Abstract
The use of cannabis can impair cognitive function, especially short-term memory. A controversial question is whether long-term cannabis use during the late-adolescence period can cause irreversible deficits in higher brain function that persist after drug use stops. In order to examine the short- and long-term effects of chronic exposure to cannabinoids, rats were administered chronic i.p. treatment with the CB1/CB2 receptor agonist WIN55,212-2 (WIN; 1.2 mg/kg) for two weeks during the late adolescence period (post-natal days 45–60) and tested for behavioral and electrophysiological measures of cognitive performance 24 hrs, 10 and 30 days after the last drug injection. The impairing effects of chronic WIN on short-term memory in the water maze and the object recognition tasks as well as long-term potentiation (LTP) in the ventral subiculum (vSub)-nucleus accumbens (NAc) pathway were temporary as they lasted only 24 h or 10 d after withdrawal. However, chronic WIN significantly impaired hippocampal dependent short-term memory measured in the object location task 24 hrs, 10, 30, and 75 days after the last drug injection. Our findings suggest that some forms of hippocampal-dependent short-term memory are sensitive to chronic cannabinoid administration but other cognitive impairments are temporary and probably result from a residue of cannabinoids in the brain or acute withdrawal effects from cannabinoids. Understanding the effects of cannabinoids on cognitive function may provide us with tools to overcome these impairments and for cannabinoids to be more favorably considered for clinical use.
Introduction
Cannabis is the most widely used illicit drug after nicotine and alcohol [1] and can impair several aspects of cognitive function [2], [3].
In humans, cannabinoids impair both encoding and recall of verbal and non verbal information depending on dose and task difficulty [2]. In animal studies, cannabinoids impair memory in a variety of experimental conditions such as the radial maze, instrumental discrimination tasks and the Morris water maze [4].
Given the well established role of the hippocampus in learning and memory processes, and its high expression of CB1 receptors [5], [6], it is likely that the adverse effects of cannabinoids on spatial learning tasks, short-term memory, and attention are attributable to their actions within this brain region. Using electrophysiological recordings from hippocampal slices, previous studies have shown that cannabinoid receptor activation inhibits LTP in the hippocampus [7]–[11]. We have shown that acute administration of the CB1/2 receptor agonist WIN55,212-2 (WIN; 0.5 mg/kg) impairs the induction of LTP in the schaffer collateral-CA1 projection of anesthetized rats [12]. Additionally, WIN administered systemically or into the CA1 (5 µg/side) impairs spatial learning in the water maze [12]. Hence, acute exposure to cannabinoids impairs both hippocampal spatial learning and LTP. Recently, we found that acute WIN administered into the ventral subiculum (vSub; 5 µg/side) also impairs acquisition and retrieval of memory in the social discrimination task [13].
A controversial question is whether long-term exposure to cannabinoids can cause irreversible deficits in higher brain function that persist after drug use stops. Cognitive deficits caused by long term exposure to cannabinoids can last for many days, and possibly for weeks [14]–[16], after discontinuing use, but it is still unclear whether long-term cannabinoids exposure causes irreversible cognitive deficits.
Results from studies in humans [1] and rats [17], [18] suggest that vulnerable periods exist during human and rat brain development up to the age of 16 years (post-natal day (PND) ∼35–40 in rats), during which cannabis can permanently compromise cognitive functions.
It is difficult to define the time course of adolescence, with no single event signaling its onset or termination [19]. During adolescence, the brain undergoes numerous changes [20]; massive loss of synapses in neocortical regions, remodeling of the prefrontal cortex, maturational changes in the hippocampus [20]–[22]. Neuroplastic modifications also include changes in dendritic spine density, synaptic rearrangements and development of myelination [23]. This remodeling process may be disrupted by cannabinoids leading to lasting adverse effects on brain and behavior [23]. Receptors for endogenous cannabinoids mature slowly during the postnatal period [24], [25], with binding peaking during adolescence at higher than adult levels in hippocampus [25]. These endogenous cannabinoid systems may reach functional maturity around adolescence [26], [27].
Evidence from both animal and human studies suggests that frequent exposure to cannabis during adolescence may have long-term effects on the development of cognition, brain structure and function [14], [15], [28], [29], [30]. However, studies on adults were not straightforward in determining whether such deficits, observed after only hours or days of abstinence, are temporary (perhaps due to a residue of cannabinoids in the brain) or long-lasting (due to a neurotoxic effect of long-term exposure). For example, Quinn et al., [23] found deficits in object recognition following repeated exposure to Δ9-THC (THC) in adolescent but not adult rats, a result consistent with other reports of cannabinoid administration in immature but not mature rats causing lasting impairments in learning [17], [18], [28].
In the current study, we aimed to examine the short- and long-term effects of chronic exposure to cannabinoids in the late-adolescence period that falls between adolescence and adulthood (PND ∼45–60). To that end, male rats were chronically injected with a cannabinoid receptor agonist during late-adolescence and behavioral and electrophysiological measures of cognitive performance were tested 24 hours, 10 and 30 days after cessation of drug treatment.
Materials and Methods
Subjects
Male Sprague-Dawley rats (45 days old, ∼200 g; Harlan, Jerusalem, Israel) were caged together (5 per cage) at 22±2°C under 12-hour light/dark cycles (lights turned on at 07:00 and turned off at 19:00). Rats had access to water and laboratory rodent chow ad libitum. The experiments were approved by the University of Haifa Ethics and Animal Care Committee, and adequate measures were taken to minimize pain or discomfort.
Drug Treatment
The CB1/2-receptor agonist WIN55,212-2 (Tocris, USA) was initially dissolved in dimethylsulfoxide (DMSO), and further diluted with 1% Tween 80 and 98% saline (0.9% NaCl). Final DMSO concentration was 1%. This DMSO and saline solution was also used as the vehicle.
WIN was administered intraperitoneally (i.p.) at a dose of 1.2 mg/kg, 0.3 ml (based on previous reports [31], [32]). For chronic experiments, WIN was administered during PND 45–60, as the rats received 14 injections i.p., one per day. For acute experiments, WIN was administered in a single i.p. injection (1.2 mg/kg) 24 h before testing.
The Morris Water Maze Task
The water maze, placed in a dimly lit room, consisted of a pool of water (diameter 1.7 m; 50 cm high rim; manufactured by the University of Haifa). For the spatial training task a submerged escape platform (12×12 cm) was placed 30 cm away from the edge in a fixed location. Each trial was initiated by placing the animal in one of three quadrants (in which there is no platform) near the wall of the tank. Animals were allowed to search for the hidden platform for a maximum of 60 s, while their latency to find the hidden platform was manually recorded by an experimenter. If a rat did not reach the platform within 60 s, an experimenter would guide it there [12]. The rat was then allowed to remain on the platform for 25 s before removal back to the home cage.
The experiment consisted of 3 days ( Figure 1a ). All trials are presented in blocks of two. On the first day, the animals went through a massed training protocol of 14 trials with inter-trial intervals of 3 min [Acq1–7] [33], and 6 more trials to assess short-term memory conducted after 30 min [STM1–3]. On the second day, the animals underwent 8 trials to further train them before assessing their performance in a reversal task, as the massed protocol is considered to be less efficient in producing learning than the spaced paradigms often used in other studies [33] [LTM1–4]. This training session can also be a measure of long-term memory retrieval as rats that have acquired the task will demonstrate better performance. On the third day, the platform was moved to the opposite quadrant of the maze. The animals went through 10 reversal trials in which they were tested for their ability to learn the new platform location [R1–R5].
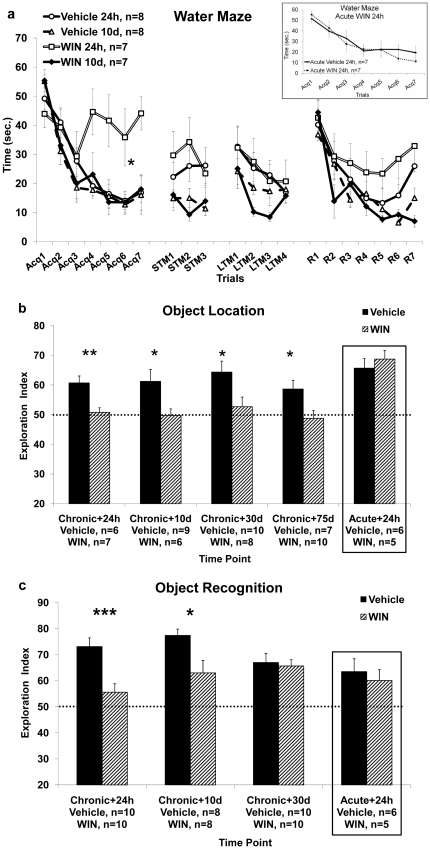
Figure 1. The effects of chronic exposure to WIN55,212-2 during late-adolescence on hippocampal and non-hippocampal dependent tasks.
1a. Rats tested in the water maze 24 h, but not 10 d, after the last WIN injection, show increased latency to locate the hidden platform compared with the vehicle group on the first training day (Acq1–7). (*, p<0.05: WIN 24 h different from Vehicle 24 h). Inset: a control experiment where rats were tested in the water maze 24 hours following a single WIN injection. 1b. Rats tested in the object location task 24 h, 10 d, 30 d and 75 d after the last vehicle injection spent significantly more time exploring the new location compared with the WIN groups. (*, p<0.05; **, p<0.01: Vehicle different from WIN). On the right square: a control experiment where rats were tested in the object location task 24 hours following a single WIN injection. 1c. Rats tested in the object recognition task 24 h or 10 d, but not 30 d, after the last vehicle injection spent significantly more time exploring the new location compared with the WIN groups (*, p<0.05; ***, p<0.001: Vehicle different from WIN). On the right square: a control experiment where rats were tested in the object recognition task 24 hours following a single WIN injection.
Object location memory task
This task measures an animal's ability to detect that an object has moved to a new location. This is a hippocampal-dependent spatial memory task [34]–[36]. The objects were two small identical ceramic dolls (10×8×7 cm; painted blue and pink) located in a squared black open-field (50×50×50 cm) under dim light, 10 cm from the walls. The open-field and the objects were thoroughly cleaned between trials with odorous clean wipes.
The rats were habituated to the experimental apparatus by allowing them to explore it for 10 min every day for 4 days without objects before the experiment was performed. In the sample phase, each rat was placed in the open-field arena and exposed to the objects for 5 min. The test phase was given 30 min after the sample trial (i.e., to test short-term memory). One object was moved to a new location and the time spent exploring the objects at the old and new locations were recorded for 5 min.
A digital camera placed above the arena and connected to a video tape was used to track rat behavior during the exploration session. Recorded data was analyzed by two judges blind to experimental conditions and inter-rater reliability was assured.
Exploration was defined as when the subject sniffed at, whisked at, or looked at the object from no more than 2 cm away. An exploration index calculated for each animal was expressed as T N/(T N+T F) (T F = time spent exploring the object in the familiar location; T N = time spent exploring the object in the novel location). Intact spatial recognition memory in the test phase was reflected in an exploration score higher than 0.5, which implies greater exploration of the object in the novel location ( Figure 2a ).
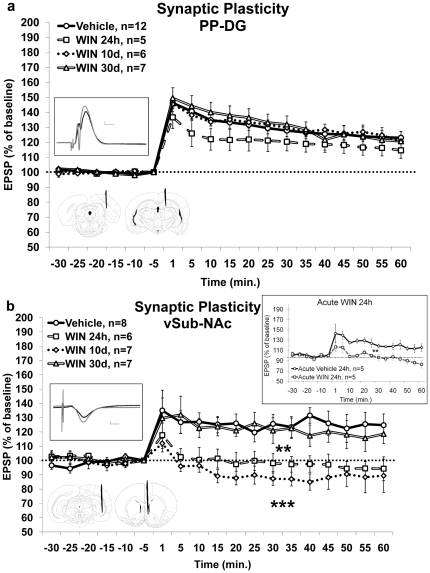
Figure 2. The effects of chronic exposure to WIN55,212-2 during late-adolescence on synaptic-plasticity in the hippocampus and nucleus-accumbens.
2a. Chronic administration of WIN had no effect on LTP levels in the PP-DG pathway measured 24 h, 10 d or 30 d after the last drug injection. Inset: Up: representative traces in the DG for the vehicle group taken before (black) and 5 min after (gray) HFS to the PP (calibration: 0.2 mV, 10 µs); Down: schematic drawing of electrodes tip positions: left - representative location of the stimulating electrode tip in the perforant path (anteroposterior, −8.0 mm; lateral, ±4.0 mm; ventral, −3.0 mm), right - representative location of the recording electrode tip in the dentate gyrus (anteroposterior, −4.0 mm; lateral, ±2.5 mm; ventral, −3.7 mm). 2b. Chronic administration of WIN impaired LTP levels in the vSub-NAc pathway measured 24 h or 10 d, but not 30 d, after the last drug injection. (**, p<0.01: WIN 24 h group different from WIN 30 d and Vehicle groups, and WIN 10 d group different from WIN 30 d group; ***, p<0.001: WIN 10 d group different from Vehicle group). Inset: Up: representative traces in the NAc for the vehicle group taken before (black) and 5 min after (gray) HFS to the vSub (calibration: 0.2 mV, 10 µs); Down: schematic drawing of electrodes tip positions: left - representative location of the stimulating electrode tip in the ventral subiculum (anteroposterior, −6.5 mm; lateral, ±5.0 mm; ventral, −6.0 mm), right - representative location of the recording electrode tip in the nucleus accumbens (anteroposterior, +1.6 mm; lateral, ±1.0 mm; ventral, −5.5 mm); On the right: a control experiment where acute administration of WIN impaired LTP levels in the vSub-NAc pathway measured 24 h after a single injection (**, p<0.01).
Object recognition memory task
This task measures the ability to discriminate the familiarity of previously encountered objects. If a rat is presented with both a familiar object and a novel object, it will direct more exploration at the novel object. This task is dependent on the prefrontal cortex and perirhinal cortex [37], [38]. In the sample phase, each rat was placed in the open-field arena and exposed to two identical objects (the same objects as in the object location memory task) for 5 min. In the test phase, thirty min after the sample trial, the rat was presented with one of the objects from the sample trial and with a novel object (ceramic triangle, 10.5×5×2 cm; painted gray) for 5 min ( Figure 1c ). The familiar and novel objects were counterbalanced during the sample and test phases. The rest of the parameters were identical to the object location task described above.
Electrophysiology
Surgical Procedure
Rats were anesthetized (with 40% urethane, 5% chloral hydrate in saline, injection volume of 4 ml/1 kg, i.p.) and placed in a stereotaxic frame. Small burr holes were drilled in the skull to allow electrodes to be inserted into the brain. A recording microelectrode (glass, tip diameter of 2–5 µm, filled with 2 M NaCl, resistance of 1–4 M) was inserted into the DG (anteroposterior, −4.0 mm; lateral, ±2.5 mm; ventral, −3.7 mm) ( Figure 2a ) or into the NAc shell (anteroposterior, +1.6 mm; lateral, ±1.0 mm; ventral, −5.5 mm) ( Figure 2b ). A bipolar 125 µm stimulating electrode was positioned in the perforant path (PP; anteroposterior, −8.0 mm; lateral, ±4.0 mm; ventral, −3.0 mm) ( Figure 2a ) or the vSub (anteroposterior, −6.5 mm; lateral, ±5.0 mm; ventral, −6.0 mm), respectively ( Figure 2b ). After positioning the electrodes, the rat was left for 60 minutes before commencing the experiment.
LTP Induction
LTP was induced by theta-like high-frequency stimulation (HFS) (three sets of 10 trains; each train consisting of 10 pulses at 200 Hz; inter-train interval, 200 ms; inter-set interval, 1 min) to the vSub or PP. Field potentials were recorded from the NAc or DG every 5 minutes for 60 minutes after HFS to the vSub/PP. LTP was measured as an increase in the amplitude of the excitatory post-synaptic potentials (EPSPs). Potentiation was measured as a percentage change from the average of the 30 min baseline before HFS.
Open Field
The apparatus consisted of a square black open-field (50×50×50 cm). The floor was divided by 1-cm-wide white lines into 25 squares measuring 10×10 cm each. A video image of the open-field was displayed on a TV monitor, and the movements of the rat were manually recorded by two ‘blind’ experimenters and analyzed in order to measure motor activity over a period of 5 min.
Recordings were made of the time the rat spent in the central and peripheral squares, the number of instances of rearing, and the total distance covered ( Figure 3 ). The open-field arena was thoroughly cleaned between trials with odorous clean wipes.
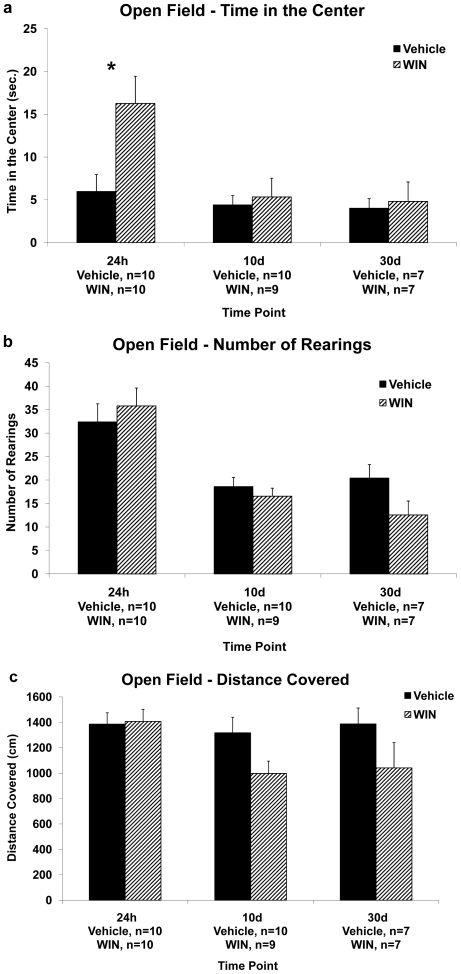
Figure 3. The effects of chronic exposure to WIN55,212-2 during late-adolescence on locomotion and anxiety in the open-field.
3a. Chronic administration of WIN caused an increase in the time the rats spent in the center when tested in the open field 24 h after the last drug injection. (*, p<0.05: Vehicle different from WIN). 3b. Chronic administration of WIN had no effect on the number of rearings the rats performed in the open field when measured 24 h, 10 d or 30 d after the last drug injection. 3c. Chronic administration of WIN had no effect on the distance the rats covered in the open field when measured 24 h, 10 d or 30 d after the last drug injection.
Sucrose intake
Water bottles were removed before the dark part of the cycle, and replaced with bottles containing a 1% sucrose solution. Sucrose consumption was measured during the 12 dark hours of the cycle and was then normalized according to every rat's specific weight ( Figure 4a ). Rats were individually housed during the sucrose intake measurement.
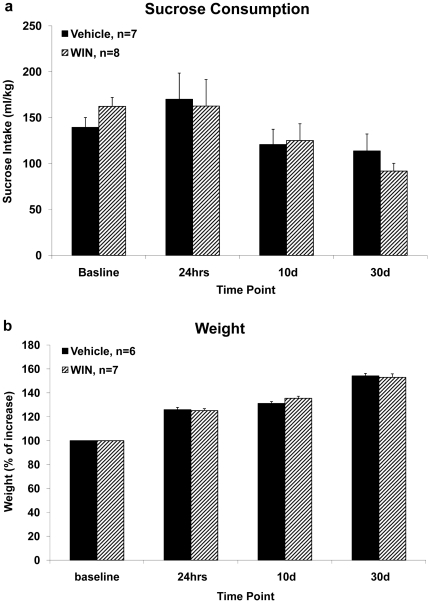
Figure 4. The effects of chronic exposure to WIN55,212-2 during late adolescence on sucrose consumption.
4a. Chronic administration of WIN had no effect on sucrose consumption when measured at baseline, 24 h, 10 d or 30 d after the last drug injection. 4b. Chronic administration of WIN had no effect on weight gain compared to the vehicle group when measured at baseline, 24 h, 10 d, or 30 d after the last drug injection.
Statistical Analysis
The results are expressed as means ±SEM. For statistical analysis, mixed design ANOVA, 3-way mixed ANOVA, 2-way ANOVA, and t-test were used as indicated. All post hoc comparisons were made using the least significant difference multiple-comparison test (LSD).
Experimental design
Rats were chronically injected for 14 days with WIN or vehicle. Every rat underwent one behavioral or electrophysiological test, to prevent carryover effects due to multiple tests. However, the sucrose consumption and weight gain measures were taken from rats that were tested behaviorally or electrophysiologically. Hence, different groups of rats were tested at the different time points post-injection.
Behavioral measures: (i) water maze– 24 h or 10 days after the last chronic injection and 24 h after a single injection (figure 1a), (ii) object location task– 24 h, 10, 30 or 75 days after the last chronic injection and 24 h after a single injection (figure 1b), (iii) object recognition task– 24 h, 10 days or 30 days after the last chronic injection and 24 h after a single injection (figure 1c).
Electrophysiological measures: (i) LTP in the PP-DG pathway– 24 h, 10 days or 30 days after the last injection (figure 2a), (ii) LTP in the vSub-NAc pathway– 24 h, 10 days or 30 days after the last chronic injection and 24 h after a single injection (figure 2b).
Control experiments: (i) locomotion and anxiety-like behavior in the open field– 24 h, 10 days or 30 days after the last injection (figure 3), (ii) sucrose consumption– 24 h, 10 days or 30 days after the last injection (figure 4a), and (iii) weights– 24 h, 10 days or 30 days after the last injection (figure 4b).
Results
The effects of chronic exposure to the cannabinoid receptor agonist WIN55,212-2 during late adolescence on hippocampal and non-hippocampal dependent tasks
To determine the effects of chronic exposure to WIN during late-adolescence on spatial learning and memory, we used two hippocampal- dependent paradigms: the aversive Morris water maze task, and the non-aversive object location task.
The Morris water maze
Rats were injected with vehicle or WIN for 2 weeks and after 24 h (Vehicle 24 h, n = 8; WIN 24 h, n = 7) or 10 days (Vehicle 10 d, n = 8; WIN 10 d, n = 7) taken to the water maze. The data were analyzed using a 3-way mixed ANOVA with treatment (Vehicle/WIN) and time of testing (24 h, 10 d) as between-subject factors, and the trials of training (Acq, STM, LTM, R) as a within-subject factor ( Figure 1a ).
On day one, analysis of the acquisition data [Acq1–7] revealed significant main effects for treatment (F(1,26) = 4.03, p = 0.05), for time of testing (F(1,26) = 5.31, p<0.05), and for trials (F(1,26) = 112.29, p<0.001). Significant interactions were found for [trials×treatment] (F(1,26) = 14.21, p = 0.01), for [trials×time of testing] (F(1,26) = 12.69, p = 0.01), and for [trials×treatment×time of testing] (F(1,26) = 14.43, p = 0.01). Post-hoc analysis revealed a significant difference in latency to find the hidden platform between the vehicle treated rats and the WIN treated rats after 24 h (p<0.05), indicating that 24 h after the last chronic injection the WIN treated rats took longer to find the hidden platform than the vehicle treated rats ( Figure 1a ).
Thirty min after training rats were tested in the maze for their short-term memory of the platform location (STM1–3). A significant main effect for time of testing (F(1,26) = 12.3, p<0.01) was found. There was no significant main effect for treatment (F(1,26)<1, NS), and no significant interaction effect (F(1,26)<1, NS).
On day two, 48 hours after the last chronic injection, rats were tested in the maze for their long-term memory of the platform location (LTM1–4). A significant main effect for time of testing (F(1,26) = 4.29, p<0.05) and for trials (F(1,26) = 9.8, p<0.01) was found. There was no significant main effect for treatment (F(1,26)<1, NS), and no significant interaction effect (F(1,26)<1, NS). The absence of a significant difference between the treatment groups indicates that on day two there were no treatment effects on performance.
On day three, 72 hours after the last chronic injection, the platform was moved to the opposite quadrant of the maze and the rats were trained to find this new location. A significant main effect for trials (F(1,26) = 128.47, p<0.001) was found. There was no significant main effect for treatment (F(1,26)<1, NS), for time of testing (F(1,26) = 2.37, NS), and no significant interaction effect (F(1,26)<1, NS).
In order to find whether the pattern of acquisition on the water maze task in the WIN treated rats 24 hours after chronic treatment is the result of an acute effect on learning, we conducted a control experiment where we treated rats with an acute dose of WIN (1.2 mg/kg) and then tested their acquisition of the water maze task after 24 h (Vehicle, n = 7; Acute WIN24h, n = 7; Figure 1a , inset). Mixed design ANOVA [treatment×trials (2×7)] of the acquisition data [Acq1–7] revealed a significant effect for trials (F(1,12) = 66.74, p<0.001), indicating the rats' improvement over trials in finding the hidden platform. There were no significant effects for treatment (F(1,12)<1, NS), or a significant interaction effect between trials and treatment (F(1,12) = 1.73, NS). This suggests that an acute single injection of WIN does not affect the performance in the water maze task 24 h after the injection.
The object location task
Next, we examined the effects of chronic exposure to WIN during late-adolescence on short term spatial memory in the non-aversive object location task. The data were analyzed using a 2-way ANOVA with treatment (Vehicle/WIN) and time of testing (24 h, 10 d, 30 d, 75 d) as independent variables, and exploration index as a dependent variable. A significant main effect was found for treatment (F(1,55) = 21.35, p<0.001), suggesting that in all time points measured, the WIN treated rats spent significantly less time exploring the novel location compared with the vehicle treated rats ( Figure 1b ). There was no significant main effect for time of testing (F(3,55)<1, NS), or a significant interaction effect (F(3,55)<1, NS).
One-sample t-test performed on each of the vehicle and WIN groups revealed a significant difference from the 50% exploration index point at all times tested in the Vehicle group [24 h: (t(5) = 4.26, p<0.01); 10 d: (t(8) = 2.82, p<0.05); 30 d: (t(9) = 3.88, p<0.01); 75 d: (t(6) = 2.97, p<0.05)], but not in the WIN group. Thus, 24 hours, 10, 30 and 75 days after withdrawal, rats still demonstrate impaired short-term memory in the object location task.
There was no significant difference in the sample phase (day 1) between any of the groups in exploration index (mean±SD) 24 h (Vehicle: 45.76±2.19; WIN: 51.3±1.84), 10 d (Vehicle: 52.07±4.01; WIN: 52.58±2.51), 30 d (Vehicle: 53.39±3.34; WIN: 52.86±2.11), or 75 d (Vehicle: 50.68±2.36; WIN: 51.69±1.42) after withdrawal.
In order to find whether the effects seen on spatial memory after 24 hours of withdrawal would also be evident after a single injection of WIN, we performed a control experiment, in which we examined the effects of an acute administration of WIN (1.2 mg/kg) on spatial short term memory in the object location task. We tested the rats in the object location task 24 hours after a single injection. Independent-samples t-test did not reveal a significant difference between the groups (Vehicle: 65.7±2.75; WIN: 68.76±2.53; t(10)<1, NS), indicating that an acute single injection of WIN does not affect the performance in the object location task 24 h after the injection ( figure 1b , right square).
In a separate experiment we aimed to test whether a lower dose of chronic WIN (0.5 mg/kg instead of 1.2 mg/kg) is enough to impair performance in the object location task. As we were interested in long-term effects, rats were tested in this task after 10 days of withdrawal (Vehicle, n = 7; WIN 10 d, n = 9). Independent-samples t-test revealed that rats treated with a lower dose of WIN also exhibited impairment in short-term memory (mean±SD) (45.34±5.11) compared with vehicle treated rats (60.67±4.64), 10 days after the last injection (t(14) = 2.16, p<0.05), suggesting that chronic treatment with a lower dose of WIN may be sufficient for the observed short term memory impairment.
The object recognition task
Since chronic exposure to WIN during late-adolescence affected non-aversive short-term hippocampal dependent spatial memory, we asked whether these effects will appear in a similar non-hippocampal dependent task. To that end we used the object-recognition task, which examines the visual and tactile properties of the explored objects rather than their spatial location.
The data were analyzed using a 2-way ANOVA with treatment (Vehicle/WIN) and time of testing (24 h, 10 d, 30 d) as independent variables and exploration index as a dependent variable ( Figure 1c ). A significant main effect was found for treatment (F(1,50) = 15.17, p<0.001), and a significant interaction effect of [treatment×time of testing] (F(2,50) = 3.23, p<0.05). There was no significant main effect for time of testing (F(2,50) = 1.38, NS).
Independent samples t-test revealed that the main effect of treatment stemmed from the fact that the WIN treated rats spent significantly less time exploring the novel object compared with the vehicle treated rats, after 24 hrs (t(18) = 3.76, p = 0.001) and 10 d (t(10.34) = 2.38, p<0.05) of withdrawal.
One-sample t-test performed on each of the vehicle and WIN groups revealed a significant difference between the Vehicle group and the 50% exploration index point at all times tested, [24 h: (t(9) = 6.92; p<0.001), 10 d: (t(7) = 10.09; p<0.001), 30 d: (t(9) = 4.89; p = 0.001)], and in the WIN group a significant difference was found at 10 d (t(7) = 2.41, p<0.05) and 30 d: (t(9) = 6.55, p<0.001).
In order to find whether the effects seen in the object recognition task after 24 hours of withdrawal would also be evident after a single injection of WIN, we conducted a control experiment, in which we examined the effects of an acute administration of WIN (1.2 mg/kg) on short term object recognition memory. Independent-samples t-test did not reveal a significant difference between the groups (Vehicle: 63.51±4.16; WIN: 60.13±3.47; t(11)<1, NS), indicating that an acute single injection of WIN does not affect the performance in the object recognition task 24 h after the injection ( figure 1c , right square).
The effects of chronic exposure to the cannabinoid receptor agonist WIN55,212-2 during late adolescence on synaptic plasticity
Synaptic plasticity in the perforant path-dentate gyrus pathway
Next we sought to examine whether chronic WIN administration would impair hippocampal LTP. Rats were anesthetized and taken for electrophysiological recording in the PP-DG pathway 24 h, 10 d or 30 d after the last chronic WIN injection and compared to a vehicle group. We found no difference in EPSP amplitude following HFS between vehicle injected rats tested 24 h (n = 4), 10 days (n = 4) or 30 days (n = 4) after the last injection (F(2,9) = 1.2, NS), and hence the vehicle group was grouped to one for reasons of clarity.
Mixed design ANOVA [treatment×time (4×13)] post-HFS did not indicate a significant effect on EPSP amplitude for the treatment (F(3,26)<1, NS), or the interaction between treatment and time (F(3,26) = 1.39, NS; Figure 2a ). There was a significant within-subject effect for the time (F(1,26) = 63.48, p<0.001), due to a decrease in potentiation levels from the post tetanic potentiation (measured 1 min after HFS) throughout the experiment in all groups. Hence, chronic exposure to WIN did not have a significant effect on the induction of LTP in the PP-DG pathway at any of the time points examined. Mixed design ANOVA on EPSP amplitude pre-HFS [treatment×time (4×6)] did not reveal significant effects for the treatment (F(3,26) = 1.22, NS), the time (F(1,26) = 2.35, NS), or the interaction between treatment and time (F(3,26) = 2.72, NS).
Synaptic plasticity in the ventral subiculum-nucleus accumbens pathway
Rats were anesthetized and taken for electrophysiological recording in the vSub-NAc 24 h, 10 d or 30 d after the last WIN injection and compared to a vehicle group. We found no difference between vehicle injected rats tested 24 h, 10 d or 30 d after the last injection, and hence the vehicle group was grouped to one for reasons of clarity (n = 8).
Mixed design ANOVA [treatment×time (4×13)] post-HFS indicated significant effects on EPSP amplitude for the treatment (F(3,28) = 6.91, p = 0.001), for the time (F(1,28) = 4.18, p = 0.05), but not for the interaction between treatment and time (F(3,28)<1, NS; Figure 2b ). Post hoc analysis revealed significantly higher levels of potentiation in the vehicle group and the WIN 30 d group compared with the WIN 24 h group (p<0.01, different from Vehicle; p<0.05, different from WIN 30 d) and with the WIN 10 d group (p<0.001, different from Vehicle; p<0.01, different from WIN 30 d). Hence, chronic exposure to WIN impaired the induction of LTP in the vSub-NAc pathway 24 h and 10 d, but not 30 d after withdrawal. Mixed design ANOVA on EPSP amplitude pre-HFS [treatment×time (4×6)] did not reveal significant effects for the treatment (F(3,28)<1, NS), or the time (F(1,28)<1, NS), or the interaction between treatment and time (F(3,28) = 1.85, NS).
In order to find whether the impairment of LTP seen after 24 h and 10 d would appear also following a single injection of WIN, rats were treated with an acute dose of WIN (1.2 mg/kg) and then taken for electrophysiological recording in the vSub-NAc after 24 h (Vehicle, n = 5; Acute WIN24h, n = 5; Figure 2b , inset on the right side). Mixed design ANOVA [treatment×time (2×13)] indicated significant effects on EPSP amplitude for the treatment (F(1,8) = 17.51, p<0.01), for the time (F(1,8) = 8.9, p<0.05), but not for the interaction between treatment and time (F(1,8)<1, NS). This suggests that an acute single injection of WIN affects LTP in the NAc 24 h after the injection. Mixed design ANOVA on EPSP amplitude pre-HFS [treatment×time (2×6)] did not reveal significant effects for the treatment (F(1,8)<1, NS), or the time (F(1,8)<1, NS), or the interaction between treatment and time (F(1,8)<1, NS).
The effects of chronic exposure to the cannabinoid receptor agonist WIN55,212-2 during late adolescence on the rats' weight, sucrose consumption and performance in an open field test
To exclude motor deficits or other non-specific alterations that might have caused the effects on learning and plasticity, rats were chronically administered with WIN and tested for locomotion and anxiety levels in the open field and their weights and sucrose consumption were monitored.
Open field
The data were analyzed using a 2-way ANOVA with treatment (Vehicle/WIN) and time of testing (24 h, 10 d, 30 d) as independent variables, and time in the center/number of rearings/distance covered as dependent variables.
Analysis of the time the rats spent in the center of the open field revealed a significant main effect for time of testing (F(2,47) = 5.39, p<0.01), a significant effect for treatment (F(1,47) = 5.6, p<0.05), and a significant interaction effect (F(2,47) = 3.85, p<0.05). Post-hoc comparisons revealed that the significant main effect of time of testing stemmed from a significant difference between the 24 h groups and the 10 d groups (p<0.01), and between the 24 h group and the 30 d group (p<0.01).
Independent-samples t-test revealed a significant difference between the treatment groups 24 h after withdrawal (t(12.83)<1, p = 0.01), suggesting that the WIN group (n = 10) spent significantly more time in the center of the open field than the vehicle group (n = 10), perhaps indicating their lower level of stress compared to the vehicle group ( Figure 3a ). There were no significant differences between the treatment groups after 10 days of withdrawal (t(17)<1, NS), or after 30 days of withdrawal (t(8.8)<1, NS).
Analysis of the number of rearings the rats performed in the open field revealed a significant main effect for time of testing (F(2,47) = 20.78, p<0.001; Figure 3b ). Post-hoc comparisons revealed that this effect stems from significant differences between the 24 h groups (mean±SD) (WIN: 35.8±2.94; Vehicle: 32.4±2.94) and the 10 d groups (WIN: 16.56±3.1; Vehicle: 18.6±2.94; p<0.001), and between the 24 h group and the 30 d group (WIN: 12.57±3.5; Vehicle: 20.43±3.52; p<0.001).
Analysis of the distance the rats covered in the open field revealed a marginally significant main effect for treatment (F(1,46) = 3.96, p = 0.053). However, independent-samples t-test did not reveal a significant difference between the treatment groups at any of the time points tested (24 h: t(18)<1, NS; 10 d: t(16)<1, NS; 30 d: t(12)<1, NS), suggesting no effect on gross motoric behavior ( Figure 3c ). This could indicate a possible tolerance effect to the chronic administration of WIN.
Sucrose consumption test
To examine the effects of chronic exposure to WIN during late-adolescence on hedonia, we used the sucrose consumption test. We measured sucrose intake before injection (baseline), 24 h, 10 d, and 30 d after the last injection ( Figure 4a ).
Two-way ANOVA with treatment (Vehicle/WIN) and time of testing (baseline, 24 h, 10 d, 30 d) as independent variables, and sucrose consumption as the dependent variable did not reveal significant effects for the treatment (F(1,13)<1, NS), or the interaction between treatment and time of testing (F(1,13)<1, NS). There was a significant main effect for time of testing (F(1,13) = 9.51, p<0.01).
Weight
We also measured the rats' weight up to 30 days after the last injection. Rats were weighed before injection (baseline), 24 hrs, 10 days, or 30 d after the last injection ( Figure 4b ). Mixed design ANOVA on weight gain [treatment×time point (2×4)] did not reveal significant effects for the treatment (F(1,11) = 3.11, NS), or the interaction between treatment and time point (F(1,11)<1, NS), but there was a significant within-subject effect for the time point (F(1,11) = 660.98, p<0.001), signifying the rats' weight gain over time.
Discussion
Our findings suggest that the effects of chronic cannabinoid exposure in the late-adolescent period in rats on learning and memory are task- and brain region-specific (see Table 1 ). The most robust effect of chronic WIN administration was the impairment of short-term memory in the spatial version of the object recognition task that persisted even after 75 days of withdrawal. However, we found a gradual recovery of behavioral and electrophysiological impairments in acquisition and short-term memory in the water maze, short-term object recognition memory, and LTP in the vSub-NAc pathway. No significant long-term effects of chronic WIN were observed on locomotion, sucrose consumption or weight gain. Hence, most of the deficits observed were temporary corroborating with previous studies showing that long-term cannabinoid administration produces CB1 receptor desensitization and down-regulation in the hippocampus that recovers to control level at 14 days after cessation of treatment [39], [40].
Table 1. Summary of results.
| Time Point | |||
| Test | 24 hours | 10 days | 30 days |
| Water maze | Impairment in acquisition | No effect | - |
| Location recognition | Impairment in short-term memory | Impairment in short-term memory | Impairment in short-term memory (also at 75 days) |
| Object recognition | Impairment in short-term memory | Attenuation of short-term memory | No effect |
| Synaptic plasticity – PP-DG | No effect | No effect | No effect |
| Synaptic plasticity – vSub-NAc | Impairment of LTP | Impairment of LTP | No effect |
| Open field | Increased time in the center | No effect | No effect |
| Sucrose consumption | No effect | No effect | No effect |
| Weight | No effect | No effect | No effect |
The table summarizes the effects of chronic i.p. treatment with the CB1/CB2 receptor agonist WIN55,212-2 (1.2 mg/kg) for two weeks during the late adolescence period (post-natal days 45–60) on behavioral and electrophysiological measures of cognitive performance tested 24 hrs, 10 and 30 days after the last drug injection.
The effects of chronic exposure to WIN55,212-2 during late adolescence on performance in behavioral tasks
The acquisition in the spatial task in the Morris water maze was impaired after 24 hrs of withdrawal corroborating with previous studies using THC and HU-210 [41], [42]. The impairment was no longer evident after 10 days of withdrawal, suggesting that the 24 hrs effect could be due to drug residue of cannabinoids in the CNS or to withdrawal effects from the drug. This is consistent with Wise et al. [43], showing that the induction of withdrawal in THC-dependent rats impaired performance in the water maze. Twenty-four hours after withdrawal, both treatment groups showed poor short-term spatial memory, perhaps due to the less efficient training in the massed protocol compared with the spaced protocol used in other studies [33]. Acute treatment with WIN did not affect the acquisition of the water maze task, excluding the possibility that an acute single injection of WIN affects spatial learning 24 h after the injection.
In the spatial version of the object recognition task, WIN-treated rats showed impairment in short-term memory that was evident up to 75 days after the last injection. This is a robust effect suggesting that this type of spatial memory is more sensitive to the effects of chronic WIN exposure during late adolescence than the water maze task. The first explanation for the differential effects of WIN on performance involves the different level of stress in each task. The water maze is a highly aversive learning task, especially when using the massed training protocol [33] whereas the object location task is considered non-aversive. The cannabinoid system and the stress system are highly interconnected [44]–[49] and it may be that the effects of WIN on performance in a stressful learning paradigm are different than in a neutral task. Several studies suggested that the cannabinoid system is not involved in the extinction of non-aversive memories [50]–[52]. Harloe et al. [50] examined extinction learning under aversive and appetitive conditions, and reported impairment of extinction learning by a cannabinoid antagonist only under aversive conditions, suggesting that the endocannabinoid system might become activated specifically in highly aversive situations. Similarly, we have recently shown that WIN microinjected into the basolateral amygdala can prevent the effects of stress exposure (elevated platform stress) on performance in an aversive learning task (i.e., conditioned avoidance and extinction of inhibitory avoidance) [53], but WIN could not prevent the effects of the same stressor on the performance in the non-aversive object location task [54]. Hence, a possible interaction between WIN and the stressfulness of the task may have a different outcome on performance. The second explanation relates to the brain areas involved in these tasks. The water maze task heavily relies on the dorsal CA1 area [55]–[57], and the object location task, although heavily relying on the hippocampus, also involves other brain areas (i.e., prefrontal cortex, perirhinal cortex; [58]–[61], [38]) that are also affected by the chronic systemic administration of WIN. As in the water maze task, chronic treatment with WIN was necessary in order to cause impairment in performance, since no impairment was evident 24 hours after a single WIN injection.
In the non-spatial object recognition task, we found a gradual recovery over time of short-term memory impairment, perhaps due to drug residue. At 10 days after withdrawal, the WIN treated rats demonstrated attenuated performance compared to the vehicle treated rats, however their exploration index was significantly higher than chance levels, suggesting that they acquired the task. After 30 days of withdrawal, when the drug components would reasonably be expected to have disappeared from the CNS [62], [63], no effect on short-term memory could be discerned. The object recognition task is to a great extent dependent on the prefrontal cortex and the perirhinal cortex [37], [38], which may suggest a greater sensitivity of the hippocampus than the cortex to the effects of chronic WIN treatment during late-adolescence. An acute single injection of WIN did not affect performance in the object recognition task 24 h after the injection suggesting that chronic treatment with WIN is required for memory impairment to occur.
The effects of chronic exposure to WIN55,212-2 during late adolescence on synaptic plasticity
Chronic WIN administration during late adolescence had no significant effect on plasticity in the DG hippocampal area although there are very high levels of CB1 receptors in all subfields of the hippocampus, including the DG [64]–[66]. A possible explanation for the lack of effect is low sensitivity or adaptation of DG neurons to high chronic levels of WIN. Yet, a recent study found that acute WIN affects miniature inhibitory postsynaptic currents in the DG without altering event amplitude, area, rise time, or decay [67]. This study showed that WIN potentiated action potential-independent release of GABA in the DG which was not mediated through a CB1or CB2 receptor mechanism [67]. This may suggest that although WIN has no effect on HFS-induced plasticity in the DG, it affects the spontaneous release of GABA.
We have recently found that acute administration of WIN (0.5 mg/kg) significantly impaired LTP in the schaffer collateral-CA1 projection [12]. Here we found that chronic administration of either vehicle or WIN impaired LTP in the schaffer collateral-CA1 pathway (data not shown). Hence, we could not differentiate, using our paradigm, between the effects of chronic WIN exposure and the stress-induced effects of chronic i.p. injections on LTP. Daily injections can constitute chronic stress for the rats, and the CA1 area is highly sensitive to stress [68], [69]. Hill et al. [70] found that rats that were chronically exposed to high levels of the CB1 receptor agonist HU-210 demonstrate impaired LTP in the CA1 region when examined 18 h following the final drug administration. There are several differences between our experiment and Hill's; drug administration began when the rats were 300 g, the same point at which we end our drug administration; electrophysiological recording and stimulating electrodes were in different coordinates than in our chronic experiment; rats injected with HU-210 were taken for electrophysiology after completing extensive behavioral training.
In the vSub-NAc pathway chronic WIN impaired LTP induced after 24 hrs or 10 days, but not after 30 days, of withdrawal. LTP in this pathway is NMDA-dependent [71], [72]. Also, it has been suggested that the NAc, by integrating vSub input of contextual information, mediates goal-directed behavior and that the hippocampal input in the NAc is well placed to influence reward and incentive systems [73], [74]. The NAc represents a critical site for mediating the rewarding and/or addictive properties of several classes of abused drugs, including ethanol, opioids, psychomotor stimulants, and marijuana [75]–[77].
Acute treatment with WIN impaired LTP after 24 hours in this pathway. This could suggest that the NAc is particularly sensitive to the effects of WIN due to its involvement in the neural processing of rewarding stimuli. The role of the NAc in behavior reinforced by both natural reward [78] and drugs of abuse is supported by vast experimental evidence [79], [80]. Moreover, in vivo experiments indicate that cannabinoids reduce excitability of NAc neurons [81]. This acute effect is consistent with Mato et al. [82] who found impairment of LTD in the NAc and CA1, 24 hours following a single in vivo injection of THC. This effect was reversible within 3 days, suggesting that the modification in the functional properties of cannabinoid receptors was transient. Together with our previous findings on the impairing effects of acute WIN on LTP in the CA1 [12] the data suggest that alterations in synaptic plasticity as a result of cannabinoid treatment can occur within 24 hours of acute exposure.
The effects of chronic exposure to WIN55,212-2 during late adolescence on non memory related measures
Chronic exposure to WIN had no effect on weight gain, sucrose consumption or gross locomotion, but it significantly affected the rats' level of anxiety, as measured in the open field, after 24 hrs of withdrawal. These findings suggest that the long-term effects of chronic WIN on learning and plasticity are probably not due to changes in sensory-motor parameters or other non-specific effects.
Since acute administration of cannabinoids results in depression of motoric activity in the open field, and chronic administration results in tolerance to these effects [83], the lack of effect here on locomotion could be the result of tolerance to WIN, developed over the two weeks of daily injections.
Increased time spent in the central part of the open field arena is an indication of a reduction in anxiety-like behavior [84]. Activation of the cannabinoid system has anxiolytic properties [45], [85] that could explain the effect in the open field 24 hrs after withdrawal when drug residue may still be present in the CNS.
Anhedonia, or the decreased ability to experience pleasure, can be examined in rats by reduction in sucrose intake. Responding to natural and artificial rewards is mediated by the NAc and its dopaminergic inputs [86]. Although we found that chronic WIN interferes with LTP in the NAc, no effect was observed in sucrose consumption. This may suggest a greater sensitivity of the LTP-mediated neural circuit in the NAc to the effects of chronic WIN than the neural circuit mediating sucrose intake.
Long-term effects of cannabinoids
Prolonged exposure to cannabinoid agonists in laboratory animals is associated with the development of tolerance to most of their pharmacological effects [87]. There is a brief ‘drug residue’ effect of 12–24 hrs after acute exposure to cannabinoids that may persist longer in chronic users [63]. The average terminal elimination half-life of the THC metabolite THCCOOH in plasma of chronic cannabis users is as long as 4.3 days and may be as long as 12.6 days [88]. WIN has a shorter half-life than THC [89]–[91] and undergoes significant metabolism similar to that of other cannabinoids [92]. Prolonged treatment with THC or with WIN resulted in cannabinoid receptor desensitization and down-regulation throughout the brain, as well as tolerance to cannabinoid-mediated effects, and attenuation of CB1 receptor-mediated G-protein activation that persisted for several days after cessation of treatment [39]–[40]. In adolescent rats, this desensitization of CB1 receptors following prolonged treatment with THC is slower than in adults, perhaps contributing to the differentiation in long-lasting cognitive effects between adolescents and adults [93].
Summary
Our results point to a gradual recovery over time rather than persistent long-lasting impairments following chronic WIN administration. Yet, WIN had a long-term impairing effect on performance in a non-aversive hippocampal-dependent short-term memory task, corroborating animal and human studies on short-term memory [94]–[98].
Studying the lasting effects of cannabinoids on cognitive function may advance our understanding of the potential harmful consequences of cannabinoids. Dissociating the short-term from the long-lasting effects of cannabinoids may indicate whether long-term exposure to cannabinoids is associated with long-lasting deficits in higher brain function that persist after drug use stops. This will help in determining whether the clinical benefits of using cannabinoids outweigh the risks, and to better cope with the deficits induced by cannabinoids.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 2.Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: A review. Psychopharmacology. 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- 3.Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handb Exp Pharmacol. 2005;168:445–477. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- 4.Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: animal studies. Curr Drug Targets CNS Neurol Disord. 2003;2:389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- 5.Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 7.Collins DR, Pertwee RG, Davies SN. The action of synthetic cannabinoids on the induction of long-term potentiation in the rat hippocampal slice. Eur J Pharmacol. 1994;259:7–8. doi: 10.1016/0014-2999(94)90666-1. [DOI] [PubMed] [Google Scholar]
- 8.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 9.Nowicky AV, Teyler TJ, Vardaris RM. The modulation of long-term potentiation by delta-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Res Bull. 1987;19:663–672. doi: 10.1016/0361-9230(87)90052-9. [DOI] [PubMed] [Google Scholar]
- 10.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 11.Terranova JP, Michaud JC, Fur G, Soubrié P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: Reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- 12.Abush H, Akirav I. Cannabinoids modulate hippocampal memory and plasticity. Hippocampus. 2010;20:1126–38. doi: 10.1002/hipo.20711. [DOI] [PubMed] [Google Scholar]
- 13.Segev A, Akirav I. Differential effects of cannabinoid receptor agonist on social discrimination and contextual fear in amygdala and hippocampus. Learn Mem. 2011;18:254–9. doi: 10.1101/lm.2110511. [DOI] [PubMed] [Google Scholar]
- 14.Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Curr Drug Abuse Rev. 2008;1:114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- 15.O'Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol. 2006;20:611–21. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- 16.Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, et al. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–10. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 17.Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- 18.Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology. 1985;85:436–439. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- 19.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 20.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 21.van Eden CG, Kros JM, Uylings HBM. The development of the rat prefrontal cortex: Its size and development of connections with thalamus, spinal cord and other cortical areas. In: Uylings HBM, van Eden CG, De Bruin JPC, Corner MA, Feenstra MGP, editors. Progress in brain research, The prefrontal cortex: its structure, function and pathology, vol. 85. Amsterdam: Elsevier; 1990. pp. 169–83. [DOI] [PubMed] [Google Scholar]
- 22.Wolfer DP, Lipp H-P. Evidence for physiological growth of hippocampal mossy fiber collaterals in the guinea pig during puberty and adulthood. Hippocampus. 1995;5:329–40. doi: 10.1002/hipo.450050406. [DOI] [PubMed] [Google Scholar]
- 23.Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–26. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 24.Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–8. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Fride E, Mechoulam R. Developmental aspects of anandamide: ontogeny of response and prenatal exposure. Psychoneuroendocrinology. 1996a;21:157–72. doi: 10.1016/0306-4530(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 27.Fride E, Mechoulam R. Ontogenetic development of the response to anandamide and D(9)-tetrahydrocannabinol in mice. Brain Res Dev Brain Res. 1996b;95:131–4. doi: 10.1016/0165-3806(96)00087-9. [DOI] [PubMed] [Google Scholar]
- 28.O'shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–8. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- 29.Rubino T, Vigano D, Realini N, Guidali C, Braida D, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 30.Schneider M, Drews E, Koch M. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol. 2005;16:447–54. doi: 10.1097/00008877-200509000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Schneider M, Koch M. The effect of chronic peripubertal cannabinoid treatment on deficient object recognition memory in rats after neonatal mPFC lesion. Eur Neuropsychopharmacol. 2007;17:180–186. doi: 10.1016/j.euroneuro.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- 33.Akirav I, Sandi C, Richter-Levin G. Differential activation of hippocampus and amygdala following spatial learning under stress. Eur J Neurosci. 2001;14:719–25. doi: 10.1046/j.0953-816x.2001.01687.x. [DOI] [PubMed] [Google Scholar]
- 34.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 35.Goodman T, Trouche S, Massou I, Verret L, Zerwas M, et al. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171:769–78. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 36.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–65. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- 38.Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2010;48:2262–72. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- 40.Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70:986–96. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- 41.Da Silva GE, Takahashi RN. SR 141716A prevents delta 9-tetrahydrocannabinol-induced spatial learning deficit in a Morris-type water maze in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:321–325. doi: 10.1016/s0278-5846(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 42.Ferrari F, Ottani A, Vivoli R, Giuliani D. Learning impairment produced in rats by the cannabinoid agonist HU 210 in a water-maze task. Pharmacol BiochemBehav. 1999;64:555–561. doi: 10.1016/s0091-3057(99)00106-9. [DOI] [PubMed] [Google Scholar]
- 43.Wise LE, Varvel SA, Selley DE, Wiebelhaus JM, Long KA, et al. Δ(9)-Tetrahydrocannabinol-dependent mice undergoing withdrawal display impaired spatial memory. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2305-5. May 11 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barna I, Zelena D, Arszovszki AC, Ledent C. The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci. 2004;75:2959–2970. doi: 10.1016/j.lfs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: Implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2009;34:791–7. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci USA. 2010;107:9406–11. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 49.Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: A novel mechanism for stress habituation. Eur J Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harloe JP, Thorpe AJ, Lichtman AH. Differential endocannabinoid regulation of extinction in appetitive and aversive Barnes maze tasks. Learn Mem. 2008;15:806–9. doi: 10.1101/lm.1113008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hölter SM, Kallnik M, Wurst W, Marsicano G, Lutz B, et al. Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol. 2005;510:69–74. doi: 10.1016/j.ejphar.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, et al. The disruptive effects of the CB 1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology. 2007;191:223–231. doi: 10.1007/s00213-006-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci. 2009;29:11078–88. doi: 10.1523/JNEUROSCI.1223-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segev A, Ramot A, Akirav I. Stress hormones receptors in the amygdala mediate the effects of stress on the consolidation, but not the retrieval, of a non aversive spatial task. PLoS One. 2011 doi: 10.1371/journal.pone.0029988. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, et al. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: Water maze performance correlates. Neuroscience. 2003;122:585–590. doi: 10.1016/j.neuroscience.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 56.Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, et al. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. Retrieval induces hippocampal-dependent reconsolidation of spatial memory. Learn Mem. 2006;13:431–440. doi: 10.1101/lm.315206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aggleton JP, Kyd RJ, Bilkey DK. When is the perirhinal cortex necessary for the performance of spatial memory tasks? Neurosci Biobehav Rev. 2004;28:611–24. doi: 10.1016/j.neubiorev.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 59.DeCoteau WE, Kesner RP. Effects of hippocampal and parietal cortex lesions on the processing of multiple-object scenes. Behav Neurosci. 1998;112:68–82. doi: 10.1037//0735-7044.112.1.68. [DOI] [PubMed] [Google Scholar]
- 60.Goodrich-Hunsaker NJ, Howard BP, Hunsaker MR, Kesner RP. Human topological task adapted for rats: Spatial information processes of the parietal cortex. Neurobiol Learn Mem. 2008;90:389–94. doi: 10.1016/j.nlm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Save E, Poucet B, Foreman N, Buhot MC. Objects exploration and reaction to a spatial and a non-spatial change in the rat following damage to the posterior parietal cortex or the dorsal hippocampus. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- 62.Iversen L. Long-term effects of exposure to cannabis. Curr Opin Pharmacol. 2005;5:69–72. doi: 10.1016/j.coph.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- 64.Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, et al. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- 67.Hofmann ME, Bhatia C, Frazier CJ. Cannabinoid receptor agonists potentiate action potential-independent release of GABA in the dentate gyrus through a CB1 receptor-independent mechanism. J Physiol. 2011;589:3801–21. doi: 10.1113/jphysiol.2011.211482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia R, Musleh W, Tocco G, Thompson RF, Baudry M. Time-dependent blockade of STP and LTP in hippocampal slices following acute stress in mice. Neurosci Lett. 1997;233:41–4. doi: 10.1016/s0304-3940(97)00621-6. [DOI] [PubMed] [Google Scholar]
- 69.Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23:4406–9. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill MN, Froc DJ, Fox CJ, Gorzalka BB, Christie BR. Prolonged cannabinoid treatment results in spatial working memory deficits and impaired long-term potentiation in the CA1 region of the hippocampus in vivo. Eur J Neurosci. 2004;20:859–63. doi: 10.1111/j.1460-9568.2004.03522.x. [DOI] [PubMed] [Google Scholar]
- 71.Dong Z, Cao J, Xu L. Opiate withdrawal modifies synaptic plasticity in subicular-nucleus accumbens pathway in vivo. Neuroscience. 2007;144:845–54. doi: 10.1016/j.neuroscience.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 72.Schotanus SM, Chergui K. NR2A-containing NMDA receptors depress glutamatergic synaptic transmission and evoked-dopamine release in the mouse striatum. J Neurochem. 2008;106:1758–65. doi: 10.1111/j.1471-4159.2008.05512.x. [DOI] [PubMed] [Google Scholar]
- 73.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–90. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci. 1994;108:456–68. doi: 10.1037//0735-7044.108.3.456. [DOI] [PubMed] [Google Scholar]
- 75.Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- 76.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, et al. Neurocircuitry targets in ethanol: Reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- 77.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 78.Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology (Berl) 2004;172:241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- 79.Wise R, Gardner E. Functional anatomy of substance-related disorders. In: D'haenen H, den Boer J, Willner P, editors. Biological psychiatry. Wiley, New York; 2002. pp. 509–522. [Google Scholar]
- 80.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 81.Pistis M, Muntoni AL, Pillolla G, Gessa GL. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. Eur J Neurosci. 2002b;15:1795–1802. doi: 10.1046/j.1460-9568.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- 82.Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, et al. A single in-vivo exposure to Delta9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;7:585–6. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- 83.Fride E. Anandamides: tolerance and cross-tolerance to delta 9-tetrahydrocannabinol. Brain Res. 1995;697:83–90. doi: 10.1016/0006-8993(95)00790-w. [DOI] [PubMed] [Google Scholar]
- 84.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 85.Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 87.González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–18. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 88.Johansson E, Halldin MM, Agurell S, Hollister LE, Gillespie HK. Terminal elimination plasma half-life of delta-1-tetrahydrocannabinol (delta-1-THC) in heavy users of marijuana. Eur J Clin Pharmacol. 1989;37:273–277. doi: 10.1007/BF00679783. [DOI] [PubMed] [Google Scholar]
- 89.Brusberg M, Arvidsson S, Kang D, Larsson H, Lindström E, et al. CB1 receptors mediate the analgesic effects of cannabinoids on colorectal distension-induced visceral pain in rodents. J Neurosci. 2009;29:1554–64. doi: 10.1523/JNEUROSCI.5166-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Croxford JL, Miller SD. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212. J Clin Invest. 2003;111:1231–40. doi: 10.1172/JCI17652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valverde O, Karsak M, Zimmer A. Analysis of the endocannabinoid system by using CB1 cannabinoid receptor knockout mice. In: Pertwee R, editor. Cannabinoids: Handbook of experimental pharmacology. Heidelburg: Springer-Verlag; 2005. pp. 117–147. Vol. 168. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Q, Ma P, Iszard M, Cole RB, Wang W, et al. In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a cannabinoid receptor agonist. Drug Metab Dispos. 2002;30:1077–86. doi: 10.1124/dmd.30.10.1077. [DOI] [PubMed] [Google Scholar]
- 93.Moore NL, Greenleaf AL, Acheson SK, Wilson WA, Swartzwelder HS, et al. Role of cannabinoid receptor type 1 desensitization in greater tetrahydrocannabinol impairment of memory in adolescent rats. J Pharmacol Exp Ther. 2010;335:294–301. doi: 10.1124/jpet.110.169359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hampson RE, Simeral JD, Kelly EJ, Deadwyler SA. Tolerance to the memory disruptive effects of cannabinoids involves adaptation by hippocampal neurons. Hippocampus. 2003;13:543–556. doi: 10.1002/hipo.10081. [DOI] [PubMed] [Google Scholar]
- 95.Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26:309–19. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- 96.Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann NY Acad Sci. 2004;1021:384–90. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- 97.Jager G, Kahn RS, Van den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) 2006;185:358–68. doi: 10.1007/s00213-005-0298-7. [DOI] [PubMed] [Google Scholar]
- 98.Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176:239–47. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]