Abstract
Background
The remarkable diversity of mating patterns and sexual systems in flowering plants has fascinated evolutionary biologists for more than a century. Enduring questions about this topic include why sexual polymorphisms have evolved independently in over 100 plant families, and why proportions of self- and cross-fertilization often vary dramatically within and among populations. Important new insights concerning the evolutionary dynamics of plant mating systems have built upon a strong foundation of theoretical models and innovative field and laboratory experiments. However, as the pace of advancement in this field has accelerated, it has become increasingly difficult for researchers to follow developments outside their primary area of research expertise.
Scope
In this Viewpoint paper we highlight three important themes that span and integrate different subdisciplines: the changes in morphology, phenology, and physiology that accompany the transition to selfing; the evolutionary consequences of pollen pool diversity in flowering plants; and the evolutionary dynamics of sexual polymorphisms. We also highlight recent developments in molecular techniques that will facilitate more efficient and cost-effective study of mating patterns in large natural populations, research on the dynamics of pollen transport, and investigations on the genetic basis of sexual polymorphisms. This Viewpoint also serves as the introduction to a Special Issue on the Evolution of Plant Mating Systems. The 15 papers in this special issue provide inspiring examples of recent discoveries, and glimpses of exciting developments yet to come.
Keywords: Biparental inbreeding, heterostyly, mate diversity, mixed-mating system, next generation sequencing, outcrossing, paternity, phenotypic plasticity, plant breeding systems, pollination, self-fertilization, sexual polymorphism
INTRODUCTION
Biologists have long been fascinated by the remarkable diversity of mating patterns and sexual systems in flowering plants. Why, for example, do proportions of self- and cross-fertilization often vary dramatically among populations and even among individuals of the same species? Why have sexual polymorphisms evolved over 100 times, yet are found in only a small minority of plant species? Do plants with high rates of self-fertilization have reduced outcross siring success? Why does the composition and diversity of mates vary markedly within and among populations? These are fundamental questions in evolutionary biology and have been the focus of a large body of productive mathematical modelling and innovative empirical study (Darwin, 1876, 1877; Fisher, 1941; Bateman, 1948; Lloyd, 1992; Schoen and Lloyd, 1992; Lloyd and Barrett, 1996; Dudash et al., 1997; Pannell and Barrett, 1998; Griffin and Eckert, 2003; Teixeira and Bernasconi, 2007; Case and Ashman, 2009).
Research on the evolution of plant mating systems has flourished over the past decade (e.g. Kalisz et al., 2004; Delph and Wolf, 2005; Goodwillie et al., 2005; Harder and Barrett, 2006; Barrett, 2010; Eckert et al., 2010; Bodbyl Roels and Kelly, 2011). However, as the pace of advancement in evolutionary biology and population genetics has accelerated, it has become increasingly difficult for researchers to continue to follow developments outside their primary area of research expertise. Therefore, a major goal of this Special Issue on Plant Mating is to showcase research that spans and integrates different subdisciplines.
Here we highlight some of the recurring themes of papers in the Special Issue. These include: the changes in morphology, phenology and physiology that accompany the transition to selfing; the evolutionary consequences of pollen pool diversity in flowering plants; and the evolutionary dynamics of sexual polymorphisms. We also highlight recent developments in molecular techniques that will facilitate more efficient and cost-effective study of mating patterns in large natural populations, research on the dynamics of pollen transport, and investigations on the genetic basis of sexual polymorphisms.
UNSOLVED MYSTERIES IN THE TRANSITION TO SELF-FERTILIZATION
Some of the most confounding puzzles in plant mating system evolution concern the suite of changes in morphology, phenology and physiology that typically accompany the transition to selfing (Darwin, 1876; Ornduff, 1969; Wyatt, 1988; Vallejo Marín and Barrett, 2009; Mazer et al., 2010; Sicard and Lenhard, 2011; Ivey and Carr, 2012, this issue; Kalisz et al., 2012, this issue). For example, do traits such as small flower size, reduced separation of anthers and stigma (Fig. 1), and decreased investment in male allocation evolve simultaneously with self-fertility, or sequentially? If sequentially, is there a predictable order? Surprisingly little is known about such questions, despite the profound biological importance of this common evolutionary transition (Stebbins, 1974; Hamrick and Godt, 1989; Goldberg et al., 2010). Although much remains to be learned, recent research has provided valuable insights. For example, changes in life history, floral display and floral morphology that accompany transitions to selfing have been identified through comparative analyses within lineages that have both selfing and outcrossing species (Barrett et al., 1996; Weller and Sakai, 1999; Ferrero et al., 2012, this issue; Kalisz et al., 2012, this issue). Furthermore, effects of key floral traits on variation in selfing rates have been measured in several species (Holtsford and Ellstrand, 1992; Karron et al., 1997; van Kleunen and Ritland, 2004; Takebayashi et al., 2006; Kennedy and Elle, 2008; Eckert et al., 2009; Dart et al., 2012, this issue; Kalisz et al., 2012, this issue). In addition, adaptive trait change (a reduction in herkogamy) has been directly observed in a five-generation greenhouse experiment that excluded pollinators from Mimulus guttatus (Bodbyl Roels and Kelly, 2011). Studies such as these have begun to provide clues about the transition to selfing, but the sequence of trait transitions and the evolutionary mechanisms driving these transitions have yet to be demonstrated for any species.
Fig. 1.
Gilia achilleifolia exhibits striking within-population variation in the degree of herkogamy, which is associated with variation in selfing rate. Individuals with high herkogamy self less than individuals with low herkogamy. Photograph by Naoki Takebayashi.
Obtaining estimates of the strength of selection on candidate mating system traits is an important first step for clarifying the mechanisms involved in their evolution (Fenster and Ritland, 1994; van Kleunen and Ritland, 2004). Such fitness estimates need to include both male and female function, as well as fitness costs of selfing (sensu Lau et al., 2008; Busch and Delph, 2012, this issue; Karron and Mitchell, 2012, this issue). Phenotypic manipulations of traits can help reveal how they influence fitness, as well as their contributions to different modes of selfing (Schoen and Lloyd, 1992; Campbell et al., 1996; Eckert, 2000; Fetscher, 2001). More generally, well-designed experiments can directly test mechanistic hypotheses about trait evolution, such as the extent to which mating system traits evolve through direct selection, as opposed to correlated evolution with other adaptive traits (Fenster and Ritland, 1994; Barrett et al., 1996; Mazer et al., 2004; Ivey and Carr, 2012, this issue).
A second area ripe for investigation concerns how environmental variation may facilitate mating system transitions (Lloyd, 1992; Ashman, 2006). Several theoretical and empirical studies have addressed the influence of pollinators on the evolution of selfing (Holsinger et al., 1984; Lloyd, 1992; Barrett and Harder, 1996; Goodwillie et al., 2005; Morgan et al., 2005; Karron et al., 2009; Karron and Mitchell, 2012, this issue). However, other environmental influences also appear to be important and warrant further study. For example, interactions with natural enemies or variation in the abiotic environment can alter the expression of inbreeding depression (Ivey et al., 2004; Armbruster and Reed, 2005; Cheptou and Donohue, 2011), male fertility (Quesada et al., 1995; Mutikainen and Delph, 1996; Caruso et al., 2005, Herlihy and Delph, 2009), selfing rates (Ivey and Carr, 2005; Steets et al., 2006), and other mating system features (Ashman, 2006; Steets et al., 2007; Eckert et al., 2010). These results suggest that a diversity of environmental factors may also influence transitions in the mating system.
A third promising area for research concerns the role of environmental variation in mating system evolution through phenotypic plasticity (e.g. Levin, 2010, 2012, this issue; Murren and Dudash, 2012, this issue). For example, plastic variation in traits correlated with selfing rate may help explain a long-standing puzzle in mating system biology: the evolutionary stability of mixed mating systems (Waller, 1980; Elle and Hare, 2002; Goodwillie et al., 2005; Ivey and Carr, 2005; Steets et al., 2006). In addition, plastic responses in mating system traits can facilitate colonization or adaptation to marginal habitats, which can pave the way for mating system transitions in stressful or novel habitats (Holtsford and Ellstrand, 1992; Levin, 2010; Cheptou, 2012, this issue; Ivey and Carr, 2012, this issue; Levin, 2012, this issue). Trait plasticity, however, need not inevitably lead to mating system transitions. For example, herkogamy can show marked plasticity (Fig. 2; Elle and Hare, 2002; Ivey and Carr, 2005; Brock and Weinig, 2007; Vallejo Marín and Barrett, 2009; de Waal et al., 2012, this issue), which may make the transition to selfing likely in some environments (e.g. where anther–stigma separation is lower) but favour outcrossing in others (Lloyd, 1992; Morgan et al., 2005). As with sex-differential phenotypic plasticity (see below), little is known about the heritability of plasticity in traits affecting selfing rates (Vallejo Marín and Barrett, 2009; Ivey and Carr, 2012, this issue). Therefore the extent to which plasticity in mating system traits might be an adaptive response to fluctuating environments deserves additional study (Levin, 2012, this issue).
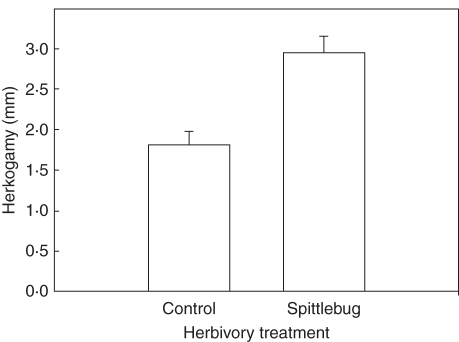
Fig. 2.
Herbivory by spittlebugs influences mating system traits in Mimulus guttatus. Plants in field populations that were experimentally exposed to spittlebug herbivory had 63 % greater herkogamy than control plants that did not receive herbivory. Redrawn from Ivey and Carr (2005).
Transitions to selfing undoubtedly occur through diverse pathways for different lineages. Nonetheless, dissection of the evolutionary mechanisms leading to these mating system traits, and their environmental and genetic influences, will help unravel the secrets behind this common feature of hermaphroditic reproduction.
EXPANDING THE CONCEPT OF MATING SYSTEM IN SELF-COMPATIBLE HERMAPHRODITES
When a pollinator visits a hermaphroditic flower it often deposits a mixture of pollen from several different sources, including self pollen, pollen from close relatives, and pollen from one or more unrelated donors (Fig. 3; Waser, 1993; Karron et al., 2006). Although this diverse pollen pool is often dichotomized as ‘self vs. outcross’, we believe that a deeper understanding of the diversity of outcross pollen, the factors influencing these mating patterns, and the fitness consequences of these mating events is needed.
Fig. 3.
(A) Bumble bees typically deposit a mixture of pollen from multiple pollen donors onto the stigma of a receptive Mimulus ringens flower. (B) Pollen grains (coloured gold) germinating on a Mimulus ringens stigma. Photographs by Jeff Karron, Randall Mitchell and John Bell.
One important component of a diverse mating pool is crossing between close relatives (biparental inbreeding), which may be especially likely in populations that have substantial genetic structure (Waller, 1993; Griffin and Eckert, 2003). When pollinators move short distances and pollen carryover is limited, a large fraction of the pollen deposited on a stigma might come from close relatives (Mitchell et al., 2009). These inbred (but non-self) matings can substantially reduce heterozygosity and, if inbreeding depression is present, lower offspring fitness (Nason and Ellstrand, 1995). The reduction in fitness due to biparental inbreeding is thought to influence the evolution of mating systems by changing the relative costs and benefits of selfing vs. outcrossing (Uyenoyama, 1986; Yahara, 1992; Waller, 1993). For example, when deleterious recessive alleles are expressed through biparental inbreeding, the fitness difference between self- and outcross progeny is reduced (Waller, 1993).
Surprisingly, the extent of biparental inbreeding (b) and the fitness consequences of biparental inbreeding depression have rarely been quantified in natural populations. Most attempts to quantify biparental inbreeding using marker genes have compared estimates of selfing based on single marker genes (ss) with estimates based on multiple marker genes (sm; Brown, 1990). Although theory suggests that ss – sm will give an estimate of b (Shaw et al., 1981), this parameter is usually substantially underestimated due to an insufficient number of marker loci (Leclerc-Potvin and Ritland, 1994; Ritland, 2002). A more powerful approach is to compare the selfing rate of experimental populations that have been randomly rearranged to break up genetic structure with the selfing rate of paired control populations where plants were returned to their original locations (Kelly and Willis, 2002). Using this approach, a study of Aquilegia canadensis demonstrated that nearly 30 % of all matings were between close relatives (Griffin and Eckert, 2003). Given the high level of inbreeding depression in A. canadensis, full sib mating is likely to have a dramatic effect on fitness (Herlihy and Eckert, 2004). More work is needed to thoroughly assess the extent of biparental inbreeding in natural populations, to quantify its fitness consequences, and to determine the ecological conditions that tend to promote biparental inbreeding.
The dichotomy of selfing versus outcrossing also masks important differences in the diversity of donors siring seeds within fruits (Bernasconi, 2003; Bernasconi et al., 2004). Theory suggests that clutches sired by multiple fathers will have greater variation in progeny performance, increasing the likelihood that favourable combinations of maternal and paternal genes will be generated (Falconer, 1981; Yasui, 1998; Whittingham and Dunn, 2006). An increase in mate diversity within fruits also decreases genetic relatedness among siblings (Ritland, 1989), potentially intensifying competition among maternal half-sibs (Karron and Marshall, 1990, 1993; Bernasconi, 2003).
Researchers studying vertebrates have suggested that females may engage in multiple mating (polyandry) to ‘hedge’ their bet that their initial mate or mates will provide a sufficient number of high-quality gametes (Double and Cockburn, 2000; Fox and Rauter, 2003; Whittingham et al., 2010). Could such a strategy occur in flowering plants? In fruits of Mimulus ringens, outcross seeds are sired by one to eight pollen donors (Mitchell et al., 2005). Karron et al. (2006) demonstrated that sequential pollinator probes to individual flowers significantly enhance mate diversity within fruits (Fig. 4). If plants can regulate the timing of stigmatic receptivity, stigma closure or floral longevity, they could potentially influence mate diversity within fruits (Bernasconi, 2003; Lankinen and Madjidian, 2011). More research is needed to understand how mate diversity varies within and among populations, the factors regulating mate diversity, and the fitness consequences of variation in mate diversity in natural populations.
Fig. 4.
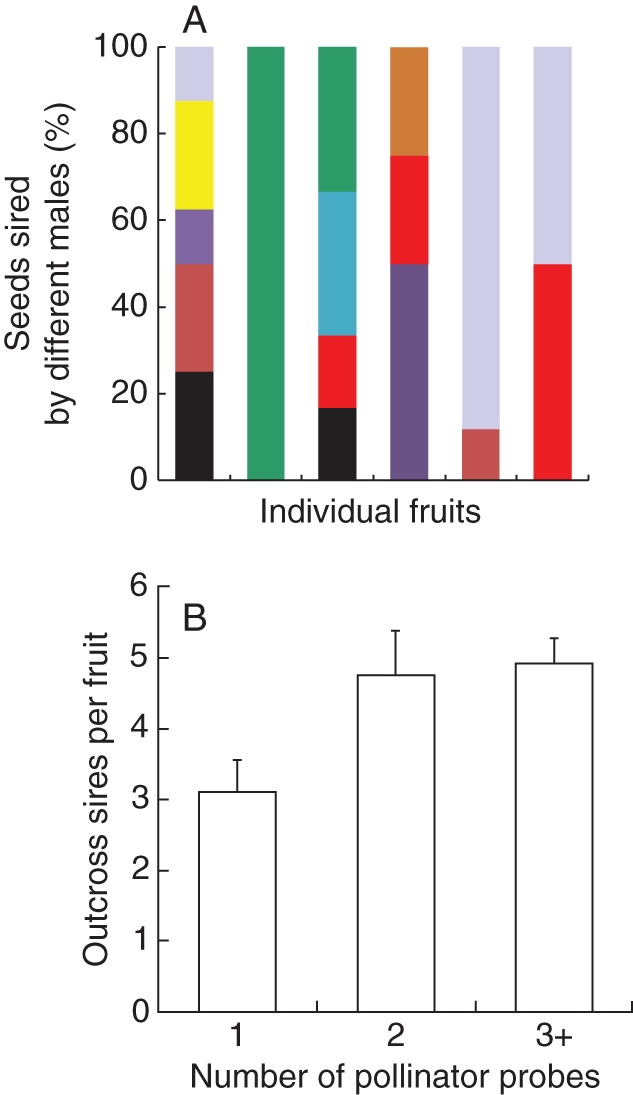
(A) Outcross sire profile for six Mimulus ringens flowers that received a single visit. Colours indicate sires of the seeds. (B) An increase in the number of visits to individual Mimulus ringens flowers increases sires per fruit (P < 0·01). Modified from Karron et al. (2006).
EVOLUTIONARY DYNAMICS OF SEXUAL POLYMORPHISMS
Studies of sexual polymorphisms, such as separate sexes and heterostyly, can provide important insights into the evolution of floral form and function. Gender and stylar polymorphisms have each evolved multiple times across the Angiosperms, and ratios of sexual morphs often vary widely within and among species (Figs 5 and 6). Thus, these polymorphisms provide natural experiments for studying how variation in sex-organ morphology influences pollinator visitation, mating success and sex-specific fitness gain while holding other aspects of the species' biology more-or-less constant. By taking advantage of natural variation within and among populations, recent work has begun to evaluate the relative strength of two contrasting processes that can influence the distribution of sexual morphs within species: natural selection (including frequency-dependent selection) and stochastic processes (such as genetic drift, extinction–colonization and metapopulation dynamics; Pannell and Dorken, 2006; Barrett et al., 2009; McCauley and Bailey, 2009).
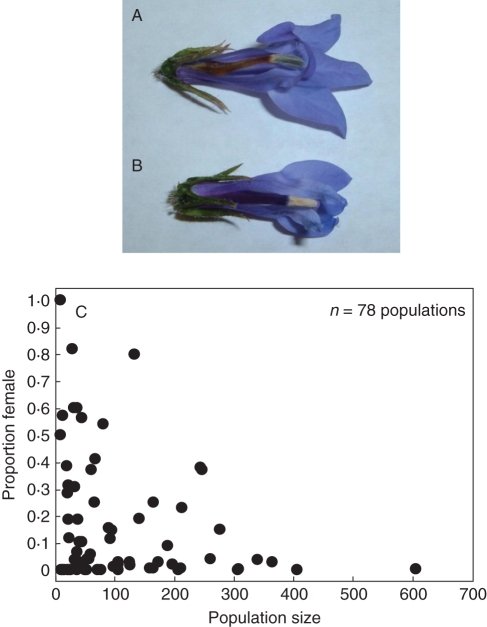
Fig. 5.
Gyndioecious Lobelia siphilitica populations contain (A) hermaphrodites and (B) females. (C) Relationship between population size (number of plants) and sex ratio in natural populations of L. siphilitica in eastern North America. Data are from Caruso and Case (2007) and Proell (2009). Smaller populations exhibit a wider range of female frequencies. Photograph by Maia Bailey.
Fig. 6.
Subdioecy in Wurmbea dioica. Populations contain three sex morphs (left-to-right): hermaphrodite, male and female. See Vaughton and Ramsey (2012). Photograph by Glenda Vaughton and Mike Ramsey.
Disentangling the outcomes of selection from the outcomes of stochastic processes is not an easy task because both processes can yield similar patterns. For example, sexual morph ratios within a population may reflect selection favouring one morph over another, but such a pattern may also result from stochastic changes in the spatial distribution of alleles that underlie or modify sexual phenotypes. Morph ratios are known to vary markedly when population size is small (Fig. 5; Caruso and Case, 2007; Brys et al., 2008), when populations have low levels of neutral genetic diversity (Cuevas et al., 2006; Ness et al., 2010; Li et al., 2012, this issue), or when extinction–colonization dynamics are clearly present (Pannell and Dorken, 2006).
In order to fully evaluate the roles of selective and stochastic processes on sexual-system variation within species, it is important to determine the distribution of morph-determining alleles within and among populations (De Cauwer et al., 2011). If morph determination has a simple genetic basis (such as a single nuclear locus), the sexual morphs themselves are reliable markers for those alleles. Thus, inferences from neutral genetic markers are effective for examining how stochastic processes affect morph ratios (e.g. Hodgins and Barrett, 2007; Ness et al., 2010). However, in many species genetic control of sexual polymorphisms is complex or is poorly understood. This is especially true for some gynodioecious species (Delph et al., 2007). In these latter cases, it may be unreasonable to assume that the distribution of neutral genetic markers clearly represents stochastic forces acting on morph-determining alleles. As described in more detail below, new technological innovations in DNA sequencing may soon help fast track progress in this area.
The role of stochasticity is of particular interest for understanding why and how sexual systems evolve from one state to another (reviewed in Barrett, 2010). Although transitions must certainly involve deterministic forces, such as selection on morph-specific fitness, they may also be facilitated by chance events affecting morph ratios. Stochastic loss of style morphs from populations can alter mating dynamics in heterostylous species, and favour transitions between sexual-system states (Fig. 7; Barrett et al., 2009). Population sex-ratio variation in some gynodioecious species may in fact be largely stochastic, driven by processes such as genetic drift, founder effects, and extinction–colonization dynamics (Bailey and Delph, 2007). However, the role of stochasticity in evolutionary transitions to and from separate sexes may depend strongly on the genetics of sex determination. Although solid empirical data are scarce, stochastic effects are predicted to matter more for species with cytonuclear sex determination compared to those with nuclear sex determination (McCauley and Bailey, 2009; Dufay and Pannell, 2010).
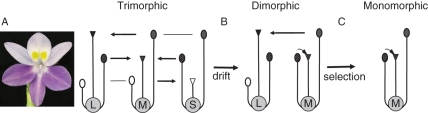
Fig. 7.
Transition from outcrossing to selfing in (A) tristylous Eichhornia paniculata occurs in two steps: (B) genetic drift results in the loss of the S morph, (C) resulting in selection for reproductive assurance in the M morph. Redrawn from Barrett et al. (2009). Photograph by Spencer Barrett.
Just as stochastic forces may influence transitions between sexual system states, phenotypic plasticity in morph-determining traits may also play a critical evolutionary role. Of particular interest is whether sexual plasticity facilitates or constrains sexual-system evolution. Plasticity may facilitate sexual-system transitions such as (a) from combined to separate sexes (Dorken and Michard, 2008; Vaughton and Ramsey, 2012, this issue), and (b) from outcrossing to selfing (Vallejo-Marin and Barrett, 2009; Dart et al., 2012, this issue; Levin, 2012, this issue). By contrast, gender plasticity may constrain transitions to full dioecy (Delph, 2003; Delph and Wolf, 2005; Ehlers and Batillon, 2007; Spigler and Ashman, 2012, this issue).
Whether or not plasticity facilitates or constrains transitions between sexual systems will depend on whether genes underlying plasticity also influence the expression of sexual polymorphisms. Without better information on the genetic basis of both plasticity and sex determination, this remains challenging to address. Studies such as Spigler and Ashman (2011) and Vaughton and Ramsey (2012, this issue) provide important insights into the consequences of sex-differential plasticity in two ways. First, they show that the probability, extent and cost of gender plasticity vary among sexual system states. In subdioecious populations, a lower proportion of hermaphrodites exhibit plasticity, their capacity to adjust their sex allocation is more limited, and plasticity is more costly compared to hermaphrodites in gynodioecious species (Vaughton and Ramsey, 2012, this issue). Second, these studies suggest genetic variation for sex-differential plasticity that should not necessarily constrain selection for increased gender specialization. However, observed plasticity itself may reflect selection to stabilize intermediate sexual systems.
Much remains to be learned about how particular types of sex determination influence sexual-system transitions. Additional insights may be gained by assessing the variability of sexual systems among close relatives. Comparative studies of sexual-system evolution within clades reveal important patterns in traits associated with sexual systems. Phylogenetic studies also provide insights concerning the order in which these traits arise (e.g. Alonso and Herrera, 2011; Ferrero et al., 2012, this issue). However, we suggest that sexual-system studies within species should also be viewed in the broader context of the evolutionary lability of sexual systems within clades. Frequently-studied gynodioecious taxa include those that are closely related to dioecious species (e.g. Fragaria, Silene, Wurmbea), as well as taxa that have no documented dioecy either within the same genus (e.g. Beta, Thymus, Daphne, Kallstroemia) or the same family (e.g. Geranium, Nemophila, Plantago; http://www.umsl.edu/~renners/Dioecy%20table%20www.pdf). Gynodioecious species may have no close dioecious relatives because they have little propensity to evolve dioecy. Thus, categorizing species based on this propensity may help to identify traits that permit or facilitate the full transition to dioecy versus traits that may constrain it.
To illustrate this point, we have reviewed data presented by Dufay and Billard (2012, this issue). In their paper, these authors assessed whether inbreeding avoidance is likely to play an important role in the maintenance of female plants, as commonly predicted for gyndioecious species (Charlesworth, 1999). Dufay and Billard (2012, this issue, table 5) identify seven out of 21 species in their survey where avoidance of inbreeding is likely to contribute to selection favouring females. However, if we look at those seven species based on the sexual systems of their relatives, all but two also show a phylogenetic association with dioecy (i.e. they have dioecious relatives). In contrast, most (11 of 14) of the species for which inbreeding avoidance was deemed unlikely to be important lack dioecious relatives. Thus, a more precise inference about the variable role of inbreeding avoidance in female advantage may be possible. Inbreeding avoidance may maintain females in gynodioecious species in clades where transitions to full dioecy have occurred. By contrast, inbreeding avoidance may be a less important component of female advantage in clades that have evolved gynodioecy but not dioecy.
AVENUES FOR FUTURE RESEARCH
The role of pollinators in the mating system
Studies of mating system patterns seldom incorporate the dynamics of the pollination process. Therefore mating system mechanisms are often divorced from mating system outcomes (Harder and Barrett, 1996). For example, few studies can directly link pollinator movements to the realized mating system of flowers visited by those pollinators (Brunet and Sweet, 2006; Karron et al., 2004; 2009; Karron and Mitchell, 2012, this issue). Yet a synthetic approach is critical to understanding fundamental questions such as: how would the loss or decline of the primary pollinator of a species influence outcrossing rates, mate diversity and patterns of pollen-mediated gene dispersal? How do reductions in pollinator visitation rates and S-allele diversity interact in effects on reproductive success (Young et al., 2012, this issue)? For plants with sexual polymorphisms, how does pollinator behaviour vary among morphs, and how does this affect the mating system and the relative success of different sexual types (Ashman, 2000; Case and Ashman, 2009)? How does sharing pollinators with co-occurring plant species affect the mating system (Bell et al., 2005; Moeller, 2006)? Understanding and answering such questions will require thorough studies of pollinator visitation patterns and precise genetic estimation of the mating system, and will provide more comprehensive insights into the evolution of plant reproduction.
Experimental studies of plasticity in selfing rates and sexual polymorphisms
Traits such as herkogamy, floral display size, self-fertility and gender expression often show striking spatial and temporal variation. Yet surprisingly few experimental studies have quantified the effects of environmental variation on the expression of mating-system traits. Additional research on phenotypic plasticity will be particularly helpful for understanding the origin and maintenance of mating-system variation. For example, does plasticity of mating-system traits differ among sites, and is this due to environmental factors such as soil moisture, herbivory and/or pollinator availability (Ashman 2006; Kameyama and Kudo, 2009; Ivey and Carr, 2012, this issue)? Does plasticity of mating-system traits vary across a species' range (Levin, 2012, this issue)? Do inbreeding species exhibit higher levels of phenotypic plasticity than outcrossing species (Bradshaw, 1965; Ivey and Carr, 2012, this issue)? Does the expression of gender plasticity vary with environmental context, and how would such environmental dependence affect the evolution of sexual polymorphisms (Vaughton and Ramsey, 2012, this issue; Spigler and Ashman, 2012, this issue)?
Addressing these questions effectively will require innovative experimental designs and strategic choice of study systems. Nonetheless, given that trait variation underpins adaptive evolution, identifying the causes of variation in mating-system traits is crucial for improving our understanding of mating-system evolution.
The future of plant mating system studies in the era of next-generation sequencing
A generation ago research on plant mating systems blossomed with the widespread availability of co-dominant genetic markers (Brown and Allard, 1970), facilitating studies of outcrossing rates and patterns of paternity. Today, plant evolutionary biologists are adapting to another revolution in molecular technology, next-generation sequencing (NGS), which again opens up fresh areas of inquiry. For example: does the genetic composition of stigmatic pollen loads differ from the genetic composition of progeny within fruits? Do subtle differences in the floral morphology of pollen donors lead to spatial heterogeneity in placement of pollen on pollinators? Can we assess frequency-dependent changes in self-incompatibility alleles in natural populations? By taking advantage of current developments in genome sequencing technology we may now be able to address these and other previously intractable questions.
With next-generation sequencing technologies, researchers are increasingly able to genotype hundreds of markers within populations of non-model organisms, and probe the genetic architecture of population and individual traits critical to answering important questions about plant mating systems. The benefits of NGS are already being seen across a wide range of fields including genomics (Hawkins et al., 2010), genetics (Metzker, 2010), human disease (Day-Williams and Zeggini, 2011), and crop genetics and breeding (Varshney et al., 2009). There is much excitement about the potential for NGS to benefit fields closely allied to plant mating systems, including genomic ecology and evolutionary biology (Hudson, 2008), molecular ecology (Ekblom and Galindo, 2011) and conservation genetics (Allendorf et al., 2010; Ouborg et al., 2010). What opportunities might NGS offer plant mating system studies?
Rapid cost-effective marker development for mating system and paternity analysis
One far-reaching benefit of NGS that is already being realised across multiple fields is rapid, cost-effective, genome-wide discovery of variable genetic markers such as microsatellites (Dalca and Brudno, 2010; Davey et al., 2011). Gardner et al. (2011) offer a helpful procedural guide for ecologists planning to use NGS for marker development. As the technology improves further, the simultaneous genotyping of large panels of microsatellites across multiple individuals will be possible in any species for a fraction of current costs. Thus NGS will enable for the first time cost-effective paternity analysis in large natural populations. This capability could even enable determination of pollen donor representation in the pollen load on a single stigma. Comparison to realized paternity for entire seed families could lead to exciting insights into pollen competition and reproductive incompatibilities.
Contributions of NGS for phylogenetic analysis
The mapping of mating system traits onto a robust phylogeny can reveal important evolutionary insights. For example, Ferrero et al. (2012, this issue) discovered phylogenetic evidence for the independent evolution of heterostyly and incompatibility in the Boraginaceae, contradicting the expectation of strong linkage between these traits. Unfortunately, a lack of phylogenetically informative markers often prevents the construction of robust phylogenies, the first step in this process. This is particularly true in recently evolved groups that may offer the most potential for exploring dynamic evolutionary processes (Fig. 8; Hodges and Derieg, 2009; Peakall et al., 2010).
Fig. 8.

The rewardless orchid Chiloglottis trapeziformis sexually attracts its specific pollinator, males of the wasp species Neozeleboria cryptoides, by a novel semiochemical identical to the sex pheromone of the female wasp. Closely related species of Chiloglottis use different chemical variants to attract their own specific male pollinators, suggesting chemical changes may trigger speciation. Next-generation sequencing is now being employed to fast track the discovery of the genes involved in speciation. Photograph by Rod Peakall.
NGS can assist phylogenetic analysis in at least two ways. First, rapid, low-cost phylogenetically informative genetic markers can be found via transcriptome sequencing that targets coding genes (Ekblom and Galindo, 2011). Genome-wide phylogenetic analysis can be achieved using restriction-site associated DNA sequencing (RAD-Seq) that offers the potential for detecting 1000s of polymorphisms simultaneously across multiple individuals in a single NGS run (Davey and Blaxter, 2011; Davey et al., 2011).
Candidate gene discovery
Beyond marker development per se, NGS offers exciting new opportunities for fast-tracking candidate gene discovery (Bräutigam and Gowik, 2010). While the genetic bases of self-incompatibility (SI) mechanisms and the loss of SI are increasingly well understood in model systems such as Arabidopsis (see Shimizu et al., 2011, for review), NGS may open the door for rapid progress in candidate SI gene discovery in non-model systems. Similarly, we anticipate NGS will facilitate progress on the genetic basis of sex determination in plants, which is central to our understanding of the evolution of separate sexes. Additionally, identifying the loci underlying variation in mating system traits is becoming increasingly accessible outside model systems through developments in NGS. Restriction-site associated DNA sequencing techniques can generate a wealth of data across the entire genome for a sample of individuals in a population. When individual sequences within the population sample are grouped by some measured phenotype, loci linked to the measured trait can be identified (Davey and Blaxter, 2011). Theory for marker-based inferences of the heritability of quantitative traits is already well developed (Ritland, 1996) and, in conjunction with the developing measures of relatedness for population data gleaned from massive batteries of markers (Ritland, 2011; Browning and Browning, 2010), the underlying genetic architecture of floral traits is now within reach.
Rapid chloroplast genome sequencing
With next-generation sequencing capability the entire chloroplast genome is becoming an increasingly accessible tool for use in both population and community studies. For example, Doorduin et al. (2011) reported the complete chloroplast genome sequence of 17 individuals within a single pest plant species, Jacobaea vulgaris. Although only recently recognized for its potential to reveal genetic variation within species (see Ebert and Peakall, 2009, for review), when combined with predominantly uniparental transmission, genetic variation at the chloroplast is ideally suited for teasing apart pollen and seed-mediated gene flow. Studies that make use of individual levels of cpDNA variation will also shed new light on patterns of both maternity and paternity in dispersed seeds and juvenile plants in natural populations.
Metzker (2010) suggests that the potential applications of NGS are primarily limited by ‘one's imagination’, and there can be little doubt that NGS offers powerful and exciting new opportunities for advancing the field of plant mating systems. Nonetheless, NGS is not a panacea, nor is it a substitute for employing simple, yet elegant experimental designs, or a justification for abandoning strategically chosen ‘model’ systems. Indeed a danger of this new revolution is that we lose sight of the important questions in biology (Tautz et al., 2010), perhaps even drowning in the gigabytes of data that will emerge from a single run! Finally, notwithstanding the promise of NGS, there remain many outstanding questions about plant mating systems that can be investigated effectively without the need to conduct genetic or molecular analysis at all, as many studies in this Special Issue demonstrate.
CONCLUSIONS
The remarkable diversity of plant mating systems provides unique opportunities for exploring the dynamics of evolutionary processes. Armed with a strong and growing theoretical basis for predictions, powerful new molecular techniques and creative field experiments, researchers are poised to make many exciting breakthroughs in the coming years. The papers in this Special Issue provide inspiring examples of recent discoveries, and glimpses of exciting developments yet to come.
ACKNOWLEDGEMENTS
We are grateful to Annals of Botany Editors Don Levin, Pat Heslop-Harrison and David Frost for their extraordinary efforts in producing this Special Issue. We would also like to thank each of the authors for contributing stimulating papers on plant mating system evolution. Finally, we acknowledge with appreciation nearly 30 reviewers who provided exceptionally insightful comments on the manuscripts.
LITERATURE CITED
- Allendorf FW, Hohenlohe PA, Luikart G. Genomics and the future of conservation genetics. Nature Reviews Genetics. 2010;11:697–709. doi: 10.1038/nrg2844. [DOI] [PubMed] [Google Scholar]
- Alonso C, Herrera C. Back-and-forth hermaphroditism: phylogenetic context of the reproductive system evolution in subdioecious Daphne laureola. Evolution. 2011;65:1680–1692. doi: 10.1111/j.1558-5646.2011.01246.x. [DOI] [PubMed] [Google Scholar]
- Armbruster P, Reed DH. Inbreeding depression in benign and stressful environments. Heredity. 2005;95:235–242. doi: 10.1038/sj.hdy.6800721. [DOI] [PubMed] [Google Scholar]
- Ashman TL. Pollinator selectivity and its implications for the evolution of dioecy and sexual dimorphism. Ecology. 2000;81:277–2591. [Google Scholar]
- Ashman TL. The evolution of separate sexes: a focus on the ecological context. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. New York: Oxford University Press; 2006. pp. 204–222. [Google Scholar]
- Bailey MF, Delph LF. A field guide to models of sex-ratio evolution in gynodioecious species. Oikos. 2007;116:1609–1617. [Google Scholar]
- Barrett SCH. Darwin's legacy: the forms, function, and sexual diversity of flowers. Philosophical Transactions of the Royal Society of London, Series B. 2010;365:351–368. doi: 10.1098/rstb.2009.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD. Ecology and evolution of plant mating. Trends in Ecology and Evolution. 1996;11:73–79. doi: 10.1016/0169-5347(96)81046-9. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Harder LD, Worley AC. The comparative biology of pollination and mating in flowering plants. Philosophical Transactions of the Royal Society of London, Series B. 1996;351:1271–1280. [Google Scholar]
- Barrett SCH, Ness RW, Vallejo-Marín M. Evolutionary pathways to self-fertilization in a tristylous plant species. New Phytologist. 2009;183:546–556. doi: 10.1111/j.1469-8137.2009.02937.x. [DOI] [PubMed] [Google Scholar]
- Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Bell JM, Karron JD, Mitchell RJ. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology. 2005;86:776–785. [Google Scholar]
- Bernasconi G. Seed paternity in flowering plants: an evolutionary perspective. Perspectives in Plant Ecology, Evolution and Systematics. 2003;6:149–158. [Google Scholar]
- Bernasconi G, Ashman T-L, Birkhead TR, et al. Evolutionary ecology of the prezygotic stage. Science. 2004;303:971–975. doi: 10.1126/science.1092180. [DOI] [PubMed] [Google Scholar]
- Bodbyl-Roels SA, Kelly JK. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution. 2011;65:2541–2552. doi: 10.1111/j.1558-5646.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Bräutigam A, Gowik U. What can next generation sequencing do for you? Next generation sequencing as a valuable tool in plant research. Plant Biology. 2010;12:831–841. doi: 10.1111/j.1438-8677.2010.00373.x. [DOI] [PubMed] [Google Scholar]
- Brock MT, Weinig C. Plasticity and environment-specific covariances: an investigation of floral-vegetative and within-flower correlations. Evolution. 2007;61:2913–2924. doi: 10.1111/j.1558-5646.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- Brown A, Allard R. Estimation of the mating system in open pollinated maize populations using isozyme polymorphisms. Genetics. 1970;66:133–145. doi: 10.1093/genetics/66.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AHD. Genetic characterization of plant mating systems. In: Brown AHD, Clegg MT, Kahler AL, Weir BS, editors. Plant population genetics, breeding, and genetic resources. Sunderland, MA: Sinauer Associates; 1990. [Google Scholar]
- Browning SR, Browning BL. High-resolution detection of identity by descent in unrelated individuals. The American Journal of Human Genetics. 2010;86:526–539. doi: 10.1016/j.ajhg.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet J, Sweet HR. Impact of insect pollinator group and floral display size on outcrossing rate. Evolution. 2006;60:234–246. [PubMed] [Google Scholar]
- Brys R, Jacquemyn H, Beeckman T. Morph-ratio variation, population size, and female reproductive success in distylous Pulmonaria officinalis (Boraginaceae) Journal of Evolutionary Biology. 2008;21:1281–1289. doi: 10.1111/j.1420-9101.2008.01569.x. [DOI] [PubMed] [Google Scholar]
- Busch JW, Delph LF. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Annals of Botany. 2012;109:553–562. doi: 10.1093/aob/mcr219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DR, Waser NM, Price MV. Mechanisms of hummingbird-mediated selection for flower width in Ipomopsis aggregata. Ecology. 1996;77:1463–1472. [Google Scholar]
- Caruso CM, Case AL. Sex ratio variation in gynodioecious Lobelia siphilitica: effects of population size and geographic location. Journal of Evolutionary Biology. 2007;20:1396–1405. doi: 10.1111/j.1420-9101.2007.01361.x. [DOI] [PubMed] [Google Scholar]
- Caruso CM, Remington DLD, Ostergren KE. Variation in resource limitation of plant reproduction influences natural selection on floral traits of Asclepias syriaca. Oecologia. 2005;146:68–76. doi: 10.1007/s00442-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Case AL, Ashman T-L. Resources and pollinators contribute to population sex-ratio bias and pollen limitation in Fragaria virginiana. (Rosaceae) Oikos. 2009;118:1250–1260. [Google Scholar]
- Charlesworth D. Theories of the evolution of dioecy. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag; 1999. pp. 33–59. [Google Scholar]
- Cheptou PO, Donohue K. Environment-dependent inbreeding depression: its ecological and evolutionary significance. New Phytologist. 2011;189:395–407. doi: 10.1111/j.1469-8137.2010.03541.x. [DOI] [PubMed] [Google Scholar]
- Cheptou PO. Clarifying Baker's Law. Annals of Botany. 2012;109:633–641. doi: 10.1093/aob/mcr127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas E, Arias DM, Domínguez CA, Castillo RA, Molina-Freaner F. The genetic structure of the gynodioecious Kallstroemia grandiflora (Zygophyllaceae): the role of male sterility and colonization history. Heredity. 2006;97:269–274. doi: 10.1038/sj.hdy.6800849. [DOI] [PubMed] [Google Scholar]
- Dalca AV, Brudno M. Genome variation discovery with high-throughput sequencing data. Briefings in Bioinformatics. 2010;11:3–14. doi: 10.1093/bib/bbp058. [DOI] [PubMed] [Google Scholar]
- Dart SR, Samis KE, Austen E, Eckert CG. Broad geographic covariation between floral traits and the mating system in Camissoniopsis cheiranthifolia (Onagraceae): multiple stable mixed mating systems across the species' range? Annals of Botany. 2012;109:599–611. doi: 10.1093/aob/mcr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The effects of cross and self-fertilization in the vegetable kingdom. London: Murray; 1876. [Google Scholar]
- Darwin C. The different forms of flowers on plants of the same species. New York: D. Appleton & Co; 1877. [Google Scholar]
- Davey JW, Blaxter ML. RADSeq: next-generation population genetics. Briefings in Functional Genomics. 2011;9:416–423. doi: 10.1093/bfgp/elq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey JW, Hohenlohe PA, Etter PD, Boone JQ, Catchen JM, Blaxter ML. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nature Reviews Genetics. 2011;12:499–510. doi: 10.1038/nrg3012. [DOI] [PubMed] [Google Scholar]
- Day-Williams AG, Zeggini E. The effect of next-generation sequencing technology on complex trait research. European Journal of Clinical Investigation. 2011;41:561–567. doi: 10.1111/j.1365-2362.2010.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cauwer I, Arnaud J-F, Corseaux A, Dufay M. Sex-specific fitness variation in gynodioecious Beta vulgaris ssp. maritima: do empirical observations fit theoretical predictions? Journal of Evolutionary Biology. 2011;24:2456–2472. doi: 10.1111/j.1420-9101.2011.02380.x. [DOI] [PubMed] [Google Scholar]
- Delph LF. Sexual dimorphism in gender plasticity and its consequences for breeding system evolution. Evolution & Development. 2003;5:34–39. doi: 10.1046/j.1525-142x.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- Delph LF, Wolf DE. Evolutionary consequences of gender plasticity in genetically dimorphic breeding systems. New Phytologist. 2005;166:119–128. doi: 10.1111/j.1469-8137.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- Delph LF, Touzet P, Bailey MF. Merging theory and mechanism in studies of gynodioecy. Trends in Ecology and Evolution. 2007;22:17–24. doi: 10.1016/j.tree.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Doorduin L, Gravendeel B, Lammers Y, Ariyurek Y, Chin-A-Woeng T, Vrieling K. The complete chloroplast genome of 17 individuals of pest species Jacobaea vulgaris: SNPs, microsatellites and barcoding markers for population and phylogenetic studies. DNA Research. 2011;18:93–105. doi: 10.1093/dnares/dsr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorken ME, Mitchard ETA. Phenotypic plasticity of hermaphrodite sex allocation promotes the evolution of separate sexes: an experimental test of the sex-differential plasticity hypothesis using Sagittaria latifolia (Alismataceae) Evolution. 2008;62:971–978. doi: 10.1111/j.1558-5646.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- Double M, Cockburn A. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proceedings of the Royal Society B: Biological Sciences. 2000;267:465. doi: 10.1098/rspb.2000.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudash MR, Carr DE, Fenster CB. Five generations of enforced selfing and outcrossing in Mimulus guttatus: inbreeding depression variation at the population and family level. Evolution. 1997;51:54–65. doi: 10.1111/j.1558-5646.1997.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Dufay M, Billard E. How much better are females? The occurrence of female advantage, its proximal causes and its variation within and among gynodioecious species. Annals of Botany. 2012;109:505–519. doi: 10.1093/aob/mcr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufay M, Pannell JR. The effect of pollen versus seed flow on the maintenance of nuclear-cytoplasmic gynodioecy. Evolution. 2010;64:772–784. doi: 10.1111/j.1558-5646.2009.00847.x. [DOI] [PubMed] [Google Scholar]
- Ebert D, Peakall R. Chloroplast simple sequence repeats (cpSSRs): technical resources and recommendations for expanding cpSSR discovery and applications to a wide array of plant species. Molecular Ecology Resources. 2009;9:673–690. doi: 10.1111/j.1755-0998.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- Eckert CG. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology. 2000;81:532–542. [Google Scholar]
- Eckert CG, Ozimec B, Herlihy CR, Griffin CA, Routley MB. Floral morphology mediates temporal variation in the mating system of a self-compatible plant. Ecology. 2009;90:1540–1548. doi: 10.1890/08-1063.1. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Kalisz S, Geber MA, et al. Plant mating systems in a changing world. Trends in Ecology and Evolution. 2010;25:35–43. doi: 10.1016/j.tree.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Ehlers BK, Bataillon T. ‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New Phytologist. 2007;174:194–211. doi: 10.1111/j.1469-8137.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- Ekblom R, Galindo J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity. 2011;107:1–15. doi: 10.1038/hdy.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elle E, Hare JD. Environmentally induced variation in floral traits affects the mating system in Datura wrightii. Functional Ecology. 2002;16:79–88. [Google Scholar]
- Falconer DS. Introduction to quantitative genetics. New York: Longman Inc; 1981. [Google Scholar]
- Fenster CB, Ritland K. Evidence for natural selection on mating system in Mimulus (Scrophulariaceae) International Journal of Plant Sciences. 1994;155:588–596. [Google Scholar]
- Ferrero V, Arroyo J, Castro S, Navarro L. Unusual heterostyly: style dimorphism and self-incompatibility are not tightly associated in Lithodora and Glandora (Boraginaceae) Annals of Botany. 2012;109:655–665. doi: 10.1093/aob/mcr222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetscher AE. Resolution of male–female conflict in an hermaphroditic flower. Proceedings of the Royal Society of London B: Biological Sciences. 2001;268:525–529. doi: 10.1098/rspb.2000.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Average excess and average effect of a gene substitution. Annals of Human Genetics. 1941;11:53–63. [Google Scholar]
- Fox CW, Rauter CM. Bet-hedging and the evolution of multiple mating. Evolutionary Ecology Research. 2003;5:273–286. [Google Scholar]
- Gardner MG, Fitch AJ, Bertozzi T, Lowe AJ. Rise of the machines – recommendations for ecologists when using next generation sequencing for microsatellite development. Molecular Ecology Resources. 2011;11:1093–1101. doi: 10.1111/j.1755-0998.2011.03037.x. [DOI] [PubMed] [Google Scholar]
- Goldberg EE, Kohn JR, Lande R, Robertson KA, Smith SA, Igic B. Species selection maintains self-incompatibility. Science. 2010;330:493–495. doi: 10.1126/science.1194513. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology and Systematics. 2005;36:47–49. [Google Scholar]
- Griffin CAM, Eckert CG. Experimental analysis of biparental inbreeding in a self-fertilizing plant. Evolution. 2003;57:1513–1519. doi: 10.1111/j.0014-3820.2003.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Hamrick JL, Godt MJ. Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS, editors. Plant population genetics, breeding, and genetic resources. Sunderland, MA: Sinauer Associates; 1989. pp. 43–63. [Google Scholar]
- Harder LD, Barrett SCH. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, editors. Floral biology. New York: Chapman and Hall; 1996. [Google Scholar]
- Harder LD, Barrett SCH. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. [Google Scholar]
- Hawkins RD, Hon GC, Ren B. Next-generation genomics: an integrative approach. Nature Reviews Genetics. 2010;11:476–486. doi: 10.1038/nrg2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy CR, Delph LF. Selection lines of Silene latifolia (Caryophyllaceae) differ in how stress affects pollen production. International Journal of Plant Sciences. 2009;170:1103–1108. [Google Scholar]
- Herlihy CR, Eckert CG. Experimental dissection of inbreeding and its adaptive significance in a flowering plant, Aquilegia canadensis (Ranunculaceae) Evolution. 2004;58:2693–2703. doi: 10.1111/j.0014-3820.2004.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Hodges SA, Derieg NJ. Adaptive radiations: from field to genomic studies. Proceedings of the National Academy of Sciences. 2009;106:9947–9954. doi: 10.1073/pnas.0901594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins KA, Barrett SCH. Population structure and genetic diversity in tristylous Narcissus triandrus: insights from microsatellite and chloroplast DNA variation. Molecular Ecology. 2007;16:2317–2332. doi: 10.1111/j.1365-294X.2007.03314.x. [DOI] [PubMed] [Google Scholar]
- Holsinger KE, Feldman MW, Christiansen FB. The evolution of self-fertilization in plants: a population genetic model. American Naturalist. 1984;124:446–453. [Google Scholar]
- Holtsford TP, Ellstrand NC. Genetic and environmental variation in floral traits affecting outcrossing rate in Clarkia tembloriensis (Onagraceae) Evolution. 1992;46:216–225. doi: 10.1111/j.1558-5646.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Hudson ME. Sequencing breakthroughs for genomic ecology and evolutionary biology. Molecular Ecology Resources. 2008;8:3–17. doi: 10.1111/j.1471-8286.2007.02019.x. [DOI] [PubMed] [Google Scholar]
- Ivey CT, Carr DE, Eubanks MD. Effects of inbreeding in Mimulus guttatus on tolerance to herbivory in natural environments. Ecology. 2004;85:567–574. [Google Scholar]
- Ivey CT, Carr DE. Effects of herbivory and inbreeding on the pollinators and mating system of Mimulus guttatus (Phrymaceae) American Journal of Botany. 2005;92:1641–1649. doi: 10.3732/ajb.92.10.1641. [DOI] [PubMed] [Google Scholar]
- Ivey CT, Carr DE. Tests for the joint evolution of mating system and drought escape in Mimulus. Annals of Botany. 2012;109:583–598. doi: 10.1093/aob/mcr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Randle A, Chaiffetz D, Faigeles M, Butera A, Beight C. Dichogamy correlates with outcrossing rate and defines the selfing syndrome in the mixed-mating genus Collinsia. Annals of Botany. 2012;109:571–582. doi: 10.1093/aob/mcr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama Y, Kudo G. Flowering phenology influences seed production and outcrossing rate in populations of an alpine snowbed shrub, Phyllodoce aleutica: effects of pollinators and self-incompatibility. Annals of Botany. 2009;103:1385–1394. doi: 10.1093/aob/mcp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Marshall DL. Fitness consequences of multiple paternity in wild radish. Raphanus sativus Evolution. 1990;44:260–268. doi: 10.1111/j.1558-5646.1990.tb05196.x. [DOI] [PubMed] [Google Scholar]
- Karron JD, Marshall DL. Effects of environmental variation on fitness of singly and multiply sired progenies of Raphanus sativus (Brassicaceae) American Journal of Botany. 1993;80:1407–1412. [Google Scholar]
- Karron JD, Mitchell RJ. Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals of Botany. 2012;109:563–570. doi: 10.1093/aob/mcr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Jackson RT, Thumser NN, Schlicht SL. Outcrossing rates of individual Mimulus ringens genets are correlated with anther-stigma separation. Heredity. 1997;79:365–370. [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. The influence of floral display size on selfing rates in Mimulus ringens. Heredity. 2004;92:242–248. doi: 10.1038/sj.hdy.6800402. [DOI] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ, Bell JM. Multiple pollinator visits to Mimulus ringens (Phrymaceae) flowers increase mate number and seed set within fruits. American Journal of Botany. 2006;93:1306–1312. doi: 10.3732/ajb.93.9.1306. [DOI] [PubMed] [Google Scholar]
- Karron JD, Holmquist KG, Flanagan RJ, Mitchell RJ. Pollinator visitation patterns strongly influence among-flower variation in selfing rate. Annals of Botany. 2009;103:1379–1383. doi: 10.1093/aob/mcp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Willis J. A manipulative experiment to estimate biparental inbreeding in monkeyflowers. International Journal of Plant Sciences. 2002;163:575–579. [Google Scholar]
- Kennedy BF, Elle E. The reproductive assurance benefit of selfing: importance of flower size and population size. Oecologia. 2008;155:469–477. doi: 10.1007/s00442-007-0924-7. [DOI] [PubMed] [Google Scholar]
- van Kleunen M, Ritland K. Predicting evolution of floral traits associated with mating system in a natural plant population. Journal of Evolutionary Biology. 2004;17:1389–1399. doi: 10.1111/j.1420-9101.2004.00787.x. [DOI] [PubMed] [Google Scholar]
- Lankinen Ã, Madjidian JA. Enhancing pollen competition by delaying stigma receptivity: pollen deposition schedules affect siring ability, paternal diversity, and seed production in Collinsia heterophylla (Plantaginaceae) American Journal of Botany. 2011;98:1191–1200. doi: 10.3732/ajb.1000510. [DOI] [PubMed] [Google Scholar]
- Lau JA, Miller RE, Rausher MD. Selection through male function favors smaller floral display size in the common morning glory Ipomea purpurea (Convolvulaceae) American Naturalist. 2008;172:63–74. doi: 10.1086/588080. [DOI] [PubMed] [Google Scholar]
- Leclerc-Potvin C, Ritland K. Modes of self-fertilization in Mimulus guttatus (Scrophulariaceae): a field experiment. American Journal of Botany. 1994;81:199–205. [Google Scholar]
- Levin DA. Environment-enhanced self-fertilitzation: implications for niche shifts in adjacent populations. Journal of Ecology. 2010;98:1276–1283. [Google Scholar]
- Levin DA. Mating system shifts on the trailing edge. Annals of Botany. 2012;109:613–620. doi: 10.1093/aob/mcr159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Koski MH, Ashman T-L. Functional characterization of gynodioecy in Fragaria vesca ssp. bracteata (Rosaceae) Annals of Botany. 2012;109:545–552. doi: 10.1093/aob/mcr279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences. 1992;153:370–380. [Google Scholar]
- Lloyd DG, Barrett SCH. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman & Hall; 1996. [Google Scholar]
- Mazer SJ, Paz H, Bell MD. Life history, floral development, and mating system in Clarkia xantiana (Onagraceae): do foral and whole-plant rates of development evolve independently? American Journal of Botany. 2004;91:2041–2050. doi: 10.3732/ajb.91.12.2041. [DOI] [PubMed] [Google Scholar]
- Mazer SJ, Dudley LS, Hove AA, Emms SK, Verhoeven AS. Physiological performance in Clarkia sister taxa with contrasting mating systems: do early-flowering autogamous taxa avoid water stress relative to their pollinator-dependent counterparts? International Journal of Plant Sciences. 2010;171:1029–1047. [Google Scholar]
- McCauley DE, Bailey MF. Recent advances in the study of gynodioecy: the interface of theory and empiricism. Annals of Botany. 2009;104:611–620. doi: 10.1093/aob/mcp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker ML. Sequencing technologies – the next generation. Nature Reviews Genetics. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- Mitchell R, Karron JD, Holmquist KG, Bell JM. Patterns of multiple paternity in fruits of Mimulus ringens (Phrymaceae) American Journal of Botany. 2005;92:885–890. doi: 10.3732/ajb.92.5.885. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD. New frontiers in competition for pollination. Annals of Botany. 2009;103:1403–1413. doi: 10.1093/aob/mcp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller DA. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology. 2006;87:1510–1522. doi: 10.1890/0012-9658(2006)87[1510:gsopcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morgan MT, Wilson WG, Knight TM. Plant population dynamics, pollinator foraging, and the selection of self-fertilization. American Naturalist. 2005;166:169–183. doi: 10.1086/431317. [DOI] [PubMed] [Google Scholar]
- Murren CJ, Dudash MR. Variation in inbreeding depression and plasticity across native and non-native field environments. Annals of Botany. 2012;109:621–632. doi: 10.1093/aob/mcr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutikainen P, Delph LF. Effects of herbivory on male reproductive success in plants. Oikos. 1996;75:353–358. [Google Scholar]
- Nason JD, Ellstrand NC. Lifetime estimates of biparental inbreeding depression in the self incompatible annual plant Raphanus sativus. Evolution. 1995;49:307–316. doi: 10.1111/j.1558-5646.1995.tb02243.x. [DOI] [PubMed] [Google Scholar]
- Ness RW, Wright SI, Barrett SCH. Mating-system variation, demographic history, and patterns of nucleotide diversity in the tristylous plant Eichhornia paniculata. Genetics. 2010;184:381–392. doi: 10.1534/genetics.109.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornduff R. Reproductive biology in relation to systematics. Taxon. 1969;18:121–133. [Google Scholar]
- Ouborg NJ, Pertoldi C, Loeschcke V, Bijlsma R, Hedrick PW. Conservation genetics in transition to conservation genomics. Trends in Genetics. 2010;26:177–187. doi: 10.1016/j.tig.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Pannell JR, Barrett SCH. Baker's law revisited: reproductive assurance in a metapopulation. Evolution. 1998;52:657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Pannell JR, Dorken ME. Colonisation as a common denominator in plant metapopulations and range expansions: effects on genetic diversity and sexual systems. Landscape Ecology. 2006;21:837–848. [Google Scholar]
- Peakall R, Ebert D, Poldy J, et al. Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchids: implications for pollinator-driven speciation. New Phytologist. 2010;188:437–450. doi: 10.1111/j.1469-8137.2010.03308.x. [DOI] [PubMed] [Google Scholar]
- Proell JM. Kent, OH, USA: Masters Thesis, Kent State University; 2009. Population sex ratio and size affect pollination, reproductive success, and seed germination in gynodioecious Lobelia siphilitica: evidence using experimental populations and microsatellite genotypes. [Google Scholar]
- Quesada M, Bollman K, Stephenson AG. Leaf damage decreases pollen production and hinders pollen performance in Cucurbita texana. Ecology. 1995;76:437–443. [Google Scholar]
- Ritland K. Correlated matings in the partial selfer Mimulus guttatus. Evolution. 1989;43:848–859. doi: 10.1111/j.1558-5646.1989.tb05182.x. [DOI] [PubMed] [Google Scholar]
- Ritland K. A marker-based method for inferences about quantitative inheritance in natural populations. Evolution. 1996;50:1062–1073. doi: 10.1111/j.1558-5646.1996.tb02347.x. [DOI] [PubMed] [Google Scholar]
- Ritland K. Extensions of models for the estimation of mating systems using n independent loci. Heredity. 2002;88:221–228. doi: 10.1038/sj.hdy.6800029. [DOI] [PubMed] [Google Scholar]
- Ritland K. Evolutionary potential in the wild: more than meets the eye. Molecular Ecology. 2011;20:3494–3495. doi: 10.1111/j.1365-294X.2011.05224.x. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Lloyd DG. Self- and cross-fertilization in plants. III. Methods for studying modes and functional aspects of self-fertilization. International Journal of Plant Sciences. 1992;153:381–393. [Google Scholar]
- Shaw DV, Kahler AL, Allard RW. A multilocus estimator of mating system parameters in plant populations. Proceedings of the National Academy of Sciences. 1981;78:1298–1302. doi: 10.1073/pnas.78.2.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu KK, Kudoh H, Kobayashi MJ. Plant sexual reproduction during climate change: gene function in natura studied by ecological and evolutionary systems biology. Annals of Botany. 2011;108:777–787. doi: 10.1093/aob/mcr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A, Lenhard M. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Annals of Botany. 2011;107:1433–1443. doi: 10.1093/aob/mcr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigler RB, Ashman T-L. Sex ratio and subdioecy in Fragaria virginiana: the roles of plasticity and gene flow examined. New Phytologist. 2011;190:1058–1068. doi: 10.1111/j.1469-8137.2011.03657.x. [DOI] [PubMed] [Google Scholar]
- Spigler RB, Ashman T-L. Gynodioecy to dioecy: are we there yet? Annals of Botany. 2012;109:531–543. doi: 10.1093/aob/mcr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. Flowering plants: evolution above the species level. Cambridge, MA: Belknap; 1974. [Google Scholar]
- Steets JA, Hamrick JL, Ashman T-L. Consequences of vegetative herbivory for maintenance of intermediate outcrossing in an annual plant. Ecology. 2006;87:2717–2727. doi: 10.1890/0012-9658(2006)87[2717:covhfm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Steets JA, Wolf DE, Auld JR, Ashman T-L. The role of natural enemies in the evolution and expression of mixed mating in hermaphroditic plants and animals. Evolution. 2007;61:2043–2055. doi: 10.1111/j.1558-5646.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- Takebayashi N, Wolf DE, Delph LF. Effect of variation in herkogamy on outcrossing within a population of Gilia achilleifolia. Heredity. 2006;96:159–165. doi: 10.1038/sj.hdy.6800780. [DOI] [PubMed] [Google Scholar]
- Tautz D, Ellegren H, Weigel D. Next generation molecular ecology. Molecular Ecology. 2010;19:1–3. doi: 10.1111/j.1365-294X.2009.04489.x. [DOI] [PubMed] [Google Scholar]
- Teixeira S, Bernasconi G. High prevalence of multiple paternity within fruits in natural populations of Silene latifolia, as revealed by microsatellite DNA analysis. Molecular Ecology. 2007;16:4370–4379. doi: 10.1111/j.1365-294X.2007.03493.x. [DOI] [PubMed] [Google Scholar]
- Uyenoyama MK. Inbreeding and the cost of meiosis: the evolution of selfing in populations practicing partial biparental inbreeding. Evolution. 1986;40:388–404. doi: 10.1111/j.1558-5646.1986.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Vallejo-Marín M, Barrett SCH. Modification of flower architecture during early stages in the evolution of self-fertilization. Annals of Botany. 2009;103:951–962. doi: 10.1093/aob/mcp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney RK, Nayak SN, May GD, Jackson SA. Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends in Biotechnology. 2009;27:522–530. doi: 10.1016/j.tibtech.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Vaughton G, Ramsey M. Gender plasticity and sexual system stability in Wurmbea. Annals of Botany. 2012;109:521–530. doi: 10.1093/aob/mcr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal C, Anderson B, Barrett SCH. The natural history of pollination and mating in bird-pollinated Babiana (Iridaceae) Annals of Botany. 2012;109:667–679. doi: 10.1093/aob/mcr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller DM. Environmental determinants of outcrossing in Impatiens capensis (Balsaminaceae) Evolution. 1980;34:747–761. doi: 10.1111/j.1558-5646.1980.tb04014.x. [DOI] [PubMed] [Google Scholar]
- Waller DM. The statics and dynamics of mating system evolution. In: Thornhill NW, editor. The natural history of inbreeding and outbreeding. Chicago: University of Chicago Press; 1993. [Google Scholar]
- Waser NM. Population structure, optimal outbreeding, and assortative mating in angiosperms. In: Thornhill NW, editor. The natural history of inbreeding and outbreeding. Chicago: University of Chicago Press; 1993. [Google Scholar]
- Weller SG, Sakai AK. Phylogenetic approaches for the analysis of plant breeding system evolution. Annual Review of Ecology and Systematics. 1999;30:167–199. [Google Scholar]
- Whittingham LA, Dunn PO. Fitness benefits of polyandry for experienced females. Molecular Ecology. 2006;19:2328–2335. doi: 10.1111/j.1365-294X.2010.04640.x. [DOI] [PubMed] [Google Scholar]
- Whittingham LA, Dunn PO, Stapleton MK. Repeatability of extra-pair mating in tree swallows. Molecular Ecology. 2010;15:841–849. doi: 10.1111/j.1365-294X.2006.02808.x. [DOI] [PubMed] [Google Scholar]
- Wyatt R. Phylogenetic aspects of the evolution of self-pollination. In: Gottlieb LD, Jain SK, editors. Plant evolutionary biology. New York: Chapman and Hall; 1988. pp. 109–131. [Google Scholar]
- Yahara T. Graphical analysis of mating system evolution in plants. Evolution. 1992;46:557–561. doi: 10.1111/j.1558-5646.1992.tb02059.x. [DOI] [PubMed] [Google Scholar]
- Yasui Y. The ‘genetic benefits’ of female multiple mating reconsidered. Trends in Ecology and Evolution. 1998;13:246–250. doi: 10.1016/s0169-5347(98)01383-4. [DOI] [PubMed] [Google Scholar]
- Young AG, Broadhurst LM, Thrall PH. Interacting effects of pollen limitation and self-incompatibility on plant reproductive success and population viability. Annals of Botany. 2012;109:643–653. doi: 10.1093/aob/mcr290. [DOI] [PMC free article] [PubMed] [Google Scholar]