Abstract
Research suggests that dysfunctional glutamatergic signalling may contribute to depression, a debilitating mood disorder affecting millions of individuals worldwide. Ketamine, a N-methyl-D-aspartate (NMDA) receptor antagonist, exerts rapid antidepressant effects in approximately 70% of patients. Glutamate evokes the release of D-serine from astrocytes and neurons, which then acts as a co-agonist and binds at the glycine site on the NR1 subunit of NMDA receptors. Several studies have implicated glial deficits as one of the underlying facets of the neurobiology of depression. The present study tested the hypothesis that D-serine modulates behaviours related to depression. The behavioural effects of a single, acute D-serine administration were examined in several rodent tests of antidepressant-like effects, including the forced swim test (FST), the female urine sniffing test (FUST) following serotonin depletion, and the learned helplessness (LH) paradigm. D-serine significantly reduced immobility in the FST without affecting general motor function. Both D-serine and ketamine significantly rescued sexual reward-seeking deficits caused by serotonin depletion in the FUST. Finally, D-serine reversed LH behaviour, as measured by escape latency, number of escapes, and percentage of mice developing LH. Mice lacking NR1 expression in forebrain excitatory neurons exhibited a depression-like phenotype in the same behavioural tests, and did not respond to D-serine treatment. These findings suggest that D-serine produces antidepressant-like effects and support the notion of complex glutamatergic dysfunction in depression. It is unclear whether D-serine has a convergent influence on downstream synaptic plasticity cascades that may yield a similar therapeutic profile to NMDA antagonists like ketamine.
Keywords: Antidepressant, D-serine, glutamate, N-methyl-D-aspartate receptor (NMDAR), NR1 knockout
Introduction
Depression is a chronic, severe mental illness, and a leading cause of disability worldwide (Belmaker & Agam, 2008; Duman, 2009; Hunsberger et al. 2009; Manji et al. 2001; Sanacora et al. 2008). Heritable genetic and epigenetic variants, emotional and socialpsychological factors, and early life events have all been implicated in the pathogenesis of depression (Belmaker & Agam, 2008; Krishnan & Nestler, 2008). While the disorder is treatable, the effectiveness of current antidepressants, and the adverse effects associated with their use, remains far from satisfactory for most patients with depression (Belmaker & Agam, 2008; Manji et al. 2001; Sanacora et al. 2008). For example, data from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, the largest longitudinal randomized antidepressant treatment trial to date, showed that citalopram, a commonly used antidepressant, was associated with a remission rate of only 36.8% and an intolerance rate of 16% (Trivedi et al. 2006; Zisook et al. 2008). Data from the STAR*D study also showed that roughly 40% of patients with depression relapsed within 1 year (Trivedi et al. 2006; Zisook et al. 2008). It is also important to note that for those patients who do respond to currently available antidepressants, clinical benefits often take weeks to occur, suggesting that many of the therapeutic effects are produced by changes in downstream signalling cascades (Krishnan & Nestler, 2008; Manji et al. 2001). Indeed, a growing body of evidence supports the hypothesis that disruptions in neuronal synaptic plasticity cascades are critical to the onset of depression (Krishnan & Nestler, 2008). The limitations of current antidepressants have motivated researchers to identify new therapeutic strategies that act more quickly, and are more effective for a greater number of patients.
Extensive clinical and preclinical evidence suggests that monoaminergic disruptions contribute to depression. Monoamine depletion can lead to relapse in patients with a history of depression (Ruhe et al. 2007) and rapidly induces depression in patients who respond to monoamine oxidase inhibitors (MAOIs) (Shopsin et al. 1976). However, studies measuring monoamines and derived metabolites in the cerebrospinal fluid (CSF) of patients with depression have obtained mixed results (Hou et al. 2006; Lambert et al. 2000; Mann et al. 1996). In addition, preclinical animal studies found that pharmacological and genetic depletion of certain monoamines can abolish the response to antidepressants in depression-related behavioural tests such as the tail suspension test (TST) and forced swim test (FST) (Cryan et al. 2004; O’Leary et al. 2007; Page et al. 1999).
From an aetiological perspective, disrupted glutamatergic neurotransmission, resulting from altered N-methyl-D-aspartate receptor (NMDAR) function and deficient glial reuptake of glutamate, has also been implicated in depression (Krishnan & Nestler, 2008; Sanacora et al. 2008). Consistent with glutamate dysfunction in the brains of some depressed patients, a sub-anaesthetic (0.5 mg/kg), intravenous infusion of the NMDA antagonist ketamine was found to result in rapid and long-lasting symptom relief in about 60–70% of patients with treatment-resistant depression or bipolar depression (Diazgranados et al. 2010a ; Zarate et al. 2006). Preclinical studies further found that ketamine significantly up-regulates prominent synaptic plasticity cascades and promotes the formation of new synapses in vivo (Li et al. 2010). Despite its role as an NMDA antagonist, electrophysiological (Li et al. 2010) and neurochemical (Maeng et al. 2008b) evidence suggests that ketamine enhances glutamatergic neurotransmission.
Glia, particularly astrocytes, play an integral role at glutamatergic synapses where they are active, highly influential participants in neuronal signalling (Haydon, 2001). Reduced density of forebrain glia has been reported in clinical (Bremner et al. 2002; Cotter et al. 2001; Ongur et al. 1998), post-mortem (Cotter et al. 2001), and preclinical studies of depression (Banasr & Duman, 2008; Banasr et al. 2010). While originally considered exclusively a gliotransmitter, D-serine was recently found to be produced, stored, and released by neurons (Kartvelishvily et al. 2006; Rosenberg et al. 2010), suggesting that neurons may actually contribute more to its forebrain concentration than astrocytes (Yoshikawa et al. 2006, 2007). Regardless, D-serine’s role in glutamatergic throughput has key implications for regulating synaptic plasticity and may provide a nexus between neuronal and glial signalling (Billard, 2008).
Astrocytes are involved in the uptake of glutamate and the release of gliotransmitters like D-serine, which has a high affinity for the glycine binding site on the obligatory NR1 NMDAR subunit (Mothet et al. 2000). Notably, reductions in the NR1 subunit – which is a target of D-serine – have been implicated in mood disorders (Law & Deakin, 2001; McCullumsmith et al. 2007; Nudmamud-Thanoi & Reynolds, 2004). In addition, chronic corticosterone exposure and isolation stress significantly reduced NR1 expression in rodents (Cohen et al. 2011; Hermes et al. 2011). However, the therapeutic potential of compounds that target glia–neuron and inter-neuronal interactions to enhance glutamatergic signalling remains relatively unknown. Here, we used a series of behavioural experiments to evaluate the antidepressant-like effects of treatment with a single, acute dose of D-serine.
Method
Animals
All animal experimental procedures were approved by the Animal Care and Use Committee of the National Institute of Mental Health (NIMH) and followed NIMH guidelines. Three sets of animals were used: (1) male Wistar Kyoto (WKY) rats obtained from Taconic Farms (USA); (2) male 129S1/SvImJ wild-type (WT) mice obtained from Jackson Laboratories (USA); and (3) conditional NR1 knockout (KO) mutant mice, which were produced by crossing an αCaM kinase II-driven Cre recombinase transgenic line, T29-1 (Tsien et al. 1996), with a loxP-flanked NR1 knock-in mouse line (Dang et al. 2006). Control animals for experiments involving NR1 KO mice were littermates where Cre recombinase was not expressed and sequences flanked by loxP (Flox) remained as homozygous (NR1 subunit expression persisted). Cre recombination was previously detected in the principal neurons of the entire forebrain, including cortex, hippocampus, amygdala, olfactory bulb, striatum, thalamus, and basal forebrain (Tsien et al. 1996), but was not observed in the Gad67-positive local interneurons (Jiang et al. 2010). Animals were housed in a facility with constant temperature (22±1 °C) and a 12-h light/dark cycle (lights on 06:00 hours), with food and water available ad libitum.
Reagents and treatment doses
4-Chloro-D,L-phenylalanine (para-chlorophenylalanine, PCPA), ketamine, and D-serine were obtained from Sigma (USA). PCPA and ketamine were dissolved in phosphate-buffered saline (PBS), while D-serine was dissolved in 0.9% sterile saline. Pilot dose–response tests (data not shown) were used to determine optimally therapeutic doses of D-serine. All agents, including vehicle controls, were delivered intraperitoneally (i.p. ; 800 mg/kg D-serine for rats and 2.7 g/kg for mice; 300 mg/kg PCPA; 2.5 mg/kg ketamine).
Open-field test (OFT)
The OFT was used with WKY rats and NR1 KO mice to monitor locomotor and exploratory activity as described previously (Shaltiel et al. 2008). Animals receiving experimental treatments were injected 30 min prior to the test. Animals were introduced into the center of a clear, Plexiglas 35 × 35 cm arena, and digital video was recorded. The videos were subsequently analysed using the Topscan video tracking system (Clever Systems Inc., USA) for total locomotor activity (distance travelled), time spent in the centre of the arena, and frequency of entries into the centre of the arena. Each arena was thoroughly cleaned with a 10% alcohol solution between testing sessions.
FST in rats
The FST was conducted with WKY rats as described previously (Einat et al. 2003). Briefly, the test involves two exposures to a cylindrical plastic tank, 60 cm high, 25 cm diameter, filled with water (22–23 °C) to a depth of 40 cm, during which the rodents cannot touch the bottom of the tank or otherwise escape. During the first exposure, rats were placed in the water and digitally recorded for 15 min. Approximately 24 h later, animals were injected with D-serine or saline and returned to their home cage. Thirty minutes later, rats were placed in their respective tanks and recorded for a 5-min test session. Immobility duration was scored using the ForcedSwimScan video tracking system (Clever Systems Inc., USA).
FST in mice
The FST was conducted with NR1 KO mice as described previously (Shaltiel et al. 2008). Briefly, transparent Plexiglas cylinders, 50 cm high, diameter 20 cm, were filled with tap water at 22–24 °C to approximately 25 cm in a way that mice were not able to touch the floor or escape. Animals receiving treatments were injected 30 min prior to the test with either D-serine or saline. Individual mice were placed in the water for a 6-minute session, and their behaviour was recorded for later analysis. Immobility duration was scored over the final 4 min of the test using the ForcedSwimScan video tracking system (Clever Systems Inc.).
Female urine sniffing test (FUST)
The FUST, a non-operant test for measuring reward-seeking behaviour in rodents based on interest in sniffing pheromone odours from the opposite sex, was conducted with WKY rats, 129S1/SvImJ WT mice, and NR1 KO mice as described previously (Malkesman et al. 2010, 2011). Animals receiving PCPA treatment or PBS (vehicle control) were injected between 09:00 and 10:00 hours on the day before the FUST experiment; animals receiving ketamine or PBS (vehicle control) were treated between 17:00 and 18:00 hours in the evening prior to FUST experiments. Animals receiving D-serine or saline (vehicle control) were injected 30–45 min before the FUST.
Prior to testing, animals were habituated to a sterile cotton-tipped applicator inserted into their home cage. At the onset of testing, animals were transferred into a dark room (3 lx lighting). The test had three phases: (1) a single exposure (3 min) to the cotton tip dipped in sterile water, during which the time spent sniffing the applicator was measured; (2) an interval of 45 min during which no applicator was presented to the animal; and (3) a single exposure (3 min) to a cotton tip infused with fresh urine collected from females of the same strain in oestrus, during which the time spent sniffing was measured.
Learned helplessness (LH) paradigm
The LH paradigm was conducted with 129S1/SvImJ WT mice using the Gemini Avoidance System (USA) as described previously (Maeng et al. 2008a), with slight modifications. The helplessness induction session was conducted on day 0 and mice were given 180 sessions of inescapable electric footshocks [0.45 mA, 24-s duration, inter-trial interval 20 s average (range 12–28 s)], accompanied by a cued light 3 s prior to footshock delivery in one of the closed compartments.
In the screening session, conducted 24 h after induction, the mice received 30 trials of a 3-s light (conditioned stimulus) followed by an additional 24 s of light plus shock [unconditioned stimulus, 0.45 mA, inter-trial interval 30-s average (range 22–38 s)]. Mice that had developed helplessness – i.e. that had failed to escape more than 24 trials during the screening – were treated with D-serine or saline (vehicle control). D-Serine and saline injections were administered 30–45 min prior to the final phase of the LH paradigm, the active avoidance test (AAT). The parameters of the AAT were identical to those found in the screening section ; latency to escape, as well as the number of escape failures, were recorded by the Gemini Avoidance System.
NR1 KO mice only underwent the AAT portion of the LH paradigm, without inducing or screening for LH first. For experiments involving D-serine and saline, mice received injections 30–45 min prior to the AAT.
Serotonin depletion and hippocampal level measurements
Serotonin levels were measured in WKY rats that received a single injection of PCPA or PBS (vehicle control) in the morning between 09:00 and 10:00 hours on the day prior to sacrifice. Serotonin amounts were measured in fresh, rapidly isolated hippocampal tissues. Samples were processed in buffer proportional to tissue mass using a glass dounce homogenizer and then centrifuged to remove nuclei and large debris (1000 g). Relative levels of serotonin in control and PCPA-treated rats were compared using a serotonin ELISA kit according to the manufacturer’s protocol (Rocky Mountain Diagnostics, USA).
In-situ hybridization histochemistry
To detect mouse NR1 mRNAs, a complementary RNA (cRNA) probe derived from the AvrII-SphI 0.4 kb antisense DNA fragment of rat NR1 cDNA from exon 13 to exon 16 was labeled with [35S]UTP. In-situ hybridization histochemistry was conducted as described previously (Young & Mezey, 2004).
Statistics
Statistical analyses were performed by either two-way ANOVA (multiple variables) or Student’s t tests (single variable, two comparable groups). When values were quantified multiple times in distinct conditions for the same animal (e.g. time spent sniffing water vs. urine in the FUST), two-way repeated-measures ANOVA was used with Bonferroni post-hoc comparison tests where appropriate. χ2 statistics were computed to assess the percentage of mice that remained helpless in the LH paradigm after D-serine or saline treatment. Data are expressed as mean±S.E.M.; significance was evaluated at p<0.05 for all statistical measures.
Results
D-Serine reduced immobility in the FST without affecting motor activity
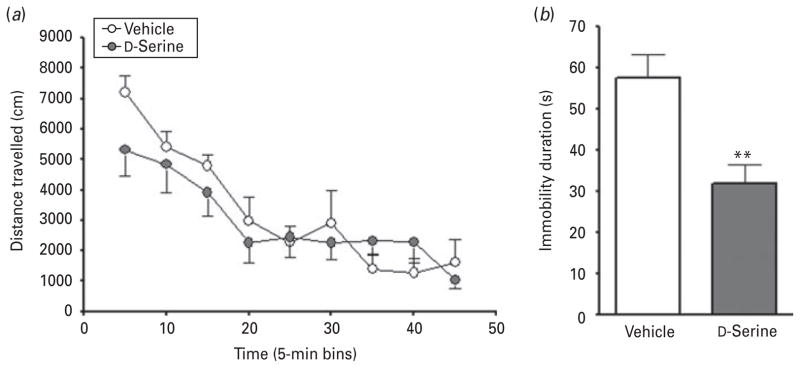
The FST is frequently used to examine the antidepressant-like properties of experimental compounds (Malkesman et al. 2009; Porsolt et al. 1977). To eliminate the potential confound of altered locomotor output, we first measured open-field activity in a stress-sensitive strain of rats (WKY) (De La Garza & Mahoney, 2004) treated with D-serine (Fig. 1a). Time significantly affected distance travelled (F8,80=18.53, p<0.0001), but D-serine treatment did not significantly alter the locomotion of rats in the OFT (F1,80=0.3840, p=0.5493; n=6 per group). No significant interaction was noted between the two variables (F8,80=1.363, p=0.2258). D-Serine treatment neither activated nor suppressed motor activity, and rats habituated to the novel environment in a similar way to animals treated with vehicle control. The same dose of D-serine used in the OFT did, however, significantly reduce immobility duration in the FST (t test : t19=3.473, p=0.0025; D-serine, n=10; vehicle, n=9; Fig. 1b).
Fig. 1.
D-Serine improved immobility duration without affecting general motor activity of rats. (a) In the open field test, D-serine had no significant effect on the locomotor activity of male Wistar Kyoto (WKY) rats. (b) In the forced swim test, D-serine treatment significantly reduced immobility duration in WKY rats. Data are mean±S.E.M. (** p<0.01).
Serotonin depletion reduced time spent sniffing urine in the FUST
Here, we used a pharmacological approach to deplete serotonin and then conducted a behavioural test to evaluate sexual reward-seeking behaviour. Working with the same rat strain (WKY), we blocked serotonin synthesis with the tryptophan hydroxylase inhibitor PCPA and conducted the FUST the following day. Previous studies from our laboratory found that sniffing urine elicited behavioural and neurochemical responses that suggest this is a rewarding activity to rodents, and the model was sensitive to environmental, genetic, and pharmacological manipulations relevant to depression (Malkesman et al. 2010).
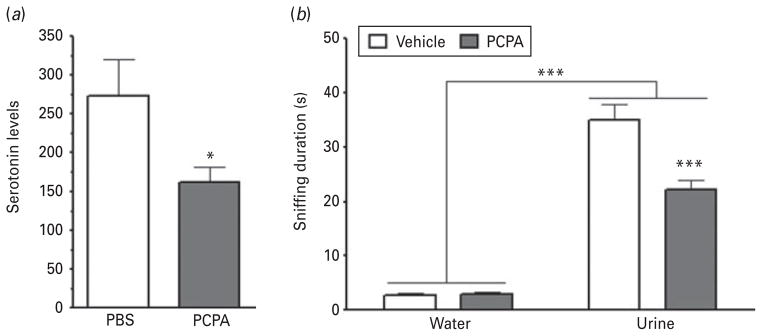
We first assessed whether a single injection of PCPA was sufficient to deplete serotonin levels in WKY rats and found that PCPA administration significantly reduced serotonin levels in hippocampal tissue compared to vehicle-treated control rats (Fig. 2a; t test : t16=2.220, p=0.0421; n=8 per group).
Fig. 2.
Serotonin depletion led to reward-seeking deficits in rats. (a) A single administration of PCPA was sufficient to significantly reduce serotonin levels in male Wistar Kyoto rats. Values are provided as arbitrary relative units. (b) In the female urine sniffing test, serotonin-depleted rats spent significantly less time sniffing urine, but not water, compared to control animals. Data are mean±S.E.M. (* p<0.05, *** p<0.001).
Next, we evaluated whether the FUST was sensitive to the effects of serotonin depletion in WKY rats (Fig. 2b). Consistent with our previous work, time spent sniffing was found to be significantly affected by odour (F1,36=277.9, p<0.0001; PCPA, n=20; vehicle, n=16). Treatment also significantly affected sniffing duration (F1,36=14.80, p=0.0005), and a significant interaction was noted between the two variables (F1,36=17.81, p=0.0002). Post-hoc comparisons indicated that PCPA significantly reduced time spent sniffing urine (t=5.502, p<0.001), but not water (t=0.06133, p>0.05).
Ketamine and D-serine rescued PCPA-induced sniffing deficits in the FUST
Given the antidepressant-like effects of D-serine in the FST, we next examined how D-serine affected reward-seeking behaviour in serotonin-depleted rats. Evidence from other behavioural measures suggested that PCPA disrupts response to certain classes of antidepressants (Page et al. 1999); therefore, we first evaluated the effects of the novel antidepressant ketamine in the FUST. Ketamine targets NMDAR-mediated signalling, increases synaptogenesis, and its therapeutic effects are probably mediated by signals downstream of glutamatergic neurotransmission (DiazGranados et al. 2010b; Li et al. 2010; Sanacora et al. 2008). Animals received an acute sub-anesthetic dose of ketamine (2.5 mg/kg), mirroring previous preclinical rodent studies from our laboratory (Maeng et al. 2008b).
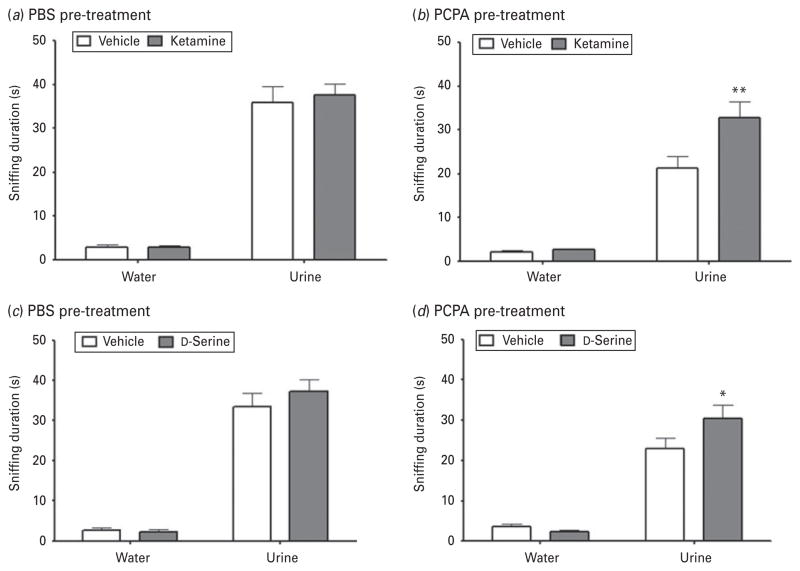
The baseline effects of ketamine on sniffing duration were measured in a group of rats that received the vehicle-control PBS (Fig. 3a). Sniffing time was significantly affected by odour (F1,17=225.1, p<0.0001; ketamine, n=8; vehicle, n=9) but not by treatment (F1,17=0.1272, p=0.7258) or by the interaction between the two variables (F1,17=0.1591, p=0.6949). However, in serotonin-depleted rats (Fig. 3b), both odour and treatment significantly affected time spent sniffing (odour: F1,19=120.9, p<0.0001; treatment: F1,19=6.522, p=0.0199; ketamine, n=10; vehicle, n=9), as did the interaction between the two variables (F1,19=5.913, p=0.0251). Post-hoc comparisons revealed that ketamine significantly increased time spent sniffing urine (t=3.471, p<0.01) but not water (t=0.1401, p>0.05) in serotonin-depleted rats.
Fig. 3.
Ketamine and D-serine both improved sexual reward-seeking deficits in serotonin-depleted rats. (a) Ketamine had no baseline effect on sniffing duration in male Wistar Kyoto rats administered PBS (vehicle control). (b) In serotonin-depleted rats, ketamine treatment significantly increased time spent sniffing urine, but not water. (c) D-Serine had no baseline effect on sniffing duration in control rats administered PBS. (d) In serotonin-depleted rats (by PCPA), D-serine significantly increased time spent sniffing urine, but not water. Data are mean±S.E.M. (* p<0.05, ** p<0.01).
Rats treated with D-serine had a similar sniffing duration profile to those treated with ketamine. In the group receiving PBS vehicle (Fig. 3c), time spent sniffing was significantly affected by odour (F1,9= 199.4, p<0.0001), but not by treatment (F1,9=0.5065, p=0.4947; n=6 per group) or by the interaction between the two variables (F1,9=0.7860, p=0.3984). In rats administered PCPA (Fig. 3d), sniffing duration was significantly affected by odour (F1,22=115.9, p<0.0001), but not by treatment (F1,22=2.161, p=0.1556; n=11 per group); there was also a trend towards significance in the interaction between the two variables (F1,22=4.040, p=0.0568). Post-hoc analysis found that in serotonin-depleted rats, D-serine significantly increased time spent sniffing urine (t=2.502, p<0.05) but not water (t=0.4220, p>0.05).
D-serine improved measures in the LH paradigm
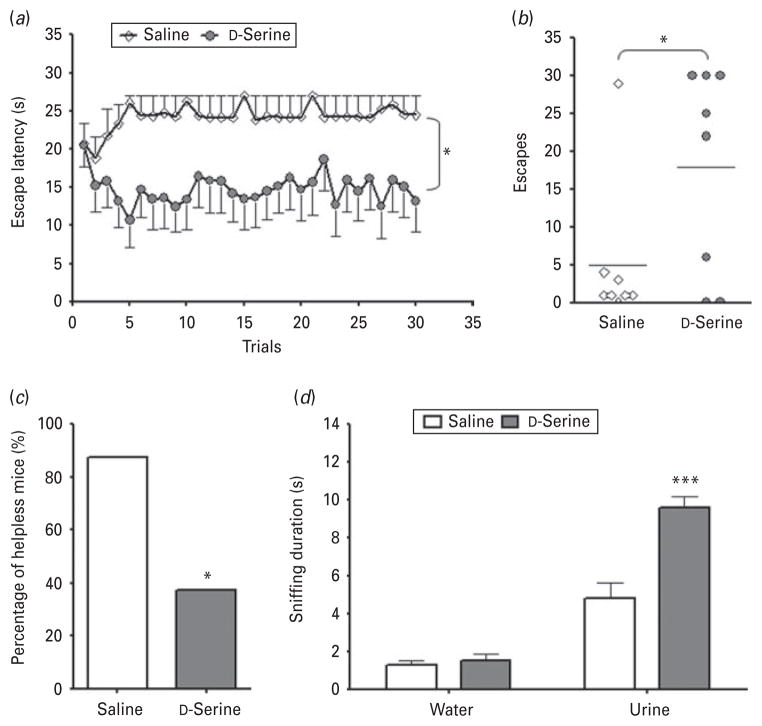
After evaluating the efficacy of D-serine in the FST and serotonin depletion model, we turned to another well-known and widely used model of antidepressant efficacy, the LH paradigm (Krishnan & Nestler, 2008). WT mice underwent a series of random, inescapable footshocks and were screened for LH behaviour the following day. Those mice that developed helplessness were randomly divided into two groups and treated with either D-serine or saline (vehicle) prior to the AAT. Escape latency (Fig. 4a) was significantly influenced by treatment (F1,406=5.812, p=0.0302; n=7 per group) but not by trial number (F29,406=0.57, p=0.9647) or by the interaction of the two variables (F29,406=1.35, p=0.1102). LH mice treated with D-serine also had a significantly greater number of escapes (Fig. 4b) than those treated with saline (t test : t14=2.177, p=0.0235). Upon screening for spontaneous recovery (Fig. 4c), it was found that D-serine significantly increased the percentage of mice that recovered from helplessness (χ2=4.267, p=0.0389).
Fig. 4.
D-Serine improved measures of helplessness and sexual reward-seeking deficits in wild-type (WT) mice that developed helplessness. (a) D-Serine treatment significantly reduced escape latencies in male WT mice that developed helplessness, (b) increased the number of escapes, and (c) increased the number of mice that recovered from helplessness. (d) D-Serine treatment significantly increased sniffing duration in learned helplessness (LH) mice vs. saline-treated LH mice. Data are mean±S.E.M. (* p<0.05, *** p<0.001).
Our previous work found that LH mice exhibited sniffing deficits in the FUST, and that such deficits could be rescued by treatment with the conventional antidepressant citalopram (Malkesman et al. 2010). We thus investigated whether D-serine altered time spent sniffing urine or water in WT mice that had developed LH (Fig. 4d). Sniffing duration was significantly affected by odour (F1,8=121.5, p<0.0001), treatment (F1,8=18.38, p=0.0027), and the interaction between the two variables (F1,8=18.39, p=0.0027; n=5 per group). Post-hoc analysis indicated that D-serine significantly increased time spent sniffing urine (t=5.798, p<0.001) but not water (t=0.2649, p>0.05) in LH mice.
NR1 ablation altered behaviour in the FUST and AAT
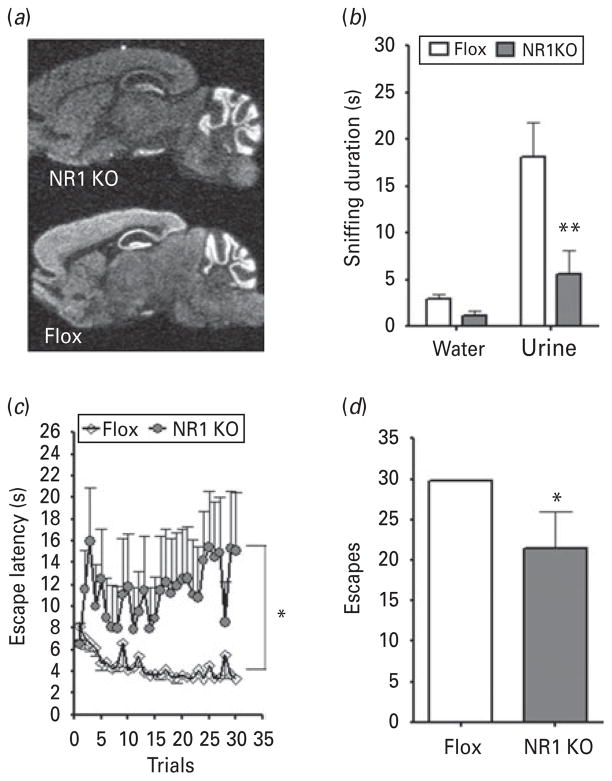
D-Serine is an endogenous agonist of the obligatory NR1 subunit of the NMDAR and has a high affinity to the glycine-binding site (Mothet et al. 2000). Because of D-serine’s antidepressant-like profile in the FST, FUST, and LH paradigm, we next investigated how selective ablation of the gene encoding the NR1 subunit would influence measures of depressive-like behaviours. Using the cre-lox system, we generated a strain of KO mice lacking expression of NR1 in excitatory neurons of the cortex, hippocampus, and other forebrain regions, including striatum, superior and inferior colliculi. Ablation was detected at age 4 months using an in-situ hybridization probe that binds specifically to NR1 gene transcripts, while gene expression persisted in control mice where sequences flanked by lox (Flox) remained (Fig. 5a).
Fig. 5.
Ablation of NR1 in forebrain excitatory neurons resulted in a depression-like phenotype. (a) By age 4 months, KO mice lacked expression of NR1 gene transcripts in the entire forebrain region. In comparison, control (Flox) littermates had persistent, normal NR1 expression throughout the forebrain. (b) In the female urine sniffing test, male NR1 KO mice exhibited a sexual reward-seeking deficit compared to control (Flox) littermates. (c) In the active avoidance test portion of the learned helplessness paradigm, male NR1 KO mice had significantly elevated escape latencies and (d) fewer escapes on average. Data are mean±S.E.M. (* p<0.05, ** p<0.01).
An initial phenotype assessment revealed that the body weight of NR1 KO mice did not differ from Flox control mice [t test : t15=1.705, p=0.1120; KO, n=7; Flox, n=8; Supplementary Fig. S1a (available online)], but they did have a hyperactive phenotype. In the OFT (Supplementary Fig. S1b, c), distance travelled was significantly affected by time (F5,65=4.190, p=0.0023), genotype (F1,65=11.06, p=0.0055), and the interaction between the two variables (F5,65=3.254, p=0.0110). NR1 KO mice were significantly more active than Flox control mice and did not habituate to the open-field arena (correlation : Flox, r=−0.9052, p=0.013; NR1 KO, r=−0.007574, p=0.9886). Time spent in the centre field of the arena (Supplementary Fig. S1d), often used as a measure of anxiety, was lower in NR1 KO mice, but the difference was not statistically significant (t test : t15=1.248, p=0.2342). In the FST (Supplementary Fig. S1e), the immobility duration of NR1 KO mice did not differ from that of Flox control mice (t test : t15=1.558, p=0.1402).
We next evaluated NR1 KO and Flox control mice in depression-related behavioural tests. In the FUST (Fig. 5b), sniffing duration was significantly influenced by odour (F1,13=719.2, p=0.0010), genotype (F1,52= 382.0, p=0.0097; KO, n=7; Flox, n=8), and the interaction between the two variables (F1,52=222.8, p=0.0349). Post-hoc analysis revealed that NR1 KO mice spent significantly less time than Flox control mice sniffing urine (t=3.778, p<0.01) but not water (t=0.506, p>0.05).
NR1 KO mice were also compared with Flox controls in the AAT portion of the LH paradigm. Escape latency (Fig. 5c) and number of escapes (Fig. 5d) were analysed in accordance with our previous analysis of WT mice. Trial number did not significantly influence escape latency (F29,435=1.265, p=0.1647; KO, n=6; Flox, n=11), but genotype (F1,435=7.255, p=0.0167) and the interaction between the two variables (F29,435= 2.068, p=0.0011) did significantly affect latency. NR1 KO mice had significantly fewer escapes than Flox controls (t test : t15=2.543, p=0.0225).
D-Serine had no antidepressant-like effects in conditional NR1 KO mice
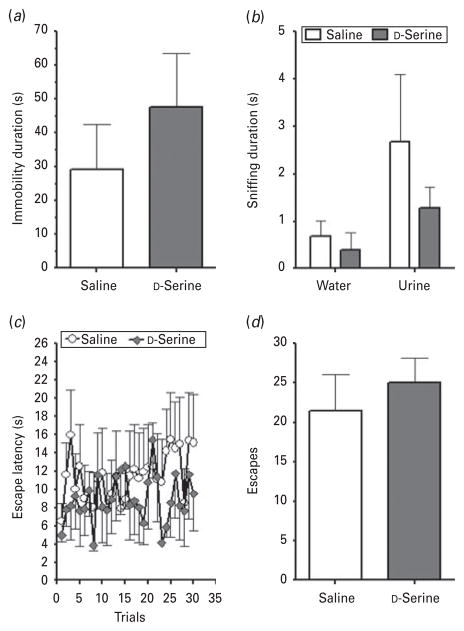
After characterizing the depression-like phenotype of the conditional NR1 KO mice, we lastly investigated whether these mice would be sensitive to D-serine treatment. We examined immobility duration in the FST (Fig. 6a), sniffing duration in the FUST (Fig. 6b), and escape latency (Fig. 6c) and number of escapes (Fig. 6d) in the AAT portion of the LH paradigm. In contrast to the results seen in WT mice, D-serine had no significant influence on immobility in the FST (t test : t10=0.8822, p=0.3984; n=5 per group). In the FUST, sniffing duration was not significantly altered by treatment (two-way repeated-measures ANOVA: F1,9=1.141, p=0.3133). In the AAT, D-serine had no significant effect on escape latency (F1,290= 0.2973, p=0.5975) or on the number of escapes in NR1 KO mice (t test : t10=0.6308, p=0.5423).
Fig. 6.
NR1 KO mice did not respond to D-serine treatment. (a) In the forced swim test, NR1 KO mice treated with D-serine had no significant difference in immobility duration vs. animals receiving saline (vehicle) treatment. (b) In the female urine sniffing test, D-serine had no impact on sniffing duration in male NR1 KO mice. (c) Animals treated with D-serine had no significant difference in escape latency, or (d) number of escapes in the active avoidance test portion of the learned helplessness paradigm. Data are mean±S.E.M.
Discussion
The present study used three distinct behavioural paradigms relevant to depression to evaluate the preclinical antidepressant-like profile of the gliotransmitter and glutamatergic NR1 subunit agonist D-serine. Acute treatment with D-serine was found to decrease immobility in the FST, increase the time that serotonin-depleted rodents spent sniffing female urine in the FUST, and improve escape measures in the LH paradigm. We then examined the behaviour of mice with forebrain-specific ablation of the NR1 subunit in excitatory neurons and found that these KO mice exhibited a depression-like phenotype in the FUST and AAT behavioural measures. Finally, we found that D-serine treatment did not rescue the deficits of NR1 KO mice in these depression-related behavioural tests. Taken together, the results of this series of experiments offer consistent evidence that D-serine – and the NR1 subunit of NMDARs – deserve further investigation as a putative pharmacological strategy for treating depression.
One of the major limitations of conventional antidepressants is that chronic treatment is needed before therapeutic benefits are seen. However, recent clinical studies have demonstrated that a single intravenous administration of ketamine (Ibrahim et al. 2011; Zarate et al. 2006), as well as some NR2B antagonists (Preskorn et al. 2008), exert rapid antidepressant effects. Preclinical studies have noted similar antidepressant effects associated with a single injection of ketamine or a NR2B antagonist (Li et al. 2010, 2011; Maeng et al. 2008b). Accordingly, and in keeping with standard practice for the initial assessment of a compound’s antidepressant effects, the current study used a single, acute injection of D-serine to assess its antidepressant-like effects in a series of preclinical tests. Previous studies demonstrated that a single i.p. injection of D-serine is sufficient to elicit a rapid (within 30 min), transient increase in D-serine levels in the prefrontal cortex (Fukushima et al. 2004), lead to a significant haemodynamic response in the hippocampus (Panizzutti et al. 2005), and trigger significant changes in gene expression in brain tissues (Davidson et al. 2009). Subcutaneous injection of D-serine also increases cortical levels of the compound, although the effect is more delayed (Smith et al. 2009).
We first used two well-established preclinical behavioural models – the FST and the LH paradigm – to examine the putative antidepressant-like effects of D-serine. To further refine our investigation, we also used a more recently developed paradigm, the FUST, to measure sniffing duration in monoamine-depleted animals; this newly developed paradigm has predictive and face validity for measuring depressive-like symptoms in rodents (Hunsberger et al. 2011; Malkesman et al. 2009, 2010). Unlike the FST and LH paradigms, which focus on despair-related measures (DiazGranados et al. 2010b; Krishnan & Nestler, 2008; Maier, 1984), the FUST measures sexual reward-seeking behaviour, which is distinct and relevant to anhedonia, a hallmark symptom of depression. The first portion of our experiment assessed the injection of PCPA and whether the FUST was sensitive to serotonin depletion, which is known to affect rodent response to certain antidepressants (O’Leary et al. 2007; Page et al. 1999) in the FST and TST. We confirmed, biochemically and behaviourally, that PCPA delivery altered serotonin levels in the hippocampus, one of many brain regions involved in reward-seeking behaviours (Malkesman et al. 2010), and one that is known to contain serotonin-sensitive downstream signalling cascades (Krishnan & Nestler, 2008). Administration of PCPA significantly reduced serotonin levels in the hippocampus and markedly reduced the time that rodents spent sniffing urine, but did not affect time spent sniffing water. Time spent sniffing urine remained significantly greater than time spent sniffing water in rodents that received PCPA, which suggests that olfactory function is unimpaired in serotonin-depleted animals. These findings suggest that serotonin depletion causes acute sexual reward-seeking deficits.
We next used the FUST to examine the antidepressant-like effects of D-serine on serotonin-depleted animals, which has not been previously investigated. Because monoamine depletion has a mixed response to conventional antidepressants in behavioural tests like the FST and TST (Cryan et al. 2004; O’Leary et al. 2007; Page et al. 1999), we used ketamine, a novel, rapid-acting compound with antidepressant properties that does not rely on traditional monoaminergic neurotransmission. Ketamine, which was found to have antidepressant-like effects in other depression-related behavioural measures such as the FST and LH paradigm (Maeng et al. 2008b), alters glutamate release. In the present study, both ketamine and D-serine significantly increased the time that serotonin-depleted rats spent sniffing urine, but not water, in the FUST. Baseline measures were not affected, and neither ketamine nor D-serine significantly affected time spent sniffing water or urine in rats with normal serotonin levels. Our findings in the FUST, in combination with the results from the FST and LH paradigm, support D-serine’s potential as an endogenously expressed antidepressant compound.
Finally, we explored the mechanisms underlying D-serine’s therapeutic profile, and found that D-serine is mediated through the NR1 subunit of NMDARs on excitatory neurons. D-serine’s role as a gliotransmitter, neurotransmitter, and allosteric modulator of NMDARs suggests that it is an important regulator of excitatory-inhibitory balance (Billard, 2008). D-Serine is a co-agonist of glutamate that binds with high affinity to the glycine site of the obligatory NR1 subunit of NMDARs. NR1 is required for the channel to function properly and regulate ion movement through the receptor complex (Nakanishi, 1992). Forebrain-specific ablation of NR1 expression in excitatory neurons led to a depression-like phenotype in mice. Compared with Flox control animals, NR1 KO mice exhibited reduced sniffing of female urine in the FUST, increased escape latencies, and reduced number of escapes in the LH paradigm. In the present series of experiments, genotype had no significant effect on the FST, but this finding is confounded by the hyperactivity observed in NR1 KO mice in the OFT; significant changes in motor activity can influence immobility performance in the FST. We find it unlikely that NR1 KO hyperactivity would significantly affect less motor-sensitive paradigms like the LH and FUST, although we cannot eliminate the possibility entirely. In the FUST, for example, no significant difference was noted between NR1 KO and control mice in time spent sniffing water; only time spent sniffing female urine was affected. It is also important to note that treatment of NR1 KO mice with D-serine did not significantly affect behavioural measures in the FST, FUST, or AAT portion of the LH paradigm. These findings strongly support the hypothesis that D-serine’s antidepressant-like effects are mediated via the NR1 subunit of NMDARs on excitatory neurons, and that disruptions in NMDAR-mediated signalling probably contribute to depressive-like behaviours.
Our overall results from this series of experiments, together with recent clinical findings in schizophrenia and post-traumatic stress disorder (PTSD) (Heresco-Levy et al. 2009; Tsai & Lin, 2010), suggest that D-serine has antidepressant-like properties in rodents as well as in humans. Nevertheless, these clinical studies also noted that while doses of D-serine ranging from 30 to 120 mg/kg.d were well tolerated in patients, adverse kidney side-effects were also reported (Heresco-Levy et al. 2005; Kantrowitz et al. 2010). Our current data from NR1 KO mice also support the hypothesis that these antidepressant-like effects occur through the NR1 subunit of NMDARs. Additional studies with D-serine-like agents capable of penetrating the blood–brain barrier (BBB) are needed in order to further explore the therapeutic potential of NR1 glycine-site agonists for the treatment of depressive symptoms.
We found that an NMDAR co-agonist as well as an NMDA antagonist produced similar antidepressant-like effects in mice, despite their opposing effects on NMDARs. Clinical findings support modulation of NMDA-dependent signalling as a possible new approach to treating depression. A recent preclinical Phase-I trial of GLYX-13, a glycine-site NMDAR partial agonist, found that this agent had antidepressant-like effects in rodent paradigms of depression (http://www.naurex.com/media/Naurex_ACNP_release_120310_FINAL-2.pdf). Clinical studies have noted that ketamine treatment significantly improved suicidal ideation (DiazGranados et al. 2010b ; Larkin & Beautrais, 2011; Price et al. 2009). Reconciling the similar effects of compounds with opposing roles on NMDARs will require further investigation of the convergent downstream effects of these putative treatments.
Recent studies have also significantly expanded our understanding of the downstream targets of NMDAR antagonists. Interestingly, these compounds promote synaptogenesis and enhance prominent synaptic plasticity cascades, which can also be activated by NMDAR stimulation. Ketamine’s transient blockade of NMDARs activates brain-derived neurotrophic factor (BDNF) by rapidly deactivating eukaryotic elongation factor 2 (eEF2) kinase (Autry et al. 2011) and induces synaptogenesis by indirectly activating the extracellular signal-regulated kinase (ERK), phosphoinositide-3 kinase-AKT (PI3K/AKT), and mammalian target of rapamycin (mTOR) pathways (Li et al. 2010). Physiological NMDAR activation requires glutamate and the co-agonists glycine or D-serine, and can activate the ERK pathway (Lenz & Avruch, 2005; Sweatt, 2004). ERK pathway deficits have also been discovered in the brains of individuals who committed suicide (Dwivedi et al. 2001, 2006, 2009) and those suffering from certain mood disorders (Yuan et al. 2010). These findings raise the possibility that ketamine and D-serine activate common, convergent downstream targets, thereby producing similar effects on protein expression and promoting comparable changes in synaptic plasticity and dendritic remodelling. Such a result may explain how both NMDA agonists and antagonists elicit a therapeutic response in rodent and clinical studies. Overall, our work supports the hypothesis that modulation of glutamatergic signalling is a promising avenue for the development of novel, rapidly acting antidepressants.
Supplementary Material
Acknowledgments
Ioline Henter (NIMH) provided invaluable editorial assistance in the preparation of the manuscript.
Footnotes
Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org/pnp).
Statement of Interest
All authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRPNIMH-NIH). H.K.M. and G.C. are currently full-time employees of Johnson and Johnson Pharmaceutical Research and Development (USA); all of this work was initiated and most was conducted while they were employees of the NIMH. A patent application for the use of ketamine in depression has been submitted listing Dr Manji and Dr Zarate among the inventors; they have assigned their rights on the patent to the U.S. Government.
References
- Autry AE, Adachi M, Nosyreva E, Na ES, et al. NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biological Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. New England Journal of Medicine. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Billard JM. D-serine signalling as a prominent determinant of neuronal-glial dialogue in the healthy and diseased brain. Journal of Cellular and Molecular Medicine. 2008;12:1872–1884. doi: 10.1111/j.1582-4934.2008.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, et al. Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Cohen JW, Louneva N, Han LY, Hodes G, et al. Chronic corticosterone exposure alters postsynaptic protein levels of PSD-95, NR1, and synaptopodin in the mouse brain. Synapse. 2011;65:763–770. doi: 10.1002/syn.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, et al. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Archives of General Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Leary OF, Jin SH, Friedland JC, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proceedings of the National Academy of Sciences USA. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, Yin HH, Lovinger DM, et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proceedings of the National Academy of Sciences USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson ME, Kerepesi LA, Soto A, Chan VT. D-Serine exposure resulted in gene expression changes implicated in neurodegenerative disorders and neuronal dysfunction in male fischer 344 rats. Archives of Toxicology. 2009;83:747–762. doi: 10.1007/s00204-009-0405-3. [DOI] [PubMed] [Google Scholar]
- De La Garza R, II, Mahoney JJ., III A distinct neurochemical profile in WKY rats at baseline and in response to acute stress : implications for animal models of anxiety and depression. Brain Research. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archives of General Psychiatry. 2010a;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. Journal of Clinical Psychiatry. 2010b;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Neuronal damage and protection in the pathophysiology and treatment of psychiatric illness : stress and depression. Dialogues in Clinical Neuroscience. 2009;11:239–255. doi: 10.31887/DCNS.2009.11.3/rsduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Pandey GN. ERK MAP kinase signaling in post-mortem brain of suicide subjects : differential regulation of upstream raf kinases raf-1 and b-Raf. Molecular Psychiatry. 2006;11:86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, et al. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. Journal of Neurochemistry. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, et al. Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain : role of ERK kinase 1 (MEK1) International Journal of Neuropsychopharmacology. 2009;12:1337–1354. doi: 10.1017/S1461145709990575. [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. Journal of Neuroscience. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Kawai J, Imai K, Toyo’oka T. Simultaneous determination of D- and L-serine in rat brain microdialysis sample using a column-switching HPLC with fluorimetric detection. Biomedical Chromatography. 2004;18:813–819. doi: 10.1002/bmc.394. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nature Reviews Neuroscience. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ebstein R, Vass A, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biological Psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Vass A, Bloch B, Wolosker H, et al. Pilot controlled trial of D-serine for the treatment of post-traumatic stress disorder. International Journal of Neuropsychopharmacology. 2009;12:1275–1282. doi: 10.1017/S1461145709000339. [DOI] [PubMed] [Google Scholar]
- Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiology and Behavior. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Jia F, Liu Y, Li L. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide y levels in severe major depressive disorder. Brain Research. 2006;1095:154–158. doi: 10.1016/j.brainres.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Hunsberger J, Austin DR, Henter ID, Chen G. The neurotrophic and neuroprotective effects of psychotropic agents. Dialogues in Clinical Neuroscience. 2009;11:333–348. doi: 10.31887/DCNS.2009.11.3/jhunsberger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger JG, Machado-Vieira R, Austin DR, Zarate C, et al. Bax inhibitor 1, a modulator of calcium homeostasis, confers affective resilience. Brain Research. 2011;1403:19–27. doi: 10.1016/j.brainres.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, et al. Rapid decrease in depressive symptoms with an N-methyl-D-aspartate antagonist in ECT-resistant major depression. Progress in Neuropsychopharmacology and Biological Psychiatry. 2011;35:1155–1159. doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Belforte JE, Lu Y, Yabe Y, et al. eIF2alpha phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. Journal of Neuroscience. 2010;30:2582–2594. doi: 10.1523/JNEUROSCI.3971-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, et al. High dose D-serine in the treatment of schizophrenia. Schizophrenia Research. 2010;121:125–130. doi: 10.1016/j.schres.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E, et al. Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. Journal of Biological Chemistry. 2006;281:14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G, Johansson M, Agren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness : evidence in support of the catecholamine hypothesis of mood disorders. Archives of General Psychiatry. 2000;57:787–793. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. International Journal of Neuropsychopharmacology. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. Journal of Biological Chemistry. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Hunsberger JG, Pearson B, Yuan P, et al. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proceedings of the National Academy of Sciences USA. 2008a;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biological Psychiatry. 2008b;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Maier SF. Learned helplessness and animal models of depression. Progress in Neuropsychopharmacology and Biological Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- Malkesman O, Austin DR, Chen G, Manji HK. Reverse translational strategies for developing animal models of bipolar disorder. Disease Models and Mechanisms. 2009;2:238–245. doi: 10.1242/dmm.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Austin DR, Tragon T, Henter ID, et al. Targeting the BH3-interacting domain death agonist to develop mechanistically unique antidepressants. Molecular Psychiatry. 2011 doi: 10.1038/mp.2011.77. Published online: 5 July 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, et al. The female urine sniffing test : a novel approach for assessing reward-seeking behavior in rodents. Biological Psychiatry. 2010;67:864–871. doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nature Medicine. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Psych MR, Sweeney JA, et al. Attempted suicide characteristics and cerebrospinal fluid amine metabolites in depressed inpatients. Neuropsychopharmacology. 1996;15:576–586. doi: 10.1016/S0893-133X(96)00102-9. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, et al. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Research. 2007;1127:108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nudmamud-Thanoi S, Reynolds GP. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neuroscience Letters. 2004;372:173–177. doi: 10.1016/j.neulet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- O’Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, et al. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berlin) 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, et al. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berlin) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Panizzutti R, Rausch M, Zurbrugg S, Baumann D, et al. The pharmacological stimulation of NMDA receptors via co-agonist site : an fMRI study in the rat brain. Neuroscience Letters. 2005;380:111–115. doi: 10.1016/j.neulet.2005.01.062. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. Journal of Clinical Psychopharmacology. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biological Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D, Kartvelishvily E, Shleper M, Klinker CM, et al. Neuronal release of D-serine : a physiological pathway controlling extracellular D-serine concentration. FASEB Journal. 2010;24:2951–2961. doi: 10.1096/fj.09-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Molecular Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature Reviews Drug Discovery. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel G, Maeng S, Malkesman O, Pearson B, et al. Evidence for the involvement of the kainate receptor subunit gluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Molecular Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsin B, Friedman E, Gershon S. Parachlorophenylalanine reversal of tranylcypromine effects in depressed patients. Archives of General Psychiatry. 1976;33:811–819. doi: 10.1001/archpsyc.1976.01770070041003. [DOI] [PubMed] [Google Scholar]
- Smith SM, Uslaner JM, Yao L, Mullins CM, et al. The behavioral and neurochemical effects of a novel d-amino acid oxidase inhibitor compound 8 [4H-thieno [3,2-b]pyrrole-5-carboxylic acid] and D-serine. Journal of Pharmacology and Experimental Therapeutics. 2009;328:921–930. doi: 10.1124/jpet.108.147884. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Current Opinion in Neurobiology. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Current Pharmaceutical Design. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Nakajima K, Takayasu N, Noda S, et al. Expression of the mRNA and protein of serine racemase in primary cultures of rat neurons. European Journal of Pharmacology. 2006;548:74–76. doi: 10.1016/j.ejphar.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Takayasu N, Hashimoto A, Sato Y, et al. The serine racemase mRNA is predominantly expressed in rat brain neurons. Archives of Histology and Cytology. 2007;70:127–134. doi: 10.1679/aohc.70.127. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Mezey E. Hybridization histochemistry of neural transcripts. Current Protocols in Neuroscience. 2004;Chapter 1(Unit 1.3) doi: 10.1002/0471142301.ns0103s25. [DOI] [PubMed] [Google Scholar]
- Yuan P, Zhou R, Wang Y, Li X, et al. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. Journal of Affective Disorders. 2010;124:164–169. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zisook S, Ganadjian K, Moutier C, Prather R, et al. Sequenced treatment alternatives to relieve depression (STAR*D): lessons learned. Journal of Clinical Psychiatry. 2008;69:1184–1185. doi: 10.4088/jcp.v69n0719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.