Abstract
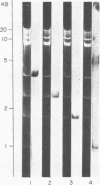
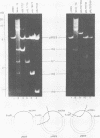
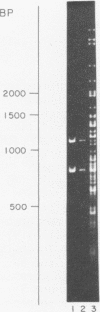
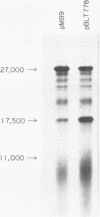
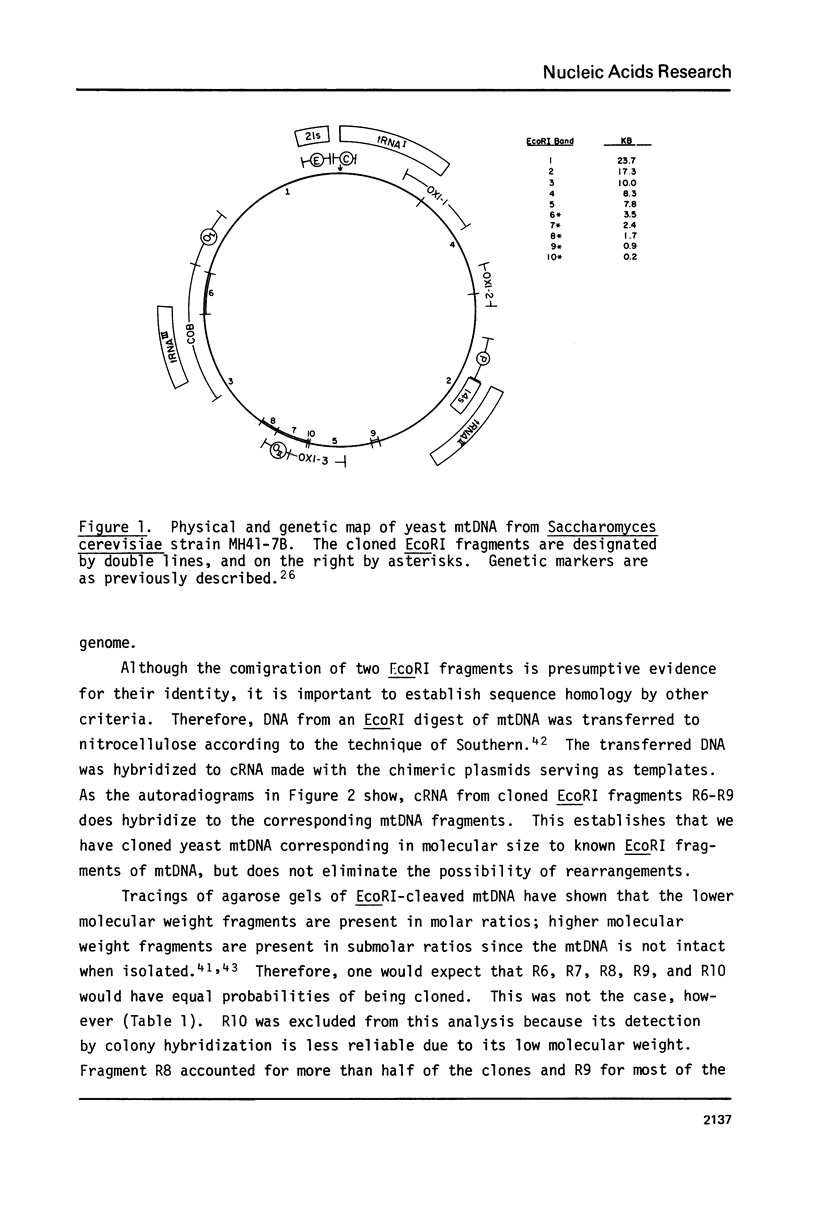
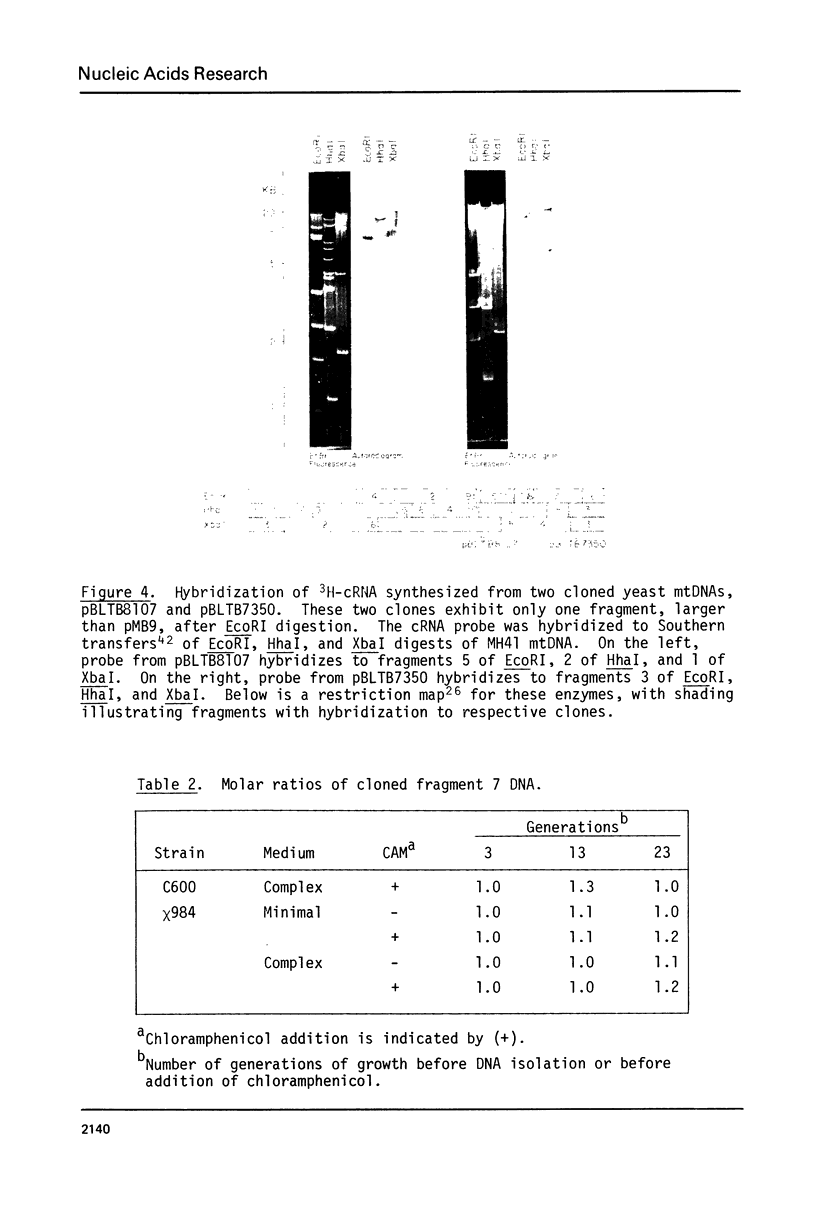
Some of the EcoRI fragments of yeast (Saccharomyces cerevisiae) mitochondrial DNA were cloned into E. coli using plasmid pMB9. The five smallest fragments in molecular weight appeared to be preferentially retained by E coli; partial fragments derived from larger mitochondrial DNA fragments were also found. One of the fragments, R7 (2.4 kb), may contain the OII gene. Cloned R7 DNA was stable under a variety of growth conditions, but showed some changes in molecular weight after transfer to different E. coli strains. Fragment R7 is transcribed in minicells, producing RNA that hybridizes specifically to mitochondrial DNA. Both DNA strands are transcribed, in contrast to the asymmetric transcription found in mitochondria. No new polypeptides were observed in minicells containing cloned fragment 7.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg P. E., Gayda R., Avni H., Zehnbauer B., Markovitz A. Cloning of Escherichia coli DNA that controls cell division and capsular polysaccharide synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):697–701. doi: 10.1073/pnas.73.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G. Organization and evolution of the mitochondrial genome of yeast. J Mol Evol. 1976 Dec 31;9(1):25–35. doi: 10.1007/BF01796120. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Watson R. M., Vinograd J., Tait K. M., Boyer H. W., Goodman H. M. The structures and fidelity of replication of mouse mitochondrial DNA-pSC 101 EcoRI recombinant plasmids grown in E. coli K12. Cell. 1976 Apr;7(4):517–530. doi: 10.1016/0092-8674(76)90202-6. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Fry K., Nelson T., Hung P. Synthesis of hybrid bacterial plasmids containing highly repeated satellite DNA. Cell. 1977 Mar;10(3):509–519. doi: 10.1016/0092-8674(77)90038-1. [DOI] [PubMed] [Google Scholar]
- Carroll D., Brown D. D. Adjacent repeating units of Xenopus laevis 5S DNA can be heterogeneous in length. Cell. 1976 Apr;7(4):477–486. doi: 10.1016/0092-8674(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Lansman R. A., Clayton D. A., Cohen S. N. Studies of mouse mitochondrial DNA in Escherichia coli: structure and function of the eucaryotic-procaryotic chimeric plasmids. Cell. 1975 Oct;6(2):231–244. doi: 10.1016/0092-8674(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Revised interpretation of the origin of the pSC101 plasmid. J Bacteriol. 1977 Nov;132(2):734–737. doi: 10.1128/jb.132.2.734-737.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. Guanidinium-CsCl density gradients for isopycnic analysis of nucleic acids. Science. 1975 Nov 7;190(4214):584–586. doi: 10.1126/science.1188358. [DOI] [PubMed] [Google Scholar]
- Faye G., Fukuhara H., Grandchamp C., Lazowska J., Michel F., Casey J., Getz G. S., Locker J., Rabinowitz M., Bolotin-Fukuhara M. Mitochondrial nucleic acids in the petite colonie mutants: deletions and repetition of genes. Biochimie. 1973;55(6):779–792. doi: 10.1016/s0300-9084(73)80030-6. [DOI] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Derepression of anthranilate synthase in purified minicells of Escherichia coli containing the Col-trp plasmid. J Bacteriol. 1973 Aug;115(2):615–622. doi: 10.1128/jb.115.2.615-622.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- Gayda R. C., Yamamoto L. T., Markovitz A. Second-site mutations in capR (lon) strains of Escherichia coli K-12 that prevent radiation sensitivity and allow bacteriophage lambda to lysogenize. J Bacteriol. 1976 Sep;127(3):1208–1216. doi: 10.1128/jb.127.3.1208-1216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gillham N. W. Genetic analysis of the chloroplast and mitochondrial genomes. Annu Rev Genet. 1974;8:347–391. doi: 10.1146/annurev.ge.08.120174.002023. [DOI] [PubMed] [Google Scholar]
- Gordon P., Casey J., Rabinowitz M. Characterization of mitochondrial deoxyribonucleic acid from a series of petite yeast strains by deoxyribonucleic acid-deoxyribonucleic acid hybridization. Biochemistry. 1974 Mar 12;13(6):1067–1075. doi: 10.1021/bi00703a002. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivell L. A., Reijnders L., Borst P. Isolation of yeast mitochondrial ribosomes highly active in protein synthesis. Biochim Biophys Acta. 1971 Sep 30;247(1):91–103. doi: 10.1016/0005-2787(71)90811-2. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler F. J., Padmanaban G., Patzer J., Ryan R., Rabinowitz M. Yeast mitochondrial RNA contains a short polyadenylic acid segment. Nature. 1975 Nov 27;258(5533):357–359. doi: 10.1038/258357a0. [DOI] [PubMed] [Google Scholar]
- Hershey N. D., Conrad S. E., Sodja A., Yen P. H., Cohen M., Jr, Davidson N., Iigen C., Carbon J. The sequence arrangement of Drosophila melanogaster 5s DNA cloned in recombinant plasmids. Cell. 1977 Jul;11(3):585–598. doi: 10.1016/0092-8674(77)90076-9. [DOI] [PubMed] [Google Scholar]
- Hirsch M., Penman S. Mitochondrial polyadenylic acid-containing RNA: localization and characterization. J Mol Biol. 1973 Nov 5;80(3):379–391. doi: 10.1016/0022-2836(73)90410-5. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Kapp L. N., Brown S. L., Klevecz R. R. Detecting small quantities of DNA on CsCl gradients. Biochim Biophys Acta. 1974 Aug 29;361(2):140–143. doi: 10.1016/0005-2787(74)90341-4. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Cohn R. H., Lowry J. C., Chang A. C., Cohen S. N. The organization of sea urchin histone genes. Cell. 1975 Nov;6(3):359–369. doi: 10.1016/0092-8674(75)90185-3. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. R factor proteins synthesized in Escherichia coli minicells: incorporation studies with different R factors and detection of deoxyribonucleic acid-binding proteins. J Bacteriol. 1974 Dec;120(3):1451–1463. doi: 10.1128/jb.120.3.1451-1463.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin A., Morimoto R., Rabinowitz M. Restriction enzyme analysis of mitochondrial DNAs of petite mutants of yeast: classification of petites, and deletion mapping of mitochondrial genes. Mol Gen Genet. 1978 Jul 25;163(3):257–275. doi: 10.1007/BF00271955. [DOI] [PubMed] [Google Scholar]
- Locker J., Rabinowitz M., Getz G. S. Electron microscopic and renaturation kinetic analysis of mitochondrial DNA of cytoplasmic petite mutants of Saccharomyces cerevisiae. J Mol Biol. 1974 Sep 15;88(2):489–507. doi: 10.1016/0022-2836(74)90497-5. [DOI] [PubMed] [Google Scholar]
- Locker J., Rabinowitz M., Getz G. S. Tandem inverted repeats in mitochondrial DNA of petite mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1366–1370. doi: 10.1073/pnas.71.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Mercereau-Puijalon O., Royal A., Cami B., Garapin A., Krust A., Gannon F., Kourilsky P. Synthesis of an ovalbumin-like protein by Escherichia coli K12 harbouring a recombinant plasmid. Nature. 1978 Oct 12;275(5680):505–510. doi: 10.1038/275505a0. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Andreone T. L., Donelson J. E. Translation in Escherichia coli mini-cells containing hamster mitochondrial DNA-Co1E1 - Ampr recombinant plasmids. Biochim Biophys Acta. 1977 Aug 16;477(4):323–333. doi: 10.1016/0005-2787(77)90251-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Gubbins E. J., Pegg E. W., 3rd, Donelson J. E. Transcription and translation of cloned Drosophila DNA fragments in Escherichia coli. Biochemistry. 1977 Mar 22;16(6):1031–1038. doi: 10.1021/bi00625a001. [DOI] [PubMed] [Google Scholar]
- Morimoto R., Lewin A., Hsu H. J., Rabinowitz M., Fukuhara H. Restriction endonuclease analysis of mitochondrial DNA from grande and genetically characterized cytoplasmic petite clones of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3868–3872. doi: 10.1073/pnas.72.10.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R., Lewin A., Rabinowitz M. Restriction cleavage map of mitochonrial DNA from the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1977 Jul;4(7):2331–2351. doi: 10.1093/nar/4.7.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R., Merten S., Lewin A., Martin N. C., Rabinowitz M. Physical mapping of genes on yeast mitochondrial DNA: localization of antibiotic resistance loci, and rRNA and tRNA genes. Mol Gen Genet. 1978 Jul 25;163(3):241–255. doi: 10.1007/BF00271954. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambach A., Hogness D. S. Translation of Drosophila melanogaster sequences in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5041–5045. doi: 10.1073/pnas.74.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner R., Rejman E., Chargaff E. Genetic implications of periodic pulsations of the rate of synthesis and the composition of rapidly labeled bacterial RNA. Proc Natl Acad Sci U S A. 1965 Sep;54(3):904–911. doi: 10.1073/pnas.54.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shineberg B., Zipser D. The ion gene and degradation of beta-galactosidase nonsense fragments. J Bacteriol. 1973 Dec;116(3):1469–1471. doi: 10.1128/jb.116.3.1469-1471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Starlinger P., Saedler H. IS-elements in microorganisms. Curr Top Microbiol Immunol. 1976;75:111–152. doi: 10.1007/978-3-642-66530-1_4. [DOI] [PubMed] [Google Scholar]
- Struhl K., Cameron J. R., Davis R. W. Functional genetic expression of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1976 May;73(5):1471–1475. doi: 10.1073/pnas.73.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. Production of a functional eukaryotic enzyme in Escherichia coli: cloning and expression of the yeast structural gene for imidazole-glycerolphosphate dehydratase (his3). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5255–5259. doi: 10.1073/pnas.74.12.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford J., Boseley P., Schaffner W., Birnstiel M. Novel screening procedure for recombinant plasmids. Science. 1977 Jan 28;195(4276):391–393. doi: 10.1126/science.318763. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Hautala J. A., Jacobson J. W., Giles N. H., Kushner S. R. Expression in Escherichia coli K-12 of the structural gene for catabolic dehydroquinase of Neurospora crassa. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3508–3512. doi: 10.1073/pnas.74.8.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]