Abstract
We investigated alexithymia, a deficit in the ability to identify and describe one’s emotions, in a sample that included patients with neurodegenerative disease and healthy controls. In addition, we investigated the relationship that alexithymia has with behavioral disturbance and with regional gray matter volumes. Alexithymia was examined with the Toronto Alexithymia Scale-20, behavioral disturbance was assessed with the Neuropsychiatric Inventory, and regional gray matter volumes were obtained from structural magnetic resonance images. Group analyses revealed higher levels of alexithymia in patients than controls. Alexithymia scores were positively correlated with behavioral disturbance (apathy and informant distress, in particular) and negatively correlated with the gray matter volume of the right pregenual anterior cingulate cortex, a region of the brain that is thought to play an important role in self and emotion processing.
The term “alexithymia” was originally coined to describe patients with psychosomatic disorders whose symptoms were thought to result from an impoverished emotional life and a restricted verbalization of affect (Sifneos, 1973; Sifneos, Apfel-Savitz, & Frankel, 1977). More recently, alexithymia has been characterized as deficient cognitive processing of emotion that results in diminished subjective feeling states (Taylor, Bagby, & Parker, 1991). Alexithymia is associated with lower quality of life (Joukamaa, Saarijärvi, Muuriaisniemi, & Salokangas, 1996; Mattila et al., 2009) and has been reported in numerous mental disorders (e.g., depression and post-traumatic stress disorder) and physical diseases (e.g., breast cancer and diabetes; Taylor & Bagby, 2004). Alexithymia is more common in men than women (Salminen, Saarijärvi, Aärelä, Toikka, & Kauhanen, 1999), and its prevalence increases across the lifespan with rates of approximately 20 to 30% in older adults (Mattila, Salminen, Nummi, & Joukamaa, 2006). Socioemotional deficits are common in alexithymia and include deficits in emotion recognition (Lane et al., 1996), perspective-taking (Moriguchi et al., 2006), and spontaneous facial affect (McDonald & Prkachin, 1990). We view alexithymia as a form of diminished self-awareness that can create profound difficulties in navigating the social world.
Neuroimaging studies of alexithymia find alterations in frontotemporal brain structures that are important for emotion (Phan, Wager, Taylor, & Liberzon, 2002). The anterior cingulate cortex (ACC), a medial frontal region that plays a prominent role in emotional awareness and experience (Craig, 2002; Lane, Ahern, Schwartz, & Kaszniak, 1997), appears to play a central role in alexithymia. Volumetric analyses have found smaller ACC volumes in individuals with and without alexithymia (Borsci et al., 2009; Gündel et al., 2004; Paradiso, Vaidya, McCormick, Jones, & Robinson, 2008). Smaller volumes in other frontotemporal regions (e.g., middle temporal gyrus, anterior insula, superior temporal sulcus, and orbitofrontal cortex) have also been reported in alexithymia (Borsci et al., 2009; Paradiso et al., 2008). Functional imaging studies also point to important roles for frontotemporal structures in alexithymia, and several studies have found decreased activation of the ACC in individuals with alexithymia (Berthoz et al., 2002; Kano et al., 2003; Karlsson, Näätänen, & Stenman, 2008; Lane et al., 1998).
In neurodegenerative diseases that target frontotemporal brain structures, changes in emotion, self-awareness, and social behavior are common. For example, in the frontotemporal dementia and semantic dementia subtypes of frontotemporal lobar degeneration, socioemotional deficits predominate, appearing quite early in the course of the disease (Caycedo, Miller, Kramer, & Rascovsky, 2009; Grossman, 2002; Rankin, Kramer, Mychack, & Miller, 2003; Seeley et al., 2007). In other diseases (e.g., corticobasal degeneration, progressive supranuclear palsy), socioemotional symptoms may arise as atrophy disrupts frontal-subcortical functioning (Litvan, Cummings, & Mega, 1998). In contrast, in Alzheimer’s disease, anterior regions may be relatively spared early on, and preservation of socioemotional functioning is observed (Bathgate, Snowden, Varma, Blackshaw, & Neary, 2001; Goodkind, Gyurak, McCarthy, Miller, & Levenson, 2010; Rankin et al., 2003; Sturm et al., 2010). Although a wide range of specific socioemotional symptoms may occur in the context of neurodegenerative disease, apathy and emotional blunting are particularly pervasive (Chow et al., 2009; Eslinger et al., 2005; Litvan et al., 1998).
In the present study, we investigated alexithymia in a heterogeneous sample that included patients with neurodegenerative disease and healthy controls. Given that alexithymia is associated with frontotemporal regions that are often disrupted in these diseases, we hypothesized that patients would have higher levels of alexithymia than controls. We also investigated the behavioral and neural correlates of alexithymia, predicting that alexithymia would be related to higher levels of behavioral disturbance and smaller ACC gray matter volumes.
Methods
Participants
Thirty-two participants were included in the present study: 25 patients with neurodegenerative disease and 7 healthy controls. Our patient groups included 5 patients with frontotemporal dementia (FTD), 4 with semantic dementia (SD), 8 with Alzheimer’s disease (AD), and 8 with corticobasal degeneration/progressive supranuclear palsy (CBD/PSP). The sample consisted of 66% men and 34% women, and 84% of the sample was right-handed. The groups did not differ in age, F(4, 27) = 0.94, n.s., or years of education, F(4, 27) = 0.83, n.s. See Table 1 for group means.
Table 1.
Participant Demographics and Clinical Status (MMSE = Mini-Mental State Examination, CDR = Clinical Dementia Rating Scale, NPI = Neuropsychiatric Inventory)
| Age | Education | MMSE | CDR | NPI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | M | SD | |
| AD | 60.5 | 6.6 | 15.6 | 3.6 | 21.6 | 6.9 | 1.0 | 0.5 | 23.3 | 15.6 |
| FTD | 66.2 | 5.0 | 16.8 | 3.0 | 27.0 | 1.6 | 0.9 | 0.7 | 43.2 | 25.9 |
| SD | 62.8 | 7.4 | 15.8 | 2.7 | 19.8 | 8.3 | 1.0 | 0.6 | 38.0 | 33.7 |
| PSP/CBD | 64.1 | 5.4 | 16.4 | 3.5 | 21.3 | 7.9 | 1.2 | 0.9 | 22.9 | 8.1 |
| Control | 56.0 | 18.4 | 18.1 | 2.0 | 29.7 | 0.5 | 0.0 | 0.0 | 1.0 | 5.7 |
Clinical Evaluation
General Procedure
Participants were evaluated by a multi-disciplinary team at the University of California, San Francisco Memory and Aging Center and were diagnosed according to standard research criteria (Boeve, Lang, & Litvan, 2003; Litvan et al., 1996; McKhann et al., 1984; Neary et al., 1998). Participants underwent an extensive evaluation that included a clinical interview, neurological exam, neuropsychological testing, and a functional assessment (informants, who accompanied the participants to clinic or were contacted by phone, were interviewed using semi-structured instruments, described below).
Measures of Disease Severity
Cognitive Status
Cognitive functioning was assessed with the Mini-Mental State Examination (MMSE), which provides an index of overall cognitive status (Folstein, Folstein, & McHugh). Scores on the MMSE range from 0 to 30 (higher scores indicate better cognitive functioning). All of the patient groups had MMSE scores that were significantly lower than the controls, F(4, 27) = 2.75, p < .05. Follow-up Bonferroni-corrected comparisons revealed no significant differences among the patient groups (see Table 1 for means).
Daily Functioning
The Clinical Dementia Rating Scale (CDR) was used to evaluate participants’ daily functioning (Morris, 1993). The CDR evaluates functioning in the domains of memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care via a structured interview that is administered to informants. The CDR is widely used as a measure of dementia severity, with higher scores indicating greater functional impairment (Morris, 1993). We examined the total CDR score (scores range from 0 to 3), which was computed using the standard CDR scoring algorithm. There was a main effect of diagnosis, F(4, 27) = 4.12, p < .05, and follow-up Bonferroni-corrected comparisons found that AD (p < .05) and PSP/CBD (p < .01), patients had significantly higher CDR scores than the controls. All patient groups were in the mild range of impairment (see Table 1 for means).
Behavioral Disturbance
The Neuropsychiatric Inventory (NPI) was used to evaluate levels of behavioral disturbance in each participant (Cummings et al., 1994). The NPI evaluates multiple domains of socioemotional behavior (e.g., disinhibition, apathy, irritability, euphoria, and apathy subscales). Frequency (0, not at all; 1, occasionally; 2, often; 3, frequently; 4, very frequently) and severity (1, mild; 2, moderate; 3, severe) ratings of problematic behaviors were made by trained interviewers who administered a semi-structured interview to each participant’s informant. Each subscale has a total score (scores range from 0 to 12), which is the product of the frequency and severity ratings. The NPI total score is the sum of all of the subscale totals (scores range from 0 to 120). Group analyses of NPI total scores revealed a main effect of diagnosis, F(4, 27) = 5.89, p < .01. Pairwise Bonferroni-corrected comparisons found that FTD patients had significantly higher NPI total scores than controls (p < .01). There were no significant differences among the patient groups (see Table 1 for means). Each informant also rated his/her own level of distress (0, not distressing at all; 1, minimal; 2, mild; 3, moderate; 4, severe; 5, extreme or very severe) around each type of behavioral disturbance in the participant.
Assessment of Alexithymia
The Toronto Alexithymia Scale-20 (TAS-20), a measure with good internal consistency and test-retest reliability (Bagby, Parker, & Taylor, 1994), was used to evaluate alexithymia in the participants. Factor analysis of the TAS-20 has found three psychometrically distinct subscales: (1) Difficulty Identifying Feelings (e.g., “I am often confused about what emotion I am feeling,” and “I have feelings that I can’t quite identify”), (2) Difficulty Describing Feelings (e.g., “I find it hard to describe how I feel about people” and “It is difficult for me to reveal my innermost feelings, even to close friends”), and (3) Externally-Oriented Thinking (e.g., “I prefer to just let things happen rather than to understand why they turned out that way” and “I find examination of my feelings useful in solving problems” [reverse-scored]). Informants completed the TAS-20 about patients and controls in their current condition, an approach that has been useful when measuring socioemotional functioning in neurodegenerative patients (Rankin et al., 2003). TAS-20 total scores can range from 0 to 100. Scores of 61 or higher were classified as alexithymic, as defined by the scale developers (Bagby & Taylor, 1997). See Table 2 for TAS-20 total and subscale scores. Missing data were minimal. However, if there were more than one-third of the items were missing from either the subscale or total score, that score was not calculated. If there were less than one-third of the items missing, the average for that subscale or total was imputed for the missing item(s).
Table 2.
Group means for the Toronto Alexithymia Scale-20
| TAS-20 Total Score | TAS-20 Difficulty Identifying Feelings | TAS-20 Difficulty Describing Feelings | TAS-20 Externally-Oriented Thinking | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| AD | 65.2 | 14.9 | 19.1 | 6.7 | 18.0 | 3.6 | 28.3 | 6.5 |
| FTD | 75.8 | 11.3 | 26.4 | 5.2 | 20.8 | 2.5 | 28.6 | 5.4 |
| SD | 64.4 | 6.5 | 20.8 | 4.4 | 17.8 | 3.3 | 25.6 | 2.7 |
| PSP/CBD | 64.8 | 8.9 | 21.6 | 3.8 | 16.4 | 4.2 | 26.9 | 4.1 |
| Control | 35.6 | 7.3 | 9.3 | 2.1 | 8.9 | 4.1 | 17.4 | 4.8 |
Brain Imaging
Structural Magnetic Resonance Imaging Acquisition
Participants underwent structural MR imaging at the San Francisco Veterans Administration Hospital. MR images were acquired on a 1.5T Magnetom VISION system (Siemens Inc., Iselin, NJ, USA) using a standard quadrature head coil. Volumetric magnetization-prepared rapid gradient echo (MP-RAGE) MRI (TR/TE/inversion time [TI] = 10/4/300 msec) to obtain T1-weighted structural images of the entire brain. The T1 images were in a coronal orientation, with a 15° flip angle, with 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness.
Freesurfer Volumes of Interest
MR images were analyzed using Freesurfer (http://surfer.nmr.mgh.harvard.edu), a surface-based structural MR image analysis tool that segments white matter and tessellates both gray and white matter surfaces (Dale, Fischl, & Sereno, 1999; Fischl, Liu, & Dale, 2001; Fischl, Sereno, & Dale, 1999; Segonne et al., 2004). Non-brain tissue was removed using a hybrid watershed/surface deformation procedure (Segonne et al., 2004) and intensity normalization (Sled, Zijdenbos, & Evans, 1998), followed by automated Talairach transformation and volumetric segmentation of cortical and subcortical gray and white matter, subcortical limbic structures, basal ganglia and ventricles, was used to calculate total intracranial volume (Fischl et al., 2002; Fischl et al., 2004). The LONI Pipeline environment (http://pipeline.loni.ucla.edu) was used to distribute Freesurfer processing tasks to an offsite CPU cluster located at UCLA -LONI. Cortical regions of interest were defined as described in Desikan et al. (Desikan et al., 2006).
In the present study, we used gray matter volumes from the frontal lobes (i.e., superior frontal gyrus; rostral and caudal divisions of the middle frontal gyrus; pars opercularis, pars orbitalis, and pars triangularis of the inferior frontal gyrus; lateral and medial divisions of the orbitofrontal cortex; frontal pole; precentral gyrus; insula; pregenual ACC 1; anterior mid-cingulate cortex 2; and paracentral lobule); temporal lobes (i.e., entorhinal cortex, parahippocampal gyrus, temporal pole, fusiform gyrus, superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, transverse temporal cortex, and banks of the superior temporal sulcus); and parietal lobes (i.e., postcentral gyrus, supramarginal gyrus, superior parietal cortex, inferior parietal cortex, posterior cingulate cortex, and precuneus cortex).
Data Analyses
Group Analyses
We ran one-way analyses of variance (ANOVAs) to compare the alexithymia total and subscale scores for the groups. Follow-up Bonferroni-corrected tests were run to examine pairwise differences, correcting for multiple comparisons.
In order to account for the possibility that differences in language functioning may explain some of the variance in alexithymia, we conducted follow-up analyses in which we used measures of language as covariates in the one-way ANOVAs described above. The covariates were neuropsychological measures of confrontational naming (i.e., total correct score on the modified Boston Naming Test out of 15), repetition (total correct out of 5 items), and sentence comprehension (total correct out of 5 items).
Partial Correlation Analyses
Behavioral Correlates of Alexithymia
To determine whether alexithymia was associated with any specific domains of behavioral disturbance, we ran two-way partial correlations (controlling for age, gender, and MMSE) between alexithymia total scores and NPI total scores, each of the NPI’s socioemotional subscales (i.e., disinhibition, apathy, euphoria, irritability, anxiety, depression, and agitation), and informant distress ratings across all participants.
Neural Correlates of Alexithymia
To examine possible relationships between alexithymia total scores and regional gray matter volumes, we first ran two-way partial correlations (controlling for age, gender, and total intracranial volume) between alexithymia total scores and a priori regions of interest (right and left pregenual ACC and anterior mid-cingulate cortex). See Figure 1 for these areas as delineated by Freesurfer. Next, to conduct a more exhaustive search for brain-alexithymia associations, we ran exploratory partial correlations between alexithymia total scores and gray matter volumes from all regions generated by Freesurfer in the frontal, temporal, and parietal lobes (for a list of the specific regions, see above).
Figure 1.
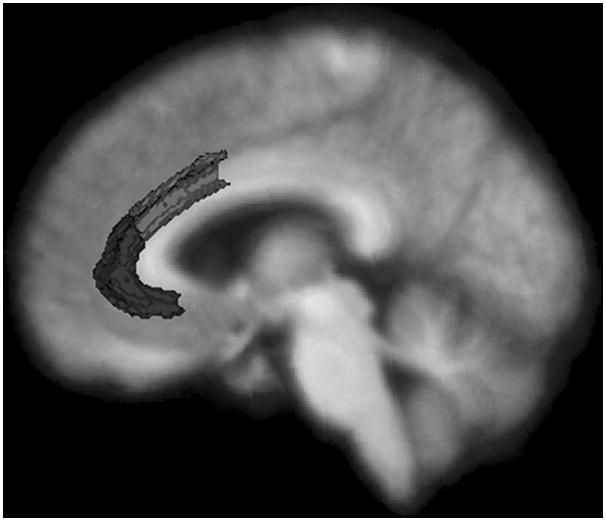
The analyses of the neural correlates of alexithymia were conducted using data only from the healthy controls. We did this because the small number of patients in each group would not enable us to control for diagnosis; thus, we would not have been able to separate the effects of diagnosis from those attributable to anatomical variability.
Results
Group Comparisons
We found a main effect of diagnosis on alexithymia total scores, F(4, 27) = 12.75, p < .001. Follow-up pairwise Bonferroni-corrected comparisons revealed that alexithymia total scores in patients with FTD (p < .001), SD (p < .001), AD (p < .001), and PSP/CBD (p < .001) were significantly higher than controls. Similar patterns were found on each of the alexithymia subscales. Main effects of diagnosis were found on the Difficulty Identifying Feelings, F(4, 27) = 11.32, p < .001; Difficulty Describing Feelings, F(4, 27) = 9.12, p < .001; and Externally-Oriented Thinking, F(4, 27) = 5.72, p < .001, subscales. Patients’ scores were significantly higher than controls on each subscale when examined with pairwise Bonferroni-corrected comparisons (see Table 2). Although no controls scored in the alexithymic range, 80% of patients were characterized as alexithymic.
Our follow-up analyses that included language variables (i.e., confrontation naming, repetition, and sentence comprehension) as covariates did not change our pattern of results. We continued to find a main effect of diagnosis on alexithymia total scores, F(4, 16) = 11.90, p < .001 and subscale scores: Difficulty Identifying Feelings, F(4, 16) = 8.98, p < .01; Difficulty Describing Feelings, F(4, 16) = 6.68, p < .01; and Externally-Oriented Thinking, F(4, 16) = 6.01, p < .01, with patients having higher levels of alexithymia than controls.
Behavioral Correlates of Alexithymia
Partial correlations (controlling for age, gender, and MMSE) revealed a significant correlation between alexithymia total scores and NPI total scores, rp(24) = .50, p< .01. Alexithymia total scores were also significantly correlated with the apathy subscale of the NPI, rp(24) = .46, p< .05, and with informants’ distress, rp(24) = .51, p< .01.
Neural Correlates of Alexithymia
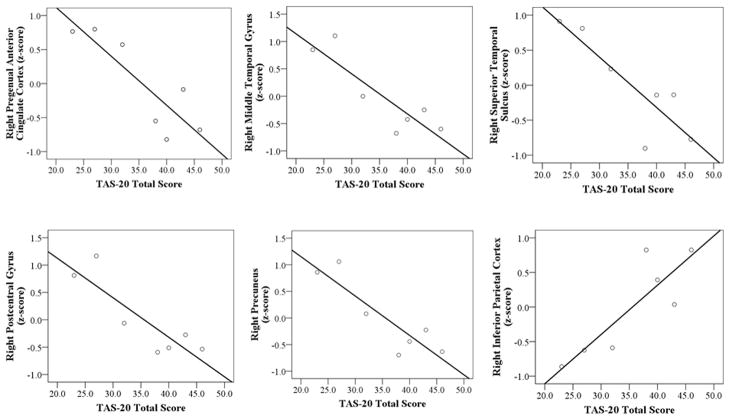
Gray matter volumes in the right pregenual ACC (controlling for age, gender, and total intracranial volume) were significantly correlated with alexithymia total scores among the controls, rp(2) = −.96, p< .05. No significant associations were found between alexithymia total scores and the left pregenual ACC, rp(2) = .31, n.s., right anterior mid-cingulate cortex, rp(2) = −.63, n.s., or left anterior mid-cingulate cortex, rp(2) = .23, n.s.
In our exploratory analyses, significant negative correlations emerged for the controls between alexithymia total scores and gray matter volumes in the right middle temporal gyrus, rp(2) = −.98, p< .05; right postcentral gyrus, rp(2) = −.97, p< .05; right precuneus, rp(2) = −.99, p<.05; and right superior temporal gyrus, rp(2) = −.96, p< .05. A positive correlation was found between alexithymia total scores and the right inferior parietal lobe, rp(2) = .95, p< .05. No significant associations were found between alexithymia and any left hemisphere regions. See Figure 2.
Figure 2.
Discussion
Alexithymia, a deficit in emotional experience and expression, has been reported across multiple psychological and medical illnesses (Taylor & Bagby, 2004). Alterations in frontotemporal brain structures, which play important roles in self and emotion processing, have been found in individuals with alexithymia (Borsci et al., 2009; Gundel et al., 2004; Paradiso et al., 2008). In the present study, we investigated levels of alexithymia in a sample of patients with neurodegenerative disease (i.e., frontotemporal dementia, semantic dementia, corticobasal degeneration, and progressive supranuclear palsy patients) and healthy controls to examine the behavioral and neural correlates of emotional self-awareness.
Neurodegenerative diseases have heterogeneous clinical presentations and neuroanatomical profiles. Despite this variability, changes in social and emotional behavior are commonly reported across diagnoses, albeit at different stages of disease progression (Chow et al., 2009; Litvan et al., 1998; Seeley et al., 2007). In the present study, we found that alexithymia was common in the neurodegenerative diseases that we sampled. Whereas none of our controls met criteria for alexithymia, 80% of our patients did. Although the FTD patients had the highest alexithymia total scores, there were no statistically significant differences in alexithymia total or subscale scores among the patient groups. Because the patients were relatively early in their disease course (mean CDR scores were approximately 1.0), these findings suggest that alexithymia may be a general feature of these neurodegenerative diseases. Controlling for language abilities did not change our pattern of results, which indicated that the alexithymia found in our patient groups could not be accounted for by a general decline in language functioning.
Past research has found impaired socioemotional skills (e.g., emotion recognition and perspective-taking) in individuals with alexithymia (Lane et al., 1996; Moriguchi et al., 2006). We found that higher levels of alexithymia were associated with greater behavioral disturbance in participants’ everyday lives (as measured by the NPI total scores). In particular, alexithymia was associated with apathy (i.e., a lack of motivation to engage with the world), which suggests that attunement to one’s inner world may facilitate interest in the social world. Along these lines, waning internal emotional experience may contribute to patients’ withdrawal from social interaction. Another indicator of these interpersonal effects was our finding that alexithymia was associated with level of distress in informants (typically spouses or family members). This finding further speaks to the importance of emotional experience and understanding for the quality of close relationships.
Frontotemporal brain structures are important for many aspects of emotion, and disruption of these systems appears to play an important role in alexithymia. Consistent with past work, we found a strong negative relationship between alexithymia and gray matter volumes of the right pregenual ACC (Borsci et al., 2009; Gündel et al., 2004; Paradiso et al., 2008). Alexithymia was not significantly associated with the volumes of the left pregenual ACC or the left or right anterior mid-cingulate cortex. It is worth noting that past studies have differed in whether the right (Gündel et al., 2004; Paradiso et al., 2008) or left (Borsci et al., 2009; Karlsson et al., 2008) hemisphere is predominantly associated with alexithymia; our findings clearly align with those that have found right hemisphere involvement.
In a set of exploratory analyses, we found significant negative associations between alexithymia and temporal (i.e., right middle and superior temporal gyri) and parietal (i.e., right postcentral gyrus and precuneus) regions. In addition, a positive association was found between alexithymia and the right inferior parietal lobe. This pattern of results is in line with past studies that have found decreased activation of the middle and superior temporal gyri (Borsci et al., 2009) and increased activation of the inferior parietal lobe (Kano et al., 2003; Moriguchi et al., 2009) in alexithymia. Given that we ran correlations between alexithymia and multiple brain regions in a small group of participants, these results are vulnerable to Type I error, and, thus we interpret them with caution.
In alexithymia, awareness, experience, and communication of emotion are impaired. Consistent with the complexity of this construct, we found associations between alexithymia and multiple regions in frontal, temporal, and parietal lobes. While frontotemporal structures are clearly important for emotion (Phan et al., 2002), frontoparietal networks have been implicated in self-awareness (Northoff et al., 2006). Thus, it is possible that networks that link the frontal lobes with the temporal and parietal lobes play important and complementary roles in self-processing and emotional experience. Although smaller volumes in the ACC and temporal lobes were consistently associated with higher levels of alexithymia, the role of the parietal lobe appears to be complex. Whereas the negative relationships between alexithymia and the postcentral gyrus and precuneus may reflect underlying deficits in bodily self-awareness (Iwamura, 1998) and autobiographical memory (Lou et al., 2004), respectively, the positive relationship between alexithymia and the inferior parietal lobe may be protective in this context. For example, a positive association has been previously reported (Moriguchi et al., 2009) between the inferior parietal lobe and neuroticism, a trait characterized by anxious and obsessive thinking about the self that runs counter to the alexithymic style.
Limitations
The present study has several limitations that should be noted. First, the measures of alexithymia and behavioral disturbance were based on informants’ retrospective reports. Although behavioral symptoms are observable, it may be more difficult for informants to report accurately on the internal emotional lives of other people (i.e., how their loved ones reflect upon their emotions). Second, to conduct a broad search for brain-alexithymia relationships, we ran multiple correlations between alexithymia and gray matter volumes in the frontal, temporal, and parietal lobes. We consider these findings to be preliminary and interpret them with due caution given that our small sample size did not enable us to correct for multiple comparisons.
Conclusions
Most studies of emotion in neurodegenerative disease have focused on patients’ ability to recognize the emotions of others. In the present study we examined how well patients reflect upon and communicate their own affective states. We found that deficits in these abilities, which are associated with alexithymia, are common across multiple neurodegenerative diseases, are related to behavioral problems, and are associated with gray matter volumes in the right pregenual anterior cingulate cortex and other frontotemporal brain structures. These findings suggest that emotional self-awareness may be yet another victim of neurodegenerative disease, one that may go unnoticed clinically. Nonetheless, deficits in subjective emotional experience, expression, and reflection likely play a major role in the difficulties that patients with frontotemporal disease have in participating successfully in the social world.
Acknowledgments
Funding
This work was supported by the National Institute on Aging [AG107766, AG19724, AG-03-006-01, AG019724-02]; the National Institute of Mental Health [MH020006]; and the State of California Alzheimer’s Disease Research Center of California [03-75271].
We thank Elizabeth Ascher for her contributions to our questionnaire data and Victor Laluz and William Irwin for their help with our Freesurfer data and figures.
Footnotes
Labeled “rostral ACC” by Freesurfer.
Labeled “caudal ACC” by Freesurfer.
References
- Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia Scale: I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994;38:33–40. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ. Measurement and validation of the alexithymia construct. In: Taylor GJ, Bagby RM, Parker JDA, editors. Disorders of Affect Regulation: Alexithymia in Medical and Psychiatric Illness. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurologica Scandinavica. 2001;103(6):367–378. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Artiges E, Van De Moortele PF, Poline JB, Rouquette S, Consoli SM, et al. Effect of impaired recognition and expression of emotions on frontocingulate cortices: an fMRI study of men with alexithymia. American Journal of Psychiatry. 2002;159(6):961–967. doi: 10.1176/appi.ajp.159.6.961. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Annals of Neurology. 2003;54(suppl 5):S15–S19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Borsci G, Boccardi M, Rossi R, Rossi G, Perez J, Bonetti M, et al. Alexithymia in healthy women: a brain morphology study. Journal of Affective Disorders. 2009;114(1–3):208–215. doi: 10.1016/j.jad.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Caycedo AM, Miller B, Kramer J, Rascovsky K. Early features in frontotemporal dementia. Current Alzheimer Research. 2009;6(4):337–340. doi: 10.2174/156720509788929255. [DOI] [PubMed] [Google Scholar]
- Chow TW, Binns MA, Cummings JL, Lam I, Black SE, Miller BL, et al. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Archives of Neurology. 2009;66(7):888–893. doi: 10.1001/archneurol.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labelling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, Grossman M. Metacognitive deficits in frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(12):1630–1635. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goodkind MS, Gyurak A, McCarthy M, Miller BL, Levenson RW. Emotion regulation deficits in frontotemporal lobar degeneration and Alzheimer’s disease. Psychology and Aging. 2010;25(1):30–37. doi: 10.1037/a0018519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. Frontotemporal dementia: A review. Journal of the International Neuropsychological Society. 2002;8:566–583. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- Gündel H, López-Sala A, Ceballos-Baumann AO, Deus J, Cardoner N, Marten-Mittag B, et al. Alexithymia correlates with the size of the right anterior cingulate. Psychosomatic Medicine. 2004;66(1):132–140. doi: 10.1097/01.psy.0000097348.45087.96. [DOI] [PubMed] [Google Scholar]
- Iwamura Y. Hierarchical somatosensory processing. Current Opinion in Neurobiology. 1998;8(4):522–528. doi: 10.1016/s0959-4388(98)80041-x. [DOI] [PubMed] [Google Scholar]
- Joukamaa M, Saarijärvi S, Muuriaisniemi ML, Salokangas RK. Alexithymia in a normal elderly population. Comprehensive Psychiatry. 1996;37(2):144–147. doi: 10.1016/s0010-440x(96)90576-3. [DOI] [PubMed] [Google Scholar]
- Kano M, Fukudo S, Gyoba J, Kamachi M, Tagawa M, Mochizuki H, et al. Specific brain processing of facial expressions in people with alexithymia: An H2 15O-PET study. Brain. 2003;126(Pt 6):1474–1484. doi: 10.1093/brain/awg131. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Näätänen P, Stenman H. Cortical activation in alexithymia as a response to emotional stimuli. British Journal of Psychiatry. 2008;192(1):32–38. doi: 10.1192/bjp.bp.106.034728. [DOI] [PubMed] [Google Scholar]
- Lane RD, Ahern GL, Schwartz GE, Kaszniak AW. Is alexythymia the emotional equivalent of blindsight? Biological Psychiatry. 1997;42:834–844. doi: 10.1016/s0006-3223(97)00050-4. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10(4):525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lane RD, Sechrest L, Reidel R, Weldon V, Kaszniak A, Schwartz GE. Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosomatic Medicine. 1996;58(3):203–210. doi: 10.1097/00006842-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. Journal of Neurology, Neurosurgery and Psychiatry. 1998;65(5):717–721. doi: 10.1136/jnnp.65.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, et al. Parietal cortex and representation of the mental self. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila AK, Saarni SI, Salminen JK, Huhtala H, Sintonen H, Joukamaa M. Alexithymia and health-related quality of life in a general population. Psychosomatics. 2009;50(1):59–68. doi: 10.1176/appi.psy.50.1.59. [DOI] [PubMed] [Google Scholar]
- Mattila AK, Salminen JK, Nummi T, Joukamaa M. Age is strongly associated with alexithymia in the general population. Journal of Psychosomatic Research. 2006;61(5):629–635. doi: 10.1016/j.jpsychores.2006.04.013. [DOI] [PubMed] [Google Scholar]
- McDonald PW, Prkachin KM. The expression and perception of facial emotion in alexithymia: a pilot study. Psychosomatic Medicine. 1990;52(2):199–210. doi: 10.1097/00006842-199003000-00007. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Decety J, Hirakata M, Maeda M, Matsuda H, et al. The human mirror neuron system in a population with deficient self-awareness: An fMRI study in alexithymia. Human Brain Mapping. 2009;30(7):2063–2076. doi: 10.1002/hbm.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, Maeda M, Mori T, Nemoto K, et al. Impaired self-awareness and theory of mind: An fMRI study of mentalizing in alexithymia. Neuroimage. 2006;32(3):1472–1482. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--A meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Vaidya JG, McCormick LM, Jones A, Robinson RG. Aging and alexithymia: association with reduced right rostral cingulate volume. American Journal of Geriatric Psychiatry. 2008;16(9):760–769. doi: 10.1097/JGP.0b013e31817e73b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan LP, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Mychack P, Miller BL. Double dissociation of social functioning in frontotemporal dementia. Neurology. 2003;60(2):266–271. doi: 10.1212/01.wnl.0000041497.07694.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen JK, Saarijärvi S, Aärelä E, Toikka T, Kauhanen J. Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. Journal of Psychosomatic Research. 1999;46(1):75–82. doi: 10.1016/s0022-3999(98)00053-1. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Allman JM, Carlin DA, Crawford RK, Macedo MN, Greicius MD, et al. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: Reciprocal networks and neuronal evolution. Alzheimer Disease and Associated Disorders. 2007;21(4):S50–S57. doi: 10.1097/WAD.0b013e31815c0f14. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sifneos PE. The prevalence of “alexithymic” characteristics in psychosomatic patients. Psychotherapy and Psychosomatics. 1973;22:255–262. doi: 10.1159/000286529. [DOI] [PubMed] [Google Scholar]
- Sifneos PE, Apfel-Savitz R, Frankel FH. The phenomenon of ‘alexithymia’. Observations in neurotic and psychosomatic patients. Psychotherapy and Psychosomatics. 1977;28(1–4):47–57. doi: 10.1159/000287043. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sturm VE, McCarthy ME, Yun I, Madan A, Yuan JW, Holley SR, et al. Mutual gaze patterns in couples with Alzheimer’s disease, frontotemporal dementia, and semantic dementia. Social Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM. New trends in alexithymia research. Psychotherapy and Psychosomatics. 2004;73(2):68–77. doi: 10.1159/000075537. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA. The alexithymia construct. A potential paradigm for psychosomatic medicine. Psychosomatics. 1991;32(2):153–164. doi: 10.1016/s0033-3182(91)72086-0. [DOI] [PubMed] [Google Scholar]