Abstract
Viewing emotional as compared with neutral images results in an increase in force production. An emotion-driven increase in force production has been associated with increased brain activity in ventrolateral prefrontal cortex and primary motor cortex (M1). In many instances, however, force production must be held constant despite changes in emotional state and the neural circuits underlying this form of control are not well understood. To address this issue, we designed a task in which subjects viewed pleasant, unpleasant, and neutral images during a force production task. We measured brain activity using functional magnetic resonance imaging and examined functional connectivity between emotion and motor circuits. Despite similar force performance across conditions, increased brain activity was evidenced in dorsomedial prefrontal cortex (dmPFC) and left ventral premotor cortex (PMv) when force was produced during emotional as compared with neutral conditions. Connectivity analyses extended these findings by demonstrating a task-dependent functional circuit between dmPFC and ventral and dorsal portions of premotor cortex. Our findings show that when force production has to be consistent despite changes in emotional context, a functional circuit between dmPFC and PMv and dorsal premotor cortex is engaged.
Introduction
Emotional states often facilitate motor function. However, human performance in the medical (Moorthy et al. 2003), military (Wallenius et al. 2004), and competitive sporting domains (Hammermeister and Burton 2001) can also be negatively altered by emotional state. In these instances, the inability to control one’s movements in highly charged emotional contexts can lead to injury and failure. The suggestion that emotional and motor neural circuits are anatomically and/or functionally linked is supported by behavioral studies and transcranial magnetic stimulation (TMS) studies which show that emotions prime the motor system for action, reduce reaction time (RT), increase the amplitude of voluntary force production, and increase excitability of the corticospinal motor tract (Frijda 1986; Flykt 2005, 2006; Coombes et al. 2006, 2007a, 2007b, 2008, 2009; Hajcak et al. 2007; van Loon et al. 2010; Elliot and Aarts 2011). Human brain imaging evidence also shows that increased activity in ventral pallidum corresponds with an increase in maximal force production following subliminally presented reward cues (Pessiglione et al. 2007), whereas activity in midbrain regions and medial prefrontal cortex (PFC) has been associated with threat detection and panic-related motor errors during a maze tracing task (Mobbs et al. 2007, 2009). Despite our knowledge of the behavioral consequences of performing motor tasks in emotional contexts and our understanding of the neural circuits that translate reward- and threat-related stimuli into motor output, the neural basis for how precise motor functions are controlled in pleasant and unpleasant emotional contexts remains poorly understood.
In the current study, we examined how memory guided force control is maintained despite changes in emotional context. Convergent evidence from brain imaging studies in humans identifies the PFC as a key region that underlies both emotional and motor processes. Up- and down-regulation of emotional reactivity to emotional images and the top-down interpretation of neutral images as aversive have each been associated with increased activity in PFC (Kim and Hamann 2007; Ochsner et al. 2009). Importantly, the reappraisal of negative scenes in unemotional terms has been associated with an increase in medial PFC activity and a corresponding decrease in amygdala activity (Ochsner et al. 2002; Kanske et al. 2011). Lateral and medial prefrontal regions are also engaged by cognitive emotion regulation strategies that inhibit amygdala activity and diminish fear (Delgado et al. 2008). Memory guided force production has also been associated with activity in regions of the human PFC including the anterior cingulate cortex, dorsolateral PFC, and ventral PFC (Vaillancourt et al. 2003). Cyclical bimanual movements performed without visual feedback also support a role for PFC in memory guided motor tasks, with activation noted in supplementary motor area (SMA), cingulate motor area, basal ganglia, inferior parietal lobe (IPL), and cerebellar lobule IV-V/dentate (Debaere et al. 2003; Heuninckx et al. 2010). These findings are consistent with neurophysiological and imaging studies that identify activity in prefrontal areas with internally regulated motor actions (Deiber et al. 1999; Jenkins et al. 2000; Ogawa et al. 2006).
A brain imaging study in humans and a neuronal recording study in rats have advanced our understanding of the role that PFC plays in controlling motor functions in emotional contexts. Viewing emotional as compared with neutral images led to increased force production and increased blood oxygen level–dependent (BOLD) activity in human ventrolateral PFC (vlPFC) (Schmidt et al. 2009). Importantly, vlPFC activity predicted increased BOLD signal in left primary motor cortex (M1). In many instances, however, force production must be held constant despite changes in emotional state and the neural circuits underlying this form of control are not well understood. In contrast to the suggested vlPFC-M1 pathway in humans which facilitates force output, evidence from a rat study has shown functional interactions between dorsomedial PFC (dmPFC) and motor cortex, which correspond with the inhibition of inappropriate motor responses (Narayanan and Laubach 2006). Albeit from different species, these findings suggest that different regions of the PFC may facilitate or inhibit the amplitude of force output. The role that PFC has in maintaining consistent force production despite changes in emotional context has not been examined in humans.
To examine this issue, human subjects viewed pleasant, unpleasant, and neutral images during a force production task. We measured force production and brain activity using functional magnetic resonance imaging (fMRI) and also examined task-dependent functional connectivity between motor and emotional circuits. Before entering the magnet, subjects were trained to consistently produce accurate force pulses to a target level. Inside the magnet, subjects tried to maintain this same level of force production despite changes in emotional context, allowing us to identify the brain circuits that regulate this behavior. Based on the study by Narayanan and Laubach (2006), the main hypothesis was that dmPFC will show increased activity when force production has to be maintained in an emotional context. Furthermore, we used connectivity analyses to determine which circuits have altered task-dependent functional connectivity with dmPFC when force is controlled in an emotional context.
Materials and Methods
Subjects
Fifteen healthy right-handed subjects with normal or corrected to normal vision participated (8 females, 7 males; M = 21.53 years, standard deviation [SD] = 3.5 years, range: 19–32 years). Each subject provided informed consent to all procedures, which were approved by the local Institutional Review Board and were in accord with the Declaration of Helsinki. All subjects were prescreened for contraindications to MRI such as pregnancy, claustrophobia, and metallic implants. In addition, because psychiatric disorders, neurological disorders, and drug use can alter reactivity to emotional stimuli (Drevets 2000; Siegle et al. 2002; Bowers et al. 2006; Asensio et al. 2010), subjects who verbally self-reported any history of these disorders were excluded during prescreening. All subjects who were recruited completed the State and Trait segments of the STAI Anxiety Inventory (STAI-S, STAI-T: Spielberger 1983) and the Beck Depression Inventory (BDI: Beck and Steer 1987). All scores were within the range of responses typically given by healthy normal subjects (STAI-S: M = 27.3, range = 26–49; STAI-T: M = 33.5, range = 20–42; BDI: M = 3.2, range = 0–14).
Experimental Protocol
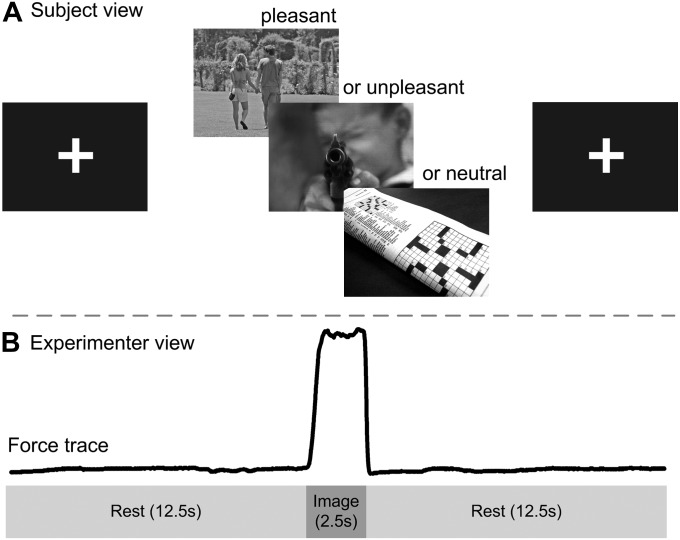
Figure 1 shows the time course for the event-related task. Subjects were asked to fixate on a white cross on a black background for 12.5 s. The cross was then replaced with a pleasant, unpleasant, or neutral image. Subjects were instructed to look at the image for the entire time it was on the screen and to produce a force pulse to a prepracticed level (15% of maximum voluntary contraction [MVC]) for the 2.5 s duration of the image presentation. At image offset, the white cross was again visible and the subject stopped producing force.
Figure 1.
The emotional processing and force production task. The figure shows the subject view (A) and the experimenter view (B) for one trial. (A) Each trial began with a 12.5-s rest period during which subjects were instructed to focus their gaze on the white fixation cross which was presented on the visual display. This fixation cross was then replaced by a pleasant, unpleasant, or neutral image for 2.5 s. Image onset was the cue for subjects to begin producing force. Image offset was the cue for subjects to stop producing force. Subjects produced force to 15% of their MVC without visual feedback. Prior to entering the scanner, all subjects were trained to produce 15% of MVC without visual feedback. (B) Force production, which coincided with image presentation was viewed by the experimenter and is shown in the time series in Figure 1B. Pleasant, unpleasant, and neutral image order were pseudorandomized for each subject to ensure that no more than 2 images from the same category were presented in a row.
Force Task
Prior to entering the magnet, all subjects completed a practice session to ensure that they could consistently produce pinch grip force pulses to 15% of their MVC without visual feedback. A graded practice session began with the calculation of each subjects’ MVC. Next, subjects produced a series of 2.5-s pulses to a target level (15% of MVC) with the aid of real-time visual feedback. Feedback was then phased out and replaced with neutral images. Neutral images were used during the practice session because we are not aware of any habituation effects to unique neutral images. The neutral images used during the practice session were not used during the experimental task. When performing the task with images instead of visual feedback, subjects received feedback (mean, SD of force) after a series of trials. The practice session took approximately 1.5 h and was complete when subjects could complete 30 trials without visual feedback, while maintaining a SD < 1.5% of MVC. All subjects reached the required level of performance.
The same custom fiber optic force transducer (Aither Engineering) was used during the practice session and the experimental task. The force transducer was constructed from rigid nonmetallic material to allow for its use inside the magnetic resonance environment. The force signal was transmitted via fiber optic wire to the Si425 Fiber Optic Interrogator (Micron Optics) outside the fMRI environment. The Si425 Fiber Optic Interrogator digitized the force data at 125 Hz and customized software written in LabView collected the force data and converted it to Newtons. The force transducer was factory calibrated by Aither Engineering and had a resolution of 0.025 N.
Subjects were required to produce force against the transducer with the index finger and thumb of their right hand in a precision grip formation (Coombes et al. 2010, 2011). Their left arm was extended down the left side of the body and their left hand remained in a relaxed and comfortable position. Subjects were instructed to produce the force pulse as quickly and accurately as possible to image onset, to maintain this level of force as accurately as possible for the duration of the image, and to stop producing force as quickly as possible to the offset of the image. In between images, subjects were instructed to relax their right hand and look at the fixation cross which appeared in the center of the screen.
Picture Stimuli
Subjects viewed a total of 90 images taken from the International Affective Picture System (Lang et al. 2008) (International Affective Picture Stimuli. Pleasant: 4608, 4651, 4652, 4656, 4658, 4659, 4668, 4670, 4672, 4681, 4683, 4687, 4689, 4693, 4694, 4695, 4698, 4810, 5621, 5629, 8030, 8158, 8163, 8179, 8180, 8185, 8200, 8370, 8400, 8490. Unpleasant: 2811, 3000, 3010, 3030, 3053, 3059, 3060, 3063, 3064, 3068, 3071, 3080, 3100, 3101, 3102, 3110, 3120, 3131, 3150, 3168, 3400, 3530, 6230, 6260, 6263, 6313, 6350, 6520, 6540, 9252. Neutral: 2102, 2104, 2190, 2200, 2210, 2215, 2221, 2397, 2411, 2480, 2495, 2499, 2512, 2570, 2595, 2870, 7004, 7006, 7009, 7040, 7041, 7052, 7059, 7080, 7090, 7150, 7234, 7235, 7705, 9260). The pleasant category included highly arousing images of erotic couples and adventure/sport scenes. The unpleasant category included highly arousing mutilation and attack images. The neutral category included low-arousing images of household objects and humans. Highly arousing emotional images and low-arousing neutral images were used to ensure polarity in emotional reactivity between emotional and neutral conditions. To control for arousal between emotional conditions, pleasant and unpleasant images were selected to be equivalent in normative ratings of emotional arousal. Images were not balanced in terms of social content. All images were converted to grayscale and matched for luminance and 90% quality jpeg file size by category using Adobe Photoshop 6 (Adobe Systems). Each picture was presented for 2.5 s, followed by a 12.5-s fixation-only period. Images were presented only once. Subjects completed three 462.5-s scans. Each scan included 10 pleasant, 10 unpleasant, and 10 neutral images. The pleasant and unpleasant images were selected to be equivalent in normative ratings of emotional arousal within and between scans. The same 30 images were always presented together within one scan. However, scan order was randomized between subjects and the image order within scans was pseudorandomized for each subject to ensure that no more than 2 pleasant, unpleasant, or neutral images were presented in a row. The visual stimuli were presented to the subject using a visual display inside the MRI scanner. The image was projected via a parallax biofeedback system (Thulborn 1999). A mirror located inside the MR environment displayed the visual stimuli onto a video screen located 35 cm from the subject’s eyes. The image was displayed on the screen at a resolution of 640 × 480 pixels and a refresh rate of 60 Hz. Once the fMRI scan session was complete, subjects viewed and rated each image for valence and arousal using a 9-point computerized version of the Self-Assessment Manikin (SAM) (Bradley and Lang 1994). Subjects used a mouse to make one rating corresponding to valence and one rating corresponding to arousal for each image. For the valence dimension, the range extended from a smiling, satisfied figure (a score of 9) to a frowning, unhappy figure (score of 1). For the arousal dimension, the range extended from a bored, sleepy figure (score of 1) to a highly aroused, frenzied figure (score of 9).
MRI Data Acquisition
Magnetic resonance images were collected using a volume head coil inside a 3-T MR Scanner (GE Healthcare 3T94 Excite 2.0). The subjects were supine in the scanner while performing the task. The subject’s head was stabilized using adjustable padding and then fitted with the projector-visor system for displaying visual stimuli. The functional images were obtained using a -sensitive, single shot, gradient-echo echo-planar pulse sequence (echo time 25 ms; repeat time 2500 ms; flip angle 90o; field of view 200 mm2; imaging matrix 64 × 64; 42 axial slices at 3-mm thickness; 0-mm gap between slices). High-resolution anatomical scans were obtained using a T1-weighted SPGR (spoiled gradient echo) pulse sequence (echo time 2.9 ms; repeat time 9.9 ms; flip angle 25o; field of view 240 mm2; imaging matrix 256 × 256; 124 axial slices at 1.5-mm thickness; 0-mm gap between slices).
Data Analysis of Self-reported Valence and Arousal Scores
Subjects viewed and rated each image for valence and arousal using the 9-point computerized version of the SAM. For each subject, valence and arousal ratings were averaged for each image category. The effect of image category (pleasant, unpleasant, and neutral) on each dependent measure (valence, arousal) was analyzed in separate one-way repeated measures analysis of variance (ANOVA) (SPSS, v.16). The probability value was set at P < 0.05 for each analysis.
Force Data Analysis
Force data were analyzed using custom algorithms in LabVIEW. The force time series data were digitally filtered by using a fourth-order Butterworth filter with a 20 Hz low-pass cutoff. Six dependent measures were calculated for each force pulse: 1) RT, 2) duration of force, 3) mean force, 4) positive rate of change of force onset, 5) negative rate of change of force offset, and 6) integral of force. Each dependent measure was then averaged within pleasant, unpleasant, and neutral image categories for each subject. The average for each image category was comprised of 30 pulses. All pulses for all subjects were included in the analysis. The effect of image category (pleasant, unpleasant, and neutral) on each dependent measure was analyzed in separate one-way repeated measures ANOVA’s (SPSS, v.16). The probability value was set at P < 0.05 for each analysis.
fMRI Data Voxelwise Analysis
All fMRI data processing was done using AFNI (http://afni.nimh.nih.gov/afni/). First, whole-brain voxelwise analyses were conducted on the fMRI data. The first 4 volumes in each scan series were discarded to allow for T1 equilibrium effects. The remaining images were then realigned to compensate for small head movements. Translational movement parameters never exceeded 1 voxel in any direction for any subject or scan. All subjects were included in all analyses. Motion-corrected individual data sets were normalized by dividing the instantaneous signal in each voxel at each point in the time series by the mean signal in that voxel across each scan. Each participant’s data were concatenated across runs and then a Gaussian filter was applied to the data sets (full-width half-maximum at 4 mm) to reduce the influence of anatomical variability among the individual maps for group-level analyses. Three separate regressors, depicting each of the 3 trial types (pleasant, unpleasant, and neutral), were created by convolving the train of stimulus events with a simulated hemodynamic response function. Next, the time series data were regressed to the simulated hemodynamic response function for the task sequence. Six additional regressors of no interest were included to account for head motion. The dependent variable at this level of analysis was the estimated ß-coefficient of the regressed time series and its associated t-statistic for each image condition versus rest. Before group analyses, each subject’s anatomical data set was normalized to the International Consortium for Brain Mapping 152 template (Montreal Neurological Institute [MNI]) using the automated function in AFNI. Each subject’s individual functional data sets were then transformed to standardized space using the standardized anatomical data set as a template. Activated regions were anatomically labeled using the basal ganglia human area template (Prodoehl et al. 2008), the human motor area template (Mayka et al. 2006), the Schmahmann MRI atlas of the human cerebellum (Schmahmann et al. 2000), the human brain anatomy in computerized images (Damasio 2005), and recent meta-analyses of emotion-related neuroimaging studies (Kober et al. 2008; Sabatinelli et al. 2011).
Separate paired t-tests were run to compare whole-brain activation for pleasant versus neutral (PvN) and unpleasant versus neutral (UvN) conditions. Statistical analyses were limited to regions that showed increased activity during emotional as compared with neutral conditions (i.e., P > N, U > N). The resulting t-maps were corrected for multiple comparisons using a Monte Carlo simulation model (AlphaSim). The data sets were thresholded to remove all voxels with t < 3.32 with an activation cluster minimum of 324 μL (P < 0.05, corrected). The independent variable in each t-test was image type. In each analysis, significant clusters of brain activity could potentially reflect 3 processes: 1) the production and maintenance of force, 2) emotional processing, and 3) the production and maintenance of force during emotional processing. Because the characteristics of force production were expected to be similar for pleasant, unpleasant, and neutral images, this analysis approach controlled for activity related to the production of force. To identify brain areas involved in emotional processing and in force production during emotional processing, we next conducted a conjunction analysis. The conjunction analysis was performed by examining the β-values from the PvN t-test and the β-values from the UvN t-test. The conjunction analysis identified areas of activation that were common to both contrasts (PvN and UvN) and that were significantly activated in each of those contrasts. Brain regions identified by the conjunction analysis were labeled as an area related to emotional processing and/or an area related to force production during emotional processing.
Control Experiment
To isolate brain areas that are involved in emotional processing from brain areas that are involved in the production of force during emotional processing, we conducted a control experiment. The objective of the control experiment was to identify brain areas related to emotional image processing. The methods largely followed the main experiment but with one critical difference; subjects did not produce force during image presentation. To maintain consistency with the primary experiment, 15 healthy right-handed subjects with normal or corrected to normal vision participated in the control experiment (7 females, 8 males; M = 25.5 years, SD = 6.16 years, range = 21–45 years). Two of the 15 subjects who completed the control experiment were also subjects in the primary experiment. Screening procedures were the same as in the primary experiment. All subjects reported levels of anxiety and depression within the range of responses typically given by healthy normal subjects (STAI-S: M = 27.2, range = 25–43; STAI-T: M = 33.27, range = 20–36; BDI: M = 4.2, range = 0–11). Subjects viewed a total of 60 images taken from the International Affective Picture System (a subset from those viewed in the primary experiment). Subjects completed two 462.5-s scans. Each scan included 10 pleasant, 10 unpleasant, and 10 neutral images. The imaging parameters were identical to those used in the primary experiment. Subjects also completed the SAM rating.
The voxelwise analysis and conjunction analysis outlined above for the primary experiment were also conducted on the control experiment data. This approach allowed us to identify areas of the brain that show increased activity during emotional image processing. We next compared the conjunction analysis from the primary experiment with the conjunction from the control experiment. The primary experiment controlled for activation related to force production and the control experiment controlled for activation related to emotional image viewing. Hence, areas that were identified in the primary conjunction analysis that were not identified in the control conjunction analysis were labeled as areas associated with force production during emotional imaging processing. Seed regions were then placed within these areas for subsequent functional connectivity psycho–physiological interaction (PPI) analyses on the primary data set (Friston et al. 1997; Mattfeld and Stark 2011).
PPI Functional Connectivity Analysis
PPI analyses examine whether the contribution of one area to another changes as a function of changes in the experimental or psychological context (Friston et al. 1997). This analysis allows one to examine context-dependent functional coupling between 2 brain areas. We defined the psychological context as producing force while viewing an emotionally arousing image as compared with producing force while viewing a neutral image. To perform the PPI analysis, we added 3 regressors to the regression model outlined above in the voxelwise analysis, one regressor representing global activity across the concatenated scans, another regressor for the time series activity in the seed region, and a third regressor representing the interaction between the context (force production during emotional or neutral conditions) and the time series from the seed region.
In the PPI analysis, we examined whether the correlation between our seed region and the rest of the brain changed as a function of image content (emotional vs. neutral). To construct our interaction regressor, we isolated a single time series of activity for all events of interest, giving TRs for emotionally arousing images a value of 1 and TRs for the neutral images a value of −1. A sphere with a 5-mm radius was placed in any area that showed increased activity when producing force while viewing an emotionally arousing image as compared with when producing force while viewing a neutral image. This sphere became our seed. Note that seeds were not placed in areas that were common to the conjunction analyses from both the primary experiment and the control experiment. For each subject, the average time series of the BOLD response within this sphere was extracted and then detrended. We then deconvolved the seed time series into its underlying neural function prior to calculating the interaction term. This deconvolution step was used to account for the temporal lag and other aspects of the hemodynamic response. We then created the interaction term by combining the physiological event (deconvolved seed time series) with the file demarcating whether the presented image was emotionally arousing or neutral. The resulting neural interaction term was then convolved with a gamma basis function using AFNI's “Waver” program. The regression analysis used in the voxelwise analysis was then rerun with the global regressor, seed regressor, and the interaction regressor added. The correlation coefficients for the interaction term were Fisher’s z transformed, converted to standardized space, and analyzed at the group level using a t-test. The resulting t-maps were then thresholded to remove all voxels with t < 3.32 with an activation cluster minimum of 324 μL (P < 0.05, corrected).
Results
Force Production
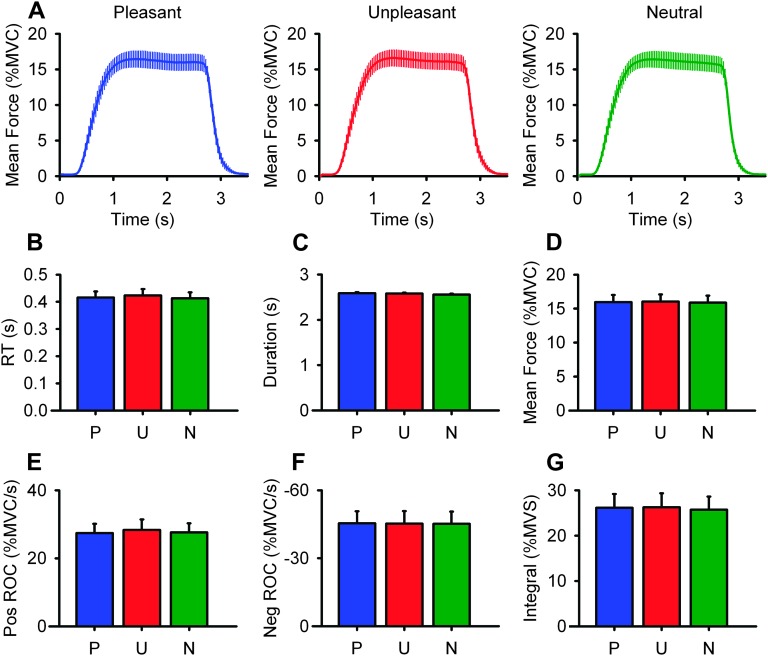
Figure 2A shows force pulses averaged over all trials (+1 standard error) for all subjects during the presentation of pleasant (blue), unpleasant (red), and neutral (green) images in the primary experiment. The force traces suggest that characteristics of force production were similar between image categories, and this was confirmed by statistical analyses. Figure 2B shows the mean RT of force production for each image category. The associated one-way repeated measures ANOVA revealed a nonsignificant effect of image category (F2,28 = 1.02, P > 0.05). Nonsignificant effects of image category were also revealed for mean duration of force, (Fig. 2C: F2,28 = 1.70, P > 0.05), force amplitude (Fig. 2D: F2,28 = 0.51, P > 0.05), peak rate of change of force onset (Fig. 2E: F2,28 = 0.69, P > 0.05), peak rate of change of force offset (Fig. 2F: F2,28 = 0.72, P > 0.05), and the integral of force (Fig. 2G: F2,28 = 1.02, P > 0.05). Together these findings show that, as intended by the experimental design, force production was similar between image categories.
Figure 2.
Force Data. (A) Mean force (+1 standard error) time series for all 15 subjects averaged for all pleasant (blue), unpleasant (red), and neutral (green) image categories. Image onset was at time point 0. Statistical analyses for the characteristics of force production were captured by RT (B), duration of force production (C), mean force production (D), positive rate of change of force (E), negative rate of change of force (F), and integral of force (G). Separate one-way ANOVAs were run for each dependent variable. No significant differences between image categories were revealed (all P’s > 0.05).
Self-reported Image Ratings
We next determined how subjects perceived each image that was presented during the experimental session. Mean and standard error data for each image category are shown in Table 1. Subject ratings were similar to normative ratings for emotional valence. Pleasant images were rated as more pleasant than neutral images and unpleasant images, and neutral images were rated as more pleasant than unpleasant images (F2,28 = 238.28, P < 0.001). Pleasant and unpleasant images were rated as similarly arousing, and each were rated as more arousing than neutral images (F2,28 = 57.70, P < 0.001). The pattern of findings in the primary experiment was repeated in the control experiment for emotional valence (F2,28 = 329.73, P < 0.001: pleasant > neutral > unpleasant) and emotional arousal (F2,28 = 40.94, P < 0.001: pleasant = unpleasant > neutral) (see Table 1). The rating data suggest that the images elicited the expected emotional reactivity in the primary experiment and in the control experiment.
Table 1.
Mean and standard error (SE) values for self-reported ratings of valence and arousal for pleasant, unpleasant, and neutral images used in the primary experiment and in the control experiment
| Self-report ratings |
||||
| Valence |
Arousal |
|||
| M | SE | M | SE | |
| Primary experiment | ||||
| Pleasant | 7.41 | 0.21 | 6.94 | 0.23 |
| Unpleasant | 1.68 | 0.17 | 6.27 | 0.68 |
| Neutral | 5.09 | 0.07 | 1.65 | 0.25 |
| Control experiment | ||||
| Pleasant | 7.18 | 0.15 | 5.86 | 0.39 |
| Unpleasant | 1.69 | 0.17 | 5.69 | 0.74 |
| Neutral | 5.20 | 0.08 | 1.56 | 0.16 |
Voxelwise Brain Imaging Analysis
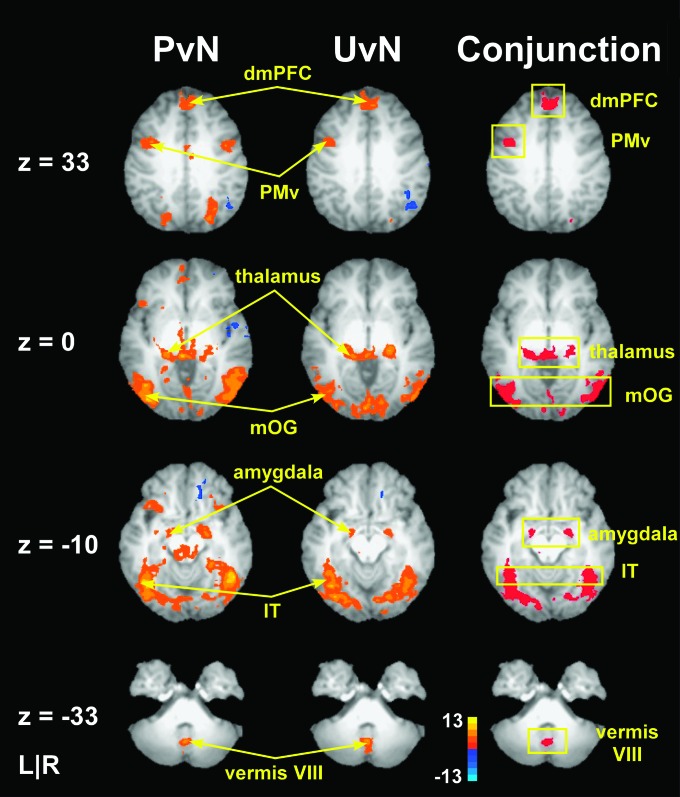
The t-test, which contrasted brain activation during force production and the presentation of pleasant as compared with neutral images (PvN), revealed an increase in BOLD signal in brain regions including areas of the extrastriate visual cortex (bilateral middle occipital gyrus [mOG], inferior temporal [IT] gyrus), bilateral thalamus, dmPFC, inferior frontal gyrus (IFG), bilateral PMv, bilateral PMd, superior frontal gyrus (SFG), bilateral amygdala, bilateral IPL, precuneus, caudate, substantia nigra, and cingulate gyrus. Cluster size, Talairach coordinates, MNI coordinates, t-values, and z-values for all significant brain activity identified in the PvN contrast are shown in Table 2. The left column in Figure 3 shows group activation maps from the PvN contrast overlaid on axial slices from one subjects’ normalized anatomical scan in Talairach space. The figure identifies clusters of activity in a subset of the regions identified in the PvN contrast including dmPFC, PMv, thalamus, mOG, IT, amygdala, and vermis VIII of the cerebellum.
Table 2.
Labels, cluster size, Talairach, and MNI coordinates (center of mass), t-values and z-values for areas identified in the primary experiment which required subjects to produce force while viewing pleasant (P), unpleasant (U), and neutral (N) images
| Primary experiment (image + force) |
|||||||||||||||||||
| P > N |
U > N |
||||||||||||||||||
| Talairach coordinates |
MNI coordinates |
T-value | Z-value | Talairach coordinates |
MNI coordinates |
T-value | Z-value | ||||||||||||
| Voxels | X | Y | Z | X | Y | Z | Voxels | X | Y | Z | X | Y | Z | ||||||
| mOG | R | 1633 | 39.3 | −65.6 | 8.5 | 43.4 | −67.2 | 10.6 | 11.09 | 3.91 | 705 | 43.7 | −65.1 | −3.2 | 48.0 | −67.7 | −2.5 | 8.52 | 3.38 |
| mOG | L | 1283 | −40.2 | −69.1 | 3.4 | −41.6 | −71.9 | 6.2 | 10.19 | 3.91 | 817 | −39.9 | −69.1 | −2.2 | −41.3 | −72.3 | 0.0 | 7.06 | 3.38 |
| Thalamus | R | 71 | 22 | −30 | 0 | 24.7 | −30.4 | −1.8 | 4.87 | 3.28 | 58 | 21.8 | −28.3 | −0.5 | 24.5 | −28.7 | −2.5 | 6.8 | 3.37 |
| Thalamus | L | 109 | −24 | −30 | 0 | −24.4 | −30.7 | −1.2 | 4.88 | 3.27 | 118 | −15.4 | −30.7 | −0.8 | −15.3 | −31.5 | −2.1 | 6.66 | 3.35 |
| dmPFC | M | 290 | −3.4 | 55.4 | 20.4 | −2.6 | 61.5 | 13.6 | 8.60 | 3.90 | 113 | −2.4 | 49.9 | 33.8 | −1.4 | 56.8 | 28.9 | 5.81 | 3.29 |
| PMv | R | 149 | 43.7 | 9.8 | 27.5 | 48.0 | 14.2 | 24.9 | 5.72 | 3.47 | 24 | 44.3 | 12.8 | 25.3 | 48.6 | 17.2 | 22.2 | 3.94 | 2.98 |
| PMv | L | 97 | −40.7 | 6.6 | 30 | −42.2 | 10.5 | 29.0 | 5.93 | 3.51 | 44 | −43.7 | 7.2 | 32.3 | −45.4 | 11.3 | 31.5 | 4.98 | 3.19 |
| PMd | R | 23 | 40.3 | −7.8 | 49.4 | 44.6 | −2.6 | 50.7 | 4.29 | 3.12 | |||||||||
| PMd | L | 34 | −43.9 | −3.1 | 52.6 | −45.4 | 2.1 | 54.9 | 5.23 | 3.37 | |||||||||
| IFG | L | 70 | −50.1 | 22.9 | 14.4 | −52.4 | 26.3 | 10.4 | 7.04 | 3.73 | 22 | −47.4 | 25.1 | 11.1 | −49.6 | 28.4 | 6.5 | 3.88 | 2.97 |
| IFG | L | 128 | −35.8 | 24.1 | −6 | −37.3 | 25.9 | −12.5 | 6.52 | 3.63 | |||||||||
| IFG | R | 31 | 29.8 | 22.4 | −8.9 | 32.8 | 24.3 | −16.4 | 4.77 | 3.25 | 17 | 52.1 | 26.9 | 8.4 | 56.7 | 30.7 | 2.1 | 5.84 | 3.3 |
| SFG | R | 24 | 16.4 | 31.1 | 54.6 | 18.9 | 38.8 | 53.3 | 5.65 | 3.46 | |||||||||
| Pre-SMA | L | 106 | −11.4 | 24.8 | 55.3 | −10.7 | 32.1 | 55.0 | 7.30 | 3.77 | 25 | −9.6 | 17.9 | 57.4 | −8.8 | 25.0 | 58.0 | 4.36 | 3.08 |
| STG | R | 18 | 66.3 | −35.9 | 20.2 | 72.2 | −34.6 | 20.5 | 5.75 | 3.48 | |||||||||
| V1/V2 | M | 96 | −2.3 | −75.6 | −3.5 | −1.1 | −79.1 | −1.3 | 5.27 | 3.37 | 426 | 3.3 | −88.8 | 1.1 | 5.0 | −92.6 | 4.9 | 11.03 | 3.51 |
| Amygdala | R | 74 | 20.8 | −6.5 | −7.8 | 23.3 | −6.2 | −12.5 | 8.57 | 3.90 | 13 | 20.9 | −6.4 | −9.8 | 23.4 | −6.3 | −14.7 | 4.63 | 3.13 |
| Amygdala | L | 28 | −15.6 | −4.6 | −8.1 | −15.6 | −4.5 | −12.5 | 6.14 | 3.56 | 12 | −19.6 | −5.8 | −9.9 | −19.9 | −5.9 | −14.4 | 4.57 | 3.12 |
| IPL | L | 24 | −31.3 | −57.5 | 42.6 | −31.8 | −56.2 | 48.5 | 5.12 | 3.34 | |||||||||
| Precuneus | M | 41 | 0.7 | −53.3 | 45.4 | 2.4 | −51.3 | 50.8 | 5.55 | 3.44 | |||||||||
| Caudate | L | 28 | −33.6 | −13.2 | −5.8 | −34.8 | −13.5 | −9.0 | 3.80 | 2.97 | |||||||||
| ACC | M | 16 | 0.1 | −1.3 | 31.8 | 1.5 | 2.5 | 31.2 | 4.63 | 3.22 | |||||||||
| SN | R | 13 | 10.5 | −21 | −7.1 | 12.3 | −21.6 | −10.3 | 5.94 | 3.52 | 13 | 8.5 | −23 | −9.1 | 10.2 | −23.9 | −12.3 | 3.52 | 2.94 |
| SN | L | 12 | −10 | −19 | −6.5 | −9.6 | −19.5 | −9.6 | 4.22 | 3.10 | |||||||||
| Vermis VIII | M | 68 | −2 | −64.8 | −30.4 | −1.1 | −70.0 | −32.1 | 7.22 | 3.76 | 43 | −4.4 | −64.4 | −32.5 | −3.6 | −69.7 | −34.4 | 5.28 | 3.23 |
| Lob VIIIA | L | 14 | −14.4 | −60.5 | −45.1 | −14.4 | −66.8 | −48.6 | 7.44 | 3.79 | |||||||||
Note: Data are reported from contrasts that identified regions where brain activity was greater when force was produced while subjects viewed pleasant as compared with neutral images (P > N) and unpleasant as compared with neutral images (U > N).
Figure 3.
Brain activity during the primary experimental scans in prefrontal cortex, thalamus, amygdala, visual cortex, and cerebellum. Axial slices showing mean BOLD activation detected by voxelwise analyses for the pleasant versus neutral contrast (PvN) and the unpleasant versus neutral contrast (UvN), and the corresponding conjunction analysis overlaid on a single subjects transformed brain in Talairach space. The color bar ranges from t = −13 to t = +13 with a group activation threshold of P < 0.05, corrected. The corrected t-statistics associated with each voxel are displayed. The PvN and UvN activation maps show that force production, when paired with emotional as compared with neutral images, corresponds with increased activity in dmPFC, PMv, thalamus, mOG, amygdala, IT, and vermis VIII of the cerebellum. Increased BOLD signal in these common areas, which are shown in the yellow boxes, was confirmed in the conjunction analysis.
The t-test which contrasted brain activation during force production and the presentation of unpleasant as compared with neutral images (UvN) revealed an increase in BOLD signal bilaterally in brain regions which included mOG, ITG, thalamus, dmPFC, IFG, PMv, PMd, SFG, amygdala, and IPL. Cluster size, Talairach coordinates, MNI coordinates, t-values, and z-values for all significant brain activity identified in the UvN contrast are shown in Table 2. The middle column in Figure 3 shows group activation maps from the UvN contrast and identifies distinct clusters of activity in dmPFC, PMv, thalamus, mOG, IT, amygdala, and vermis VIII of the cerebellum.
The third column in Figure 3 shows areas that were identified in the conjunction analysis. The conjunction analysis identified common brain areas that showed increased activity when force production was paired with emotionally arousing pleasant and unpleasant images as compared with when force production was paired with neutral images. Areas found in the conjunction analysis are shown surrounded by yellow boxes and include dmPFC (0, 47, 34), left PMv (−43, 6, 31), bilateral thalamus (left: −11, −31, −1; right: 22, −28, −1), bilateral mOG (left: −36, −68, −8; right: 44, −70, −6) which extended to IT gyrus, bilateral amygdala (left: −19, −5, −9; right: 22, −8, −8), and vermis VIII in the cerebellum (−3, −62, −33). Other areas identified by the conjunction analysis were right PMv, left IFG, right SN, and left pre-SMA. Cluster size, Talairach coordinates, and MNI coordinates for all areas identified in the conjunction analysis are shown in Table 4. All characteristics of force production were similar between image conditions, suggesting that differences in force production could not account for the activation differences in regions identified in the conjunction analysis. Accordingly, activation within these regions corresponded with processes related to emotional processing and to processes related to the production and maintenance of force while viewing emotional images.
Table 4.
Labels, cluster size, and Talairach, and MNI coordinates for areas identified by the conjunction analysis for the primary and control experiment
| Conjunction—primary experiment |
Conjunction—control experiment |
||||||||||||||
| Talairach coordinates |
MNI coordinates |
Talairach coordinates |
MNI coordinates |
||||||||||||
| Voxels | X | Y | Z | X | Y | Z | Voxels | X | Y | Z | X | Y | Z | ||
| mOG | R | 713 | 44.3 | −70.1 | −6.3 | 48.6 | −73.2 | −5.5 | 175 | 46 | −62.4 | −5.2 | 50.4 | −65.0 | −5.0 |
| mOG | L | 672 | −38.6 | −68 | −8.1 | −40.0 | −71.7 | −6.6 | 215 | −44.7 | −67.4 | −1.3 | −46.4 | −70.5 | 0.9 |
| Thalamus | R | 31 | 22.5 | −28.2 | −1.3 | 25.2 | −28.6 | −3.4 | 11 | 19.3 | −33.1 | 2.9 | 21.9 | −33.5 | 1.7 |
| Thalamus | L | 82 | −11.8 | −31.3 | −1.8 | −11.4 | −32.2 | −3.2 | 5 | −21.6 | −29.6 | −0.9 | −21.9 | −30.3 | −2.3 |
| PMv | R | 21 | 43.9 | 12.6 | 25 | 48.1 | 16.9 | 21.8 | 21 | 38.9 | 16.6 | 27.7 | 42.8 | 21.3 | 24.5 |
| IFG | L | 15 | −48.5 | 23.3 | 13.1 | −50.7 | 26.6 | 8.9 | 6 | −36.2 | 24.6 | 8.4 | −37.6 | 27.7 | 3.4 |
| Amygdala | R | 12 | 22.7 | −8.3 | −8.9 | 25.3 | −8.2 | −13.6 | 4 | 23.9 | −4.8 | −9.2 | 26.6 | −4.5 | −14.3 |
| Amygdala | L | 9 | −19.3 | −5 | −9 | −19.6 | −5.0 | −13.5 | 3 | −22 | −10 | −8 | −22.5 | −10.2 | −11.9 |
| SN | R | 5 | 10.1 | −22.3 | 6.5 | 12.0 | −21.8 | 4.9 | 8 | 9.6 | −15.1 | −8.4 | 11.3 | −15.5 | −12.3 |
| dmPFC | M | 66 | 0 | 47 | 34.1 | 1.2 | 53.8 | 29.4 | |||||||
| PMv | L | 37 | −43.4 | 6.7 | 31.6 | −45.1 | 10.7 | 30.8 | |||||||
| Pre-SMA | L | 14 | −9.2 | 17.4 | 58.5 | −8.3 | 24.5 | 59.2 | |||||||
| Vermis VIII | M | 23 | −3.3 | −62.6 | −33 | −2.5 | −67.9 | −35.1 | |||||||
Note: For each experiment, the conjunction analysis identified areas of activation that were common to both contrasts (PvN and UvN) and that were significantly activated in each of those contrasts.
To delineate these 2 processes, we analyzed the data of the control experiment. In the control experiment, subjects completed a passive viewing paradigm that replicated the main experiment, but subjects did not produce force. Cluster size, Talairach coordinates, MNI coordinates, t-values, and z-values for all significant brain activity identified in the PvN and the UvN t-tests are shown in Table 3. Brain areas identified in the subsequent conjunction analysis are shown in Table 4. The conjunction analysis used in the primary experiment was replicated for the control experiment and demonstrates that the passive viewing of emotional images is associated with activity in bilateral mOG, bilateral amygdala, bilateral thalamus, bilateral IFG, and right SN. A comparison of the conjunction analyses from the primary experiment and the control experiment revealed 4 brain regions that only showed increased activity when force production was paired with the presentation of emotionally arousing as compared with neutral images. As shown in Table 4, these areas were dmPFC, left PMv, left pre-SMA, and vermis VIII in the cerebellum. To further ensure that activity in these areas was not related to motor memory, we conducted an additional experiment in which 15 right-handed healthy adults produced force to 15% of their MVC without visual feedback while functional brain activity was recorded. We contrasted force production with rest. Consistent with previous findings (Vaillancourt et al. 2003), no activity was identified in dmPFC, left PMv, left pre-SMA, or vermis VIII. This additional control experiment confirmed that activity within these regions in the primary experiment corresponded with force production in emotional contexts rather than force production alone. These 4 areas were used as seed regions in functional connectivity PPI analyses.
Table 3.
Labels, cluster size, Talairach and MNI coordinates (center of mass), t-values and z-values for areas identified in the control experiment which required subjects to view pleasant (P), unpleasant (U), and neutral (N) images
| Control experiment (image only) |
|||||||||||||||||||
| P > N |
U > N |
||||||||||||||||||
| Talairach coordinates |
MNI coordinates |
T-value | Z-value | Talairach coordinates |
MNI coordinates |
T-value | Z-value | ||||||||||||
| Voxels | X | Y | Z | X | Y | Z | Voxels | X | Y | Z | X | Y | Z | ||||||
| mOG | R | 978 | 41.7 | −67.7 | 4.5 | 45.9 | −69.8 | 6.3 | 14.92 | 4.06 | 349 | 46 | −64.3 | −4.8 | 50.4 | −66.9 | −4.4 | 6.83 | 3.04 |
| mOG | L | 811 | −45.1 | −67.7 | 2.5 | −46.8 | −70.5 | 5.2 | 8.55 | 3.61 | 269 | −43 | −68.3 | −2.3 | −44.6 | −71.5 | −0.1 | 6.28 | 3.05 |
| Thalamus | R | 97 | 22.5 | −34.6 | 3.1 | 25.3 | −35.0 | 2.0 | 7.31 | 3.57 | 64 | 20.3 | −34 | 4.3 | 22.9 | −34.3 | 3.3 | 4.73 | 2.99 |
| Thalamus | L | 156 | −24.6 | −30.2 | 2 | −25.1 | −30.8 | 1.0 | 6.01 | 3.43 | 45 | −20.8 | −28.1 | −2.6 | −21.1 | −28.9 | −4.3 | 4.44 | 2.95 |
| IFG (PMv) | R | 119 | 40.5 | 9.5 | 27.6 | 44.5 | 13.8 | 25.0 | 5.58 | 3.36 | 64 | 44.1 | 22.6 | 19.1 | 48.3 | 27.0 | 14.4 | 4.73 | 2.98 |
| PMd | R | 119 | 32.9 | 5.8 | 50.1 | 36.6 | 11.8 | 50.4 | 6.11 | 3.45 | |||||||||
| PMd | L | 108 | −26.5 | 6.7 | 50.8 | −26.8 | 12.4 | 51.9 | 5.88 | 3.41 | |||||||||
| IFG | L | 22 | −33.4 | 24.2 | 8.9 | −34.6 | 27.3 | 4.0 | 4.13 | 3.03 | 30 | −37 | 25 | 11.9 | −38.5 | 28.4 | 7.3 | 7.41 | 3.05 |
| IFG | R | 38 | 29.1 | 19.5 | −15 | 32.0 | 20.7 | −22.9 | 6.20 | 3.46 | |||||||||
| SFG | L | 21 | −11.6 | 61.7 | −0.1 | −11.6 | 66.3 | −9.6 | 5.48 | 3.34 | |||||||||
| Amygdala | R | 38 | 20.6 | −1.3 | −14.7 | 23.0 | −1.3 | −20.6 | 5.57 | 3.35 | 13 | 24.9 | 4.2 | −12 | 27.6 | 4.7 | −18.2 | 4.83 | 2.99 |
| Amygdala | L | 12 | −28.9 | −8.5 | −12.6 | −29.9 | −9.1 | −17.0 | 4.61 | 3.15 | 12 | −26.5 | −6.5 | −15 | −27.3 | −7.2 | −19.9 | 4.09 | 2.9 |
| IPL | R | 55 | 31 | −50.1 | 42.1 | 34.8 | −48.0 | 46.5 | 5.70 | 3.38 | |||||||||
| IPL | L | 159 | −60.8 | −25.3 | 30 | −63.5 | −23.4 | 32.1 | 6.93 | 3.54 | |||||||||
| Precuneus | L | 402 | −3.8 | −56.7 | 51.7 | −2.3 | −54.4 | 58.2 | 6.15 | 3.45 | |||||||||
| Caudate | L | 78 | −32.8 | −27.5 | −4.3 | −33.9 | −28.5 | −6.1 | 5.94 | 3.42 | |||||||||
| SN | R | 12 | 10.5 | −15.7 | −8.1 | 12.3 | −16.1 | −11.9 | 4.43 | 3.10 | 14 | 11.5 | −15.3 | −11.7 | 13.3 | −15.9 | −15.9 | 4.24 | 2.92 |
| ACC | M | 47 | 0.5 | 1.3 | −5 | 1.6 | 2.1 | −9.8 | 7.65 | 3.59 | 14 | 5.5 | −20.9 | 26.6 | 7.3 | −18.6 | 27.1 | 5.21 | 3.03 |
| SI | R | 19 | 51 | −24.9 | 36.7 | 56.0 | −21.7 | 38.0 | 5.59 | 3.36 | |||||||||
| Lob VIII | L | 23 | −16.5 | −60.8 | −44.7 | −16.7 | −67.1 | −48.1 | 4.93 | 3.22 | |||||||||
| Lob VIII | R | 25 | 14.8 | −60.4 | −46.1 | 16.8 | −66.6 | −50.1 | 5.12 | 3.26 | |||||||||
| Insula | L | 16 | −36.4 | 7.6 | −4.5 | −37.9 | 8.6 | −9.4 | 4.92 | 3.21 | |||||||||
| Cingulate gyrus | L | 17 | −8.6 | −25.6 | 24.2 | −7.8 | −23.9 | 25.0 | 8.38 | 3.04 | |||||||||
| STN | R | 17 | 6.6 | −11.8 | −5 | 8.1 | −11.7 | −8.8 | 4.52 | 2.96 | |||||||||
| Red nucleus | L | 15 | −3.6 | −24.3 | −2.1 | −2.7 | −24.7 | −4.3 | 5.06 | 3.01 | |||||||||
Note: Data are reported from contrasts that identified regions where brain activity was greater while subjects viewed pleasant as compared with neutral images (P > N) and unpleasant as compared with neutral images (U > N).
Functional Connectivity PPI Analysis
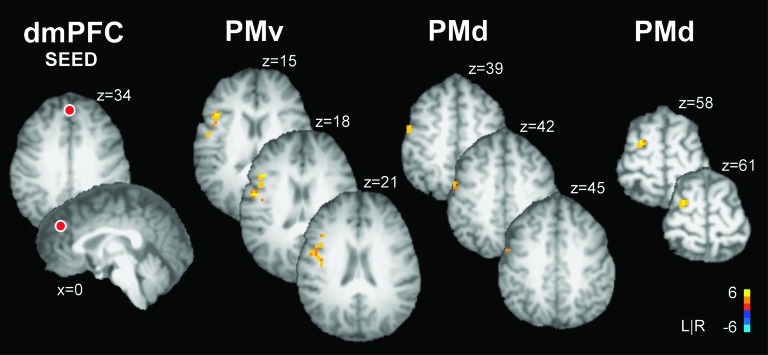
The PPI analysis was conducted to examine context-dependent changes in the functional coupling of activity in a seed region and a target region. Table 5 shows Talairach coordinates, volume size, and peak t-values for each seed region and its associated target regions. A positive t-value represents an increase in context-dependent functional coupling between the seed and target region, and a negative t-value represents a decrease in context-dependent functional coupling between the seed and target region. Figure 4 shows data from the PPI analysis that used a sphere in dmPFC as the seed region. The location of the dmPFC seed region is shown overlaid in red on axial and sagittal brain slices. When force production was paired with emotional as compared with neutral images an increase in functional coupling was evidenced between dmPFC and left PMv and 2 regions in left PMd (see Fig. 4). In contrast, a decrease in functional coupling was evidenced between dmPFC and cingulate gyrus and dmPFC and SFG (see negative t-value in Table 5).
Table 5.
Talairach and MNI coordinates of seed and corresponding target regions identified in the primary experiment using task-dependent functional connectivity analyses
| Talairach coordinates |
MNI coordinates |
Voxels | Peak t-value | |||||||
| Seed | Target | X | Y | Z | X | Y | Z | |||
| dmPFC | M | 0.0 | 47.0 | 34.1 | 1.2 | 53.8 | 29.4 | |||
| PMv | L | −47.2 | 7.9 | 18.2 | −49.2 | 10.8 | 15.9 | 50 | 5.37 | |
| PMd | L | −55.0 | −2.9 | 43.0 | −57.3 | 1.4 | 44.4 | 16 | 3.51 | |
| PMd | L | −27.2 | −4.2 | 61.6 | −27.5 | 1.8 | 64.8 | 16 | 5.21 | |
| Cingulate gyrus | R | 1.8 | 8.3 | 28.2 | 3.2 | 12.4 | 26.3 | 13 | −4.9 | |
| SFG | R | 6.9 | 31.5 | 49.2 | 8.7 | 38.7 | 47.5 | 13 | −3.52 | |
| PMv | L | −43.4 | 6.7 | 31.6 | −45.1 | 10.7 | 30.8 | |||
| Frontal gyrus | M | −3.0 | 52.0 | 3.0 | −2.3 | 56.4 | −5.4 | 55 | 3.82 | |
| Lobule VI | L | −39.4 | −59.2 | −20.1 | −41.0 | −63.4 | −20.7 | 13 | −4 | |
| Middle FG | L | −24.9 | 22.2 | 47.9 | −25.2 | 28.6 | 47.2 | 13 | 5.77 | |
| Pre-SMA | L | −9.2 | 17.4 | 58.5 | −8.3 | 24.5 | 59.2 | |||
| Caudate tail | L | −23.7 | −35.2 | 22.1 | −23.9 | −34.3 | 23.7 | 22 | −5.21 | |
| PMv | R | 53.2 | −5.0 | 14.9 | 58.1 | −2.5 | 12.1 | 12 | 4.66 | |
| Vermis VIII | M | −2.5 | −67.9 | −35.1 | −1.6 | −73.7 | −37.0 | |||
| M1/S1 | R | 42.6 | −22.3 | 49.2 | 47.1 | −17.9 | 51.8 | 31 | 5.65 | |
| M1 | R | 54.4 | −5.7 | 31.5 | 59.5 | −1.8 | 30.5 | 25 | 3.81 | |
| Middle FG | L | −25.6 | 24.4 | 47.6 | −26.0 | 30.9 | 46.7 | 17 | 4.61 | |
| Insula | R | 40.3 | 11.4 | 5.3 | 44.1 | 13.9 | 0.2 | 16 | −4.06 | |
Note: Cluster size and peak t-values are shown for each target region.
Figure 4.
Brain activity during the experimental task as revealed by the PPI functional connectivity analysis with the seed region placed in dmPFC. The location of the dmPFC seed is shown as a red sphere (not to scale) overlaid on an axial and sagittal slice of a single subjects transformed brain in Talairach space. The color bar ranges from t = −6 to t = +6 with a group activation threshold of P < 0.05, corrected. The corrected t-statistics associated with each voxel are displayed. PPI analysis revealed a task-dependent increase in functional coupling between activity in dmPFC and left PMv and dmPFC and left PMd. In each target region, activity is shown in a series of axial slices that are separated by 3 mm in the inferior to superior direction. When force production was paired with emotional as compared with neutral images, the BOLD signal increased in dmPFC and this increase was significantly coupled with increased activity in PMv and PMd. The findings suggest that a cortico-cortical network between motor and prefrontal regions regulates force control in emotional contexts.
When placing a seed region in left PMv (−43.4, 6.7, 31.6), Table 5 shows that the PPI analysis identified target regions in medial frontal gyrus and left middle frontal gyrus, and the positive t-values for clusters of voxels within each of these regions indicated a context-dependent increase in functional coupling between the seed and target. In contrast, a context-dependent decrease in the functional coupling was evidenced between left PMv and lobule VI of the cerebellum. Placing a seed in left pre-SMA (−9.2, 17.4, 58.5) revealed a significant context-dependent increase in functional coupling with right PMv, whereas a decrease in functional coupling was found between left pre-SMA and left caudate. The analysis that used a seed region in vermis VIII in the cerebellum (−2.5, −67.9, −35.1) revealed increased functional coupling with regions in the right M1/S1 and left middle frontal gyrus. In contrast, a significant context-dependent decrease in functional coupling was found between activity in vermis VIII of the cerebellum and right insula.
Discussion
The central finding of this study is that despite similar force performance across emotional contexts, increased brain activity was evidenced in dmPFC, left PMv, left pre-SMA, and vermis VIII of the cerebellum when force was produced during emotional as compared with neutral conditions. Connectivity analyses extended our findings by demonstrating a task-dependent functional circuit between 1) dmPFC and left PMv, 2) left pre-SMA and right PMv, and 3) between vermis VIII in the cerebellum and right M1. Our findings confirm that when the same amount of force has to be produced despite changes in emotional context, a functional circuit between dmPFC and premotor cortex is engaged. Identification of this functional circuit translates experimental work on the rodent PFC to the human brain.
PFC Activity during Force Production in Emotional Contexts
Ventrolateral rather than dorsomedial areas of PFC have previously been shown to influence force production in emotional contexts. Schmidt et al. (2009) demonstrated that priming individuals with emotional as compared with neutral images led to an increase in maximal grip force production, and this increase in force production coincided with increased activity in vlPFC and M1. The authors suggested that vlPFC, by driving the motor cortex, constitutes a brain pathway that allows emotional arousal to facilitate physical effort. This interpretation is in accord with the suggestion that lateral prefrontal regions are involved in selecting and maintaining action selection rules according to the immediate context and/or the ongoing temporal episode in which the person is acting (Koechlin et al. 2003; Koechlin and Hyafil 2007). In contrast to allowing the magnitude of force production to vary, as was the case in the Schmidt et al. (2009) study, the present study required subjects to produce the same level of force despite the presentation of emotional images. Our findings demonstrate that dmPFC, and not vlPFC, had increased activity when force was produced to the same level during emotional as compared with neutral conditions.
Our findings are consistent with the idea that controlling behaviors that are shaped by internal states relies on regions in the prefrontal cortex (Dolan 2002; Miller and D'Esposito 2005; Narayanan and Laubach 2006). Functional brain imaging studies have consistently associated the regulation of behavior with activity in dmPFC. Activity in this region corresponds with the generation and regulation of emotional processes (Kim and Hamann 2007; Ochsner et al. 2009), and the making of mental state attributions such as when monitoring one’s own moment-to-moment feelings (Dolcos et al. 2004). Dolcos et al. (2004) presented participants with emotional images but instructed them to experience any feelings or thoughts the pictures might elicit and to then rate each picture. The authors reported increased dmPFC activity during the presentation of pleasant and unpleasant images as compared with neutral images. Importantly, however, in the present study, subjects were not explicitly instructed to generate, regulate, or evaluate their emotions (Pavuluri et al. 2010). They were instructed simply to produce force output to a submaximal target level to the onset and offset of images. Moreover, because the control study also required subjects to passively view emotional and neutral images, our brain activity data cannot be attributed to unconscious image evaluation processes. As such, the current findings show for the first time that dmPFC is not only important in emotion regulation and nonmotor cognitive tasks but also plays a significant role in the control of motor function in emotional contexts.
Brain Activity in Emotion and Motor Circuits
A large meta-analysis of emotion-related neuroimaging studies associated activity in dmPFC, pre-SMA, and bilateral IFG with emotional processing, but the authors noted that activation within these regions was most likely not specific to emotion (Kober et al. 2008). Instead, the authors suggested that activation in these regions may correspond with a general motivational state which influences attention and the selection of intentional action. Our findings, which identify functional links between dmPFC and motor control brain areas, are consistent with this suggestion. Our findings also show that increased activity in dmPFC, left pre-SMA, left PMv, and vermis VIII of the cerebellum could not be accounted for by differences in the perceptual processes that underlie the passive viewing of emotional as compared with neutral images, the production of force while viewing images (neutral condition in the primary experiment) or force production alone without visual feedback (force only-control experiment). It is important to note, however, that brain activity related to emotional processing in the primary and control experiments was consistent with previous studies that have assessed emotional processing in general (Phelps and LeDoux 2005; Kober et al. 2008) and, specifically, during picture-viewing paradigms (Sabatinelli et al. 2005, 2009; Ochsner et al. 2009). The expected perceptions of emotion stimuli were also confirmed via self-report data.
Functional Connectivity between Cortical Regions
PFC activity may have reflected the regulation of emotional processing in order to limit the prepotent effect of emotion on the production of force. The functional connectivity analyses addressed this issue. If dmPFC activity identified in the current study was reflective of emotion regulation, we would have expected the connectivity analysis to link increased dmPFC activity with decreased amygdala activity, as has been shown previously in the down-regulation of positive and negative affect (Kanske et al. 2011) and fear extinction (Delgado et al. 2008). This was not the case. Hence, an alternative interpretation is that force production rather than emotional reactivity was regulated so that the force output was held constant. This interpretation is supported by the connectivity analysis that revealed increased functional coupling between dmPFC and left premotor regions. This increased coupling suggests a functional cortico-cortical circuit that allows force output to remain constant in emotional contexts. This suggestion is consistent with a recent model of PFC function (O'Reilly 2010), which predicts that controlling motor actions in emotional contexts should be associated with activity in the medial–dorsal–caudal region of PFC. This functional circuit may be distinct from the circuit that regulates emotion during tasks that do not involve the control of movement. Indeed, in both rats and humans, in addition to dmPFC, vmPFC has also been associated with emotional control (Milad and Quirk 2002; Phelps et al. 2004).
Our findings also demonstrate increased BOLD signal in left pre-SMA and contralateral PMv during force production in emotional contexts. Single neuron recordings in monkeys during gripping and reaching tasks have demonstrated the role that PMv plays in preparing and executing movements (Hoshi and Tanji 2000, 2007), and this has been corroborated in humans using single neuron recordings (Ojakangas et al. 2006), TMS (Davare et al. 2006), and functional brain imaging (Coombes et al. 2010). The connectivity analysis revealed that increased activity in left PMv was functionally coupled with increased activity in medial frontal gyrus, left middle frontal gyrus, and a decrease in activity in lobule VI of the cerebellum. In addition to PMv being used as a seed region, it is also noteworthy that the connectivity analysis that used dmPFC and left pre-SMA as seeds identified target regions in the premotor cortex. These findings further support the idea that premotor cortex, which has previously been associated with the preparation and execution of movements, is also involved in the production of force output in emotional contexts.
Seminal retrograde tracing studies in animals identified pre-SMA and SMA as components of distinct cortico-subcortico neural circuits (Luppino et al. 1991; Akkal et al. 2007; for a review, see Picard and Strick 1996), and these findings have been corroborated by a detailed meta-analysis of brain imaging studies in humans (Mayka et al. 2006). Activation in pre-SMA has been associated more strongly with nonmotor cognitive tasks which require attention to time (Coull et al. 2004), attention to changing visual stimuli (Hon et al. 2006), and “attention to intention” rather than “attention to movement” (Lau et al. 2004). Thus, pre-SMA activity increases during cognitively demanding tasks that require increased attentional processes. This fits in well with the current finding in pre-SMA because producing force in emotional as compared with neutral contexts may be a more demanding task that requires increased attention.
The potential increase in task demands when force was produced in emotional contexts may reflect inhibitory processes. Given previous evidence which shows a facilitation of motor system activity in emotional contexts (Flykt 2005; Coombes et al. 2006, 2007a, 2007b; Flykt and Caldara 2006; Hajcak et al. 2007; Schmidt et al. 2009; van Loon et al. 2010), the brain activity noted in the current study may reflect the inhibition of this facilitation. This interpretation is consistent with evidence, which identifies pre-SMA, along with inferior frontal cortex, and subthalamic nucleus as components of a cortico-subcortical network that inhibits action (Aron and Poldrack 2006). The association between pre-SMA activity and the regulation (or potential inhibition) of force production in emotional contexts identified in the current study is noteworthy because increased pre-SMA activity has been associated with stopping an ongoing response followed by a switch in direction of one hand during bimanual circular drawing (Coxon et al. 2010). Furthermore, using the stop-signal paradigm, increased pre-SMA activity has been associated with the successful inhibition of motor responses in humans (Aron and Poldrack 2006), with error and posterror processing related to cognitive control in humans (Hendrick et al. 2010), and with the proactive control of motor readiness and the reactive inhibition of unwanted movements in monkeys (Chen et al. 2010). Identifying the brain circuits which underlie the inhibition of motor responses in emotional contexts may be important to our understanding of impulse control disorders which have been associated with treatment in Parkinsons Disease (Broen et al. 2011). We raise the idea of inhibition and its clinical implications with caution, however, because the cognitive control of action that may have emerged in the current study was not explicitly manipulated and as a result was much more subtle that the typical motor inhibition and response switching paradigms. Given that the emotional and neutral images were not balanced in terms of social content, an additional explanation for our findings, especially in PMv, is that emotional images that depicted human activity may have activated the mirror mechanism (di Pellegrino et al. 1992). However, this explanation is unlikely because the emotion only-control experiment used the same images as the primary experiment, and therefore, any activation related to the mirror mechanism should have been present in both experiments and therefore controlled for.
Functional Connectivity between Cortical and Cerebellar Regions
In addition to regions in prefrontal and premotor cortex, vermis VIII in the cerebellum also showed increased activity when force production was paired with emotional as compared with neutral images. Connectivity analyses further qualified the role of this cerebellar region by associating its activity with activation of right M1/S1, left middle frontal gyrus, and right insula. Activity in vermis VIII, which is considered part of the posterior vermis (Stoodley and Schmahmann 2010), has previously been associated with emotional processing (Heath 1977; Schmahmann 1991, 2000). For instance, stimulating the cerebellar vermis modulates firing patterns in the amygdala and septum (Zanchetti and Zoccolini 1954; Berman 1997; Bobee et al. 2000) and has been shown to attenuate aggression in patients (Heath et al. 1978). The link between emotional processing and cerebellar activity is further supported by evidence which has documented pathological laughing and crying in patients with cerebellar pathology from stroke (Parvizi et al. 2001) and by evidence which shows increased posterior vermis activation in substance abusers during reward-related tasks (Anderson et al. 2006). Additional support comes from studies which show that vermis damage following cerebellar tumor removal in children is associated with abnormal affective symptoms and personality change (Levisohn et al. 2000) and with behavioral disturbances ranging from irritability to behaviors which are suggestive of autism (Riva and Giorgi 2000).
The current findings corroborate this previous work by showing that the posterior vermis is indeed related to aspects of emotional processing. We also extend the findings by showing that emotional processing alone is not enough to activate this region (as shown in the control study). Rather, our findings suggest that activity in this area may be related to the integration of emotion and motor responses. Given the functional connectivity analysis which revealed coupling between vermis VIII of the cerebellum and right M1/S1, our findings provide empirical support for the proposal that the cerebellum plays a role in translating emotional states into autonomic and motor responses (Sacchetti et al. 2009). Moreover, the suggestion that this region may be involved in regulating motor responses is supported by evidence which links the posterior vermis (vermis IX: 0, −56, −40) with button press movements during a stop-signal task in humans (Ide and Li 2011), and their coordinate is positioned anterior and inferior to the coordinate for the posterior vermis region found in the current study (vermis VIII: −2.5, −67.9, −35).
Conclusions
An emotion-driven increase in force production has been associated with increased human brain activity in vlPFC and M1. In many instances, however, force production must be held constant despite changes in emotional context. Work in rats suggests that controlling motor output in emotional contexts engages dmPFC and motor cortex. Here, we translate these findings to humans by demonstrating that when force is precisely controlled at the same level despite altered emotional contexts, increased brain activity occurs in dmPFC and PMv and not vlPFC or M1. Connectivity analyses extended these findings by revealing a task-dependent functional circuit between dmPFC and ventral and dorsal portions of premotor cortex.
Funding
National Institutes of Health (F32-MH-83424, R01-NS-58487, R01-NS-52318, R01-NS-40902, R01-NS-28127).
Acknowledgments
Conflict of Interest : None declared.
References
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Maas LC, Frederick B, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF, et al. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31:1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, Carcelen R, Romero FJ. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict Biol. 2010;15:504–516. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck depression inventory manual. San Antonio (TX): The Psychological Corp; 1987. [Google Scholar]
- Berman AJ. Amelioration of aggression: response to selective cerebellar lesions in the rhesus monkey. Int Rev Neurobiol. 1997;41:111–119. [PubMed] [Google Scholar]
- Bobee S, Mariette E, Tremblay-Leveau H, Caston J. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112:107–117. doi: 10.1016/s0166-4328(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Bowers D, Miller K, Mikos A, Kirsch-Darrow L, Springer U, Fernandez H, Foote K, Okun M. Startling facts about emotion in Parkinson's disease: blunted reactivity to aversive stimuli. Brain. 2006;129:3356–3365. doi: 10.1093/brain/awl301. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Broen M, Duits A, Visser-Vandewalle V, Temel Y, Winogrodzka A. Impulse control and related disorders in Parkinson's disease patients treated with bilateral subthalamic nucleus stimulation: a review. Parkinsonism Relat Disord. Forthcoming. 2011 doi: 10.1016/j.parkreldis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci. 2010;30:14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes SA, Cauraugh JH, Janelle CM. Emotion and movement: activation of defensive circuitry alters the magnitude of a sustained muscle contraction. Neurosci Lett. 2006;396:192–196. doi: 10.1016/j.neulet.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Cauraugh JH, Janelle CM. Dissociating motivational direction and affective valence: specific emotions alter central motor processes. Psychol Sci. 2007a;18:938–942. doi: 10.1111/j.1467-9280.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Cauraugh JH, Janelle CM. Emotional state and initiating cue alter central and peripheral motor processes. Emotion. 2007b;7:275–284. doi: 10.1037/1528-3542.7.2.275. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Corcos DM, Sprute L, Vaillancourt DE. Selective regions of the visuomotor system are related to gain induced changes in force error. J Neurophysiol. 2010;103:2114–2123. doi: 10.1152/jn.00920.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes SA, Corcos DM, Vaillancourt DE. Spatiotemporal tuning of brain activity and force performance. Neuroimage. 2011;54:2226–2236. doi: 10.1016/j.neuroimage.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes SA, Gamble KM, Cauraugh JH, Janelle CM. Emotional states alter force control during a feedback occluded motor task. Emotion. 2008;8:104–113. doi: 10.1037/1528-3542.8.1.104. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Tandonnet C, Fujiyama H, Janelle CM, Cauraugh JH, Summers JJ. Emotion and motor preparation: a transcranial magnetic stimulation study of corticospinal motor tract excitability. Cogn Affect Behav Neurosci. 2009;9:380–388. doi: 10.3758/CABN.9.4.380. [DOI] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Goble DJ, Van Impe A, De Vos J, Wenderoth N, Swinnen SP. Reduced basal ganglia function when elderly switch between coordinated movement patterns. Cereb Cortex. 2010;20:2368–2379. doi: 10.1093/cercor/bhp306. [DOI] [PubMed] [Google Scholar]
- Damasio H. Human brain anatomy in computerized images. Cary (NC): Oxford University Press; 2005. [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/JNEUROSCI.3386-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage. 2003;19:764–776. doi: 10.1016/s1053-8119(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 1999;81:3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Aarts H. Perception of the color red enhances the force and velocity of motor output. Emotion. 2011;11:445–449. doi: 10.1037/a0022599. [DOI] [PubMed] [Google Scholar]
- Flykt A. Visual search with biological threat stimuli: accuracy, reaction times, and heart rate changes. Emotion. 2005;5:349–353. doi: 10.1037/1528-3542.5.3.349. [DOI] [PubMed] [Google Scholar]
- Flykt A. Preparedness for action: responding to the snake in the grass. Am J Psychol. 2006;119:29–43. [PubMed] [Google Scholar]
- Flykt A, Caldara R. Tracking fear in snake and spider fearful participants during visual search: a multi-response domain study. Cogn Emot. 2006;20:1075–1091. [Google Scholar]
- Frijda NH. The emotions. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Molnar C, George MS, Bolger K, Koola J, Nahas Z. Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology. 2007;44:91–97. doi: 10.1111/j.1469-8986.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Hammermeister J, Burton D. Stress, appraisal, and coping revisited: examining the antecedents of competitive state anxiety with endurance athletes. Sport Psychologist. 2001;15:66–90. [Google Scholar]
- Heath RG. Modulation of emotion with a brain pacemamer. Treatment for intractable psychiatric illness. J Nerv Ment Dis. 1977;165:300–317. [PubMed] [Google Scholar]
- Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar stimulation: effects on septal region, hippocampus, and amygdala of cats and rats. Biol Psychiatry. 1978;13:501–529. [PubMed] [Google Scholar]
- Hendrick OM, Ide JS, Luo X, Li CS. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PLoS One. 2010;5:e13155. doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Age-related reduction in the differential pathways involved in internal and external movement generation. Neurobiol Aging. 2010;31:301–314. doi: 10.1016/j.neurobiolaging.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Hon N, Epstein RA, Owen AM, Duncan J. Frontoparietal activity with minimal decision and control. J Neurosci. 2006;26:9805–9809. doi: 10.1523/JNEUROSCI.3165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Integration of target and body-part information in the premotor cortex when planning action. Nature. 2000;408:466–470. doi: 10.1038/35044075. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage. 2011;54:455–464. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville (FL): University of Florida; 2008. [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol. 1991;311:463–482. doi: 10.1002/cne.903110403. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Stark CE. Striatal and medial temporal lobe functional interactions during visuomotor associative learning. Cereb Cortex. 2011;21:647–658. doi: 10.1093/cercor/bhq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy K, Munz Y, Dosis A, Bann S, Darzi A. The effect of stress-inducing conditions on the performance of a laparoscopic task. Surg Endosc. 2003;17:1481–1484. doi: 10.1007/s00464-002-9224-9. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC. The What and How of prefrontal cortical organization. Trends Neurosci. 2010;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gabrieli JD, Gross JJ. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci. 2009;20:1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Inui T, Sugio T. Separating brain regions involved in internally guided and visual feedback control of moving effectors: an event-related fMRI study. Neuroimage. 2006;32:1760–1770. doi: 10.1016/j.neuroimage.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Ojakangas CL, Shaikhouni A, Friehs GM, Caplan AH, Serruya MD, Saleh M, Morris DS, Donoghue JP. Decoding movement intent from human premotor cortex neurons for neural prosthetic applications. J Clin Neurophysiol. 2006;23:577–584. doi: 10.1097/01.wnp.0000233323.87127.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124:1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Parnes SA, Fitzgerald JM, Sweeney JA. A pharmacological functional magnetic resonance imaging study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2010;20:395–406. doi: 10.1089/cap.2009.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE. Region of interest template for the human basal ganglia: comparing EPI and standardized space approaches. Neuroimage. 2008;39:956–965. doi: 10.1016/j.neuroimage.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Bradley MM, Costa VD, Keil A. The timing of emotional discrimination in human amygdala and ventral visual cortex. J Neurosci. 2009;29:14864–14868. doi: 10.1523/JNEUROSCI.3278-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Scelfo B, Strata P. Cerebellum and emotional behavior. Neuroscience. 2009;162:756–762. doi: 10.1016/j.neuroscience.2009.01.064. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neurolinguistics. 2000;13:189–214. [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI atlas of the human cerebellum. San Diego (CA): Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Clery-Melin ML, Lafargue G, Valabregue R, Fossati P, Dubois B, Pessiglione M. Get aroused and be stronger: emotional facilitation of physical effort in the human brain. J Neurosci. 2009;29:9450–9457. doi: 10.1523/JNEUROSCI.1951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) PaloAlto (CA): Consulting Psychologists Press; 1983. [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR. Visual feedback to stabilize head position for fMRI. Magn Reson Med. 1999;41:1039–1043. doi: 10.1002/(sici)1522-2594(199905)41:5<1039::aid-mrm24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol. 2003;90:3330–3340. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- van Loon AM, van den Wildenberg WP, van Stegeren AH, Hajcak G, Ridderinkhof KR. Emotional stimuli modulate readiness for action: a transcranial magnetic stimulation study. Cogn Affect Behav Neurosci. 2010;10:174–181. doi: 10.3758/CABN.10.2.174. [DOI] [PubMed] [Google Scholar]
- Wallenius C, Larsson G, Johansson CR. Military observers’ reactions and performance when facing danger. Mil Psychol. 2004;16:211–229. [Google Scholar]
- Zanchetti A, Zoccolini A. Autonomic hypothalamic outbursts elicited by cerebellar stimulation. J Neurophysiol. 1954;17:475–483. doi: 10.1152/jn.1954.17.5.475. [DOI] [PubMed] [Google Scholar]