff, 2003). Since inbreeding unmasks deleterious recessive alleles and reduces heterozygosity, progeny fitness is expected to decrease with increased inbreeding (reviewed in Charlesworth and Willis, 2009). Indeed, seed quality decreases with increased inbreeding (e.g. Richards, 2000), and differences in quality (i.e. germination timing and rate, seedling size and survival) of seeds produced by females and hermaphrodites have been observed in many gynodioecious species (Shykoff et al., 2003; Dufay and Billard, 2011). The frequency of females in a population is thus predicted to be a function of selfing rate, relative seed production and inbreeding depression (Darwin, 1877; Lloyd, 1974; Charlesworth and Charlesworth, 1978).
Features of the sexual system not only influence the sex ratio but also influence the distribution of genetic diversity within gynodioecious populations (Tarayre and Thompson, 1997). First, because hermaphrodites are capable of mixed mating whereas females are obligately outcrossed, genetic diversity will depend on sex morph. Specifically, when male sterility is dominant (e.g. females are heterozygous ‘Fh’ and hermaphrodites are homozygous ‘hh’), females will always be the product of outcrossing, whereas hermaphrodites may be the product of female outcrossing, hermaphrodite outcrossing or hermaphrodite selfing. When females derive exclusively from female lineages, they are expected to have lower inbreeding coefficients than hermaphrodites (Ashman, 1992; Ashley et al., 2003; Collin et al., 2009). Secondly, if there is inbreeding depression, then individuals produced by inbreeding may be less likely to survive to adulthood. For these reasons, females are expected to be more heterozygous than hermaphrodites, and so the overall population heterozygosity will increase as female frequency increases (Gouyon and Couvet, 1987; Tarayre and Thompson, 1997; Cuevas et al., 2006). While there have been numerous studies of selfing rates of the sex morphs (reviewed in Shykoff et al., 2003), much less attention has been paid to the effects of gender dimorphism on the distribution of genetic diversity within populations (but see Tarayre and Thompson, 1997; Akimoto et al., 1999; Medrano et al., 2005; Cuevas et al., 2006).
Fragaria vesca ssp. bracteata is reported to be gynodioecious, and is the only diploid Fragaria species that is not hermaphroditic (Staudt, 1999). Recent phylogenetic analyses based on whole chloroplast genome sequencing (Njuguna, 2010) implicate it as the maternal donor to the octoploid Fragaria (F. virginiana and F. chiloensis) which subsequently evolved sub-dioecy and dioecy, respectively (W. Njuguna et al., unpubl. res.). Thus, Fragaria vesca ssp. bracteata is in a key position to provide insight into the evolution of dioecy from gynodioecy (Goldberg et al., 2010; Spigler et al., 2010). However, characterization of gynodioecy in F. vesca ssp. bracteata has been based solely on floral morphology and segregation of progeny from controlled crosses (Ahmadi and Bringhurst, 1991; Staudt, 1999). In this work we sought to determine whether F. vesca ssp. bracteata exhibits the functional and population genetic hallmarks of a gynodioecious species. Specifically, we predicted that females would produce more seeds than hermaphrodites. We also predicted that hermaphrodites would have higher selfing rates than females and that this would contribute to the higher quality of female's seeds relative to those of hermaphrodites. Lastly, given that male sterility is reported to be determined by a dominant nuclear allele (Ahmadi and Bringhurst, 1991), we predicted that adult females would be more heterozygous and have lower inbreeding coefficients than hermaphrodites.
MATERIALS AND METHODS
Study system
Fragaria vesca ssp. bracteata is a herbaceous perennial plant that inhabits shady damp localities such as forest edges, meadows, rivers and roadsides in mountainous regions from British Columbia to Mexico (Staudt, 1999). It blooms in the spring and is insect pollinated. It reproduces clonally by plantlets on runners and sexually by seeds. Hermaphrodites are self-compatible (Staudt, 1999). Flowers of females are smaller (petal area: 70·0 ± 4·0 vs. 85·1 ± 5·4 mm2; t = –2·6; P < 0·01) and have fewer (19·8 ± 0·3 vs. 21·1 ± 0·4; t = –2·5; P < 0·01) shorter stamens (2·5 ± 0·1 vs. 3·8 ± 0·2 mm; t = –5·9; P < 0·01) than hermaphrodites (M. Koski, R. Dalton and T.-L. Ashman, unpubl. res.).
Plant material and sampling
For use in the following investigations we collected 87 adult plants of F. vesca ssp. bracteata from a roadside population on Mary's Peak, Oregon, USA (N44°29′18·4″, W123°32′14·7″) in the spring of 2010. We collected plants at least 1 m apart along three transects on two occasions during the flowering season (12 May 2010 and 7 July 2010). These were transplanted into 266 mL pots with a 2:1 mixture of Fafard #4 to sand. Plants received fertilizer and water as needed. Sex was determined by visual inspection of 2–3 flowers per plant. Females were identified on the basis of flower and anther size and the absence of pollen in their anther sacs. This population is 30 % female (T.-L. Ashman, unpubl. res.).
Reproductive allocation and seed production
In the greenhouse, we produced one clone from each of 43 of the field-collected plants (22 females and 21 hermaphrodites). These were grown in 266 mL pots in a 2:1 mix of Fafard #4 soil and sand for 4 months and then were subjected to a month-long winterization period (total dark and 4 °C) before returning to the greenhouse where they experienced 10 h daylength and 16–18 °C daytime temperatures for the approx. 3 month duration of flowering and fruiting. To ensure full potential seed set, we hand-pollinated all plants three times per week with outcross pollen. Once flowering ceased, we counted flower and fruit number, and collected the second fruit on each plant. On these we scored the number of fertilized ovules (achenes) and unfertilized ovules per fruit. Seed set was determined as the number of fertilized ovules divided by the number of total ovules. We calculated the proportion of fruit set as the number of fruits divided by the total number of flowers, and estimated seed production per plant as the number of fertilized ovules/fruit multiplied by fruit number for each plant. We also scored plant size as the product of leaf number and the diameter of the central leaflet (measured in mm), and vegetative reproduction as the number of plantlets and runners produced by the end of flowering. For two hermaphrodites, fruit set was missed and thus total plant seed fertility was not estimated for these plants.
Mating system and open-pollinated seed quality
For 28 (16 female and 12 hermaphrodite) of the field-collected adults we harvested a single open-pollinated fruit per plant. From each of these, up to 16 seeds were planted individually in 96-well trays filled with Sunshine #3 germination mix. These were placed in a growth chamber under 14/10 h light and 20/15·5 °C day/night conditions. We recorded germination daily for 43 d. For each maternal family we calculated the germination rate as the number of germinated seeds divided by the number of seeds planted, and mean time to germination for germinated seedlings.
DNA extraction and simple sequence repeat (SSR) genotyping
Genomic DNA was extracted from young leaf tissue of the 87 field-collected adults and 393 progeny from the 28 fruits following a procedure modified by Penet et al. (2008). DNA was quantified using a Nanodrop 2000 spectrophotometer (ThermoScientific) and was diluted to 3 ng μL−1 with sterilized ddH2O.
We screened 11 SSR primer pairs on the 47 females and 40 hermaphrodite adult plants to estimate population genetic parameters. We selected primers from published studies of congeners [CX661264, CX661603, CX662180, CO816938 (Spigler et al., 2008, 2010); EMFv160AD (Hadonou et al., 2003); UDF002 (Sargent et al., 2004); EMFn153 (Sargent et al., 2006); ARSFL7, ARSFL27 (Lewers et al., 2005); Fvi11 (Ashley et al., 2003); and BFACT10 (Rousseau-Gueutin et al., 2008)]. For all of these, we followed the ‘poor man's PCR’ protocol (Schuelke, 2000) and the reaction conditions described in Spigler et al. (2008). Prior to capillary electrophoresis on an ABI 3730 DNA Analyzer (Applied Biosystems), 1 µL of each reaction was mixed with 0·5 µL of LIZ500 standard and 8·5 µL of Hi-Di formamide. Electrophoretic products were visualized and product sizes assessed using GeneMapper (Applied Biosystems). In addition, we scored four SSRs (ARSFL7, EMFv160AD, UDF002 and CX661264) that were polymorphic and in linkage equilibrium (see the Results) in the progeny to estimate the family-level mating system for 16 female and 12 hermaphrodites.
Data analyses
Reproductive allocation
We determined whether reproductive or vegetative traits differed between the sex morphs using two-tailed t-tests (PROC TTEST, SAS Institute, 2008).
Genetic diversity and mating system
We calculated several population genetic parameters for females and hermaphrodites separately. We determined the observed (na) and effective number of alleles (ne) and Shannon information index (I) as a measure of allelic diversity for each SSR locus using POPGEN32 software ver. 1·31. In addition, for each locus and across all loci, we used Fisher's exact test to determine whether females and hermaphrodites differed in genotypic diversity using GENEPOP ver. 4·0 (Raymond and Rousset, 1995). We calculated PIC (polymorphic information content) values and observed heterozygosity (Ho) using Microsatellite Toolkit ver. 3·1·1 (Park, 2001). We tested for differences between the sexes in Ho with t-tests. We also calculated the inbreeding coefficient FIS (Wright, 1951) for the whole population and for the sex morphs separately using GENEPOP ver. 4·0.
We used ARLEQUIN ver. 3·1 (Excoffier et al., 2005) to test for linkage disequilibrium between each pair of polymorphic SSR loci and determined significance of deviations from equilibrium via randomization. Significance values were Bonferroni adjusted to account for multiple tests (Rice, 1989).
We used MLTR ver. 3·2 (Ritland and Jain, 2002) to estimate family-level multilocus (tm) and single locus (ts) outcrossing rates and converted these to selfing rates using s = 1 – t. We used the ‘method of moments’ procedure for small sample sizes, and estimated standard errors by bootstrapping. We compared mean family-level selfing rates between the sexes with a t-test. We estimated biparental inbreeding as tm – ts for each sex and compared it with 0 with a t-test.
Progeny seed quality
We tested the effect of maternal sex on progeny germination rate using a Generalized Linear Model with a binomial distribution (PROC GENMOD, SAS Institute, 2008) and on germination time using an analysis of variance (ANOVA, PROC GLM, SAS Institute, 2008). In the ANOVA, maternal family was defined as a random variable and was nested within maternal sex. In addition, for hermaphrodite families only, we used linear regression (PROC REG, SAS Institute, 2008) to test the prediction that a higher family-level selfing rate leads to a lower family-mean germination rate and longer time to germination. For the regression analyses we used a one-tailed probability for significance testing.
RESULTS
Reproductive allocation
Females and hermaphrodites did not differ significantly in any of the reproductive or vegetative traits measured, except for the estimated number of seeds per plant (Table 1). Hermaphrodites produced approx. 30 % more flowers than females.
Table 1.
Mean ± s.e. and t-tests for reproductive traits in female and hermaphrodite Fragaria vesca ssp. bracteata grown in the greenhouse
| Trait | Female | Hermaphrodite | t |
|---|---|---|---|
| Plant size (mm) | 207·3 ± 31·4 | 238·4 ± 30·4 | –0·7 |
| Flower number/plant | 4·2 ± 0·3 | 5·6 ± 0·7 | –1·7 |
| Proportion of fruit set | 0·95 ± 0·02 | 0·87 ± 0·05 | 1·4 |
| Ovule number/flower | 71·7 ± 3·7 | 75·5 ± 5·9 | –0·6 |
| Seed set/fruit | 0·73 ± 0·04 | 0·76 ± 0·06 | 0·7 |
| Seed number/plant | 202·6 ± 19·8 | 313·4 ± 47·4 | –2·2* |
| Plantlet number/plant | 5·4 ± 1·1 | 5·6 ± 1·1 | –0·2 |
| Runner number/plant | 2·1 ± 0·23 | 2·1 ± 0·36 | –0·01 |
*P < 0·05.
Genetic diversity
The observed numbers of alleles, effective numbers of alleles, polymorphic information content and Shannon information index were all generally lower in females than in hermaphrodites (Table 2).
Table 2.
Indices of genetic diversity in female (F) and hermaphrodite (H) individuals of F. vesca ssp. bracteata
| SSR locus | Sex morph | na | ne | PIC | I | Genotypic differentiation (P-value) | Ho | FIS |
|---|---|---|---|---|---|---|---|---|
| CX661264 | F | 2 | 1·77 | 0·3402 | 0·6262 | * | 0·3830 | 0·2039 |
| H | 3 | 2·10 | 0·4221 | 0·8185 | 0·5676 | –0·1800 | ||
| EMFn153 | F | 7 | 3·26 | 0·6604 | 1·3272 | NS | 0·7708 | –0·0460 |
| H | 8 | 3·18 | 0·7208 | 1·5405 | 0·6410 | 0·1300 | ||
| ARSFL 7 | F | 3 | 1·64 | 0·3219 | 0·6172 | NS | 0·4792 | –0·2330 |
| H | 2 | 1·62 | 0·3086 | 0·5693 | 0·4615 | –0·1880 | ||
| CX661603 | F | 7 | 4·69 | 0·7579 | 1·7078 | NS | 0·3750 | 0·5313 |
| H | 8 | 4·79 | 0·7609 | 1·7217 | 0·1538 | 0·8078 | ||
| EMFv160AD | F | 10 | 5·80 | 0·8048 | 1·9052 | *** | 0·7917 | 0·0872 |
| H | 13 | 7·86 | 0·8600 | 2·2469 | 0·8205 | 0·0540 | ||
| ARSFL 27 | F | 2 | 1·02 | 0·0204 | 0·0579 | NS | 0·0208 | –0·8100 |
| H | 1 | 1·00 | 0·0000 | 0·0000 | 0·0000 | – | ||
| CX662180 | F | 1 | 1·00 | 0·0000 | 0·0000 | – | 0·0000 | – |
| H | 1 | 1·00 | 0·0000 | 0·0000 | 0·0000 | – | ||
| UDF002 | F | 1 | 1·00 | 0·0000 | 0·0000 | NS | 0·0000 | – |
| H | 2 | 1·08 | 0·0712 | 0·1630 | 0·0769 | –1·2560 | ||
| BFACT 010 | F | 7 | 3·15 | 0·6268 | 1·3167 | * | 0·5417 | 0·2632 |
| H | 10 | 4·45 | 0·7490 | 1·7974 | 0·5897 | 0·1979 | ||
| CO816938 | F | 3 | 1·04 | 0·0406 | 0·1157 | NS | 0·0417 | 0·0838 |
| H | 2 | 1·05 | 0·0487 | 0·1192 | 0·0513 | –0·1270 | ||
| Fvi 11 | F | 12 | 5·65 | 0·8026 | 1·9946 | NS | 0·6458 | 0·2241 |
| H | 12 | 4·99 | 0·7789 | 1·9486 | 0·3421 | 0·5890 | ||
| All loci (s.d.) | F | 5·00 (3·79) | 2·73 (1·90) | 0·3978 (0·3430) | 0·8790 (0·7945) | ** | 0·3682 (0·3098) | 0·1789 |
| H | 5·63 (4·63) | 3·07 (2·27) | 0·4291 (0·3551) | 0·9932 (0·8717) | 0·3368 (0·2947) | 0·2489 |
Number of observed alleles (na), effective number of alleles (ne), polymorphic information content (PIC), Shannon information index (I), observed heterozygosity (Ho) and the inbreeding coefficient (FIS) are given for each locus and for all loci combined for each sex morph.
*P < 0·05; **P <0·01; ***P < 0·001; NS, not significant.
Considering all loci, the sex morphs were genotypically differentiated, and this was most pronounced at EMFv160AD and BFACT010 (Table 2). Observed heterozygosities were lower in hermaphrodites than in females, but not significantly so (Table 2; across all loci: paired t-test, t = 0·75, d.f. = 10, P = 0·47). Across all loci, the inbreeding coefficient, FIS, for the population was 0·213, and was 50 % higher in hermaphrodites than in females (Table 2).
After Bonferroni correction for multiple tests, a few loci showed significant linkage disequilibrium, specifically between CX661264 and CX661603 (χ2 = 53·84, d.f. = 21, P = 0·0001), EMFn153 and EMFv160AD (χ2 = 323·37, d.f. = 120, P < 0·00001), CX661603 and EMFv160AD (χ2 = 136·37, d.f. = 84, P < 0·0003), and CX661603 and Fvi 11 (χ2 = 191·05, d.f. = 112, P < 0·00001).
Selfing rate
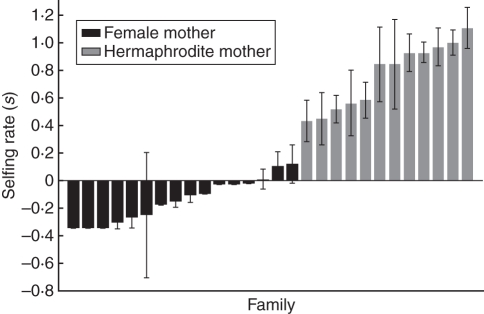
The selfing rate of F. vesca ssp. bracteata females was low and not different from zero (mean s = –0·140; range 0·120 to –0·347), whereas that of hermaphrodites was high and variable (s = 0·764; range 1·108–0·434) (Fig. 1), and these were significantly different (t = 11·37, n = 28, d.f. = 1, P < 0·0001). Estimated biparental inbreeding (tm – ts) was significantly different from zero for hermaphrodites (0·093 ± 0·117; t = 2·74, d.f. = 1, P = 0·019), but not females (–0·0003 ± 0·172; t = –0·01, d.f. = 1, P = 0·99).
Fig. 1.
Selfing rate (mean s ± s.e.) for open-pollinated seeds of female and hermaphrodite mothers.
Seed quality
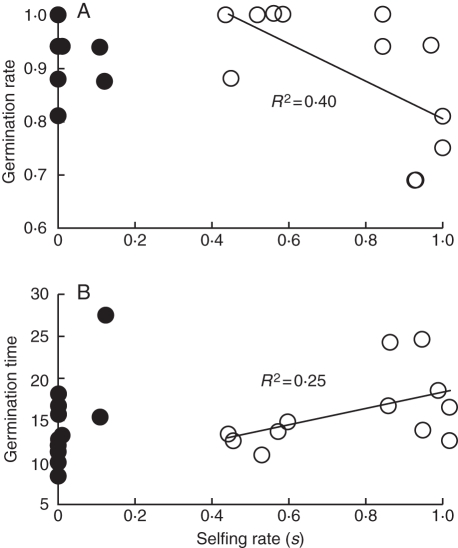
Seeds from female mothers were more likely to germinate than those from hermaphrodites (mean ± s.e.: 0·95 ± 0·02 vs. 0·89 ± 0·04; Wald χ2 = 5·23, P = 0·022) and germinated slightly but non-significantly faster (14·5 ± 0·36 vs. 15·9 ± 0·44 d; F1,26 = 0·91, P = 0·35). Among hermaphrodite families, those with higher selfing rates had lower germination success (b = –0·35; t1,10 = –2·59, P1 – tail = 0·014, Fig. 2A) and germinated more slowly (b = 9·8; t1,10 = 1·83, P1 – tail = 0·049, Fig. 2B).
Fig. 2.
Relationship of hermaphrodite selfing rate (s) with (A) seed germination rate (proportion of seeds germinating) and (B) mean germination time (days from planting). Data for female families (filled circles) were not included in regression analyses but are shown for comparative purposes.
DISCUSSION
We provide the first functional and population genetic evidence of gynodioecy in F. vesca ssp. bracteata, a key diploid congener of the sexually polymorphic octoploid Fragaria (F. virginiana and F. chiloensis). In the following paragraphs, we evaluate whether reproductive allocation and mating system jointly reflect sufficient compensation to maintain females given the current understanding of sex determination. We also compare features of F. vesca ssp. bracteata with those of other gynodioecious species and against other sexually polymorphic members of the genus Fragaria.
Do females of F. vesca ssp. bracteata show compensation?
When male sterility is determined by nuclear genes, females must have a 2-fold advantage to compensate for their loss of pollen fertility and be maintained (Lewis, 1941; Lloyd, 1974; Charlesworth and Charlesworth, 1978). We found that females of F. vesca ssp. bracteata, which allocate less per flower (in terms of stamens and petals), did not produce more seeds than hermaphrodites under greenhouse conditions with abundant pollination. Rather, they produced 30 % fewer seeds per plant which was due to lower flower production, as other components of seed production (ovule number, seed set and fruit set) showed only minor differences between the sex morphs (Table 1). We have recorded a similar sex differential for flower production per plant (female vs. hermaphrodite: 3·73 ± 0·36 vs. 5·77 ± 0·51; n = 45; d.f. = 1, t = 2·89, P = 0·006), and absence of a differential for proportion of fruit set (female vs. hermaphrodite: 0·86 ± 0·04 vs. 0·81 ± 0·05; n = 45; d.f. = 1, t = 1·59, P = 0·25) for plants under their native pollinator and resource conditions, suggesting that the differences observed in the greenhouse are likely to be reliable.
In seven out of 24 species reviewed by Shykoff et al. (2003) females produced fewer flowers than hermaphrodites, but in the vast majority of these species females had higher fruit and/or seed set than conspecific hermaphrodites (Shykoff et al., 2003). Females with lower seed production than hermaphrodites have been found in a handful of other species [e.g. Daphne laureaola (Alonso and Herrera, 2001); Kallstroemia grandiflora (Garcia et al., 2005); Trifolium hirtum (Molina-Freaner and Jain, 1992)] and, in these, alternative mechanisms of female maintenance were sought, such as seed quality differences.
Seed quality could differ between females and hermaphrodites either because of environmental or genetic maternal effects that differ between the sex morphs (e.g. Ashman, 1992; Delph and Mutikainen, 2003) or because seeds of hermaphrodites are more inbred than those of females and the former suffer inbreeding depression (reviewed in Shykoff and Collin, 2003). Our mating system analysis indicates that under natural conditions, hermaphrodites self on average 76 % of the time and that females are highly outcrossed with very little biparental inbreeding (Fig. 1). Relative to the species reviewed by Shykoff et al. (2003), F. vesca ssp. bracteata hermaphrodites are on the extreme end of selfing; only two other species are reported to have higher hermaphrodite selfing rates [Trifolium hirtum (Molina-Freaner and Jain, 1992) and Chionographis japonica (Maki, 1993)].
Charlesworth and Charlesworth (1978) showed that, for nuclear male sterility to spread, the inequality sd > (1 – k)/2 must be met, where k represents relative seed fertility of females over hermaphrodites [(mean seed production by females – mean seed production by hermaphrodites)/mean seed production of hermaphrodites], s is the selfing rate of hermaphrodites and d is inbreeding depression. Our estimates for seed fertility differential (k = –0·35) and selfing rate (s = 0·764) in F. vesca ssp. bracteata predict that inbreeding depression must be approx. 0·87 or greater in the field for females to spread. Inbreeding depression was detected at the germination stage here (Fig. 2), but this value could be an underestimate as it only reflects the early life stage and was assessed under benign conditions (see recent reviews by Fox and Reed, 2011; Cheptou and Donohue, 2011). We can also estimate inbreeding depression from the inbreeding coefficient of the in situ adult plants (FIS; Table 2) and the frequency of selfing (s), i.e. d = 1 – 2 [F(1 – s)/s(1 – F)] (Ritland, 1990; see also Kohn and Biardi, 1995). This estimator has the advantage of integration of inbreeding across the life cycle in the natural environment, but also relies on several assumptions, such as inbreeding equilibrium and selfing as the only form of inbreeding (see discussion in Goodwillie et al., 2005), and the underlying model may not be valid when the population involves two morphs (Medrano et al., 2005), so the estimate of inbreeding depression needs to be viewed with caution. Using this approach, we estimate inbreeding depression as 0·75, near average for hermaphroditic plant species with mixed mating systems (d = 0·81 ± 0·71; Goodwillie et al., 2005), and nearly high enough to account for maintenance of females. Alternatively, we can assume the population is at equilibrium and use the observed frequency of females (0·30) and Charlesworth and Charlesworth's (1978) formula for the predicted equilibrium frequency of females [Z = (k + 2sd – 1)/2(k + sd)] to solve for inbreeding depression (d). This approach gives an estimate of inbreeding depression of 0·67, fairly close to that obtained above. It is thus unclear whether female's seed quality advantage is sufficient to account for the maintenance of females under the assumption of nuclear inheritance of male sterility. Experimental estimates of the magnitude of inbreeding depression should be obtained. In addition, the genetic determination of male sterility in F. vesca ssp. bracteata (dominant nuclear male sterility; Ahmadi and Bringhurst, 1991) should be confirmed via larger scale studies that include crosses between, as well as, within populations (Bailey and Delph, 2007). These would reveal whether cytoplasmic components are involved in male sterility. If they were, then models other than those used above (that assume nuclear inheritance) would be appropriate (e.g. Lewis, 1941; Lloyd, 1975; reviewed in Bailey and Delph, 2007; Delph et al., 2007).
How does gynodioecy in F. vesca ssp. bracteata compare with sub-dioecious and dioecious congeners?
The hermaphrodite selfing rate found in this diploid gynodioecious strawberry is similar to the estimate for hermaphrodites of octoploid sub-dioecious F. virginiana (s = 0·72 ± 0·004; population ‘PR’; Rohde and Ashman, 2010). In addition, females were less inbred than hermaphrodites (FIS = 0·043 vs. 0·120) in this population of F. virginiana (Ashley et al., 2003), much as observed here in F. vesca ssp. bracteata, although in the former mean inbreeding was lower. The seed fertility differential, on the other hand, was much higher in F. virginiana (females produce 4–8 times more seeds than hermaphrodites; Ashman, 1999a; Spigler and Ashman, 2011). This difference could indicate that female sterility factors evolve only after polyploidization in the octoploid (Goldberg et al., 2010, W. Njuguna et al., unpubl. res.) or the two species may differ totally in their genetic determination of sex. The infrequency with which hermaphrodites contribute seeds to the adult population of the octoploid could also account for the lower inbreeding coefficients in hermaphrodite adults of F. virginiana compared with F. vesca ssp. bracteata, as it would appear that their progeny do not experience very high inbreeding depression (Rohde and Ashman, 2010), as might be expected in a polyploid (e.g. Soltis and Soltis, 2000; Galloway and Etterson, 2007).
Sexual dimorphism is expected to evolve in species with separate sexes when optimal values of a trait differ for male and female fertility (Lande, 1980). One might predict greater dimorphism in species with complete separation of the sexes (e.g. dioecious F. chiloensis) than in those with less complete separation of the sexes, i.e. populations still contain an hermaphrodite morph – as in sub-dioecious F. virginiana – or do not have males at all – as is the case in gynodioecious F. vesca ssp. bracteata (reviewed in Eckhart, 1999). In accordance with this prediction, sexual dimorphism was less pronounced in F. vesca ssp. bracteata than in F. virginiana, especially with respect to floral characteristics. Hermaphrodites of F. vesca ssp. bracteata had 18 % larger petals and 34 % larger stamens than females, but these differentials were 28 and 65 %, respectively, in F. virginiana (Ashman and Hitchens, 2000). In addition, there is increasing dimorphism with increasing separation of the sexes for ovule and anther number across all three species (T.-L. Ashman et al., unpubl. res.). Also, while there was more sexual dimorphism in fruit set in F. virginiana than in F. vesca ssp. bracteata (see above) there was less dimorphism in flower number (F. virginiana females produce 17 % vs. 25 % fewer flowers than hermaphrodites; Ashman, 1999a, b). These comparisons indicate that for most traits, gynodioeicous F. vesca ssp. bracteata exhibits less dimorphism than its dioecious and sub-dioecious congeners, a pattern that is predicted by theory, but has not often been shown for closely related taxa (e.g. Yakimowski et al., 2011).
Population genetic insights into gynodioecy in F. vesca ssp. bracteata
Given the proposed genetic mechanism and the potential avenues for mating, we expected females to be more heterozygous and less inbred than hermaphrodites. Adult females did have lower inbreeding coefficients than adult hermaphrodites but they were not significantly more heterozygous than hermaphrodites. This may reflect severe inbreeding depression in the field, i.e. the absence of highly inbred hermaphrodites surviving to adulthood (see above), and/or that adult hermaphrodites mostly have female mothers. It is also possible that historical colonization dynamics affected the sex morphs differently, i.e. perhaps females have undergone a bottleneck (Charlesworth, 2003). The fact that females had fewer alleles/locus on average might lend support to this latter possibility. One must also consider that null alleles can create false homozygotes and thus bias estimates of inbreeding; however, these generally have only small effects (Carlsson, 2008) and to explain the results here null alleles would have to be associated with sex type which seems unlikely.
Although there are far fewer studies for comparison, some have shown lower inbreeding in the unisexual morph relative to the cosexual one. For instance, Molina-Freaner and Jain (1992) found that females had higher levels of heterozygosity than hermaphrodites in one of two populations of Trifolium hirtum which has cytonuclear inheritance of sex phenotype. Likewise, in an androdioecious Schizopepon bryoniaefolius, males had lower inbreeding coefficients than hermaphrodites (Akimoto et al., 1999). However, no significant difference in inbreeding was found between females and hermaphrodites in Thymus vulgaris (Tarayre and Thompson, 1997). Additional insight into the effects of the sexual system on genetic diversity can be achieved through among-population comparisons, as inbreeding is expected to decrease (and heterozygosity to increase) with increasing frequencies of unisexuals, and this has been shown in several species (e.g. Schizopepon bryoniaefolius, Akimoto et al., 1999; Daphne laureola, Medrano et al., 2005; Kallstroemia gradiflora, Cuevas et al. 2006; but see T. vulgaris, Tarayre and Thompson, 1997). When such work is combined with information on sex ratios and historical gene flow, additional insight into sexual system evolution can be gained. Such work is underway in F. vesca ssp. braceata.
ACKNOWLEDGEMENTS
We thank R. Dalton, A. Liston, B. McTeague, M. Parks, S. Straub and E. York for assistance in the greenhouse, field and laboratory, G. Meindl and anonymous reviewers for comments on the manuscript, and the Ashman lab members for discussion. This work was supported by the National Science Foundation (DEB 0449488 and 1020523 to T.L.A.), the University of Pittsburgh (to M.H.K.) and visiting scholar funding to J.L. from Taizhou University, China.
LITERATURE CITED
- Ahmadi H, Bringhurst RS. Genetics of sex expression in Fragaria species. American Journal of Botany. 1991;78:504–514. [Google Scholar]
- Akimoto J, Fukuhara T, Kikuzawa K. Sex ratios and genetic variation in a functionally androdioecious species, Schizopepon bryoniaefolius (Cucurbitaceae) American Journal of Botany. 1999;86:880–886. [PubMed] [Google Scholar]
- Alonso C, Herrera CM. Neither vegetative nor reproductive advantages account for high frequency of male-steriles in southern Spanish gynodioecious Daphne laureola (Thymelaeaceae) American Journal of Botany. 2001;88:1016–1024. [PubMed] [Google Scholar]
- Ashley MV, Wilk SMN, Craft KJ, et al. High variability and disomic segregation of microsatellites in the octoploid Fragaria virginiana Mill. (Rosaceae) Theoretical and Applied Genetics. 2003;107:1201–1207. doi: 10.1007/s00122-003-1370-5. [DOI] [PubMed] [Google Scholar]
- Ashman TL. The relative importance of inbreeding and maternal sex in determining progeny fitness in Sidalcea oregana ssp. spicata, a gynodioecious plant. Evolution. 1992;46:1862–1874. doi: 10.1111/j.1558-5646.1992.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Ashman TL. Reproductive allocation in hermaphrodite and female plants of Sidalcea oregana ssp. spicata (Malvaceae) using four currencies. American Journal of Botany. 1994;81:433–438. [Google Scholar]
- Ashman TL. Determinants of sex allocation in a gynodioecious wild strawberry: implications for the evolution of dioecy and sexual dimorphism. Journal of Evolutionary Biology. 1999a;12:648–661. [Google Scholar]
- Ashman TL. Quantitative genetics of floral traits in a gynodioecious wild strawberry Fragaria virginiana: implications for the independent evolution of female and hermaphrodite floral phenotypes. Heredity. 1999b;83:733–741. doi: 10.1046/j.1365-2540.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Ashman TL, Hitchens MS. Dissecting the causes of variation in intra-inflorescence allocation in a sexually polymorphic species, Fragaria virginiana (Rosaceae) American Journal of Botany. 2000;87:197–204. [PubMed] [Google Scholar]
- Bailey MF, Delph LF. A field guide to models of sex ratio evolution in gynodioecious species. Oikos. 2007;116:1609–1617. [Google Scholar]
- Berry PE, Hahn WJ, Sytsma KJ, Hall JC, Mast A. Phylogenetic relationships and biogeography of Fuchsia (Onagraceae) based on noncoding nuclear and chloroplast DNA data. American Journal of Botany. 2004;91:601–614. doi: 10.3732/ajb.91.4.601. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Effects of microsatellite null alleles on assignment testing. Journal of Heredity. 2008;99:616–623. doi: 10.1093/jhered/esn048. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. Model for evolution of dioecy and gynodioecy. American Naturalist. 1978;112:975–997. [Google Scholar]
- Charlesworth D. Effects of inbreeding on the genetic diversity of populations. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358:1051–1070. doi: 10.1098/rstb.2003.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D, Willis JH. The genetics of inbreeding depression. Nature Reviews Genetics. 2009;10:783–796. doi: 10.1038/nrg2664. [DOI] [PubMed] [Google Scholar]
- Cheptou PO, Donohue K. Environment-dependent inbreeding depression: its ecological and evolutionary significance. New Phytologist. 2011;189:395–407. doi: 10.1111/j.1469-8137.2010.03541.x. [DOI] [PubMed] [Google Scholar]
- Collin CL, Penet L, Shykoff JA. Early inbreeding depression in the sexually polymorphic plant Dianthus sylvestris (Caryophyllaceae): effects of selfing and biparental inbreeding among sex morphs. American Journal of Botany. 2009;96:2279–2287. doi: 10.3732/ajb.0900073. [DOI] [PubMed] [Google Scholar]
- Collin CL, Shykoff JA. Outcrossing rates in the gynomonoecious–gynodioecious species Dianthus sylvestris (Caryophyllaceae) American Journal of Botany. 2003;90:579–585. doi: 10.3732/ajb.90.4.579. [DOI] [PubMed] [Google Scholar]
- Cuevas E, Arias DM, Dominguez CA, Castillo RA, Molina-Freaner F. The genetic structure of the gynodioecious Kallstroemia grandiflora (Zygophyllaceae): the role of male sterility and colonization history. Heredity. 2006;97:269–274. doi: 10.1038/sj.hdy.6800849. [DOI] [PubMed] [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. Chicago: The University of Chicago Press; 1877. [Google Scholar]
- Delph LF, Mutikainen P. Testing why the sex of the maternal parent affects seedling survival in a gynodioecious species. Evolution. 2003;57:231–239. doi: 10.1111/j.0014-3820.2003.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Delph LF, Touzet P, Bailey MF. Merging theory and mechanism in studies of gynodioecy. Trends in Ecology and Evolution. 2007;22:17–24. doi: 10.1016/j.tree.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Dufay M, Billard E. How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Annals of Botany. 2011;109:505–519. doi: 10.1093/aob/mcr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart VM. Sexual dimorphism in flowers and inflorescences. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag; 1999. pp. 175–215. [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin (version 3·0): an integrated software package for population genetics data analysis. Evolutionary Bioinformatics. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Fox CW, Reed DH. Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution. 2011;65:246–258. doi: 10.1111/j.1558-5646.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- Galloway LF, Etterson JR. Inbreeding depression in an autotetraploid herb: a three cohort field study. New Phytologist. 2007;173:383–392. doi: 10.1111/j.1469-8137.2006.01909.x. [DOI] [PubMed] [Google Scholar]
- Garcia EC, Marquez J, Dominguez CA, Molina-Freaner F. Evidence of gynodioecy in Kallstroemia grandiflora (Zygophyllaceae): microsporogenesis in hermaphrodite and female plants and lack of reproductive compensation. International Journal of Plant Sciences. 2005;166:481–491. [Google Scholar]
- Goldberg MT, Spigler RB, Ashman TL. Comparative genetic mapping points to different sex chromosomes in sibling species of wild strawberry (Fragaria) Genetics. 2010;186:1425–1433. doi: 10.1534/genetics.110.122911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution and Systematics. 2005;36:47–79. [Google Scholar]
- Gouyon PH, Couvet D. A conflict between two sexes, females and hermaphrodites. Experientia Supplementum. 1987;55:245–261. doi: 10.1007/978-3-0348-6273-8_11. [DOI] [PubMed] [Google Scholar]
- Hadonou M, Sargent DJ, Walden R, Simpson DW. Characterization of Fragaria vesca single sequence repeat (SSR) markers. Acta Horticulturae. 2003;649:99–102. [Google Scholar]
- Jacobs MS, Wade MJ. A synthetic review of the theory of gynodioecy. American Naturalist. 2003;161:837–851. doi: 10.1086/375174. [DOI] [PubMed] [Google Scholar]
- Kohn J, Biardi JE. Outcrossing rates and inferred levels of inbreeding depression in gynodioecious Cucurbita foetidissima (Cucurbitaceae) Heredity. 1995;75:77–83. [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- Lewers KS, Styan SMN, Hokanson SC. Strawberry GenBank-derived and genomic simple sequence repeat (SSR) markers and their utility with strawberry, blackberry, and red and black raspberry. American Society for Horticultural Science. 2005;130:102–115. [Google Scholar]
- Lewis D. Male sterility in natural populations of hermaphrodite plants: the equilibrium between females and hermaphrodites to be expected with different types of inheritance. New Phytologist. 1941;40:56–63. [Google Scholar]
- Lloyd DG. Theoretical sex-ratios of dioecious and gynodioecious angiosperms. Heredity. 1974;32:11–34. [Google Scholar]
- Lloyd DG. Maintenance of gynodioecy and androdioecy in angiosperms. Genetica. 1975;45:325–339. [Google Scholar]
- Maki M. Outcrossing and fecundity advantage in gynodioecious Chionographis japonica var. kurohimensis (Liliaceae) American Journal of Botany. 1993;71:775–786. [Google Scholar]
- Maurice S, Belhassen E, Couvet D, Gouyon PH. Evolution of dioecy – can nuclear–cytoplasmic interactions select for maleness? Heredity. 1994;73:346–354. doi: 10.1038/hdy.1994.181. [DOI] [PubMed] [Google Scholar]
- Medrano M, Alonso C, Herrera CM. Mating system, sex ratio, and persistence of females in the gynodioecious shrub Daphne laureola L. (Thymelaeaceae) Heredity. 2005;94:37–43. doi: 10.1038/sj.hdy.6800550. [DOI] [PubMed] [Google Scholar]
- Molina-Freaner F, Jain SK. Female frequencies and fitness components between sex phenotypes among gynodioecious populations of the colonizing species Trifolium hirtum All. in California. Oecologia. 1992;92:279–286. doi: 10.1007/BF00317376. [DOI] [PubMed] [Google Scholar]
- Njuguna W. Development and use of molecular tools in Fragaria. OR: PhD thesis, Oregon State University, Corvallis; 2010. [Google Scholar]
- Park SDE. Trypanotolerance in West African cattle and the population genetic effects of selection. Dublin: PhD thesis, University of Dublin; 2001. [Google Scholar]
- Penet L, Collin CL, Ashman TL. Florivory increases selfing: an experimental study in the wild strawberry, Fragaria virginiana. Plant Biology. 2008;11:38–45. doi: 10.1111/j.1438-8677.2008.00141.x. [DOI] [PubMed] [Google Scholar]
- Raymond M, Rousset F. GENEPOP (version 1·2): population genetics software for exact tests and ecumenicism. Journal of Heredity. 1995;86:248–249. [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:233–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Richards AJ. Plant breeding systems. London: Chapman & Hall; 1997. [Google Scholar]
- Richards CM. Inbreeding depression and genetic rescue in a plant metapopulation. American Naturalist. 2000;155:383–394. doi: 10.1086/303324. [DOI] [PubMed] [Google Scholar]
- Ritland K. Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution. 1990;44:1230–1241. doi: 10.1111/j.1558-5646.1990.tb05227.x. [DOI] [PubMed] [Google Scholar]
- Ritland K, Jain SK. Extensions of models for the estimation of mating systems using n independent loci. Heredity. 2002;88:221–228. doi: 10.1038/sj.hdy.6800029. [DOI] [PubMed] [Google Scholar]
- Rohde AS, Ashman TL. Effects of florivory and inbreeding on reproduction in hermaphrodites of the wild strawberry Fragaria virginiana. International Journal of Plant Sciences. 2010;171:175–184. [Google Scholar]
- Rousseau-Gueutin M, Lerceteau-Köhler E, Barrot L, Sargent DJ, Monfort A, Simpson D, Arùs P, Denoyes-Rothan B. Comparative genetic mapping between octoploid and diploid Fragaria species reveals a high level of colinearity between their genomes and the essentially disomic behavior of the cultivated octoploid strawberry. Genetics. 2008;179:2045–2060. doi: 10.1534/genetics.107.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent DJ, Davis TM, Tobutt KR, Wilkinson WJ, Battey NH, Simpson DW. A genetic linkage map of microsatellite, gene-specific and morphological markers in diploid Fragaria. Theoretical and Applied Genetics. 2004;109:18–20. doi: 10.1007/s00122-004-1767-9. [DOI] [PubMed] [Google Scholar]
- Sargent DJ, Clarke J, Simpson DW, et al. An enhanced microsatellite map of diploid Fragaria. Theoretical and Applied Genetics. 2006;112:1349–1359. doi: 10.1007/s00122-006-0237-y. [DOI] [PubMed] [Google Scholar]
- SAS Institute. 2008 SAS version 9·2. [Google Scholar]
- Schuelke M. An economic method for the fluorescent labeling of PCR fragments. Nature Biotechnology. 2000;18:233–234. doi: 10.1038/72708. [DOI] [PubMed] [Google Scholar]
- Shykoff JA, Kolokotronis SO, Collin CL, Lopez-Villavicencio M. Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia. 2003;135:1–9. doi: 10.1007/s00442-002-1133-z. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences, USA. 2000;97:7051–7057. doi: 10.1073/pnas.97.13.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigler RB, Lewers KS, Main DS, Ashman TL. Genetic mapping of sex determination in a wild strawberry, Fragaria virginiana, reveals earliest form of sex chromosome. Heredity. 2008;101:507–517. doi: 10.1038/hdy.2008.100. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Lewers KS, Johnson AL, Ashman TL. Comparative mapping reveals autosomal origin of sex chromosome in octoploid Fragaria virginiana. Journal of Heredity. 2010;101:S107–S117. doi: 10.1093/jhered/esq001. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Ashman TL. Sex ratio and subdioecy in Fragaria virginiana: the roles of plasticity and gene flow examined. New Phytologist. 2011;190:1058–1068. doi: 10.1111/j.1469-8137.2011.03657.x. [DOI] [PubMed] [Google Scholar]
- Staudt G. Systematics and geographic distribution of the American strawberry species. Taxonomic studies in the genus Fragaria (Rosaceae: Potentilleae). Berkeley: University of California Press; 1999. [Google Scholar]
- Tarayre M, Thompson JD. Population genetic structure of the gynodioecious Thymus vulgaris L. (Labiatae) in southern France. Journal of Evolutionary Biology. 1997;10:157–174. [Google Scholar]
- Weller SG, Sakai AK. The genetic basis of male-sterility in Schiedea (Caryophyllaceae), an endemic Hawaiin genus. Heredity. 1991;67:265–273. [Google Scholar]
- Wu X, Wang J, Na J-K, et al. The origin of the non-recombining region of sex chromosomes in Carica and Vasconellea. The Plant Journal. 2010;63:801–810. doi: 10.1111/j.1365-313X.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- Yakimowski SB, Glaettli M, Barrett SCH. Floral dimorphism in plant populations with combined versus separate sexes. Annals of Botany. 2011;108:765–776. doi: 10.1093/aob/mcr025. [DOI] [PMC free article] [PubMed] [Google Scholar]