Abstract
We demonstrate rapid mixing of sub-microliter droplets (250nl) using miniaturized magnetic stir bars (400 μm by 200 μm by 15 μm). The stir bars are fabricated using laser micromachining and placed on the substrate on which the drops are manipulated. They are activated by an externally applied magnetic field and used in combination with on-demand drop merging in enthalpy arrays. This technique results in a 10-fold increase in mixing rate, and a mixing time constant of about 2 seconds. Drop mixing times are measured by Förster resonance energy transfer (FRET) and verified by thermodynamic measurements of binding and enzymatic reactions.
Introduction
Rapid mixing of species in liquids is an important requirement for many lab-on-a-chip applications, and becomes a challenge due to the low Reynolds numbers and highly laminar flow encountered on the micro-scale [1,2,3]. In these conditions mixing relies mainly on the relatively slow process of molecular diffusion. Many physical principles to increase the rate of mixing in various microfluidic systems have been explored [4,5,6]. These principles typically rely on techniques which reduce the characteristic length scale over which the different species in the liquid must diffuse towards their equilibrium state. Active and passive mixing techniques can be identified [7]: the former rely on energy supplied by external forces or fields to break up the laminar flow and enhance the mixing rate, whereas the latter make use of passive means such as geometric features to reduce the diffusion path. Electrowetting [8], electrokinetic actuation [9], acoustic actuation [10] and magnetic actuation [11,12] are just a few of the many physical principles that have been exploited in active microfluidic mixers. Examples of passive mixing techniques include the use of structured microchannels and surfaces to increase contact area [5], induce chaotic advection [13] or enhance drop coalescence [14].
Here we present a new active mixing technique based on magnetic actuation using micromachined magnetic stir bars. It is a relatively simple and low-cost technique and particularly suited for discrete microfluidic systems. Discrete, or ‘digital’ microfluidic systems are based on the manipulation of small drops of liquid and have become a popular tool for lab-on-a-chip type applications [15,16]. Advantages over more conventional continuous flow based systems include the ability to confine and transport precise amounts of chemicals and biomaterials at relatively low cost and the ability to initiate and schedule reactions by on-demand merging and mixing of drops. Cost reductions result from simpler construction and microfabrication compared to continuous flow systems. A number of different formats of digital microfluidics can be identified: single-plate (or open), with drops confined on a single solid surface; two-plate (or closed) with drops sandwiched between two solid surfaces; and free, with drops suspended in (or on top of) an immiscible liquid [17,18].
The magnetic mixing technique described in this paper is suitable for any single-plate discrete microfluidic system and results in a significant improvement in mixing time. Its performance is demonstrated in mixing of pairs of sub-microliter drops for thermodynamic characterization on enthalpy arrays, where it is used in conjunction with on-demand electrostatic drop merging. Drop mixing times are measured by Förster resonance energy transfer (FRET), and sensitivity improvements due to rapid mixing were verified by thermodynamic measurements.
Rapid drop mixing on enthalpy arrays
Enthalpy arrays
An enthalpy array is an example of a single-plate discrete microfluidic system. Enthalpy arrays enable label-free, solution-based calorimetric detection of biomolecular interactions in a 96-detector array format [19,20,21]. Paired drops of sample (each drop measuring about 250 nl) are dispensed and then merged and mixed on the surface of the array. Each merged drop pair results in a single thermodynamic experiment (e.g. enzyme activity, protein-ligand binding) [22].
Each detector cell on the array consists of two identical thermistor sensing elements that provide a differential temperature measurement between a sample specimen and a reference specimen. Sample and reference thermistors are connected in a Wheatstone bridge configuration to reduce measurement artifacts caused by common-mode changes in temperature. Each site incorporates an electrostatic drop merging mechanism, which enables simultaneous isothermal merging of the sample and reference specimens on-demand. While the electrostatic energy from the drop merging coupled with the surface tension of the drops does cause some mixing, the time constant is large (more than 20 seconds) because diffusive mixing still dominates. This method is sufficient for performing simple measurements of the total enthalpy for some reactions, including strong binding reactions in the presence of excess ligand and reactions monitoring enzymatic activity, but it does not provide the complete, rapid mixing necessary to perform full kinetic characterization of enzymatic reactions. This paper describes a means to more thoroughly mix the sub-microliter drop pairs to substantially reduce the mixing time.
Rapid mixing by magnetic micro-stirring
The mixing method was developed to meet several requirements of array calorimetry: 1) the drop mixing must occur on a time scale no longer than the thermal dissipation time constant of the enthalpy array device (~2.3 sec), 2) the mixing should not give rise to a differential temperature increase of the mixed drops (or result in differential eddy current heating in materials), 3) the mixing technique has to be scalable – i.e. it can be performed on array- or row-level, 4) the technique should not complicate the array fabrication (avoid building mixing structures on top of array), 5) the mixing device(s) should not affect the biochemistry of the drops and reactions occurring in the merged drop, and 6) the mixing device(s) should not interfere with the sensor/electronics or give rise to excess noise.
Taking all of the above requirements into consideration, we developed a magnetic micro-stirring approach to achieve rapid drop mixing. Magnetic micro-stirring has been described and implemented before: for instance, micromachined impeller-like devices have been realized [12,23], as well as methods based on ferrofluids and magnetorheological fluids [24] and a technique based on the stirring action of a self-assembled chain of magnetic micro-spheres suspended in the liquid [25]. The approach we present here is similar to the micromachined impeller method but requires no complex and expensive wafer processing or microfabrication of the substrates on which the drops are manipulated. In our approach, miniaturized stir bars are fabricated separately from the arrays (Figure 1) and applied to the surface of the array by a pick-and-place technique before the samples are deposited (Figure 2). Stirring is remotely activated by a rotating magnetic field generated by a small magnet mounted on a motor below the array.
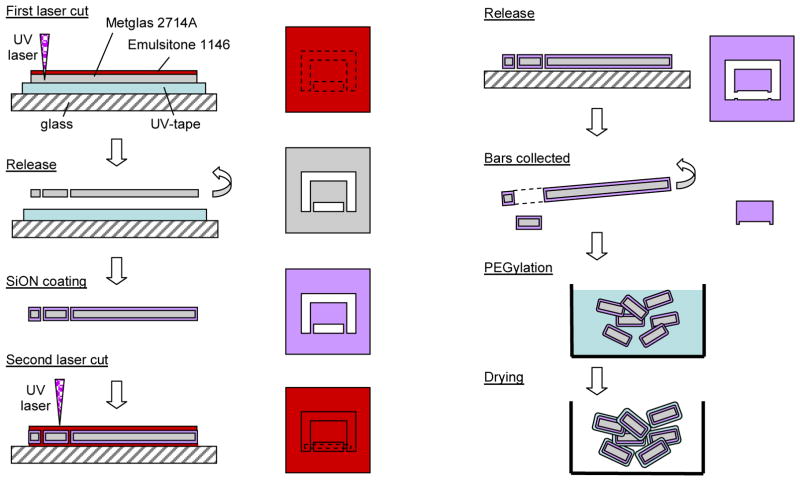
Fig. 1.
Fabrication of miniaturized stir bars
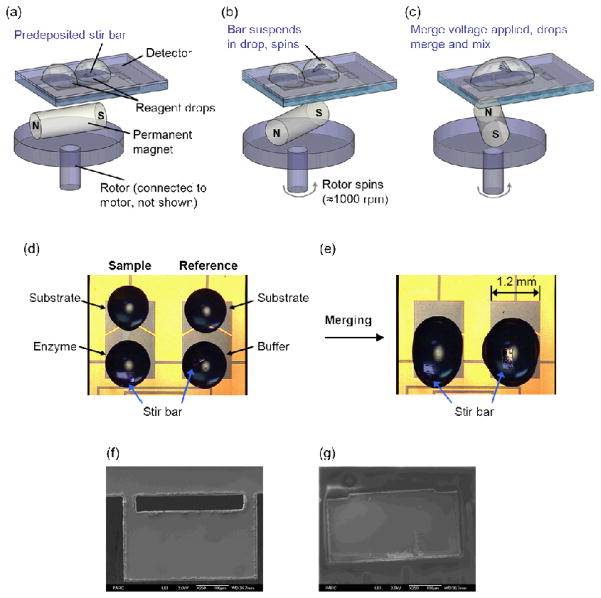
Fig. 2.
(a–c) Schematic of magnetic mixing apparatus. (d–e) Picture of stir bars in enzymatic reaction samples before (d) and after (e) measurement of enzymatic reaction (f–g) SEM images of stir bars, after the initial laser cut (f) and after PEGylation (g).
This stir bar design met several requirements of array calorimetry. First, the bars fit within drops that have diameter of 850–1000 μm. Second, the mass of the bar is limited to avoid excessive heating of the sample. Third, the surface area is small to avoid adsorption of proteins. Finally, the surface of the bars is coated to prevent diffusion of contaminants into the drop and such that the bars go reliably in suspension when the drops are deposited. Furthermore a deposition method was developed to deposit the bars at the proper location on the surface of the array. We designed and tested a few sizes and shapes of bars before selecting bars that are 400 μm long, 200 μm wide and 15 μm thick. The bars are fabricated in large batches (thousands of devices) using laser micromachining. A surface coating was applied with PECVD (Plasma Enhanced Chemical Vapour Deposition) and wet chemical processing. These micro-bars proved to reliably go in suspension in the drops upon activation of the external magnetic field. This is in contrast to our previous attempts with magnetic micro-spheres (similar to the method described in [25]), in which the micro-spheres tended to sediment and/or stick to the surface of the array. Heat flow calculations were used to estimate the amount of heat dissipated in the drops as a result of viscous energy dissipation during mixing (see electronic supplementary information (ESI)). An estimated upper limit for the temperature increase in the drop is about 30 μK per second of mixing. This is much smaller than the energy dissipated in the drops by the self-heating of the thermistors (equivalent to a temperature rise of 1–2 mK) and becomes negligible after the common-mode rejection by the Wheatstone bridge. The calculations were verified by thermodynamic measurements described in a subsequent section, which indicate that for practical purposes this mixing method can be considered isothermal.
Materials and methods
Microfabrication (Figure 1)
A cobalt-based magnetic alloy, Metglas R 2714A (Metglas, Conway, SC, USA), was used to form the bars. This material is available in sheets approximately 15 μm thick and about 1 inch wide.
The Metglas sheet was mounted on double sided adhesive UV-tape (Furukawa Electric Co., Japan, product id UC-228W-110). Emulsitone 1146 Laser Scribing Solution (Emulsitone Company, Whippany, NJ) was spun on the surface of the sheet, and then the sheet was patterned using a laser micromachining tool (Photomachining, Inc., Pelham NH) which uses a 266nm tripled Nd:YAG laser system. In this patterning step (“First laser cut” in Figure 1), the section that later became a bar was still held to the larger sheet by two small tabs. After laser cutting, UV-irradiation (365 nm, 15 minutes) was performed to remove the UV-tape, and the sheet of Metglas with patterned bars was rinsed with water and dried at 60° to 80°C. An oxygen plasma (2 minutes at 200W at 200 mTorr partial oxygen pressure) further cleaned the surface of the material (Figure 2f). The laser micromachining heats the material as it cuts through it, so there is some flow of material into bulging ridges along the cuts, as seen in Figure 2f. At this stage of the process, the bars consist of bare amorphous magnetic metal material. To reduce the dissolution of materials from the bars (e.g., metal atoms or ions) into the drop that could poison chemical reactions, a 200 nm thick conformal layer of silicon oxynitride (SiON) was deposited on the Metglas sheet of patterned bars using a PECVD process.
Next, Emulsitone 1146 solution was spread on a glass substrate, and the SiON coated Metglas strip was mounted on the Emulsitone 1146 layer and dried for approximately ten minutes at room temperature. A second layer of Emulsitone 1146 was spun onto and over the coated Metglas sheet and then again dried for approximately ten minutes at room temperature. The individual bars were then released by a second laser cutting step using the laser micromachining tool.
The Emulsitone 1146 was rinsed away with water, the bars were collected, and a PEG coating was applied to the bars both to promote suspension of the bars in aqueous solvent and to reduce the adsorption of proteins on the surface of the bars.
To apply the PEG coating, batches of approximately 3000 bars were cleaned by rinsing in 50% sulfuric acid for 3–5 minutes followed by a rinse in de-ionized water. Next, the bars were dried and put into a 20 ml glass vial, into which was added, in order, 20ml of toluene, 20 microliters of hexylamine, and 0.054g mPEG silane 1kDa (Creative PEGworks, Winston Salem, NC). The reaction was mixed at room temperature for 12–16 hours using an end-over-end rotator. The toluene was decanted, and then the bars were rinsed serially for 5 minutes with 20 ml of the following solvents in order: toluene, acetone, isopropylalcohol, and deionized water. The bars were dried under vacuum and stored in the vial until use. Figure 2g shows a single stir bar following PEGylation.
FRET measurements
For the FRET assay, complementary 10-mer oligonucleotides, 5′-AlexaFluor 555-d(TTGGTGATCC) and 5′-AlexaFluor 647-d(GGATCACCAA), were synthesized with the AlexaFluor labels added during the synthesis (Invitrogen). The sequences are identical to those described by Morrison and Stols [26], but have 5′-AlexaFluor labels rather than 5′-fluorescein and 3′-sulforhodamine 101 labels. Concentrations of oligomers were calculated from the absorbance at 260 nm using the following extinction coefficients: 102,700 M−1 cm−1 for 5′-AlexaFluor 555-d(TTGGTGATCC) and 116,300 M−1 cm−1 for 5′-AlexaFluor 647-d(GGATCACCAA). The distance between the 5′-ends of the base paired 10-mer duplex is approximately 3.4 nm, placing the donor and acceptor fluorophores within the Förster radius.
Hybridization reactions were performed at room temperature in 10 mM sodium phosphate (pH 8.0), 1 M NaCl. Association was initiated by electrostatic merging of a 250 nl drop containing 20-μM 5′-AlexaFluor 555-d(TTGGTGATCC) with a 250 nl drop containing 20 μM 5′-AlexaFluor 647-d(GGATCACCAA). Fluorescence measurements were performed using a custom-built setup consisting of a 532 nm laser, a photomultiplier tube (PMT) with an attached 675/50 nm filter and a 4x microscope objective lens placed above the surface of the array. The voltage from the PMT was recorded every 1/100 sec.
Neodymium cylinder magnets (D16, 0.0625” diameter x 0.375” thick, axially magnetized, surface field = 6570 Gauss; K&J Magnetics, Inc., Jamison, PA) were mounted on top of a motor and placed 1–2 mm below the bottom surface of the array. The axis of rotation of the magnet was centered below the stir bar to reduce unwanted lateral motion of the bar that could cause spreading of the drop. A standard magnetic stir plate was also tested as the means to rotate the bar. The stir plate could be placed further from the array, but it also produced unacceptable spreading of the drop, particularly if the center of the axis of rotation was not directly below the stir bar.
Enthalpy array measurements
Two well characterized reactions, the binding of barium chloride (BaCl2) to 18-crown-6 and the enzymatic reaction of trypsin (EC 3.4.21.4) with Nα-Benzoyl-L-arginine ethyl ester (BAEE) were selected to demonstrate the improvement in the signal-to-noise and information content of measurements enabled by incorporation of magnetic mixing. All reagents were from Sigma-Aldrich and were used without further purification.
Depositing the drops was performed with the Deerac Spot-on liquid dispensing system (Labcyte/Deerac Fluidics, Dublin, Ireland). For both types of reactions, the drops were allowed to come to thermal equilibration (2–3 min) prior to activating the magnetic mixing device under the site to be measured. On activation, the magnetic stirring mechanism was rotated at approximately 1000 rpm and allowed to equilibrate for 30 s. After equilibration, the reaction was initiated by applying a voltage (180 V) across the merging electrodes to electrostatically merge the drops of interest. The output voltage of the Wheatstone bridge was recorded for 30 s prior to the merge time and 3.5 min following initiation of the reaction. Magnetic mixing continued during the entire time.
Results and discussion
Characterization of bars and mixing rates
In these experiments we compared results from mixing caused solely by electrostatic merging with mixing enhanced by miniature magnetic stir bars. Previously, we observed that drops mixed solely by electrostatic merging did mix sufficiently to enable measurement of the total enthalpy of reaction for a strong binding reaction with excess of a low molecular weight ligand [19]. However, measuring more detailed information about reactions requires faster mixing, as does measurement of weaker reactions, providing the motivation for the work reported here. Several methods were used to assess the mixing rate. Initially we used microscopy to observe mixing of a dyed drop with a colorless drop. This approach has limitations, as the flows at various depths are optically superimposed, which can make separated fluids appear to be mixed and cause overestimation of the mixing efficiency [27]. Indeed, we found that the apparent mixing rate observed by microscopy of dyed drops was inconsistent with the thermodynamic observations from array calorimetry.
To make more accurate measurements, we developed a FRET assay that monitors the mixing of complementary DNA strands in drops. FRET assays measure the radiationless transfer of energy from a donor fluorophore to an appropriate acceptor fluorophore.
The efficiency of energy transfer varies inversely with the sixth power of the distance separating the donor and acceptor fluorophores, limiting the distance over which FRET can occur to between 1–10 nm. The donor-acceptor pair (Alexa Fluor 555, Alexa Fluor 647; Förster radius (R0) = 5.1 nm; Invitrogen) was chosen so that the DNA strands must pair to generate emission at acceptor wavelength. Thus, the FRET signal indicates the degree to which the complementary DNA chains originating in separate drops have been able to find each other and bind. Although this method still does not fully address the superposition of flows at various depths, the requirement that the DNA strands pair to generate a signal ensures that we observe mixing down to the 3–5 nm length scale.
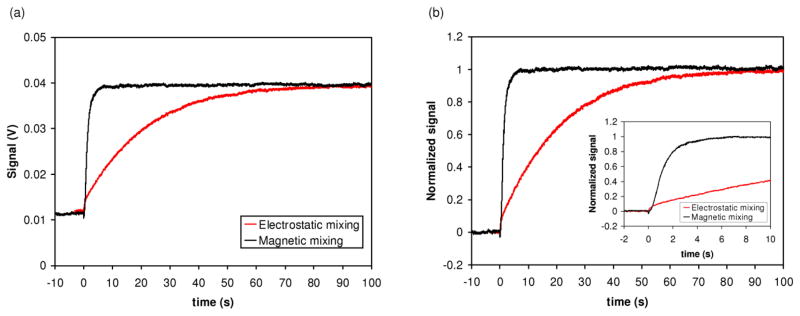
Figure 3 shows examples of the FRET data for mixing experiments for drops mixed using electrostatic merging only (“electrostatic mixing” in Figure 3) and magnetic mixing at 1500 rpm. With magnetic mixing the FRET signal rises much faster, as desired. Table 1 summarizes the rate of mixing for drops mixed using solely electrostatic merging, magnetic mixing at 600 rpm, and magnetic mixing at 1500 rpm. Magnetic mixing produced an order of magnitude faster mixing of the drops compared to electrostatic mixing. We did not observe a significant difference in the mixing rate when the stirring rate was increased from 600 rpm to 1500 rpm. For thermal measurements, we chose to use 1000 rpm to provide some latitude for mixing samples with slightly higher viscosity than buffer or dilute protein solutions.
Fig. 3.
Comparison of rate of mixing for electrostatic mixing (i.e. mixing caused solely by the electrostatic merging of drops) and magnetic mixing. (a) FRET signal as measured by photomultiplier tube. Drops were merged at t=0 s. Magnetic mixing was performed at 1500 rpm. (b) Normalized data. The inlay shows the data for the first 10 seconds following the merge.
Table 1.
Comparison of mixing solely by electrostatic merging to magnetic mixing. All experiments with magnetic mixing used the D16 magnet. The values were averaged over the indicated number of replicates, and the standard error of the mean is reported in parentheses.
| Experiment | Stirring rate | τ (s) | 90% maximum signal (s) |
|---|---|---|---|
| No stirring (n=5) | NA | 29.5 (±3.8) | 64.3 (±6.7) |
| Stir bar (n=2) | 600 rpm | 1.8 (±0.1) | 3.0 (±0.1) |
| Stir bar (n=3) | 1500 rpm | 2.1 (±0.5) | 3.5 (±0.4) |
The use of the PEG coating improved the reliability by which the bar, when inside a drop, overcame friction and other surface forces upon application of the magnetic actuation. Without this coating, we observed in some cases that the bar remained stuck to the surface of the array after a drop was deposited.
For some applications, protein adsorption to the surface of the bar is a concern. For proteins the size of serum albumin or trypsin, the maximum concentration of protein that could bind to the surface area of a bar (1.8×10−7 m2) is 15–30 nM for a 500 nl reaction volume (see ESI). This degree of adsorption will not cause significant problems for reactions that involve much higher protein concentrations (>200 nM) than the maximum possible adsorbed concentration, as is the case with many enthalpy array measurements. Nonetheless, PEGylation of bars reduces the potential of adsorption, which generally is not desirable and may be a significant concern for applications using a low protein concentration (< 100 nM).
Contamination from metals released from the bars is problematic for some applications. The concentration of various elements released from the bars was measured using inductively coupled plasma mass spectrometry (ICP-MS), and the results are shown in Table 2. For uncoated bars, the concentrations of the elements in a 500 nl reaction are between 3 × 10−6 M and 1.6 × 10−4 M. After coating the bars with SiON and PEGylation, we observed a decrease in released element concentration ranging from <2 × 10−6 M to 3.5 × 10−5 M. Although these concentrations of metals will not have a significant effect on many reactions, metalloenzymes and enzymes with cysteine residues in the active site could be affected at these concentrations of metals. The use of a chelator (e.g., ETDA or EGTA at 0.1 mM) in the reaction buffer might be employed in cases where the enzyme requires Mg(II) or Ca(II) for activity or when the presence of any of these metals inhibits the activity. As it is not always feasible to include a chelator in the reaction buffer, work is continuing to implement a coating solution that further reduces the release of metals. The increase of the concentration of boron may be attributed to the presence of boron doped silicon residue in the PECVD reactor.
Table 2.
Analysis of elements released from stir bars incubated in buffer. Tris buffer. 71.2 mm2 Metglas (400 bars; 400 μm × 200 μm × 15 μm each) was incubated with 200 μl buffer (50 mM Tris-HCl (pH 8.0), 100 mM NaCl) for 15 minutes. Values in table following subtraction of the concentration of each element in a control sample containing only buffer.
| Element | Uncoated bars, concentration (μM) | SiON coated/PEGylated bars, concentration (μM) |
|---|---|---|
| B | < 0.93 | 35 |
| Si | 28.5 | < 3.5 |
| Fe | < 1.79 | < 1.79 |
| Co | 160 | 10.5 |
| Ni | 3.58 | < 1.7 |
Enthalpy array implementation
The magnetic mixing approach was implemented in the enthalpy array system and characterized for used in thermodynamic characterization of reactions. Here, PEGylated magnetic stir bars (400 × 200 × 15 μm3) were manually placed on array detector elements using an X–Y–Z micromanipulator (model 7372E, West Bond, Anaheim, CA, USA). One bar was used for each pair of drops and was placed on the region where the enzyme or buffer (or bovine serum albumin (BSA)) drop would be placed during drop deposition (see Fig. 2d).
The presence of the bars did not appear to interfere with the drop dispensing system or impede accurate drop placement. Also, surface forces and/or stiction appeared to be sufficient to hold the bars in place during the simple wafer handling operations prior to the deposition of drops, which enables populating multiple rows of the array with bars prior to depositing the drops. Once the bars were in place, drops of reagents were deposited on measurement sites.
The array was then moved robotically into a temperature controlled measurement chamber on a block containing eight individually addressable magnetic stirring mechanisms, and a polymer cap was applied over the array to minimize drop evaporation. The neodymium cylinder magnets (D16, K&J Magnetics, Inc., Jamison, PA) were approximately 2.5 mm below the top surface of the array. A single magnet is responsible for stirring the two bars required for each pair of reactions (sample and reference) on the array. The axis of rotation of each magnet was centered below the center of each site on the array to reduce spreading of the drop.
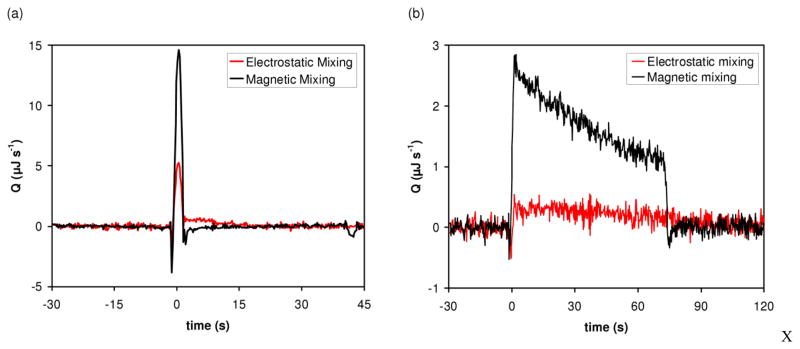
Figure 4a shows the effect of adding magnetic mixing on the rate of heat generation (Q) for binding of BaCl2 to 18-crown-6, both reagents being present in stoichiometric amounts. We observed a 1.6-fold increase in signal for binding reactions with magnetic mixing. The reaction using electrostatic merging alone yields an integrated heat of 26.8 μJ, while the combination of electrostatic merging and magnetic mixing yields an integrated heat of 42.0 μJ.
Fig. 4.
Comparison of thermal measurements using electrostatic mixing (i.e. mixing caused solely by the electrostatic merging of drops) and magnetic mixing. (a) Binding of BaCl2 to 18-crown-6 measured at 21°C in unbuffered water. The merged drops contained 2 mM BaCl2 and 2 mM 18-crown-6. (b) Enzymatic reaction. Trypsin hydrolysis of BAEE measured at 21°C in 200 mM Tris–HCl (pH 8.0), 50 mM CaCl2, and 0.2% polyethylene glycol (PEG) 8000. The merged drops contained 5 μM trypsin and 5 mM BAEE at the beginning of the reaction. Differential temperature as a function of time was converted to rate of heat generation (Q) as a function of time as described in Recht et al [21].
In addition to an increase in signal, faster mixing enables enzyme kinetics to be measured, as described in detail in Recht et al [22]. Figure 4b shows the difference in the signals obtained for trypsin hydrolysis of BAEE with electrostatic mixing and with magnetic mixing. The reaction performed with electrostatic mixing yielded a low signal-to-noise and no information about the kinetics of the enzymatic reaction. Using the same concentrations of substrate and enzyme as the electrostatic mixing experiment, the reaction with magnetic mixing produced a rapid increase in the signal (BAEE hydrolysis is exothermic). As trypsin converted substrate to product, the rate of heat generation showed a roughly linear behavior with a negative slope for approximately 70 s, after which time substrate depletion caused a decrease in rate and the signal returned to the baseline.
Conclusions and outlook
We have described the design and fabrication of miniaturized stir bars and implementation of magnetic mixing for thermodynamic measurements using enthalpy arrays. The use of the mixing method resulted in a 10-fold improvement in mixing of two 250 nl drops, corresponding to a mixing time constant of approximately 2 seconds. Mixing times were measured using a FRET assay, and the advantages of rapid mixing were confirmed by thermodynamic measurements. The stir bars were placed on the surface of an enthalpy array with an x-y-z micromanipulator, the sample drops were applied using a non-contact dispensing system, and stirring was activated remotely by a rotating magnetic field. The placement of the bars can be automated using a state-of-the-art automated pick-and-place machine. Although designed to function in the context of the enthalpy array technology, the miniaturized stir bars could be used to mix drops in any single-plate discrete microfluidic system. Thus, the technique is applicable to various chemical and biochemical assays based on different detection techniques.
Supplementary Material
Acknowledgments
The authors thank Lai Wong and Siv Kor for assistance with device fabrication, Jens Böhme for assistance with the laser micromachining tool, Doug Curry for his help on the optical measurement setup and Sasha Tuganov for his work on the magnetic motor fixture design. This work was supported by National Institutes of Health (NIH) Grant R01GM077435.
References
- 1.Squires TM, Quake SR. Reviews of Modern Physics. 2005;77(3):977–1026. [Google Scholar]
- 2.Stone HA, Stroock AD, Ajdari A. Annual Review of Fluid Mechanics. 2004;36:381–411. [Google Scholar]
- 3.Paik P, Pamula VK, Fair RB. Lab on a Chip. 2003;3:253–259. doi: 10.1039/b307628h. [DOI] [PubMed] [Google Scholar]
- 4.Ottino JM. Annual Review of Fluid Mechanics. 1990;22(1):207–254. [Google Scholar]
- 5.Nguyen NT, Wu Z. Journal of Micromechanics and Microengineering. 2005;15(2):R1–R16. [Google Scholar]
- 6.Campbell CJ, Grzybowski BA. Phil Trans R Soc Lond A. 2004;362:1069–1086. doi: 10.1098/rsta.2003.1363. [DOI] [PubMed] [Google Scholar]
- 7.Hessel V, Löwe H, Schonfeld F. Chemical Engineering Science. 2005;60(8–9):2479–2501. [Google Scholar]
- 8.Mugele F, Baret J, Steinhauser D. Applied Physics Letters. 2006;88(20):204106. [Google Scholar]
- 9.Lastochkin D, Zhou R, Wang P, Ben Y, Chang H. Journal of Applied Physics. 2004;96(3):1730. [Google Scholar]
- 10.Vivek V, Zeng Y, Kim E. Proceedings IEEE Thirteenth Annual International Conference on Micro Electro Mechanical Systems; 2000. pp. 668–673. [Google Scholar]
- 11.Lu LH, Ryu KS, Liu C. Journal of Microelectromechanical Systems. 2002;11(5):462–469. [Google Scholar]
- 12.Ryu KS, Shaikh K, Goluch E, Fan Z, Liu C. Lab on a chip. 2004;4(6):608–13. doi: 10.1039/b403305a. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh S, Huang Y. Journal of Micromechanics and Microengineering. 2008;18:065017. [Google Scholar]
- 14.Lai Y, Hsu M, Yang J. Lab on a Chip. 2010;10(22):3149–3156. doi: 10.1039/c003729j. [DOI] [PubMed] [Google Scholar]
- 15.Teh SY, Lin R, Hung LH, Lee AP. Lab on a Chip. 2008;8:198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- 16.Surenjav E, Herminghaus S, Priest C, Seemann R. Applied Physics Letters. 2009;95(15):154104. [Google Scholar]
- 17.Cho SK, Moon H, Kim C. Journal of Microelectromechanical Systems. 2003;12(1):70–80. [Google Scholar]
- 18.Abdelgawad M, Park P, Wheeler AR. Journal of Applied Physics. 2009;105(9):094506. [Google Scholar]
- 19.Torres FE, Kuhn P, De Bruyker D, Bell AG, Wolkin MV, Peeters E, Williamson JR, Anderson GB, Schmitz GP, Recht MI, Schweizer S, Scott LG, Ho J, Elrod SA, Schultz PG, Lerner RA, Bruce RH. Proceedings of the National Academy of Sciences of the USA. 2004;101:9517–9522. doi: 10.1073/pnas.0403573101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bruyker D, Wolkin MV, Recht MI, Torres FE, Bell AG, Anderson GB, Peeters E, Kolatkar A, Kuhn P, Bruce RH. Technical Digest of the 14th International Conference on Solid State Sensors, Actuators and Microsystems (Transducers ‘07); 2007. pp. 1757–1760. [Google Scholar]
- 21.Recht MI, De Bruyker D, Bell AG, Wolkin MV, Peeters E, Anderson GB, Kolatkar A, Bern MW, Kuhn P, Bruce RH, Torres FE. Analytical Biochemistry. 2008;377:33–39. doi: 10.1016/j.ab.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recht MI, Torres FE, De Bruyker D, Bell AG, Klumpp M, Bruce RH. Anaytical Biochemistry. 2009;388:204–212. doi: 10.1016/j.ab.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian Y, Zhang Y, Ku J, He Y, Xu B, Chen Q, Xia H, Sun H. Lab on a Chip. 2010;10:2902–2905. doi: 10.1039/c005277a. [DOI] [PubMed] [Google Scholar]
- 24.Song W, Ding Z, Son C, Ziaie B. Applied Physics Letters. 2007;90:092501. [Google Scholar]
- 25.Roy T, Sinha A, Chakraborty S, Ganguly R, Puri I. Physics of Fluids. 2009;21(2):27101. [Google Scholar]
- 26.Morrison LE, Stols LM. Biochemistry. 1993;32:3095–3104. doi: 10.1021/bi00063a022. [DOI] [PubMed] [Google Scholar]
- 27.Xi C, Marks DL, Parikh DS, Raskin L, Boppart SA. Proceedings of the National Academy of Sciences of the USA. 2004;101:7516–7521. doi: 10.1073/pnas.0402433101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.