Abstract
Background
Combination therapy with deferoxamine and oral deferiprone is superior to deferoxamine alone in removing cardiac iron and improving left ventricular ejection fraction (LVEF). The right ventricle (RV) is also affected by the toxic effects of iron and may cause additional cardiovascular perturbation. We assessed the effects of combination therapy on the RV in thalassaemia major (TM) using cardiovascular magnetic resonance (CMR).
Methods
We retrieved imaging data from 2 treatment trials and re-analyzed the data for the RV responses: Trial 1 was a randomized controlled trial (RCT) of 65 TM patients with mild-moderate cardiac siderosis receiving combination therapy or deferoxamine with placebo; Trial 2 was an open label longitudinal trial assessing combination therapy in 15 TM patients with severe iron loading.
Results
In the RCT, combination therapy with deferoxamine and deferiprone was superior to deferoxamine alone for improving RVEF (3.6 vs 0.7%, p = 0.02). The increase in RVEF was greater with lower baseline T2* 8-12 ms (4.7 vs 0.5%, p = 0.01) than with T2* 12-20 ms (2.2 vs 0.8%, p = 0.47). In patients with severe cardiac siderosis, substantial improvement in RVEF was seen with open-label combination therapy (10.5% ± 5.6%, p < 0.01).
Conclusions
In the RCT of mild to moderate cardiac iron loading, combination treatment improved RV function significantly more than deferoxamine alone. Combination treatment also improved RV function in severe cardiac siderosis. Therefore adding deferiprone to deferoxamine has beneficial effects on both RV and LV function in TM patients with cardiac siderosis.
Keywords: thalassaemia major, deferiprone, deferoxamine, right ventricular function
Background
In transfusion-dependent thalassaemia major (TM) patients, iron chelation therapy is mandatory to prevent or reverse iron accumulation caused by excess intake from transfusional iron and the increased gastrointestinal absorption. Deferoxamine was the first clinically available iron chelating agent, introduced over 40 years ago, and life expectancy in TM increased dramatically with its use. [1,2] However, its beneficial effects are tempered by the cumbersome treatment regimes required, which may be a contributor to the frequently observed long term complications of heart failure and cardiac death. [3]
Deferiprone is an orally active chelator with a lower molecular weight that is uncharged at physiological pH, and which is both hydrophilic and lipophilic enabling it to readily penetrate myocardial cells. It has been shown to be superior to deferoxamine in removing iron from the myocardium, and is associated with improved cardiac outcomes. [4-9] Due to differences in their access to body iron pools, the use of a combination of the two chelators seems to have a synergistic effect on removal of excess iron. [9,10] A recent randomised controlled trial comparing combination therapy with subcutaneous deferoxamine and oral deferiprone against deferoxamine monotherapy showed combination treatment to be superior in removing cardiac iron and improving left ventricular ejection fraction (LVEF). [11] The beneficial effects of combination therapy on LVEF have also been confirmed in patients with TM and severe iron loading. [12]
However, despite this success for LV function, the importance of combination therapy on right ventricular (RV) function has not been reported, even though the RV can be affected by the toxic effects of myocardial iron. [13,14] Cardiovascular magnetic resonance (CMR) provides highly reliable and reproducible measurements of RV volumes and function as well as myocardial iron using the T2* method. [15,16] We therefore compared the effects of combination treatment (deferoxamine and deferiprone) with deferoxamine monotherapy on RV function in TM patients with cardiac iron overload.
Methods
Study population
In order to examine the effects of combination treatment on the RV, we reanalyzed imaging data from 2 previously reported trials. The first was a randomized, double-blind, placebo controlled trial (RCT) comparing combined therapy of deferoxamine with deferiprone against deferoxamine with placebo in mild-moderate myocardial siderosis. [11] The second trial was a longitudinal open-label study of combination treatment (no comparison arm) in patients with severe cardiac siderosis and impaired LV function. [12] Both trials were run simultaneously in Cagliari Italy (Figure 1). The study protocol was approved by ethics committees in London and Cagliari. Patient information and consent forms were in Italian and all patients gave written informed consent. [11,12] Brief details of the trials are given below.
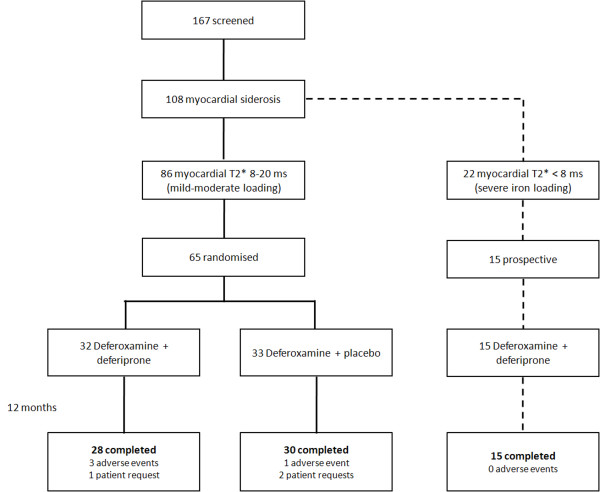
Figure 1.
Study flow-chart.
In the RCT, 167 adult TM patients (75 males, mean age 30 ± 5.3 years) were screened for quantification of myocardial iron loading using myocardial T2*. Inclusion criteria for patient screening were: diagnosis of TM currently maintained on subcutaneous deferoxamine monotherapy; age > 18 years; and maintaining pre-transfusion haemoglobin > 9 g/dL. Exclusion criteria were: patients who had received deferiprone for a total of > 6 months over the last 5 years; patients with previous reaction to deferiprone; neutropenia (absolute neutrophil count < 1.5 × 109/L) at screening; thrombocytopenia ( < 50 ×109/L) at screening; liver enzymes > 3 times upper limit of normal; any condition making CMR impossible or inadvisable. Of the 167 patients screened, 108 had significant myocardial siderosis (T2* < 20 ms), of whom 22 (13%) had severe myocardial loading (T2* < 8 ms). Patients with mild to moderate cardiac iron loading who satisfied the trial entry criteria (myocardial T2* 8-20 ms, n = 86) were invited for further detailed assessment by CMR. Of these, 65 were subsequently randomized to receive either deferoxamine plus deferiprone (combined group; n = 32) or deferoxamine plus placebo (deferoxamine group; n = 33), and were followed-up for 12 months.
Patients with severe cardiac siderosis (T2* < 8 ms) were excluded from the RCT and it was at the treating clinician's discretion to determine best clinical practice for chelation therapy. Of the 22 patients with severe myocardial siderosis, 15 (9 females, 28.9 ± 4.8 years) received open-label combination therapy according to locally developed protocols, and were followed prospectively over one year. These patients were used in a secondary comparative analysis against patients from the randomised trial who were on combination therapy.
Cardiovascular magnetic resonance
A mobile 1.5 Tesla CMR scanner (Sonata, Siemens Medical Systems, Erlangen, Germany) was transported to Cagliari for this research. Myocardial and hepatic T2* were assessed using the bright-blood single breath-hold multi-echo technique as previously described. [17] T2* analysis was performed using Thalassaemia-Tools (a plug-in of CMRtools, Cardiovascular Imaging Solutions, London, UK) with curve truncation to account for background noise. [18] Right ventricular volumes and ejection fraction were determined at baseline and at 12 months of treatment with steady state free precession cines using contiguous short-axis slices from base to apex. [15] CMRtools was used for RV volume analysis. These measurements were performed by observers blinded to the patient's clinical details and chelation regime.
Echocardiography
Doppler echocardiography studies were performed at baseline and at 12 months to look for pulmonary hypertension. Pulmonary artery systolic pressures (PAP) were determined by peak velocity of the tricuspid regurgitation jet plus estimation of right atrial pressures using standard methodology. Pulmonary hypertension was defined as PAP > 40 mmHg.
Biochemistry
Laboratory measures included weekly full blood count (due to the risk of agranulocytosis with deferiprone), serum ferritin (Abbott AXSYM System), B-type natriuretic peptide (BNP-Biosite Diagnostics Inc, San Diego, California), and liver function tests (alanine aminotransferase - ALT).
Statistical Analysis
Categorical data are presented as frequency and percentage (%). Continuous variables are presented as mean ± standard deviation (SD), except for BNP, which is displayed in median and interquartile range; and for T2* and ferritin, which use the geometric mean (anti-log of the mean of the log data) ± coefficient of variation (CV). Baseline characteristics of both treatment groups were compared using an unpaired two-tailed t-test for continuous variables (except for BNP, which was compared by a non-parametric test) and a chi-squared test for categorical variables. Analysis of variance (ANOVA) was used to compare changes in T2* and RVEF over 12 months with treatment and baseline measures entered as covariates. Changes in RVEF over 12 months within individual groups were compared with a paired t-test. Correlations of myocardial T2* with ventricular function were performed using the Spearman's rank test. Subgroup analysis was performed according to severity of myocardial iron loading with cut-offs of 8 ms and 12 ms used to define patients with mild (T2* 12-20 ms), moderate (T2* 8-12 ms) and severe (T2* < 8 ms) iron loading. Intraobserver and interobserver variability was assessed using the method of Bland and Altman. [19] The coefficient of variability was calculated as the SD of the differences between two sets of measurements divided by the mean value of the parameter under consideration. Statistical significance was set at p < 0.05. All statistical analysis was performed using Stata 10.1 software (StataCorp, Texas, USA).
Results
RCT in mild to moderate cardiac siderosis
The baseline findings and the results of the RCT comparing combination treatment against deferoxamine alone for changes in myocardial T2* and LV EF have been previously published,[11] and are briefly summarized here (table 1). The patients randomized to combination therapy or deferoxamine alone were evenly matched at baseline. The prescribed dose of deferiprone in the combination arm was 75 mg/kg/day. The average dose of deferoxamine in the deferoxamine alone arm (40.5 mg/kg/day for 5 days/week) was comparable to the combination arm (40.6 mg/kg/day for 5 days/week, p = 1.0). Four patients in the combination arm withdrew from the study (3 due to adverse events), and 3 patients in the deferoxamine arm withdrew (1 due to an adverse event). Thus, 28 patients in the combination arm and 30 patients in the deferoxamine alone arm completed the study. Over 12 months, the combination treatment group showed superior improvement in myocardial T2* compared with the deferoxamine group (ratio of change in geometric means 1.50 vs. 1.24, p = 0.02).
Table 1.
Baseline characteristics of the randomized controlled trial population, according to treatment arm.
| Combined | Deferoxamine | p-value | |
|---|---|---|---|
| Number of patients | 32 | 33 | - |
| Age (years) | 28.8 ± 4.2 | 28.7 ± 5.3 | 0.9 |
| Gender (male) | 14 (44%) | 13 (39%) | 0.5 |
| Body surface area | 1.53 ± 0.15 | 1.56 ± 0.16 | 0.5 |
| Heart rate | 78 ± 10 | 81 ± 16 | 0.3 |
| Deferoxamine dose (mg/kg/day) | 40.6 ± 13.2 (5 days/week) | 40.5 ± 14.0 5 days/week) | 1.0 |
| CMR measures | |||
| Myocardial T2* | 11.7 (0.08) | 12.4 (0.11) | 0.3 |
| Liver T2* | 4.9 (0.52) | 4.2 (0.62) | 0.5 |
| RVEDV (mL) | 129.5 ± 30.8 | 131.8 ± 34.4 | 0.8 |
| RVESV (ml) | 52.5 ± 17.5 | 52.3 ± 18.5 | 1.0 |
| RVEF (%) | 60.2 ± 7.2 | 61.0 ± 7.1 | 0.7 |
| Echo measures | |||
| PAP (mmHg) | 22.3 ± 5.0 | 20.9 ± 5.4 | 0.4 |
| Blood measures | |||
| Transfusional red blood cell input (mL/kg/year) | 133.4 ± 34.9 | 130.2 ± 38.6 | 0.7 |
| Haemoglobin (g/L) | 106 ± 9.6 | 102 ± 9.5 | 0.1 |
| Hepatitis C positive | 23 (72%) | 26 (79%) | 0.4 |
| Serum ferritin (μg/L) | 1574 (11) | 1379 (10) | 0.5 |
| BNP (pmol/L) | 13.6 (5.6, 30.1) | 15.2 (7.3, 26.2) | 0.7 |
| Creatinine (mg/dL) | 0.77 ± 0.21 | 0.74 ± 0.23 | 0.6 |
| Cardiac medication | |||
| Any | 5 (16%) | 8 (24%) | 0.4 |
| Digoxin | 3 (9%) | 4 (12%) | 0.7 |
| ACEi/ARB | 5 (16%) | 8 (24%) | 0.4 |
| Diuretics | 3 (9%) | 5 (15%) | 0.5 |
Values are presented as n (%), mean ± SD, geometric mean (coefficient of variation), or as median (25th - 75th percentile). RV = right ventricular; EDV = end-diastolic volume; ESV = end-systolic volume; PAP = pulmonary artery systolic pressure; BNP = B-type natriuretic peptide. ACEi = angiotensin-converting enzyme inhibitors; ARB = angiotensin II receptor blockers.
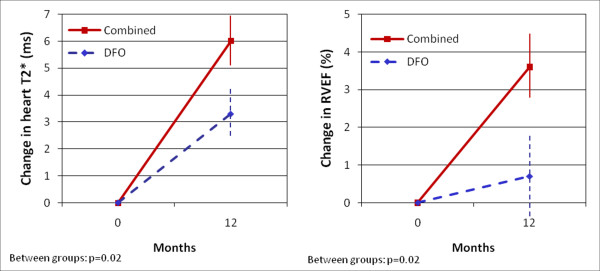
In the combination group RVEF increased from 60.2 ± 7.2% at baseline to 63.8 ± 5.9% at 12 months (p < 0.01), whereas in the deferoxamine group RVEF did not change significantly (61.0 ± 7.1% at baseline vs. 61.7 ± 6.4% at 12 months, p = 0.49). There was a significant difference in the RVEF response between groups favouring combination therapy (3.6 vs. 0.7%, p = 0.02; Figure 2). The improvement in RVEF in the combined group was mainly driven by a decrease in RV end-systolic volumes (52.5 ± 17.5 ml to 46.4 ± 14.9 ml, p < 0.01) rather than a change in RV end-diastolic volumes (129.5 ± 30.8 ml to 126.1 ± 29.8 ml, p = 0.20). There was no significant change in PAP between the combination vs. the deferoxamine arm from baseline to one year (-1.8 mmHg vs. +0.3 mmHg, p = 0.19).
Figure 2.
Change in myocardial T2* (left panel) and in RVEF (right panel) over 12 months according to treatment arm. Vertical lines represent standard error.
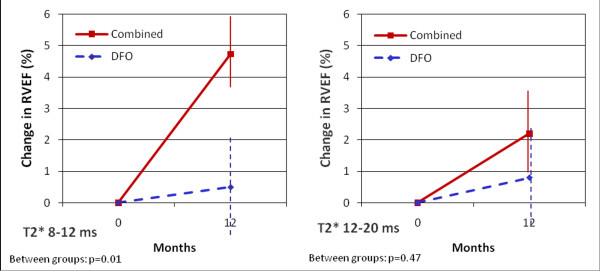
The median myocardial T2* in the RCT at baseline was 12.0 ms. This value was used as a cut-off to define patients with mild (myocardial T2* 12-20 ms) and moderate iron loading (myocardial T2* 8-12 ms), and this was in accord with the cut-offs used in the original trials. Both subgroups were then analysed according to the chelation regime. Comparing the individual treatment arms, we observed a significant improvement in RVEF in patients with T2* between 8 and 12 ms on combination therapy (58.5 ± 6.9% at baseline vs 63.3 ± 6.0% at 12 months, p < 0.01), and borderline significant improvement in patients with T2* between 12 and 20 ms (62.1 ± 7.3% at baseline vs. 64.3 ± 6.0% at 12 months, p = 0.08). Conversely, patients on deferoxamine alone had no significant improvement in RVEF, whether the baseline T2* was 8-12 ms (59.3 ± 6.8% at baseline vs. 59.8 ± 6.4% at 12 months, p = 0.70) or 12-20 ms (62.5 ± 7.2% at baseline vs. 63.3 ± 6.1% at 12 months, p = 0.58). Comparison of the between groups effects showed that combination therapy was superior to deferoxamine alone in improving RVEF in patients with baseline T2* below 12 ms (4.7% vs. 0.5%, p = 0.01) but not in those with T2* above 12 ms (2.2% vs. 0.8%, p = 0.47; Figure 3).
Figure 3.
Change in RVEF over 12 months according to treatment arm and myocardial T2* at baseline (T2* 8-12 ms on left panel, T2* 12-20 ms on right panel). Vertical lines represent standard error.
Intraobserver and interobserver variability of the right ventricular volumes and ejection fraction was derived from the first 20 subjects participating in the study (table 2). The coefficient of variability for the right ventricular measurements was small, in keeping with previous publications on the same area. [20,21]
Table 2.
Intraobserver and interobserver reproducibility for the right ventricle.
| Intraobserver | Interobserver | |||
|---|---|---|---|---|
| Mean difference (± SD) | CoV (%) | Mean difference (± SD) | CoV (%) | |
| RV end-diastolic volume (mL) | 0.6 ± 5.0 | 3.9 | 6.7 ± 8.9 | 7.1 |
| RV end-systolic volume (mL) | -0.1 ± 3.6 | 6.7 | 2.0 ± 4.7 | 8.9 |
| RV ejection fraction (%) | 0.5 ± 1.9 | 3.2 | 1.0 ± 3.0 | 5.2 |
CoV = coefficient of variability; RV = right ventricle.
Longitudinal open-label study in severe cardiac siderosis
The baseline findings and the results of the longitudinal study in the 15 patients with severe iron loading (myocardial T2* < 8 ms) evaluating combination treatment for changes in myocardial T2* and LVEF have been previously published,[12] and are briefly summarized in table 3. Two patients were in clinical heart failure, and both had BNP levels > 100 pmol/l. The mean prescribed doses of deferoxamine and deferiprone at baseline were 38.0 mg/kg for 5.3 days/week (equivalent to 40.7 mg/kg for 5 days/week) and 73.9 mg/kg/day, respectively. During the trial, doses were reduced to 20.3 mg/kg for 4.5 days/week and 65.7 mg/kg/day respectively, primarily due to reductions in ferritin. [11] All 15 patients received unblinded combination therapy with deferoxamine and deferiprone throughout the study period. Over 12 months, there was a significant improvement in myocardial T2* (ratio of change in geometric means 1.31, p < 0.01).
Table 3.
Baseline characteristics of the randomized controlled trial population (mild and moderate myocardial iron loading) vs. open-label combination therapy population (severe myocardial iron loading). Values and abbreviations presented as in table 1.
| Mild-moderate siderosis | Severe siderosis | p-value | |
|---|---|---|---|
| RCT | Longitudinal trial | ||
| Number | 65 | 15 | - |
| Age (years) | 28.8 ± 4.7 | 27.8 ± 4.8 | 0.5 |
| Gender (male) | 27 (41.5%) | 6 (40.0%) | 0.9 |
| Body surface area | 1.55 ± 0.15 | 1.56 ± 0.13 | 0.8 |
| Heart rate | 79 ± 13 | 97 ± 18 | < 0.01 |
| Deferoxamine (mg/kg/day) | 40.6 ± 13.5 (5 days/week) |
40.7 ± 12.0 (5 days/week) |
0.9 |
| CMR measures | |||
| Myocardial T2* | 12.0 (0.13) | 6.0 (0.09) | N/A |
| Liver T2* | 4.5 (0.57) | 2.9 (0.67) | 0.06 |
| RVEDV (mL) | 130.7 ± 32.4 | 146.7 ± 35.1 | 0.2 |
| RVESV (mL) | 52.4 ± 17.8 | 76.5 ± 27.6 | < 0.01 |
| RVEF (%) | 60.6 ± 7.1 | 49.0 ± 9.4 | < 0.01 |
| Echo measures | |||
| PAP (mmHg) | 21.6 ± 5.2 | 22.0 ± 5.9 | 0.8 |
| Blood measures | |||
| Hepatitis C positive | 49 (75%) | 11 (73%) | 0.9 |
| Serum ferritin (μg/L) | 1472 (0.11) | 2057 (0.08) | 0.1 |
| BNP (pmol/L) | 15.0 (7.1, 28.8) | 26.0 (15.6, 40.5) | 0.01 |
| Cardiac medication | |||
| Any | 13 (20%) | 7 (47%) | 0.03 |
| Digoxin | 7 (11%) | 4 (27%) | 0.1 |
| ACEi/ARB | 13 (20%) | 7 (47%) | 0.03 |
| Diuretics | 8 (12%) | 4 (27%) | 0.2 |
In 12 of the 15 patients (80%), the baseline RVEF was low compared to a reference TM population with a normal T2*. [22] The baseline RVESV was significantly raised and there was a trend for a higher RVEDV, resulting in a significantly reduced mean RVEF (49.0 ± 9.4%) when compared to the population with less severe iron loading (table 2). The open-label group was more frequently medicated for heart failure, particularly angiotensin-converting enzyme inhibitors (ACEi)/angiotensin II receptor blockers (ARB). No patients in the RCT or open-label cohorts were on beta-blockers. Pulmonary artery systolic pressures were similar in both groups (22.0 ± 5.9 mmHg vs 21.6 ± 5.2 mmHg, p = 0.82). Over 12 months, there was a significant improvement in RVEF (10.5 ± 5.6%, p < 0.01), with no significant change in PAP (22.0 mmHg vs 24.4 mmHg at 12 months, p = 0.16). No new cardiac medications were commenced during the study (except for the 2 patients presenting with heart failure, who were asymptomatic by the end of the study). Despite a positive trend, neither cardiac medication as a whole (increase RVEF 12 ± 6% vs 9 ± 5%, p = 0.2), nor individual medications (digoxin: 14 ± 3% vs 10 ± 6%, p = 0.2; ACEi/ARB: 12 ± 6% vs 9 ± 5%, p = 0.2; diuretics: 13 ± 5% vs 10 ± 6%, p = 0.4) were significantly associated with a higher increase in RVEF compared with those without cardiac medication.
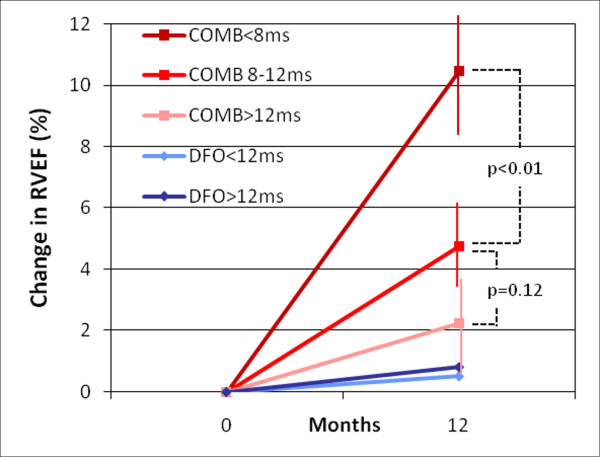
When grouping the patients on combination therapy (the 32 patients from the combination arm in the RCT plus the 15 unblinded patients on open-label combined therapy), we found an inverse relation between myocardial T2* at baseline and improvement in RVEF over one year (Figure 4). Patients with severe iron loading had a greater improvement in RVEF than patients with moderate iron loading (10.5% vs. 4.7%, p < 0.01).
Figure 4.
Breakdown of improvement in RVEF (%) for different groups according to chelation therapy and myocardial T2* baseline. Vertical lines represent standard error.
Entire study cohort
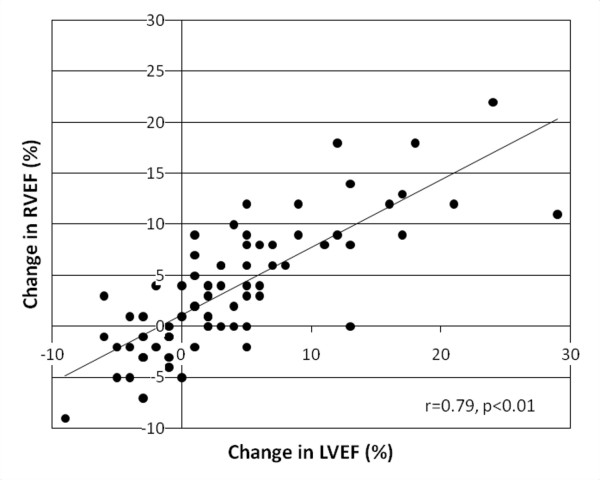
In the cohort of 80 patients with myocardial iron loading, a significant correlation was observed between baseline myocardial T2* and baseline RVEF (r = 0.46, p < 0.01) and baseline LVEF (r = 0.50, p < 0.01). Accordingly, there was a strong correlation between RVEF and LVEF at baseline (r = 0.82, p < 0.01). The improvement in RVEF during the study period also correlated with the improvement in LVEF (r = 0.79, p < 0.01; Figure 5). We also analyzed the change in RVEF with the change in cardiac iron as derived from recent human cardiac iron calibration data from Carpenter et al. [23] There was a significant inverse correlation between the improvement in myocardial iron concentration and the improvement in RVEF (r = -0.42, p < 0.01).
Figure 5.
Correlation of change in RVEF with change in LVEF over 12 months.
Discussion
With the development of the T2* technique, CMR has provided new insights into iron-overload cardiomyopathy, as the myocardial iron concentration and its toxic effect on ventricular function can be assessed at the same time with the same high-fidelity technique. In the first T2* publication by Anderson et al, normal T2* levels were associated with normal LVEF, but when T2* fell below 20 ms, there was a progressive fall in LVEF, showing that increasing iron loading is associated with worsening of LV function. [16] Similar observations have recently been made for the RV,[14] suggesting RV dysfunction may be a contributor to heart failure and cardiac mortality in TM patients, as has been found in other cardiac conditions. [24-28] However, to date, there has been little published data on the response of the right ventricle to chelation therapy, and no data at all on the most appropriate chelation regime in the presence of right ventricular dysfunction.
Chelation with deferoxamine has been one of the cornerstones for the treatment of TM. It has been extensively studied over the past decades and has shown to decrease the total body iron burden, prevent complications of iron overload and improve survival in TM. [1,2,29] However, long-term deferoxamine monotherapy has been hampered by poor compliance and failure of long term prevention of myocardial iron deposition, heart failure and cardiac deaths. [4,5] Deferiprone is a more recent iron chelator which has a lower molecular weight, is more lipophilic, is uncharged at physiologic pH and consequently appears better able to penetrate cells and organelles than deferoxamine. This may in part explain why deferiprone is superior to deferoxamine for removing iron from the heart. [6] The combined use of these two chelating agents which exploits the relative merits of each drug, has been supported in animal models, and has become an attractive therapeutic option in severe cardiac iron loading or when negative iron balance has not been achieved by other methods. [11,30,31] Observational, prospective and randomised controlled studies have demonstrated the efficacy of combined therapy in removing iron from the liver and heart, improving endothelial function and left ventricular function,[11,12] as well as endocrine function. [32] Our current study compared the effects of combination therapy on RV function in TM patients with myocardial iron loading. Our findings from the RCT show combination therapy to be superior to subcutaneous deferoxamine alone in improving RV function in patients with mild and moderate iron loading. In addition, our data from the longitudinal open-label study show that RV dysfunction is reversible even in patients with severe iron overload. It is of interest that the recovery in RV function was greatest in patients with a more severe degree of myocardial siderosis and RV dysfunction.
It is well recognized that RV performance depends not only on intrinsic contractility but also on RV afterload, which is the resistance that the RV has to overcome during ejection. Increased pulmonary artery pressures reflecting increased pulmonary vascular resistance may thus impair RV function. [33] However, none of the participants in this study had pulmonary hypertension, in line with previous studies suggesting a low prevalence of pulmonary hypertension in TM. [34,35] Whilst an improvement in myocardial T2* was associated with an improvement in RVEF, no significant changes in pulmonary artery pressures were observed throughout the study. Therefore, the improvement in RV function seen in this population was independent of pulmonary artery pressure thus eliminating a potential confounding factor for the evaluation of RV function.
Our data are unusual, because reversible RV dysfunction in the setting of a cardiomyopathy is a rarely documented phenomenon. The magnitude of recovery in RV function parallels the recovery in LV function, and this correlates with the response to chelation therapy. This is in keeping with the findings of other studies in non-ischaemic cardiomyopathies, where it has been observed that LV and RV function is usually affected in a similar way and to a similar extent. [24,36] The close association seen between RVEF and LVEF and the increase of these parameters over time following successful myocardial iron chelation supports the concept that intrinsic RV myocardial contractility is predominantly affected by intracellular iron and plays a key role in RV performance in this toxic-induced cardiomyopathy model. [14] Finally, as right ventricular ejection fraction has incremental prognostic value which is additional to LVEF,[24-27] it is reasonable to suggest that the improvement in RVEF seen in this study with the combined use of deferiprone and deferoxamine may contribute to improved outcomes.
Limitations
This is a retrospective analysis of 2 trials designed to assess the change on myocardial T2* with iron chelation regimes in which RV parameters were not planned as primary end-points. Nevertheless, all data was prospectively collected and the current RV analysis was blinded to the patients' details and chelation regimes.
Conclusions
In the RCT of mild to moderate cardiac iron loading, combination treatment improved RV function significantly more than deferoxamine alone. Combination treatment also improved RV function in severe cardiac siderosis. Therefore adding deferiprone to deferoxamine has beneficial effects on both RV and LV function in TM patients with cardiac siderosis.
Competing interests
FA has received speaker's honoraria from Novartis. GCS is a consultant to Novartis. DJP is a consultant to Novartis and ApoPharma, and a director of Cardiovascular Imaging Solutions. DJP has received research support and speaker's honoraria from Siemens, Novartis, and ApoPharma. JPC has received speaker's honoraria from Swedish Orphan and ApoPharma. SN has received financial assistance from both Novartis and Swedish Orphan for attendance at conferences.
Authors' contributions
FA and GCS participated in the design of the study, data collection, and drafted the manuscript. JPC, SVN and MAT participated in the data collection. WB performed the statistical analysis. CD, RG, JMW helped to draft the manuscript. DJP conceived of the study, participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Francisco Alpendurada, Email: f.alpendurada@rbht.nhs.uk.
Gill C Smith, Email: g.smith@rbht.nhs.uk.
John-Paul Carpenter, Email: j.carpenter@rbht.nhs.uk.
Sunil V Nair, Email: sunilnair1973@hotmail.com.
Mark A Tanner, Email: mark.tanner@imperial.nhs.uk.
Winston Banya, Email: w.banya@imperial.ac.uk.
Carlo Dessi, Email: carlo.dessi@mcweb.unica.it.
Renzo Galanello, Email: renzo.galanello@mcweb.unica.it.
John Malcolm Walker, Email: malcolm.walker@uclh.nhs.uk.
Dudley J Pennell, Email: d.pennell@ic.ac.uk.
Acknowledgements
This project was supported by the NIHR Cardiovascular Biomedical Research Unit at the Royal Brompton & Harefield NHS Foundation Trust and Imperial College London.
References
- Zurlo MG, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, Di Gregorio F, Burattini MG, Terzoli S. Survival and causes of death in thalassaemia major. Lancet. 1989;8653:27–30. doi: 10.1016/s0140-6736(89)90264-x. [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, Martin M, Koren G, Cohen AR. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–8. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–193. [PubMed] [Google Scholar]
- Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron levels and ventricular function in beta thalassemia. Lancet. 2002;360:516–20. doi: 10.1016/S0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet. 2000;355:2051–2. doi: 10.1016/S0140-6736(00)02357-6. [DOI] [PubMed] [Google Scholar]
- Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, Gotsis ED, Tanner MA, Smith GC, Westwood MA, Wonke B, Galanello R. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–44. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- Piga A, Caglioti C, Fogliacco E, Tricta F. Comparative effects of deferiprone and deferoxamine on survival and cardiac disease in patients with thalassaemia major: a retrospective analysis. Haematologica. 2003;88:489–96. [PubMed] [Google Scholar]
- Borgna-Pignatti C, Cappellini MD, De Stefano P, Del Vecchio GC, Forni GL, Gamberini MR, Ghilardi R, Piga A, Romeo MA, Zhao H, Cnaan A. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood. 2006;107:3733–7. doi: 10.1182/blood-2005-07-2933. [DOI] [PubMed] [Google Scholar]
- Kolnagou A, Kontoghiorghes GJ. Effective combination therapy of deferiprone and deferoxamine for the rapid clearance of excess cardiac iron and the prevention of heart disease in thalassemia. The Protocol of the International Committee on Oral Chelators. Hemoglobin. 2006;30:239–49. doi: 10.1080/03630260600642567. [DOI] [PubMed] [Google Scholar]
- Wonke B, Wright C, Hoffbrand AV. Combined therapy with deferiprone and desferrioxamine. Br J Haematol. 1998;103:361–4. doi: 10.1046/j.1365-2141.1998.01002.x. [DOI] [PubMed] [Google Scholar]
- Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Roughton M, Assomull R, Nair SV, Walker JM, Pennell DJ. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassaemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–84. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Pibiri M, Nair SV, Walker JM, Pennell DJ. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J Cardiovasc Magn Reson. 2008;10:12. doi: 10.1186/1532-429X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahalis G, Manolis AS, Apostolopoulos D, Alexopoulos D, Vagenakis AG, Zoumbos NC. Right ventricular cardiomyopathy in beta-thalassaemia major. Eur Heart J. 2002;23:147–56. doi: 10.1053/euhj.2001.2709. [DOI] [PubMed] [Google Scholar]
- Alpendurada F, Carpenter JP, Deac M, Kirk P, Walker JM, Porter JB, Banya W, He T, Smith GC, Pennell DJ. Relation of Myocardial T2* to Right Ventricular Function in Thalassaemia Major. Eur Heart J. 2010;31:1648–54. doi: 10.1093/eurheartj/ehq106. [DOI] [PubMed] [Google Scholar]
- Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J. 2006;27:2879–88. doi: 10.1093/eurheartj/ehl336. [DOI] [PubMed] [Google Scholar]
- Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B, Pennell DJ. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18:33–9. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- He T, Gatehouse PD, Smith GC, Mohiaddin RH, Pennell DJ, Firmin DN. Myocardial T2* measurements in iron-overloaded thalassemia: An in vivo study to investigate optimal methods of quantification. Magn Reson Med. 2008;60:1082–9. doi: 10.1002/mrm.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–23. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7(5):775–82. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- Carpenter JP, Alpendurada F, Deac M, Maceira A, Garbowski M, Kirk P, Walker JM, Porter JB, Shah F, Banya W, He T, Smith GC, Pennell DJ. Right ventricular volumes and function in thalassemia major patients in the absence of myocardial iron overload. J Cardiovasc Magn Reson. 2010;12:24. doi: 10.1186/1532-429X-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, Sheppard MN, Porter JB, Walker JM, Wood JC, Galanello R, Forni G, Catani G, Matta G, Fucharoen S, Fleming A, House MJ, Black G, Firmin DN, St Pierre TG, Pennell DJ. On T2* magnetic resonance and cardiac iron. Circulation. 2011;123:1519–28. doi: 10.1161/CIRCULATIONAHA.110.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juilliere Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–80. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–53. doi: 10.1016/0735-1097(94)00511-N. [DOI] [PubMed] [Google Scholar]
- Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–8. doi: 10.1016/S0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- Larose E, Ganz P, Reynolds HG, Dorbala S, Di Carli MF, Brown KA, Kwong RY. Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction. J Am Coll Cardiol. 2007;49:855–62. doi: 10.1016/j.jacc.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib M, Dell'Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010;121:252–8. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- Link G, Ponka P, Konijn AM, Breuer W, Cabantchik ZI, Hershko C. Effects of combined chelation treatment with pyridoxal isonicotinoyl hydrazone analogs and deferoxamine in hypertransfused rats and in iron-loaded rat heart cells. Blood. 2003;101:4172–9. doi: 10.1182/blood-2002-08-2382. [DOI] [PubMed] [Google Scholar]
- Origa R, Bina P, Agus A, Crobu G, Defraia E, Dessì C, Leoni G, Muroni PP, Galanello R. Combined therapy with deferiprone and desferrioxamine in thalassemia major. Haematologica. 2005;90:1309–14. [PubMed] [Google Scholar]
- Farmaki K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br J Haematol. 2010;148:466–75. doi: 10.1111/j.1365-2141.2009.07970.x. [DOI] [PubMed] [Google Scholar]
- Shekerdemian LS, Bush A, Lincoln C, Shore DF, Petros AJ, Redington AN. Cardiopulmonary interactions in healthy children and children after simple cardiac surgery: the effects of positive and negative pressure ventilation. Heart. 1997;78:587–93. doi: 10.1136/hrt.78.6.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derchi G, Fonti A, Forni GL, Galliera EO, Cappellini MD, Turati F, Policlinico OM. Pulmonary hypertension in patients with thalassemia major. Am Heart J. 1999;138:384. doi: 10.1016/s0002-8703(99)70129-8. [DOI] [PubMed] [Google Scholar]
- Aessopos A, Farmakis D, Hatziliami A, Fragodimitri C, Karabatsos F, Joussef J, Mitilineou E, Diamanti-Kandaraki E, Meletis J, Karagiorga M. Cardiac status in well-treated patients with thalassemia major. Eur J Haematol. 2004;73:359–66. doi: 10.1111/j.1600-0609.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- La Vecchia L, Zanolla L, Varotto L, Bonanno C, Spadaro GL, Ometto R, Fontanelli A. Reduced right ventricular ejection fraction as a marker for idiopathic dilated cardiomyopathy compared with ischemic left ventricular dysfunction. Am Heart J. 2001;142:181–9. doi: 10.1067/mhj.2001.116071. [DOI] [PubMed] [Google Scholar]