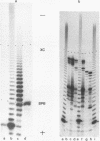
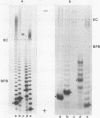
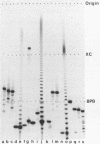
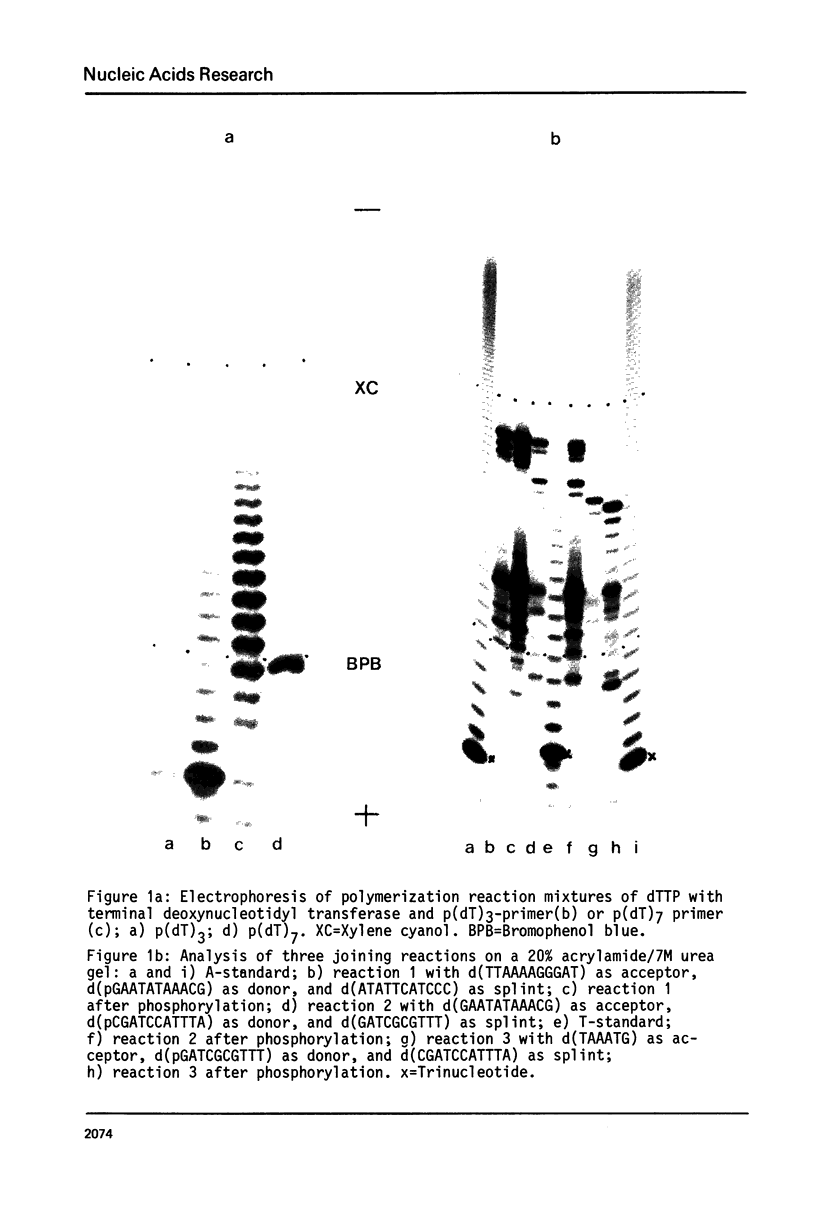
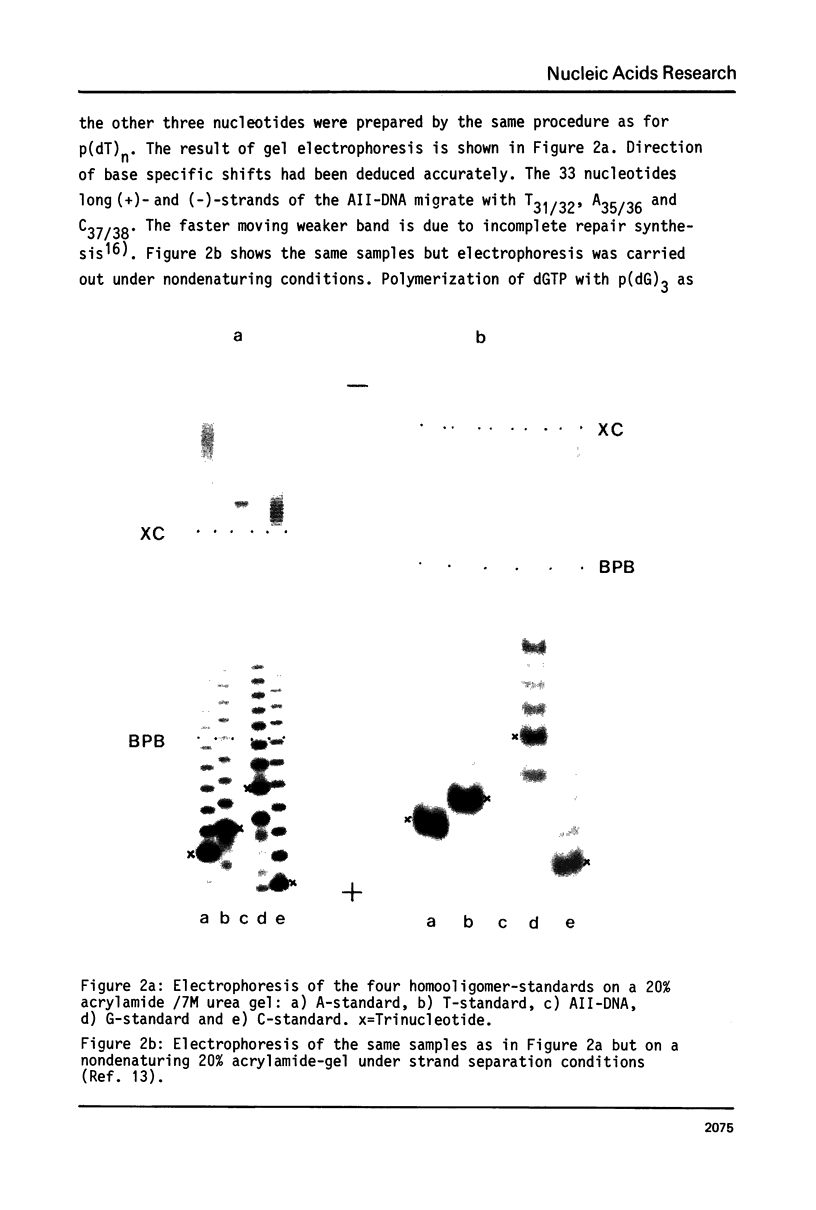
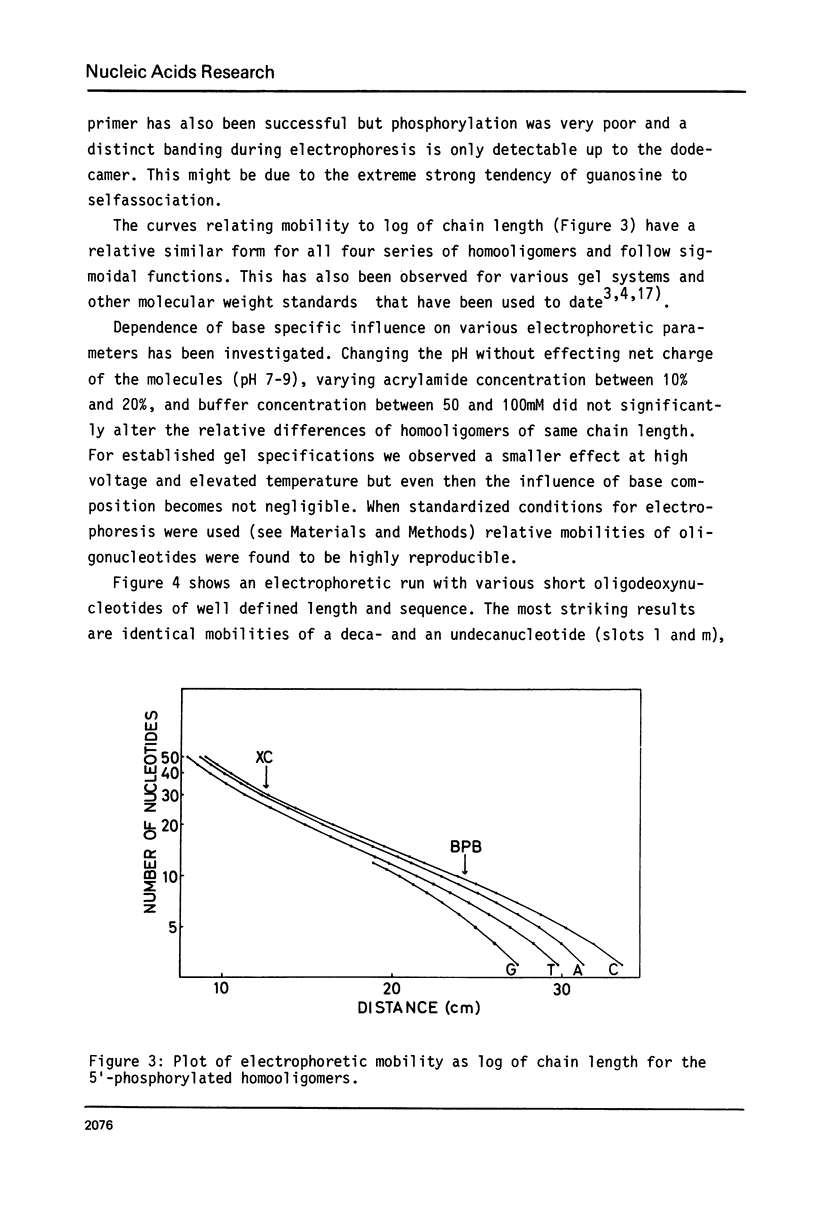
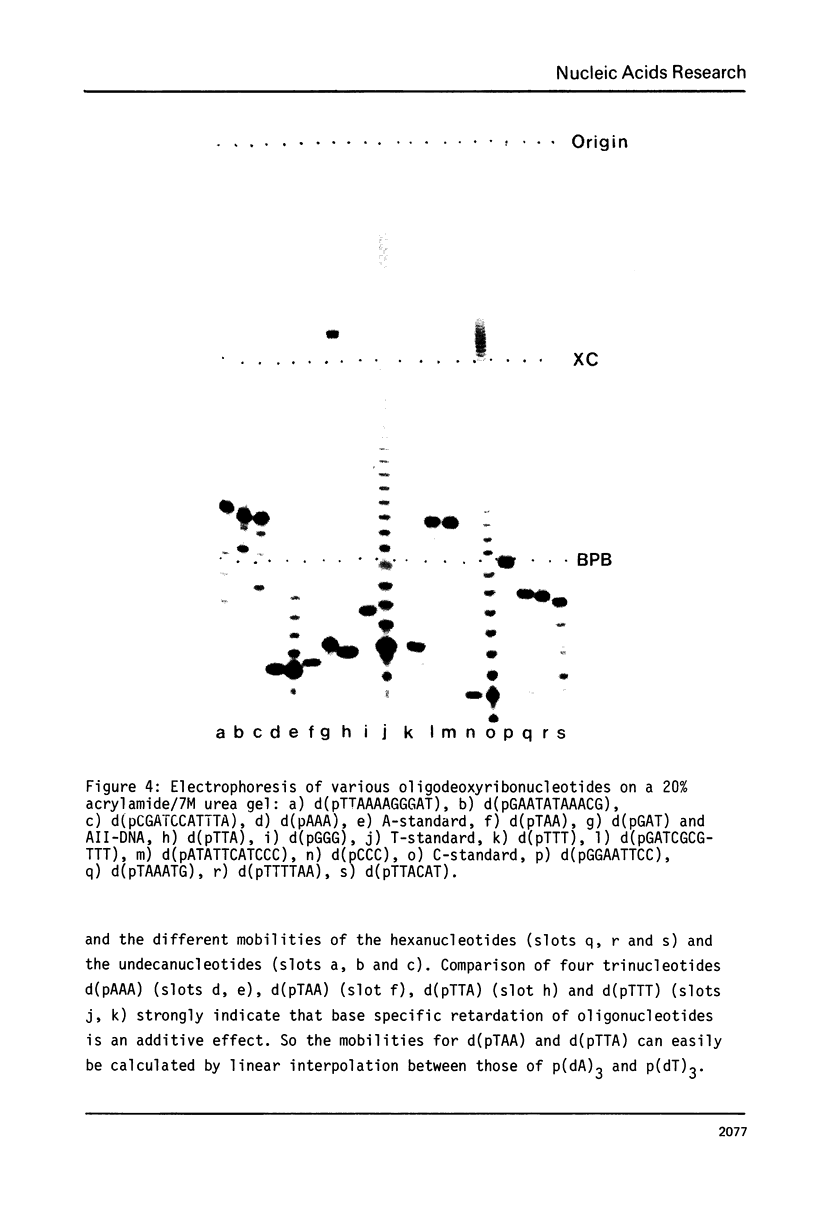
Abstract
The specific influence of the four nucleobases on electrophoretic mobility of oligodeoxyribonucleotides in polyacrylamide-gels under denaturing and nondenaturing conditions has been investigated using homooligomers from the four deoxyribonucleotides as chain length standards. Homooligomers of same chain lengths exhibit remarkable differences in mobility. Specific retardation of any other oligonucleotide investigated was found to be mainly dependent on base composition but not on sequence. A simple procedure is presented for calculating mobilities relative to the standards on denaturing gels. This allows a reliable identification of oligonucleotides on acrylamide-gels by exact chain length determination with respect to base composition and furthermore a detailed interpretation of complex reaction mixtures. The homooligomers also show the same differences in mobility on nondenaturing gels. The significance of this effect for strand separation is discussed.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asteriadis G. T., Armbruster M. A., Gilham P. T. Separation of oligonucleotides, nucleotides, and nucleosides on columns of polystyrene anion-exchangers with solvent systems containing ethanol. Anal Biochem. 1976 Jan;70(1):64–74. doi: 10.1016/s0003-2697(76)80048-6. [DOI] [PubMed] [Google Scholar]
- Flint D. H., Harrington R. E. Gel electrophoresis of deoxyribonucleic acid. Biochemistry. 1972 Dec 5;11(25):4858–4864. doi: 10.1021/bi00775a034. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yansura D. G., Caruthers M. H. Studies on gene control regions. 1. Chemical synthesis of lactose operator deoxyribonucleic acid segments. Biochemistry. 1977 May 3;16(9):1765–1772. doi: 10.1021/bi00628a001. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yansura D. G., Caruthers M. H. Studies on gene control regions. VI. The 5- methyl of thymine, a lac repressor recognition site. Nucleic Acids Res. 1977 Sep;4(9):3039–3054. doi: 10.1093/nar/4.9.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Köster H., Blöcker H., Frank R., Geussenhainer S., Kaiser W. Total synthesis of a structural gene for the human peptide hormone angiotensin II. Hoppe Seylers Z Physiol Chem. 1975 Oct;356(10):1585–1593. doi: 10.1515/bchm2.1975.356.2.1585. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Perlman D., Huberman J. A. Preparation of large quantities of separated strands from simian virus 40 DNA restriction fragments by low-temperature low-salt agarose gel electrophoresis. Anal Biochem. 1977 Dec;83(2):666–677. doi: 10.1016/0003-2697(77)90071-9. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Electrophoresis of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5373–5378. doi: 10.1021/bi00723a019. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay A. A., Grohmann K., Sinsheimer R. L. Separation of the complementary strands of DNA fragments on polyacrylamide gels. Nucleic Acids Res. 1977;4(5):1569–1578. doi: 10.1093/nar/4.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMLINSON R. V., TENER G. M. THE EFFECT OF UREA, FORMAMIDE, AND GLYCOLS ON THE SECONDARY BINDING FORCES IN THE ION-EXCHANGE CHROMATOGRAPHY OF POLYNUCLEOTIDES OF DEAE-CELLULOSE. Biochemistry. 1963 Jul-Aug;2:697–702. doi: 10.1021/bi00904a013. [DOI] [PubMed] [Google Scholar]
- Yansura D. G., Goeddel D. V., Caruthers M. H. Studies on gene control regions. 2. Enzymatic joining of chemically synthesized lactose operator deoxyribonucleic acid segments. Biochemistry. 1977 May 3;16(9):1772–1780. doi: 10.1021/bi00628a002. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Kalisch B. W. Polymerization of oligodeoxythymidylates and oligoriboadenylates catalyzed by T4 polynucleotide ligase and their use as analytical markers in polyacrylamide-gel electrophoresis. Anal Biochem. 1976 Oct;75(2):509–521. doi: 10.1016/0003-2697(76)90106-8. [DOI] [PubMed] [Google Scholar]