Abstract
The putative visual word form area (pVWFA) is the most consistently activated region in single word reading studies (i.e., Vigneau et al. 2006), yet its function remains a matter of debate. The pVWFA may be predominantly used in reading or it could be a more general visual processor used in reading but also in other visual tasks. Here, resting-state functional connectivity magnetic resonance imaging (rs-fcMRI) is used to characterize the functional relationships of the pVWFA to help adjudicate between these possibilities. rs-fcMRI defines relationships based on correlations in slow fluctuations of blood oxygen level–dependent activity occurring at rest. In this study, rs-fcMRI correlations show little relationship between the pVWFA and reading-related regions but a strong relationship between the pVWFA and dorsal attention regions thought to be related to spatial and feature attention. The rs-fcMRI correlations between the pVWFA and regions of the dorsal attention network increase with age and reading skill, while the correlations between the pVWFA and reading-related regions do not. These results argue the pVWFA is not used predominantly in reading but is a more general visual processor used in other visual tasks, as well as reading.
Keywords: orthography, reading, resting-state, rs-fcMRI, specialization
Introduction
Functional neuroimaging has helped make great strides in understanding the neural underpinnings of reading. Single studies and meta-analyses have led to a general consensus regarding the brain regions used in reading processes and some understanding of the developmental changes in the use of these regions (Schlaggar and McCandliss 2007). For example, regions in the left supramarginal gyrus (SMG; Church et al. 2008, 2010; Graves et al. 2010) and inferior frontal gyrus (IFG; Fiez and Petersen 1998; Mechelli et al. 2003) have been reported in a number of studies involving phonological processing on visual words, and several studies have reported decreased use of these regions with increasing reading skill (Sandak et al. 2004; Church et al. 2008). Regions in the left angular gyrus (AG; Binder et al. 2005, 2009) are thought by some to relate to semantic processing. A region in the left fusiform cortex at the occipitotemporal junction purported to be involved in orthographic processing (see details below) is the most consistently reported region in the reading literature (Jobard et al. 2003; Mechelli et al. 2003; Turkeltaub et al. 2003; Vigneau et al. 2006). Reliance on this region seems to increase with development (Sandak et al. 2004), possibly through some interaction with decreased reliance on phonological processing mentioned above (Sandak et al. 2004; Schlaggar and McCandliss 2007).
While the proposed orthographic processing region in left occipitotemporal cortex is consistently activated in reading studies of skilled adult readers, the role of this region is a matter of considerable debate. Some investigators have shown word-related activity in this region that is case, size, and font invariant (Cohen et al. 2002) and some studies report more activity in the region for words than for consonant strings (Cohen et al. 2002; McCandliss et al. 2003), digits (Polk et al. 2002), or false fonts (Vinckier et al. 2007), leading these researchers to designate this left occipitotemporal region the visual word form area (VWFA). However, multiple studies have shown the VWFA is also active when processing visual stimuli other than words, including pictures (Price and Devlin 2003; Ben-Shachar et al. 2007; Ploran et al. 2007; Starrfelt and Gerlach 2007; Kherif et al. 2011; Van Doren et al. 2010), faces (Mei et al. 2010), and false fonts (Xue et al. 2006; Xue and Poldrack 2007). Moreover, while damage to this region can sometimes result in acquired alexia or letter by letter reading (Dejerine 1892; Cohen et al. 2003; Gaillard et al. 2006), there is evidence that such lesions do not produce alexia exclusively (Behrmann et al. 1990, 1998; Price and Devlin 2003) and may instead cause a more general deficit in simultaneous visual processing (Starrfelt et al. 2009). In order to acknowledge the conflicting data regarding the specificity of processing performed in this region but still restrict our discussion to the piece of occipitotemporal cortex located near the classic VWFA coordinates (−45, −57, −12 in Montreal Neurological Institute (MNI) coordinates), we hereinafter refer to the region under study as the putative VWFA (pVWFA).
Despite this controversy, it seems likely that the pVWFA is involved in reading in some way, allowing several possibilities. The pVWFA could be dedicated for use in reading, either as its inherent function or due to extensive training (as described in Dehaene and Cohen 2007). Alternatively, the pVWFA could be a more general visual processor used in reading but also maintaining its involvement in other visual tasks. Here, we use resting-state functional connectivity magnetic resonance imaging (rs-fcMRI) to characterize the functional network relationships between the pVWFA and other regions to help adjudicate between these possibilities.
rs-fcMRI uses correlations in low-frequency (approximately 0.01–0.1 Hz) fluctuations of the blood oxygen level–dependent (BOLD) signal present at rest to define functional relationships between regions. rs-fcMRI has been used extensively to study functional networks including the default mode network (Greicius et al. 2003; Fox et al. 2005), attentional control networks (Fox et al. 2005; Dosenbach et al. 2007; Seeley et al. 2007), and reading networks (Koyama et al. 2010), among others. One plausible account of the resting correlations are that they reflect a long history of coactivation (Dosenbach et al. 2006; Fair, Dosenbach, et al. 2007; Fair et al. 2009) that is somewhat malleable with short-term experience (Lewis et al. 2009; Stevens et al. 2010; Tambini et al. 2010). If the correlations relate to consistent coactivations, then the descriptions of a region's correlation-based “neighbors” should provide insight into whether this region is predominantly used in reading or is a more general visual processor. Investigating how these correlations change with development may give insight into whether these relationships are already in place in early readers or change with age and/or reading skill.
If the pVWFA is used predominately for orthographic processing in reading, its rs-fcMRI defined neighbors might be expected to include potential phonological processors such as the left SMG, left IFG, and auditory association cortex, semantic processors possibly including the left AG or left middle temporal gyrus (Booth et al. 2007; Binder et al. 2009; Simmons et al. 2010) and possibly regions related to articulation such as mouth pre- and motor cortex and supplementary motor area (Alario et al. 2006). While there has been some debate about whether this region is “specific” for word processing (i.e., wholly devoted to processing words or letter strings and not involved in other processing) or “preferential” for word-related processing (i.e., responsive to multiple types of stimuli but most responsive to words or letter strings), these results would not differentiate between the two. In either case, if reading is the predominant use of the pVWFA one should expect rs-fcMRI correlations with reading-related regions.
If, on the other hand, the pVWFA is a more general visual processor that is not used predominantly for words or word-like stimuli but is used more generally for many other visual processing demands, we would instead expect to see functional connections to other visual and visual attention regions in the absence of preferential functional connections to putative reading-related regions.
Even if the data support the latter hypothesis (that the pVWFA is a more general visual processor), the location of regions with the strongest rs-fcMRI relationships to the pVWFA should still inform our understanding of the type of processing done in this region. As the pVWFA is consistently activated during reading, even if it is not used predominantly in this task, it must have some properties that make it particularly useful for reading. In reading, the ability to “group” stimuli into various sized “chunks” ranging from single letters to graphemes to whole words is important. Computing grapheme to phoneme correspondences used in the phonologic decoding of pseudowords or unfamiliar words requires grouping letters into small grapheme based chunks such as bigrams or trigrams. However, when adults read familiar words fluently, they appear to process words in much larger groups and have relatively little variability in their response latencies to name words that range in length from 3 to 7 letters (e.g., Weekes 1997; Cohen et al. 2003). Recently, Schurz et al. (2010) have shown that BOLD activity in the pVWFA shows a length by lexicality effect, whereby activity increases with length when reading pseudowords but not real words in this single region (Schurz et al. 2010). Separate studies have also shown activity in this region is modulated by bigram frequency (Kronbichler et al. 2004; Graves et al. 2010) and seems responsive to whole words (Vinckier et al. 2007; Schurz et al. 2010). Together these results argue that the pVWFA may represent letter-based stimuli in chunks of various sizes.
A region processing chunks of the various sizes used in reading might develop preferentially strong functional connections with regions that direct attention to the appropriate group of features or spatial locations. After all, it would do little good to represent highly familiar real words as a whole if attention could not be directed to the whole group of letters and likewise it would serve little use to represent pseudowords in grapheme sized chunks if attention was only directed to larger letter groups. Since regions in the dorsal attention network contribute to the direction of attention to the appropriate spatial or feature chunk (Corbetta and Shulman 2002), we hypothesize there may be functional relationships between the pVWFA and dorsal attention regions.
Again, investigating whether the functional relationships between the pVWFA and regions of the dorsal attention network increase with age and/or reading level can also help inform our understanding of these relationships. Children are more likely to read by converting graphemes into phonemes, and the ability to process words in larger groups is related to age and/or reading ability (Backman et al. 1984; Defior et al. 1996; Bijeljac-babic et al. 2004; Sandak et al. 2004; Martens and de Jong 2008). Thus, we hypothesize that there may be a relationship between any rs-fcMRI correlations between the pVWFA and dorsal attention regions with age or reading skill.
Materials and Methods
Subjects
Main Analysis
Subjects included 25 children (8 males) ages 6–9 years and 23 adults (11 males) ages 21–26 years. All subjects were right-handed mono-lingual native English speakers. All were screened for neurologic and psychiatric diagnoses and chronic use of medications by telephone interview and questionnaire. All gave written informed consent and were compensated for their time per the Washington University Human Studies Committee guidelines. Subjects were tested for IQ using the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999) and for reading age using 3 subtests of the Woodcock–Johnson III (Letter-Word ID, Passage Comprehension, and Word Attack) (Woodcock and Johnson 2002). Further information about the standard reading age and IQ for all subjects can be found in Table 1.
Table 1.
Subject characteristics
| Subject group | Chronological age |
Movement (mm r.m.s.) |
Full scale IQ |
Reading age |
||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Original data set | ||||||||
| Children (n = 22) | 8.15 | 0.84 | 0.70 | 0.31 | 117 | 15.7 | 9.5 | 3.3 |
| Adults (n = 23) | 24.2 | 1.65 | 0.26 | 0.10 | 127 | 7.8 | 24.4 | 0.58 |
| Movement matched data set | ||||||||
| Children (n = 23) | 8.5 | 1.0 | 0.41 | 0.18 | 119 (n = 23) | 15 | 10 (n = 13) | 2.8 |
| Adults (n = 23) | 24.0 | 1.4 | 0.39 | 0.12 | 132 (n = 12) | 4.8 | 24.6 (n = 6) | 0 |
Note: IQ was computed from the 2 subtest version of the Wechsler Abbreviated Scale of Intelligence. Estimated reading ages were computed from 3 Woodcock–Johnson III subtests (Letter-Word ID, Passage Comprehension, and Word Attack). IQ and reading level were only collected on a portion of the movement-matched group; the number of subjects contributing to each measurement is noted. r.m.s., root mean square; SD, standard deviation.
After further evaluation, 3 children were excluded from the final analysis. As correlations with standard reading age were an analysis of interest, the 2 children with reading ages above 2 standard deviations from the mean (reading ages of 17.6 and 18.5 years old) were excluded. One other child was excluded for showing a majority of outlier rs-fcMRI correlation values in region-wise analyses (falling more than 2 standard deviations from the mean). Thus, the final child data set included 22 children (7 males) (ages 6–9 years) with an average age of 8.2 years. Behavioral data for this final analysis group are presented in Table 1.
Movement-Matched Analysis
As seen in Table 1, the children used in the main analysis moved significantly more than the adults. Increased movement increases the noise in the rs-fcMRI signal, affecting the strength of rs-fcMRI correlations and thus potentially leading to spurious group differences. As an attempt to at least partially correct for this difference in movement, we repeated the developmental analyses with groups of children and adults matched for average root mean square movement across runs, making use of some subject data obtained from additional data sets from our laboratory. The movement-matched group of 23 children (age 7–10 years, mean 8.5) and 23 adults (age 21–26 years, mean 24.0 years) were also right-handed, native monolingual English speakers screened for neurologic and psychiatric diagnoses similar to the main group. This movement-matched group included 13 children and 6 adults from the main analysis. Unfortunately, not all of the remaining subjects in this group were tested for reading level and IQ; the numbers of subjects contributing to each measurement are listed in Table 1. Age and movement measures are also reported for this group in Table 1.
Region Definition
The coordinates of the pVWFA (−45, −62, −8, MNI coordinates), left IFG (−53, 27, 16, MNI coordinates), left SMG (−49, −57, 28, MNI coordinates), and left AG (−56, −43, 31, MNI coordinates) regions were defined from an in-house meta-analysis of 5 adult single word reading studies and a single developmental study described in further detail below. The 5 adult studies required subjects to read a word aloud; 3 of the studies also manipulated written frequency and 2 each manipulated spelling-to-sound consistency and lexicality. Regions were defined either by showing a main effect of reading in 4 of the 5 studies, an effect of frequency in 2 of the 3 studies manipulating that variable or an effect of consistency or lexicality in both of the studies manipulating each variable. The developmental study compared 24 children and 24 adults matched for performance (also reported in Church et al. 2008). Of note, these meta-analyses did not show a region in the middle temporal gyrus, where semantic effects are often found (Binder et al. 2009). However, a meta-analytic region was identified in the inferior temporal gyrus (ITG) region (−61, −33, −15), which does overlap with the large swath of activity in a meta-analysis of semantics (Binder et al. 2009). Please note that while these analyses were performed using an in-house atlas based on Talairach and Tournoux (1988), all coordinates have been converted to MNI space for reporting purposes.
In addition to testing the rs-fcMRI correlations between the pVWFA and reading-related regions, the correlations between the pVWFA and regions of the dorsal attention network were specifically investigated. Coordinates for regions in the dorsal attention network were obtained from a meta-analysis of 4 published studies, detailed in the supplementary material of Carter et al. (2010) (Table 2).
Table 2.
Dorsal attention network regions
| MNI coordinates |
Region | ||
| X | Y | Z | |
| −26 | −5 | 50 | Left frontal eye fields |
| 32 | −6 | 39 | Right frontal eye fields |
| −25 | −62 | 51 | Left aIPS |
| 25 | −64 | 51 | Right aIPS |
| −25 | −69 | 34 | Left posterior IPS |
| 32 | −80 | 18 | Right ventral IPS |
| −45 | −71 | −1 | Left MT+ |
| 44 | −68 | −6 | Right MT+ |
Note: Regions were obtained from a meta-analysis published in Carter et al. (2010). All coordinates have been converted to MNI space for reporting purposes.
In order to determine the specificity of pVWFA rs-fcMRI correlations, we compared the resting-state correlations of the left pVWFA with those of 2 other regions purportedly specialized for processing specific types of visual stimuli—the fusiform face area (FFA) and extrastriate body area (EBA) (for further discussion, see Grill-Spector and Malach 2004 and Kanwisher 2010). The coordinates of the FFA (35, −49, −14) were obtained from a published meta-analysis (Berman et al. 2010) and the coordinates of the EBA (−53, 27, 16), from a literature search (see below). For the purpose of this meta-analysis, the peak coordinates for the face localizer from the 50 studies identified in Berman et al. (2010) were transformed into our in-house atlas space and averaged. This average coordinate was converted back into MNI space for reporting purposes. Putative EBA coordinates were obtained from a literature search of papers that reported exact coordinates of a body localizer task. In order to most stringently compare the functional relationships of the pVWFA and EBA, we used the left hemisphere EBA region, as this is located quite close to the pVWFA. All coordinates were transformed into our in house atlas space and then averaged. This average region was used for the analyses reported here and the coordinates converted back to MNI space for reporting purposes only. The 12 papers used to define this region, the localizer task that was used and the reported coordinates (in MNI space) can all be found in Table 3.
Table 3.
Literature-based meta-analysis of EBA coordinates
| Citation | Coordinates |
Localizer contrast | ||
| X | Y | Z | ||
| Downing et al. (2001) | −51 | −72 | 8 | Body parts > objects |
| Astafiev et al. (2004) | −50 | −69 | 9 | Body parts > objects |
| Chan et al. (2004) | −45 | −76 | 8 | Bodies > tools |
| Spiridon et al. (2006) | −58 | −72 | 5 | Bodies > objects |
| Morris et al. (2006) | −42 | −82 | 9 | Bodies |
| Saxe et al. (2006) | −45 | −72 | 3 | Bodies and body parts > objects |
| Peelen et al. (2007) | −49 | −74 | 7 | Body parts > tools |
| Myers and Sowden (2008) | −52 | −64 | 14 | Bodies > objects |
| −50 | −63 | 17 | Bodies > objects | |
| Pinsk et al. (2009) | −52 | −72 | 14 | Body parts > objects |
| Bracci et al. (2010) | −48 | −70 | 4 | Bodies and body parts > chairs |
| Calvo-Merino et al. (2010) | −55 | −75 | 8 | Bodies > scrambles |
| Aleong and Paus (2010) | −43 | −70 | 4 | Bodies > scrambles |
| Average coordinates | −49 | −72 | 8 | |
| Standard deviation | 4.8 | 5.1 | 4.5 | |
Note: All coordinates have been converted to MNI space using icbm2tal found on brainmap.org
MR Data Acquisition and Preprocessing
Each subject was scanned for 1–4 runs, composed of 132 or 133 continuous frames with a 2.5 s time repetition (TR). During the runs, subjects looked at a black screen with a white central fixation cross. The subjects were instructed to look at the crosshair and relax but remain still with their eyes open. All subjects were fitted with a thermoplastic mask to facilitate their efforts to remain still.
A Siemens 3-T Trio scanner (Erlanger, Germany) with a 12-channel Siemens Matrix head coil was used to collect all functional and anatomical scans. A single high-resolution structural scan was acquired using a sagittal magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence (slice time echo = 3.08 ms, TR = 2.4 s, inversion time = 1 s, flip angle = 8°, 176 slices, 1 × 1 × 1 mm voxels). All functional runs were acquired parallel to the anterior–posterior commissure plane using an asymmetric spin-echo echo-planar pulse sequence (TR = 2.5 s, T2* evolution time 27 ms, flip angle 90°). Complete, or near complete, brain coverage was achieved by collecting 32 contiguous interleaved 4 mm axial slices (4 × 4 mm in-plane resolution).
Preliminary image processing included removal of a single pixel spike caused by signal offset, whole-brain normalization of signal intensity across frames, movement correction within and across runs, and slice by slice normalization to correct for differences in signal intensity due to collecting interleaved slices. For detailed description see Miezin et al. (2000).
After preprocessing, data were transformed into a common stereotactic space based on Talairach and Tournoux (1988) but using an in-house atlas composed of the average anatomy of 12 healthy young adults ages 21–29 years old and 12 healthy children ages 7–8 years old (for methods, see Lancaster et al. 1995; Snyder 1996; Brown et al. 2005). As part of the atlas transformation, the data were resampled isotropically at 3 × 3 × 3 mm. Registration was accomplished via a 12 parameter affine warping of each individual's MP-RAGE to the atlas target using difference image variance minimization as the objective function. The atlas-transformed images were checked against a reference average to ensure appropriate registration.
Several additional steps (also described in Fox et al. 2005, 2009; Fair, Dosenbach, et al. 2007) were taken in processing the rs-fcMRI data in an attempt to reduce the likelihood that the relationships between regions were due to sources such as heart rate or respiration. To mitigate such effects, the data were band-pass filtered for frequencies between 0.009 Hz and 0.08 Hz and spatially smoothed (6 mm full-width half max). Additionally, motion correction was performed via regression of the 6 parameters obtained from the rigid body head motion correction, regression of the signal derived from averaging across the whole brain, regression of signal from ventricular regions of interest (ROIs), and regression of signal from white matter ROIs.
Extraction of rs-fcMRI Time Courses and Generation of Seed Maps
A resting-state time course was extracted for 10 mm spheres centered on the pVWFA, FFA, and EBA coordinates on an individual subject basis. The regional time course was composed of the average time course of all voxels within the 10 mm sphere. These time courses were then correlated with the rs-fcMRI time course of all other voxels in the brain to create individual subject seed maps. These maps were then averaged together for the children and adults separately. The average maps were projected on the brain surface using CARET (Van Essen et al. 2001; http://brainmap.wustl.edu/CARET), thresholded at Z = ±3.5. A peak-finding algorithm (courtesy of Avi Snyder) was used to identify specific regions functionally related to the pVWFA by identifying peaks of activity with Z ≤ −3.5 or Z ≥ 3.5 at least 10 mm apart.
Comparison of pVWFA rs-fcMRI Correlations to Reading-Related and Dorsal Attention Network Regions
The resting-state time course was also extracted for 10 mm spheres centered on the coordinates of each reading-related region (left IFG, SMG, AG, and ITG) and dorsal attention network region (bilateral MT+, anterior inferior parietal sulcus (aIPS), frontal eye fields (FEF), left posterior IPS, and right ventral IPS) described above. The correlation between each of these regions and the pVWFA are plotted in Figure 2. To compare directly whether the pVWFA is more closely related to reading-related or dorsal attention regions, we calculated the average rs-fcMRI correlation between the pVWFA and all reading-related regions and the average rs-fcMRI correlation between the VWFA and all dorsal attention regions. A one-tailed paired t-test was then performed on these average values for the 23 adults.
Figure 2.
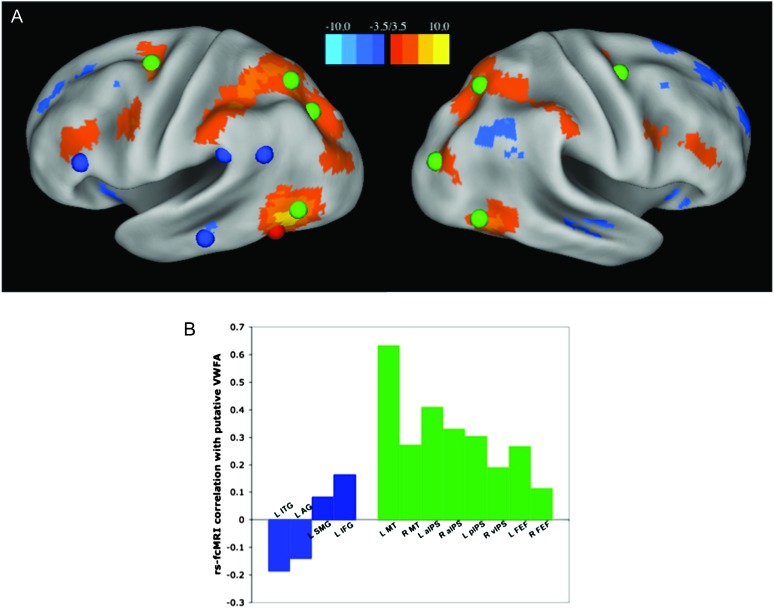
Comparison of pVWFA rs-fcMRI correlations with reading and dorsal attention regions. (A) The seed map shown in Figure 1 is overlaid with reading-related regions in blue (left IFG, ITG, SMG, and AG regions from anterior to posterior), dorsal attention network regions in green, and the location of the pVWFA seed in red. (B) The correlations between the pVWFA and reading-related regions in blue and dorsal attention regions in green. rs-fcMRI correlations to the dorsal attention regions are significantly stronger than correlations to the reading regions (P < 0.00001) when calculated as group averages. The statistical difference remains even when the bilateral MT+ regions are removed (P < 0.00001).
Comparison of pVWFA Seed Maps with FFA and EBA Seed Maps
The adult average seed map for the pVWFA was compared with the average seed maps of the FFA and EBA. This comparison was done by performing a t-test contrasting the average correlation value for each voxel with the pVWFA versus the average correlation value for voxel with the FFA and EBA separately, each t-test corrected for multiple comparisons using false discovery rate (FDR). Those voxels showing a difference with Z ≥ 3.5 were projected onto the surface of the brain using CARET.
Developmental Analysis of VWFA rs-fcMRI Relationships
Child and adult seed maps for the pVWFA region were compared directly, by performing a voxel-wise t-test similar to that described above. A t-test was performed for each voxel to determine whether there was a significant difference in the average adult correlation value versus the average child correlation value, correcting for multiple comparisons using FDR. Voxels showing a difference with Z ≥ 2.5 (P < 0.01) were projected on the brain surface using CARET.
Specific developmental comparisons of the rs-fcMRI correlations between the VWFA and dorsal attention network and reading-related region correlations were also performed. The average rs-fcMRI time course for a 10 mm spherical ROI centered on the pVWFA coordinate was correlated with the average rs-fcMRI time course for a 10 mm spherical ROI centered on each of the dorsal attention and reading-related region coordinates. These correlation values were obtained for each region pair in each subject individually. A t-test was then performed on each of these pairwise correlations, comparing children and adults. The pairwise comparisons were performed for both the original data set and the movement-matched data set.
The correlations between reading age and pVWFA/dorsal attention and pVWFA/reading-related region were also investigated. A correlation between the standard reading age and pVWFA/dorsal attention or pVWFA/reading-related region rs-fcMRI correlations, as well as a partial correlation determining the relationship between standard reading age and pVWFA/dorsal attention region rs-fcMRI correlation, controlling for chronological age and movement, was performed for each region of the dorsal attention network individually. These correlations and partial correlations were performed for all subjects together and for the children separately, as there was little variability in adult reading age.
Results
Whole-Brain Analysis of pVWFA rs-fcMRI Correlations Shows Overlap with Dorsal Attention but Not Reading -Related Regions
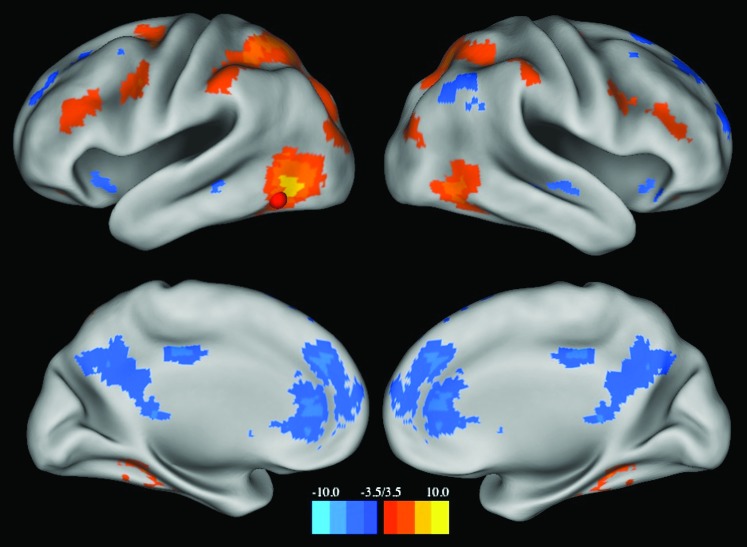
A seed map analysis of rs-fcMRI connections with the pVWFA reveals a distributed pattern of activity in adults (Fig. 1). The seed map represents those voxels whose rs-fcMRI time courses were significantly correlated (Z ≤ −3.5 or Z ≥ 3.5) with the average time course of the pVWFA seed region; the regions identified from a peak finding algorithm run on this seed-map image can be found in Table 4. The pVWFA seed map does not overlap with most regions thought to be important in reading, including the left SMG, thought to be involved in phonological processing (Church et al. 2011) and the left AG and ITG regions, purported to be involved in semantic processing (Binder et al. 2009) (reading-related regions shown in blue in Fig. 2A). As seen in Figure 2A, there is a left middle frontal gyrus (MFG) region showing significant correlations with the pVWFA, though this region is about 10 mm Euclidean distance from our meta-analysis defined IFG region, generally thought to be related to phonological processing (Mechelli et al. 2003). Moreover, the region identified from the pVWFA seed map is even further from the opercular IFG region found in some reading meta-analyses (Fiez and Petersen 1998; Jobard et al. 2003). There is also no relationship with mouth sensorimotor cortex (note the lack of correlations with pre- and post-central gyrus in Fig. 1) or auditory cortex (note the lack of correlations with superior temporal gyrus regions in Fig. 1).
Figure 1.
pVWFA seed map. Map displays voxels showing significant correlations (Z ≤ −3.5 or Z ≥ 3.5) with the rs-fcMRI time course of the pVWFA in 23 adults. The location of the pVWFA seed is marked with a red sphere.
Table 4.
Regions defined from the pVWFA seed map
| MNI coordinate |
Z-score | Number of voxels | Approximate location | ||
| X | Y | Z | |||
| Positive correlations | |||||
| Left | |||||
| −31 | −88 | 23 | 4.99 | 150 | Occipital cortex |
| −26 | −79 | 35 | 4.84 | 149 | Occipital cortex |
| −24 | −73 | −7 | 4.42 | 158 | Posterior fusiform gyrus |
| −34 | −43 | −16 | 4.62 | 155 | Anterior fusiform/inferior temporal cortex |
| −20 | −52 | −7 | 3.62 | 151 | Medial fusiform gyrus |
| −25 | −60 | 54 | 5.35 | 136 | aIPS |
| −32 | −51 | 53 | 5.44 | 139 | Anterior SPL |
| −44 | −40 | 43 | 5.13 | 144 | Lateral IPS |
| −57 | −35 | 44 | 4.63 | 140 | aIPS |
| −14 | −78 | 54 | 3.98 | 152 | Precuneus |
| −26 | −6 | 49 | 4.99 | 157 | Frontal eye fields |
| −44 | 3 | 31 | 4.94 | 154 | Premotor cortex |
| −42 | 30 | 20 | 4.62 | 148 | MFG |
| −49 | 20 | 29 | 3.91 | 140 | MFG |
| −25 | 36 | −13 | 3.54 | 151 | Medial inferior frontal cortex |
| −53 | 43 | 8 | 3.69 | 151 | Anterior insula |
| Right | |||||
| 40 | −83 | 7 | 3.52 | 149 | Occipital cortex |
| 38 | −78 | 22 | 4.61 | 148 | Occipital cortex |
| 30 | −70 | 37 | 4.14 | 149 | Occipital cortex |
| 51 | −61 | −8 | 5.84 | 158 | Occipitotemporal fusiform gyrus |
| 35 | −36 | −19 | 4.32 | 143 | Anterior fusiform gyrus |
| 25 | −65 | 52 | 5.30 | 148 | aIPS |
| 34 | −50 | 50 | 4.33 | 150 | Anterior SPL |
| 45 | −37 | 47 | 4.52 | 151 | Lateral IPS |
| 64 | −26 | 47 | 4.44 | 152 | aIPS |
| 27 | −43 | −11 | 4.01 | 143 | Medial temporal lobe |
| 28 | −7 | 57 | 4.12 | 156 | Frontal eye fields |
| 47 | 7 | 25 | 4.80 | 131 | Premotor cortex |
| 41 | −1 | 34 | 4.37 | 144 | Premotor cortex |
| 59 | 16 | 32 | 4.12 | 146 | MFG |
| 46 | 32 | 16 | 4.44 | 153 | MFG |
| 26 | 36 | −15 | 3.65 | 157 | Middle inferior frontal cortex |
| Negative correlations | |||||
| Left | |||||
| −53 | −63 | 49 | −4.09 | 122 | AG |
| −57 | −70 | 36 | −4.36 | 141 | Posterior AG |
| −48 | −74 | 49 | −3.98 | 126 | Posterior AG |
| −7 | −58 | 34 | −4.67 | 132 | Precuneus |
| −63 | −33 | −10 | −3.75 | 156 | Middle temporal gyrus |
| −35 | 18 | −10 | −4.13 | 152 | Anterior inferior insula |
| −9 | 51 | 8 | −4.88 | 140 | Anterior cingulate cortex |
| −21 | 30 | 40 | −3.95 | 151 | Middle frontal cortex |
| −23 | 50 | 32 | −4.06 | 149 | Medial frontal cortex |
| −7 | 31 | 58 | −3.61 | 137 | Superior anterior frontal cortex |
| −19 | −104 | −11 | −3.86 | 127 | Posterior cerebellum |
| −50 | −72 | −30 | −4.09 | 156 | Cerebellum |
| −38 | −84 | −35 | −3.52 | 146 | Cerebellum |
| −23 | −90 | −30 | −4.38 | 150 | Cerebellum |
| Right | |||||
| 53 | −56 | 46 | −4.69 | 143 | AG |
| 56 | −50 | 27 | −3.73 | 150 | AG |
| 51 | −67 | 38 | −4.57 | 143 | Posterior AG |
| 7 | −65 | 36 | −4.51 | 113 | Precuneus |
| 20 | −43 | 20 | −3.74 | 150 | Posterior cingulate cortex |
| 8 | −36 | 11 | −4.36 | 151 | Inferior posterior cingulate cortex |
| 57 | −20 | −11 | −4.27 | 157 | Middle temporal gyrus |
| 36 | 21 | −11 | −3.98 | 154 | Anterior inferior insula |
| 20 | 59 | 15 | −4.42 | 157 | Anterior frontal cortex |
| 20 | 41 | 40 | −4.61 | 157 | Medial frontal cortex |
| 17 | 25 | 60 | −4.43 | 148 | Superior frontal cortex |
| 24 | −85 | −33 | −4.13 | 158 | Cerebellum |
| 8 | −101 | −14 | −3.85 | 150 | Posterior cerebellum |
| Interhemisphere | |||||
| −1 | −75 | 38 | −4.70 | 138 | Precuneus |
| 5 | −55 | 32 | −4.98 | 122 | Precuneus |
| 2 | −26 | 40 | −5.52 | 158 | Posterior cingulate gyrus |
| 3 | 37 | −1 | −5.00 | 143 | Inferior anterior cingulate cortex |
| 4 | 47 | 10 | −5.56 | 129 | Anterior cingulate cortex |
| 5 | 42 | 31 | −5.41 | 151 | Medial prefrontal cortex |
| 5 | 37 | 57 | −3.74 | 139 | Superior anterior frontal cortex |
Note: Correlations between the pVWFA and dorsal attention network regions do not generalize to all stimulus-specific visual processing regions.
In contrast, the pVWFA seed map does overlap with regions from the dorsal attention network, as defined by a published meta-analysis (Carter et al. 2010) (green regions in Fig. 2A). A plot of the actual rs-fcMRI correlation values between the pVWFA and reading-related relations (shown in blue in Fig. 2B) and dorsal attention network regions (shown in green in Fig. 2B) shows the VWFA to dorsal attention region correlations are clearly stronger than the VWFA to reading-region correlations, which in some cases are even negative (Fig. 2B). A one-tailed paired t-test comparing the average correlation values between the pVWFA and all reading regions with the average correlation value between the pVWFA and all dorsal attention regions shows the latter to be significantly stronger (P < 0.0001). The difference in correlations between reading-related and dorsal attention regions remains significant (P < 0.0001) even when the bilateral MT+ regions, which are both very close to the pVWFA region and should be considered visual processing regions, are removed.
Correlations Between the pVWFa and Dorsal Attention Network Regions Do Not Generalize to all Stimulus-Specific Visual Processing Regions
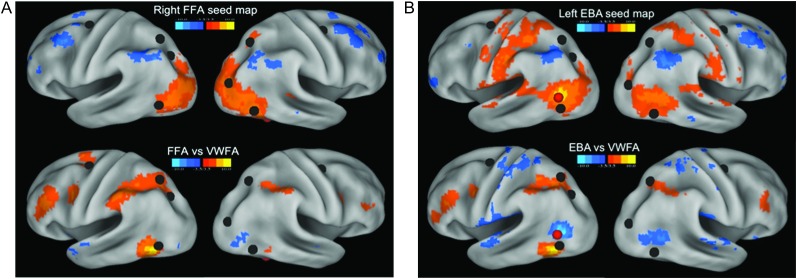
To test whether the relationship between the pVWFA and the dorsal attention network is a specific relationship or a general a property of regions thought to be stimulus-specific, we made seed maps showing all voxels significantly correlated with the rs-fcMRI time course of 2 other visual processing regions purportedly specialized for specific categories of stimuli—the right FFA and the left EBA. These seed maps were then directly compared with the pVWFA seed maps (Fig. 3).
Figure 3.
Specificity of rs-fcMRI correlations between the pVWFA and dorsal attention network regions. (A) Top panel shows the seed map of voxels significantly correlated (Z ≤ −3.5 or Z ≥ 3.5) with the right FFA rs-fcMRI time course in 23 adults. Bottom panel shows all voxels significantly different between the pVWFA and FFA seed maps. Positive Z-scores (in warm colors) indicate those voxels with significantly stronger correlations with the pVWFA; negative Z-scores (in cool colors) indicate those voxels with significantly stronger correlations with the FFA. Both are overlaid with locations of dorsal attention network regions in black. (B) Top panel shows the seed map of voxels significantly correlated (Z ≤ −3.5 or Z ≥ 3.5) with the left EBA rs-fcMRI time course in 23 adults. Bottom panel shows all voxels significantly different between the pVWFA and EBA seed maps. Positive Z-scores (in warm colors) indicate those voxels with significantly stronger correlations with the pVWFA; negative Z-scores (in cool colors) indicate those voxels with significantly stronger correlations with the left EBA. Both are overlaid with locations of dorsal attention network regions in black and the location of the EBA seed in red.
A seed placed on the right FFA shows some correlations with the right parietal dorsal attention network in adults (top panel, Fig. 3A). However, directly comparing the seed maps or the pVWFA and FFA with a paired t-test of each voxel shows significantly stronger correlations between the pVWFA and dorsal attention regions than between the FFA and dorsal attention regions (warm colors in bottom panel of Fig. 3A).
A seed placed on the left EBA also shows some correlation with the dorsal attention network in adults (top panel, Fig. 3B). However, a t-test of the pVWFA and EBA seed maps showed pVWFA to be significantly more correlated to the dorsal attention regions than is the EBA (warm colors in bottom panel of Fig. 3B).
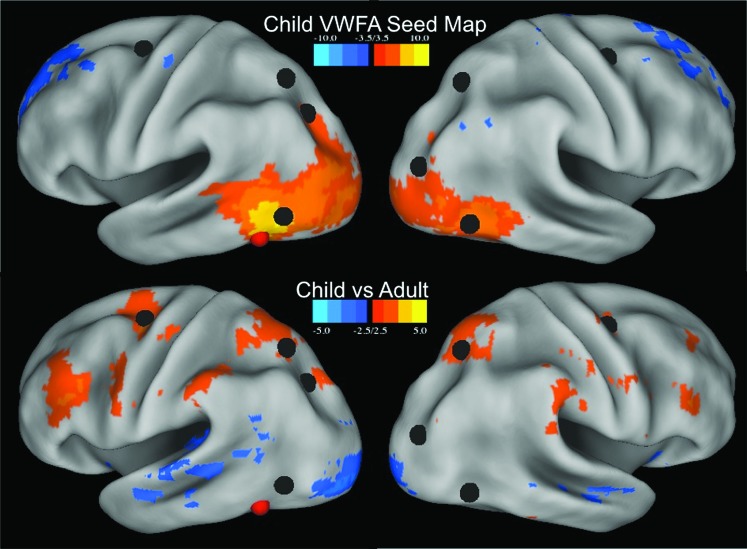
rs-fcMRI Based Functional Connectivity of the pVWFA Changes with Age
A seed map was also constructed for the pVWFA in 22 children (ages 7–9 years). The child pVWFA seed map shows some overlap with the dorsal attention network (top panel, Fig. 4). However, a direct comparison of the children and adults via a paired t-test of the 2 seed maps shows that adults have significantly stronger correlations between the pVWFA and dorsal attention regions than children (bottom panel, Fig. 4). Directly comparing the correlation values between the pVWFA and each of the dorsal attention regions individually shows significant differences (P < 0.05) between correlations with the left and right FEF, left aIPS, and right aIPS regions. While there was also a significant age-related difference between the relationship between the pVWFA and reading-related regions including the left IFG, AG, and ITG regions (P = 0.0002, P = 0.012, and P = 0.0017, respectively), in the case of the left AG and ITG, this difference was due a shift from nonsignificant correlations in children to negative correlations in adults (left AG children average r = 0.015, adults average r = −0.14; left ITG children average r = −0.019, adults average r = −0.19).
Figure 4.
Developmental differences in pVWFA rs-fcMRI correlations. Top panel shows the seed map of all voxels correlated with the pVWFA in 22 children (7–9 years old) with Z ≤ −3.5 and Z ≥ 3.5. Bottom panel shows the difference map of all voxels with a significant difference (Z ≤ −2.5 or Z ≥ 2.5, P < 0.01) in VWFA correlations between children (n = 22) and adults (n = 23). Correlations stronger in adults are shown in warm colors and those stronger in children in cool colors. The locations of the dorsal attention network regions are shown in black and the location of the pVWFA seed in red.
When a movement-matched group of children and adults was used, only the age-related differences in pVWFA to left FEF correlations remained significant (P = 0.023), though the mean correlation value was still qualitatively increased in adults relative to children in the remaining regions and the pVWFA/left aIPS correlation difference approached trend level (P = 0.15). When the age-related differences in rs-fcMRI correlations between the pVWFA and reading-related regions were tested in the movement-matched groups, the change in correlations between the pVWFA/left AG and pVWFA/left ITG relationship maintained trend level significance (P = 0.054 and P = 0.048, respectively), but, again, these correlations change from a nonsignificant r value in children to a negative r value in adults.
The rs-fcMRI Relationships between the pVWFA and aIPS Regions of the Dorsal Attention Network Are Correlated with Reading Level, though the Relationship between the pVWFA and Reading-Related Regions Are Not
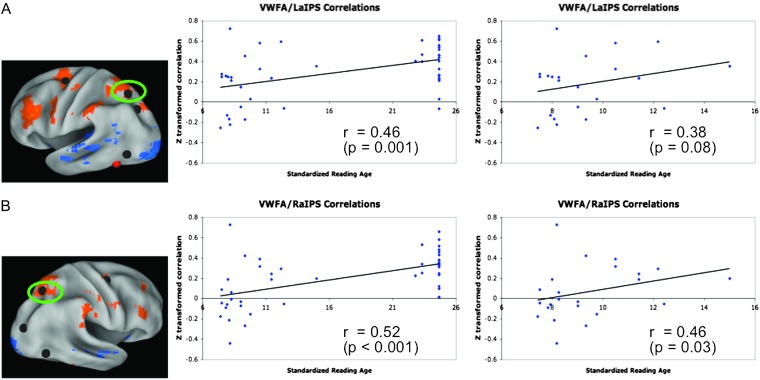
Correlations between standard reading age and the correlations between the pVWFA and dorsal attention regions were calculated across the combined group of children and adults. Only the pVWFA to left and right aIPS correlations were significantly correlated with reading age in this combined group (Fig. 5). The correlations between standardized reading age and left pVWFA and left and right IPS across the age groups were 0.462 (P = 0.001) and 0.542 (P < 0.0001), respectively. When chronological age and movement were controlled for using partial correlations, these correlations were r = 0.185 (P = 0.234) for the VWFA/left aIPS correlation and r = 0.340 (P = 0.026) for the VWFA/right aIPS correlation.
Figure 5.
Relationship between reading age and pVWFA functional correlations. (A) Relationships between reading age and pVWFA to left aIPS rs-fcMRI correlations. The location of the left aIPS region is shown on the left, the plot of all subjects (n = 23 adults and 22 children) and the line of best fit are shown in the middle panel, and the plot of child subjects only with the line of best fit is shown on the right. Note that when a partial correlation controlling for chronological age and movement is performed the correlation for the combined child/adult group drops to r = 0.19 (P = 0.23), but the correlation for the child only group increases to r = 0.43 (P = 0.06). (B) Relationships between reading age and pVWFA to right aIPS rs-fcMRI correlations. The location of the right aIPS region is shown on the left, the plot of all subjects (n = 23 adults and 22 children) and the line of best fit are shown in the middle panel, and the plot of child subjects only with the line of best fit is shown on the right. Note that when a partial correlation controlling for chronological age and movement is performed the correlation for the combined child/adult group drops to r = 0.14 (P = 0.03), but the correlation for the child only group increases to r = 0.53 (P = 0.02).
Given the narrow range of adult reading ages, we repeated the above-described correlation analysis in the child only group. For children, only the pVWFA/left and right aIPS correlations were significantly correlated with reading age. When the correlation between pVWFA and left aIPS was correlated directly with the reading age, a trend-level significance was obtained (r = 0.383, P = 0.079). When age and movement were controlled for in a partial correlation, the correlation between the pVWFA/left aIPS correlation and reading age was 0.431 (P = 0.057). Similarly, when the pVWFA/right aIPS correlation was directly correlated with reading age, the pearson's r was 0.460 (P = 0.031) and when age and movement were controlled for in a partial correlation the r was 0.526 (P = 0.017).
The correlation between standardized reading age and rs-fcMRI correlation strength between the pVWFA and all reading-related regions were also calculated. There was no significant correlation between standardized reading age and any pVWFA to reading region fc-MRI connectivity (pVWFA/left IFG: r = −0.115, P = 0.601; VWFA/left SMG: r = 0.009, r = 0.969; VWFA/left AG: r = 0.248, P =0.253; VWFA/left ITG: r = 0.341, P = 0.112). When chronological age and movement were controlled for in a partial correlation analysis, these relationships remained nonsignificant (pVWFA/left IFG: r = −0.028, P = 0.905; VWFA/left SMG: r = −0.046, P = 0.841; VWFA/left AG: r = 0.211, P = 0.360; VWFA/left ITG: r = 0.353, P = 0.116). While the correlation between standardized reading age and the pVWFA/left ITG connection approaches trend level, this correlation is in the positive direction, while the age-related change in the pVWFA/left ITG relationship is in the opposite direction, developing from a nonsignificant relationship in children to a negative relationship in adults.
Discussion
We have demonstrated that the pVWFA has resting-state functional correlations with regions in the dorsal attention network (left and right aIPS, MT+, and FEF regions) with minimal or absent correlations with reading-related regions (left SMG, AG, and ITG regions). These pVWFA to dorsal attention correlations are not a general property of all regions in the fusiform cortex or even of all specialized visual processing regions; the pVWFA shows more significant connectivity with dorsal attention regions than the right FFA and the left EBA (despite the latter's close proximity to left MT+). The rs-fcMRI correlations between pVWFA and some regions of the dorsal attention network appear to increase with age and correlations between the pVWFA and bilateral aIPS regions of the dorsal attention network also increase with reading skill. There are no such skill-related changes between the pVWFA and reading-related regions. Together, these results point to a role for the pVWFA in processing visual stimuli in general, presumably a role shaped by its relationship with regions of the dorsal attention network. Thus, while the VWFA may be considered an important region for reading, the data do not support the notion that “reading-related processing” should be its predominant functional ascription.
Resting-State Functional Connections of the pVWFA
The seed map of regions showing voxels with rs-fcMRI time courses significantly correlated to the pVWFA does not overlap significantly with regions thought to be preferentially important for reading but does overlap with regions of the dorsal attention network. There is a region in left MFG correlated to the pVWFA. As mentioned previously, this region is 10 mm Euclidean distance anterior and superior to our reading meta-analysis defined IFG region and even further from left IFG regions reported in previous meta-analyses of single-word reading studies (23.4 mm Euclidean distance from Fiez and Petersen 2008; 25.1 mm Euclidean distance from Jobard et al. 2003). While the relationship between the pVWFA and dorsal attention regions was not the focus of previously reported studies that included resting-state functional connectivity of the pVWFA, it is apparent from viewing figures presented in Koyama et al. 2010 and Zhao et al. 2011 that these groups also see resting-state correlations between the pVWFA and dorsal attention regions. The correlations between the pVWFA and dorsal attention regions were not commented upon in these papers, perhaps because the authors were investigating locations of consistent activity across reading-related regions. Recently, Wang et al. (2011) demonstrated a similar correlation between the pVWFA and regions of the dorsal attention network in task-related time courses. Taken together, the pattern of pVWFA rs-fcMRI functional connectivity suggests that the processing done in this region is more closely related to spatial attention than reading, per se.
There is the possibility that the lack of correlations between the pVWFA and reading-related regions could be due to the application of a 10 mm sphere centered on the meta-analysis derived coordinates. By using a sphere as the seed region, nonselective voxels may be intermixed with voxels that are selective for reading and/or words. However, this accounting of the results is unlikely, as there are not only statistically stronger correlations between the VWFA and dorsal attention regions than reading regions but a very different pattern of correlations between the 2 region groups. The pVWFA is consistently positively correlated with regions of the dorsal attention system, while there are negative correlations between pVWFA and 2 of the 4 reading-regions—making it unlikely that there are “weak” correlations being overpowered by spatial averaging. Moreover, if the pVWFA seed was confounded by the effects of spatial averaging, the expected result would be to have reduced correlation strength with regions across the brain. The pVWFA, though, does have strong correlations with regions of the dorsal attention network, correlations that are even stronger than nearby EBA and FFA regions. Therefore, the likelihood is low that the lack of correlations between the pVWFA and reading-related regions is due to the size of the seed region sphere.
The pVWFA as a General-Use Region
The pattern of pVWFA rs-fcMRI connectivity is not consistent with a special role for this region in reading and instead supports the notion that the pVWFA acts more generally across stimulus types. However, the results are consistent with the contention that the VWFA contributes to reading as a more general-use region with properties useful for reading. rs-fcMRI correlations are thought to reflect a history of coactivation (Dosenbach et al. 2006; Fair, Dosenbach, et al. 2007; Lewis et al. 2009; Stevens et al. 2010; Tambini et al. 2010). In our seed map analyses, we are potentially seeing the outcome of the strongest and most consistent of those coactivations.
The argument that the pVWFA is not used exclusively or predominantly for reading is consistent with much of the literature. Numerous studies report activity in this region for nonword and nonletter stimuli (Price and Devlin 2003; Xue et al. 2006; Ben-Shachar et al. 2007; Ploran et al. 2007; Starrfelt and Gerlach 2007; Xue and Poldrack 2007; Kherif et al. 2011; Mei et al. 2010; Van Doren et al. 2010). We argue that rather than processing word or letter stimuli in particular, the pVWFA is more likely to be a general-use region with properties particularly useful for reading, such as processing visual stimuli in groups or chunks of various sizes, as described in the Introduction.
If this description were true, the pVWFA would be often activated with dorsal attention regions (due to its ability to process stimuli in variably sized groups) occasionally with reading-related regions and sometimes with other nonreading task- or stimulus-specific region sets. In this case, the history of coactivation would emphasize the correlations between the pVWFA and other more task-general regions with which it is often coactivated (like the dorsal attention system regions), with the statistical remainder spread among many differing combinations of regions.
While we do not argue that the VWFA is exclusively or even predominantly involved in reading, for the remainder of this discussion we will frame the significance of the functional connections between the pVWFA and the dorsal attention network in the context of letter and word processing. While the same relationships could apply to other kinds of visual stimuli, we contend that using letters and words as examples of the utility of this relationship will be most illuminating given the long history of studying the VWFA in reading, its likely involvement in reading at some level, and the ease of describing these effects on words, which are a well-defined type of visual stimulus.
Properties of the Dorsal Attention System
Previous studies have shown the dorsal attention network is involved in overt (Petit et al. 1997; Connolly et al. 2000) and covert (Gitelman et al. 1999; Sylvester et al. 2007; Fairhall et al. 2009) spatial attention and orienting. These regions show increased activity in the preparatory period of cued spatial and feature-based attention tasks (for a review, see Corbetta and Shulman 2002) and visual search tasks (Leonards et al. 2000; Egner et al. 2008; Fairhall et al. 2009). Concomitant with these dorsal attention responses, there are changes in BOLD activity in visual regions representing the attended spatial location (Sylvester et al. 2007) and suppression of BOLD activity in regions outside the attended spatial location (Sylvester et al. 2008). It is thought that the posterior parietal regions of the dorsal attention system are related to posterior parietal cortex regions in macaque, which contain cells responsive to spatial and feature attention cues that also modulate activity in visual processing regions like MT+ (Saalmann et al. 2007). Therefore, it has been hypothesized that the dorsal attention network plays a role in visual attention by amplifying the “lower level” visual responses to specific spatial locations and features and dampening the responses to locations and features outside of the attentional window (Corbetta and Shulman 2002).
Role of the Dorsal Attention System in Reading
As described in the Introduction, we argue the pVWFA process groups of letters (and other visual items) in variously sized chunks. These variably sized chunks result in preferentially strong functional connections with regions that direct attention to the appropriate “chunk” of features or spatial locations. If this is the case, and regions in the dorsal attention network direct attention to the appropriate spatial group, activity in dorsal attention system regions should also be modulated by properties that affect letter “chunking.” As mentioned in the Introduction, reading pseudowords requires processing letters in smaller chunks as compared with reading words and so reading pseudowords should require more changes in spatial attention. In fact, the bilateral aIPS regions of the dorsal attention network do show more activity for reading pseudowords than for reading words (Church et al. 2011). Furthermore, aIPS regions show a length effect, whereby longer words and nonwords, which should require more attention shifts, show more activity than shorter words and nonwords (Church et al. 2011; Schurz et al. 2010).
Additionally, any manipulation that presents words in a format that decreases the ability of the visual system to chunk the letters or requires more shifts of spatial attention should increase dorsal attention–related parietal activity. Words presented in unusual formats of many kinds—including mixed-case stimuli (Mayall et al. 2001), rotated words, words with many spaces between the letters, words presented to the left of fixation (Cohen et al. 2008), and vertically presented words (Rosazza et al. 2009)—have all been shown to increase lateral parietal activity. Pammer et al. (2004) uses magnetoencephalography to determine the time course of activity for reading shifted-case stimuli (where every other letter is presented superior to the normal line of text) and report that there is mutual feedback between the parietal and fusiform regions when words are presented in this unique form. Moreover, while all of the manipulations described here increase the response time to read words, increased parietal activity was not seen when subjects performed the same tasks on words with low visual contrast, even though the response time to process these stimuli was just as long as the mixed case stimuli (Mayall et al. 2001). Recently, an analysis of task-based functional connectivity in older children demonstrated a relationship between the pVWFA and bilateral IPS and SPL regions overlapping the dorsal attention regions (Van der Mark et al. 2011).
Directed attention is not only important for reading single words; it is perhaps even more important for reading connected text. Data from eye-movement studies indicate that fluent reading is associated with a particular pattern of eye movements, whereby subjects land consistently to the left of center in a word and have generally one or fewer eye movements per word (for a review, see Rayner 1998). The dorsal attention network has been implicated in directing eye movements (Petit et al. 1997; Connolly et al. 2000), and Lee and Newman (2010) recently found increased activity in inferior and superior parietal lobule regions during whole sentence presentation, which requires directed eye movements, relative to rapid serial visual presentation, in which words are presented one at a time. Further investigations into the relationships between the pVWFA and dorsal attention regions during fluent reading of connected text should more directly inform this question.
Developmental Changes in pVWFA to Dorsal Attention System Connectivity
Developmental changes have been reported for both within-word letter processing and in reading connected text. Children appear to rely more on making orthographic to phonological conversions of individual word chunks than adults (Schlaggar and McCandliss 2007). Unlike adults, children show response times to read words that are dependent on word length (Defior et al. 1996; Bijeljac-babic et al. 2004; Martens and de Jong 2008). Additionally, children are significantly slower to read words with irregular orthographic to phonologic conversions than words with regular mapping, a reflection of their increased use of assembled phonology (Backman et al. 1984; Sandak et al. 2004). Children also have shorter saccades and longer fixations, indicative of less fluent eye movements, when reading connected text (Rayner 1998). These effects could indicate a less efficient relationship between the pVWFA and the dorsal attention system in early as compared with skilled readers. The development of this relationship may be reflected, at least partially, in both the age-related increases in correlations between the pVWFA and some dorsal attention regions and the reading-related increases in correlations between the pVWFA and bilateral aIPS regions.
While our data do not directly bear on the question of how the relationship between pVWFA and dorsal attention regions might develop, we speculate that interactions between these regions as children gain familiarity with the statistical regularities of real words are important. Computational models of reading (e.g., Harm and Seidenberg 2004) indicate that words become processed as a group or in large chunks due to the experience of seeing certain groups of letters presented together many times. We speculate that as the pVWFA becomes tuned to such statistical regularities, these larger chunks come to capture attention even more efficiently than single letters. This attentional “capture” may then feedforward into regions directing spatial attention. An interplay between these feedforward effects and feedback effects of attentional processing and task control could shape not only the processing of words and letter groups in the pVWFA but also the relationship between the pVWFA and dorsal attention regions.
However, there were limitations in our ability to see developmental differences related to age or reading level in this study. First, the children in this study are already relatively good readers (average reading age 9.5 years), which restricts our ability to see the earliest developmental effects. Additionally, we have no direct measure of either process we purport may be related to the VWFA to dorsal attention connectivity. We do not know to what extent the children are still reading with a phonological strategy, converting graphemes into phonemes rather than processing words as a whole. We also have no measure of connected text reading fluency. Standardized reading age can act as a surrogate of both, as both improve with increased reading ability, but further studies should be done to determine whether either of these measures relates to pVWFA/dorsal attention network correlations specifically.
Dorsal Attention System Processing and Dyslexia
Dyslexia—or reading deficits that result in a reading level that is significantly reduced relative to IQ despite access to the opportunity to learn to read—has generally been thought of as a phonological processing deficit (for a review, see Shaywitz 1998). However, there is increasing evidence that deficits in visual attention may also play a role in dyslexia (for reviews, see Valdois et al. 2004 and Vidyasagar and Pammer 2010). Dyslexic children show impairments in matching symbol strings, a visual processing task that requires no lexical processing but does require processing spatial relationships (Pammer et al. 2004). Dyslexic children with and without obvious phonological impairments also show deficits in simultaneous processing of consonant strings (Lassus-Sangosse et al. 2008). More specific attentional deficits are seen in impairments in exogenous orienting tasks exhibited by a subset of dyslexic children (Facoetti et al. 2010). Though the relationship between dyslexia and visual processing or attention is a matter of debate at present (i.e., Shovman and Ahissar 2006; Ziegler et al. 2010), the results presented here indicate a role for the dorsal attention system in visual specialization of the type used in fluent reading. Moreover, a recent study of task based functional connectivity in typical and dyslexic child readers shows reduced task-based connectivity between the pVWFA and bilateral parietal regions close to those found in the dorsal attention network (Van der Mark et al. 2011). Further study of resting-state correlations in dyslexic subjects may increasingly shed light on whether visual attention impairments are contributing to some subjects' disordered reading.
Summary and Conclusions
This study demonstrates relatively weak rs-fcMRI relationships between the pVWFA (thought to be involved in visual processing of words and letters) and regions thought to be integral to reading, including the left SMG and AG and potentially the left IFG. In contrast, we observed strong rs-fcMRI correlations between the pVWFA and regions in the dorsal attention network. This pattern of functional connectivity indicates the pVWFA may well not be predominantly used for reading, but instead, is likely to be a more general-use visual region that is able to process stimuli in groups. The relationship between the pVWFA and the dorsal attention network may be related to the ability of the pVWFA to group stimuli, which, in turn, may be used to parse visual stimuli, like words, into appropriate visual components and interact with dorsal attention networks to direct eye movements to the appropriate spatial locations. Just as these skills develop with age and reading level, we see increased correlations between the pVWFA and parts of the dorsal attention system with increases in age and reading level.
Funding
National Institutes of Health (grant numbers: NS0534425 and HD057076 to B.L.S. and NS61144 and NS6144 to S.E.P.); National Science Foundation (Interactive Graduate Education and Research Training grant number 0548890 to A.C.V.).
Acknowledgments
The authors would like to gratefully acknowledge Jessica Church, Kelly Barnes, and Jonathan Power for their careful reading and critiques of this manuscript. We would also like to thank Rebecca Coalson, Kelly McVey, and Becca Lepore for their assistance in data collection. Conflict of Interest: None declared.
References
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Res. 2006;1076:129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Aleong R, Paus T. Neural correlates of human body perception. J Cogn Neurosci. 2010;22:482–495. doi: 10.1162/jocn.2009.21211. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci. 2004;7:542–548. doi: 10.1038/nn1241. [DOI] [PubMed] [Google Scholar]
- Backman J, Bruck M, Hebert M, Seidenberg MS. Acquisition and use of spelling-sound correspondences in reading. J Exp Child Psychol. 1984;38:114–133. [Google Scholar]
- Behrmann M, Black SE, Bub D. The evolution of pure alexia: a longitudinal study of recovery. Brain Lang. 1990;39:405–427. doi: 10.1016/0093-934x(90)90148-a. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Nelson J, Sekuler EB. Visual complexity in letter-by-letter reading: “pure” alexia is not pure. Neuropsychologia. 1998;36:1115–1132. doi: 10.1016/s0028-3932(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. Differential sensitivity to words and shapes in ventral occipito-temporal cortex. Cereb Cortex. 2007;17:1604–1611. doi: 10.1093/cercor/bhl071. [DOI] [PubMed] [Google Scholar]
- Berman MG, Park J, Gonzalez R, Polk TA, Gerhke A, Knaffla S, Jonides J. Evaluating functional localizers. the case of the FFA. NeuroImage. 2010;50:56–71. doi: 10.1016/j.neuroimage.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijeljac-babic R, Millogo V, Farioli F, Grainger J. A developmental investigation of word length effects in reading using a new on-line word identification paradigm. Read Writ Interdiscip J. 2004;17:411–431. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Booth JR, Cho S, Burman DD, Bitan T. Neural correlates of mapping from phonology to orthography in children performing an auditory spelling task. Dev Sci. 2007;10:441–451. doi: 10.1111/j.1467-7687.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci S, Ietswaart M, Peelen MV, Cavina-Pratesi C. Dissociable neural responses to hands and non-hand body parts in human left extrastriate visual cortex. J Neurophysiol. 2010;103:3389–3397. doi: 10.1152/jn.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Urgesi C, Orgs G, Aglioti SM, Haggard P. Extrastriate body area underlies aesthetic evaluation of body stimuli. Exp Brain Res. 2010;204:447–456. doi: 10.1007/s00221-010-2283-6. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Peelen MV, Downing PE. The effect of viewpoint on body representation in the extrastriate body area. Neuroreport. 2004;15:2407–2410. doi: 10.1097/00001756-200410250-00021. [DOI] [PubMed] [Google Scholar]
- Church JA, Balota DA, Petersen SE, Schlaggar BL. Manipulation of length and lexicality localizes the functional neuroanatomy of phonological processing in adult readers. J Cogn Neurosci. 2011;23:1475–1493. doi: 10.1162/jocn.2010.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb Cortex. 2008;18:2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NUF, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 2008;41:45–57. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehericy S, Samson Y, Obadia M, Slachevsky A, Dehaene S. Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex. 2003;13:1313–1333. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol. 2000;84:1645–1655. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Defior S, Justicia F, Martos FJ. The influence of lexical and sublexical variables in normal and poor Spanish readers. Read Writ Interdiscip J. 1996;8:487–497. [Google Scholar]
- Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56:384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Contribution a l'étude anatomoclinique et clinique des differentes varietes de cecite verbal. C R Hebd Séances Mém Soc Biol. 1892;4:61–90. [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Egner T, Monti JM, Trittschuh EH, Wieneke CA, Hirsch J, Mesulam MM. Neural integration of top-down spatial and feature-based information in visual search. J Neurosci. 2008;28:6141–6151. doi: 10.1523/JNEUROSCI.1262-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facoetti A, Trussardi AN, Ruffino M, Lorusso ML, Cattaneo C, Galli R, Molteni M, Zorzi M. Multisensory spatial attention deficits are predictive of phonological decoding skills in developmental dyslexia. J Cogn Neurosci. 2010;22:1011–1025. doi: 10.1162/jocn.2009.21232. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Indovina I, Driver J, Macaluso E. The brain network underlying serial visual search: comparing overt and covert spatial orienting, for activations and for effective connectivity. Cereb Cortex. 2009;19:2946–2958. doi: 10.1093/cercor/bhp064. [DOI] [PubMed] [Google Scholar]
- Fiez J, Petersen S. Neuroimaging studies of word reading. Proc Natl Acad Sci U S A. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clemenceau S, Volle E, Hasboun D, Dupont S, Baulac M, Dehaene S, Adam C, et al. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. Neural systems for reading aloud: a multiparametric approach. Cereb Cortex. 2010;20:1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M, Krasnow B, Reiss A, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. The human visual cortex. Annu Rev Neurosci. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. Computing the meanings of words in reading: cooperative division of labor between visual and phonological processes. Psychol Rev. 2004;111:662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Functional specificity in the human brain: a window into the functional architecture of the mind. Proc Nat Acad Sci USA. 2010;107:11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kherif F, Josse G, Price CJ. Automatic top-down processing explains common left cccipito-temporal responses to visual words and objects. Cereb Cortex. 2011;1:103–114. doi: 10.1093/cercor/bhq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP. Reading networks at rest. Cereb Cortex. 2011;20:2549–2559. doi: 10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapp. 1995;3:209–223. [Google Scholar]
- Lassus-Sangosse D, N'Guyen-Morel MA, Valdois S. Sequential or simultaneous visual processing deficit in developmental dyslexia? Vision Res. 2008;48:979–988. doi: 10.1016/j.visres.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Lee D, Newman SD. The effect of presentation paradigm on syntactic processing: an event-related fMRI study. Hum Brain Mapp. 2010;31:65–79. doi: 10.1002/hbm.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonards U, Sunaert S, Van Hecke P, Orban GA. Attention mechanisms in visual search—an fMRI study. J Cogn Neurosci. 2000;12(Suppl 2):61–75. doi: 10.1162/089892900564073. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens VEG, de Jong PF. Effects of repeated reading on the length effect in word and pseudoword reading. J Res Read. 2008;31:40–54. [Google Scholar]
- Mayall K, Humphreys GW, Mechelli A, Olson A, Price CJ. The effects of case mixing on word recognition: evidence from a PET study. J Cogn Neurosci. 2001;13:844–853. doi: 10.1162/08989290152541494. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J Cogn Neurosci. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Mei L, Xue G, Chen C, Xue F, Zhang M, Dong Q. The “visual word form area” is involved in successful memory encoding of both words and faces. Neuroimage. 2010;52:371–378. doi: 10.1016/j.neuroimage.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Occipitotemporal activation evoked by the perception of human bodies is modulated by the presence or absence of the face. Neuropsychologia. 2006;44:1919–1927. doi: 10.1016/j.neuropsychologia.2006.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A, Sowden PT. Your hand or mine? The extrastriate body area. Neuroimage. 2008;42:1669–1677. doi: 10.1016/j.neuroimage.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Pammer K, Lavis R, Hansen P, Cornelissen PL. Symbol-string sensitivity and children's reading. Brain Lang. 2004;89:601–610. doi: 10.1016/j.bandl.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Atkinson AP, Andersson F, Vuilleumier P. Emotional modulation of body-selective visual areas. Soc Cogn Affect Neurosci. 2007;2:274–283. doi: 10.1093/scan/nsm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Clark VP, Ingeholm J, Haxby JV. Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. J Neurophysiol. 1997;77:3386–3390. doi: 10.1152/jn.1997.77.6.3386. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, Kastner S. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J Neurophysiol. 2009;101:2581–2600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploran EJ, Nelson SM, Velanova K, Petersen SE, Wheeler ME. Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J Neurosci. 2007;27:11912–11924. doi: 10.1523/JNEUROSCI.3522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D'Esposito M, Detre JA, Farah MJ. Neural specialization for letter recognition. J Cogn Neurosci. 2002;14:145–159. doi: 10.1162/089892902317236803. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Rayner K. Eye movements in reading and information processing: 20 years of research. Psychol Bull. 1998;124:372–422. doi: 10.1037/0033-2909.124.3.372. [DOI] [PubMed] [Google Scholar]
- Rosazza C, Cai Q, Minati L, Paulignan Y, Nazir TA. Early involvement of dorsal and ventral pathways in visual word recognition: an ERP study. Brain Res. 2009;1272:32–44. doi: 10.1016/j.brainres.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Rueckl JG, Katz L, Moore DL, Mason SA, Fulbright RK, Constable RT, Pugh KR. The neurobiology of adaptive learning in reading: a contrast of different training conditions. Cogn Affect Behav Neurosci. 2004;4:67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Saxe R, Jamal N, Powell L. My body or yours? The effect of visual perspective on cortical body representations. Cereb Cortex. 2006;16:178–182. doi: 10.1093/cercor/bhi095. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schurz M, Sturm D, Richlan F, Kronbichler M, Ladurner G, Wimmer H. A dual-route perspective on brain activation in response to visual words: evidence for a length by lexicality interaction in the visual word form area (VWFA) Neuroimage. 2010;49:2649–2661. doi: 10.1016/j.neuroimage.2009.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE. Dyslexia. N Engl J Med. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shovman MM, Ahissar M. Isolating the impact of visual perception on dyslexics' reading ability. Vis Res. 2006;46:3514–3525. doi: 10.1016/j.visres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20:813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AZ. Difference image vs. ratio image error function forms in PET-PET realignment. In: Myer R, Cunningham VJ, Bailey DL, Jones T, editors. Quantification of brain function using PET. San Diego (CA): Academic Press; 1996. pp. 131–137. [Google Scholar]
- Spiridon M, Fischl B, Kanwisher N. Location and spatial profile of category-specific regions in human extrastriate cortex. Hum Brain Mapp. 2006;27:77–89. doi: 10.1002/hbm.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrfelt R, Gerlach C. The visual what for area: words and pictures in the left fusiform gyrus. Neuroimage. 2007;35:334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Habekost T, Leff AP. Too little, too late: reduced visual span and speed characterize pure alexia. Cereb Cortex. 2009;19:2880–2890. doi: 10.1093/cercor/bhp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Jack AI, Corbetta M, Shulman GL. Anticipatory suppression of nonattended locations in visual cortex marks target location and predicts perception. J Neurosci. 2008;28:6549–6556. doi: 10.1523/JNEUROSCI.0275-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Shulman GL, Jack AI, Corbetta M. Asymmetry of anticipatory activity in visual cortex predicts the locus of attention and perception. J Neurosci. 2007;27:14424–14433. doi: 10.1523/JNEUROSCI.3759-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Valdois S, Bosse ML, Tainturier MJ. The cognitive deficits responsible for developmental dyslexia: review of evidence for a selective visual attentional disorder. Dyslexia. 2004;10:339–363. doi: 10.1002/dys.284. [DOI] [PubMed] [Google Scholar]
- Van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in reading. Neuroimage. 2011;54:2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Van Doren L, Dupont P, De Grauwe S, Peeters R, Vandenberghe R. The amodal system for conscious word and picture identification in the absence of a semantic task. Neuroimage. 2010;49:3295–3307. doi: 10.1016/j.neuroimage.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;41:1359–1378.. doi: 10.1136/jamia.2001.0080443. See also http://brainmap.wustl.edu/caret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyasagar TR, Pammer K. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends Cogn Sci. 2010;14:57–63. doi: 10.1016/j.tics.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang J, Shu H, Zevin JD. Left fusiform BOLD responses are inversely related to word-likeness in a one-back task. Neuroimage. 2011;55:1346–1356. doi: 10.1016/j.neuroimage.2010.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio (TX): The Psychological Corporation; 1999. [Google Scholar]
- Weekes BS. Differential effects of number of letters on word and nonword naming latency. Q J Exp Psychol Sect A Hum Exp Psychol. 1997;50:439–456. [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock–Johnson-revised tests of achievement. Itasca (IL): Riverside Publishing; 2002. [Google Scholar]
- Xue G, Chen C, Jin Z, Dong Q. Language experience shapes fusiform activation when processing a logographic artificial language: an fMRI training study. Neuroimage. 2006;31:1315–1326. doi: 10.1016/j.neuroimage.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Xue G, Poldrack RA. The neural substrates of visual perceptual learning of words: implications for the visual word form area hypothesis. J Cogn Neurosci. 2007;19:1643–1655. doi: 10.1162/jocn.2007.19.10.1643. [DOI] [PubMed] [Google Scholar]
- Zhao J, Liu J, Li J, Liang J, Feng L, Ai L, Lee K, Tian J. Intrinsically organized network for word processing during the resting state. Neurosci Lett. 2011;1:27–31. doi: 10.1016/j.neulet.2010.09.067. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Pech-Georgel C, Dufau S, Grainger J. Rapid processing of letters, digits and symbols: what purely visual-attentional deficit in developmental dyslexia? Dev Sci. 2010;13:F8–F14. doi: 10.1111/j.1467-7687.2010.00983.x. [DOI] [PubMed] [Google Scholar]