Abstract
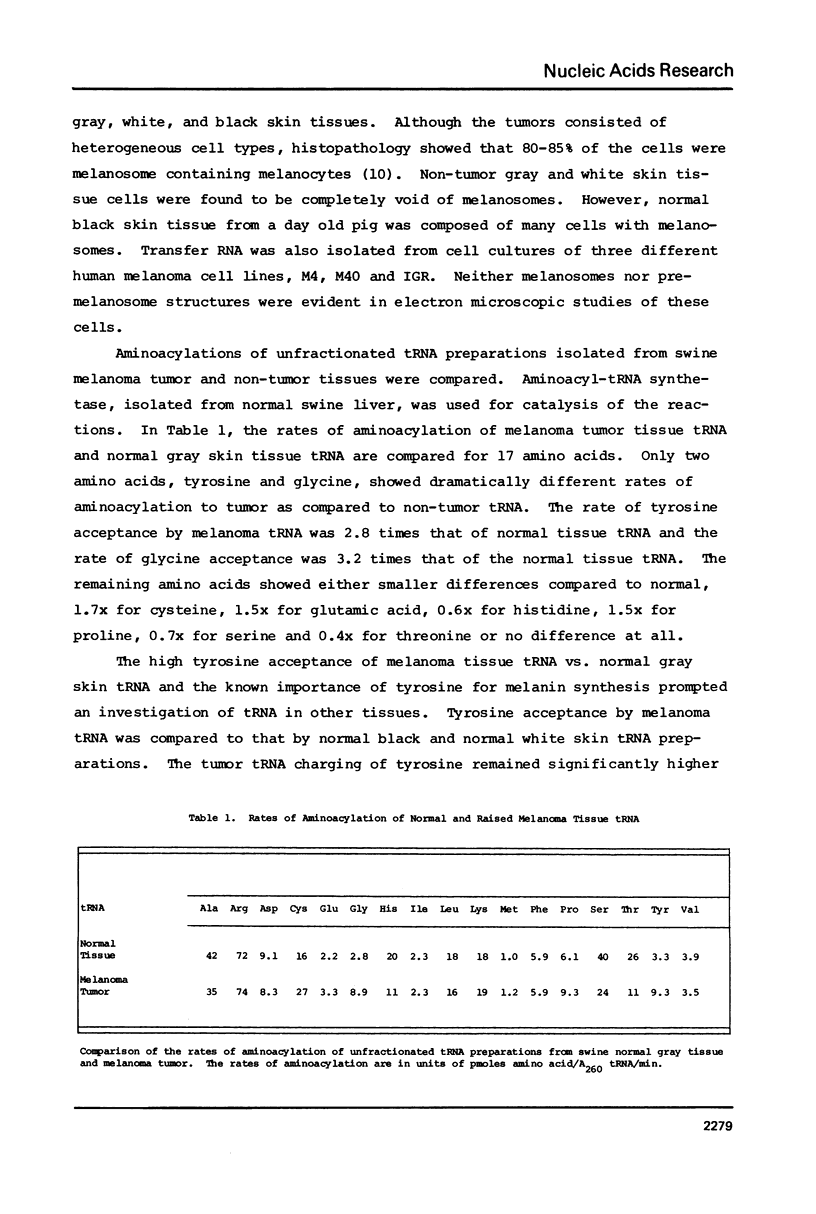
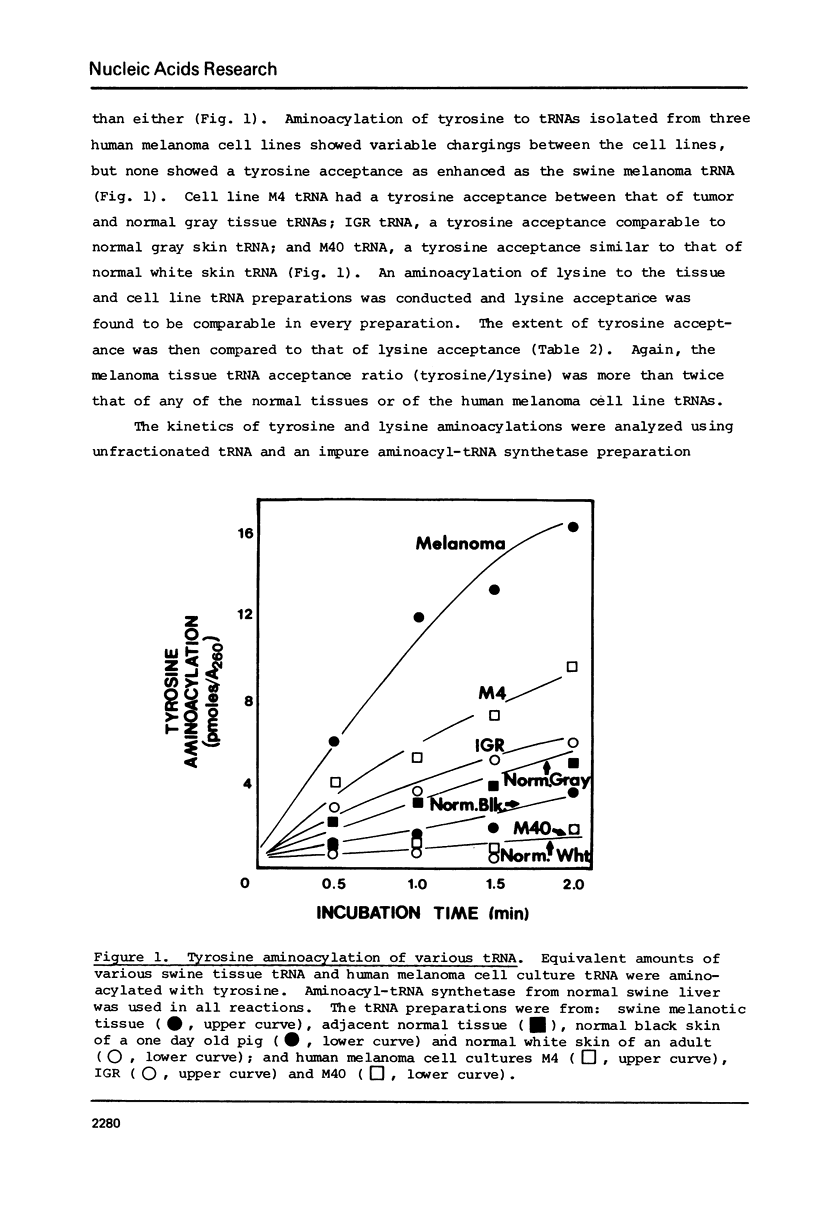
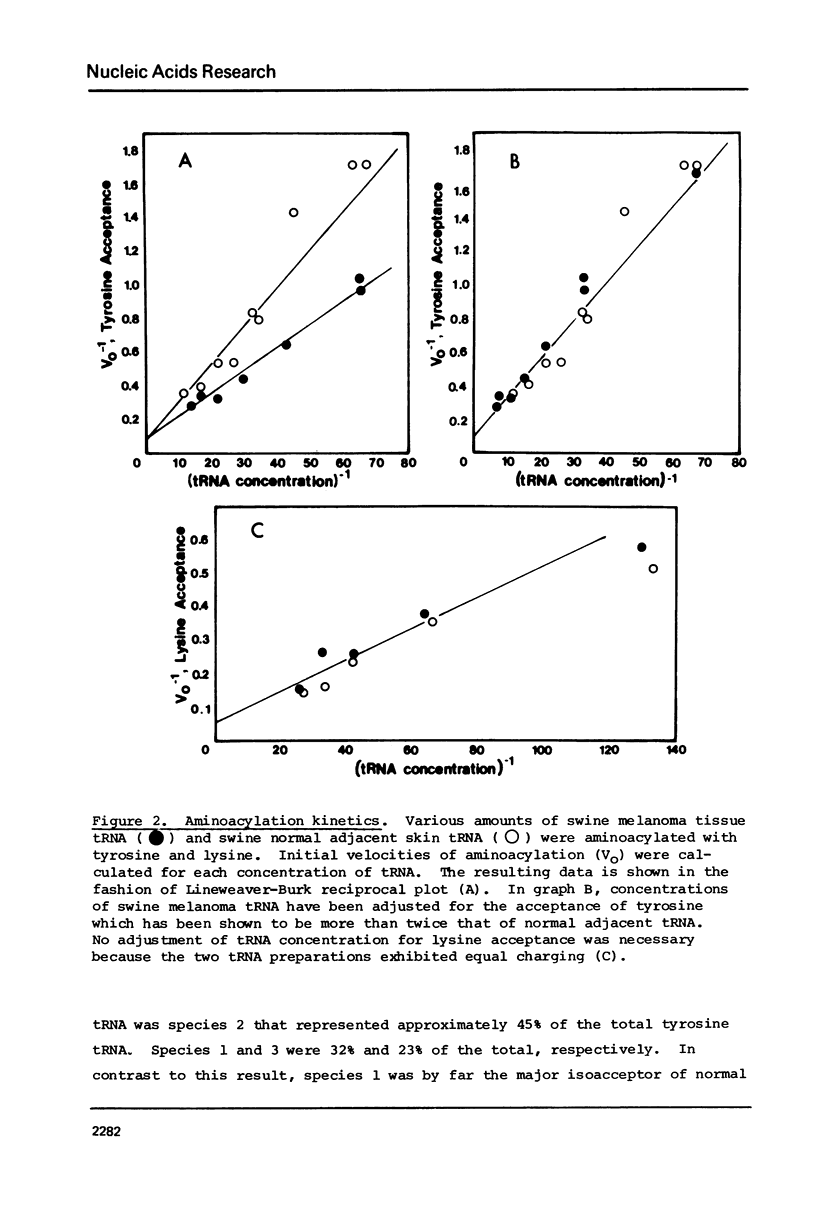
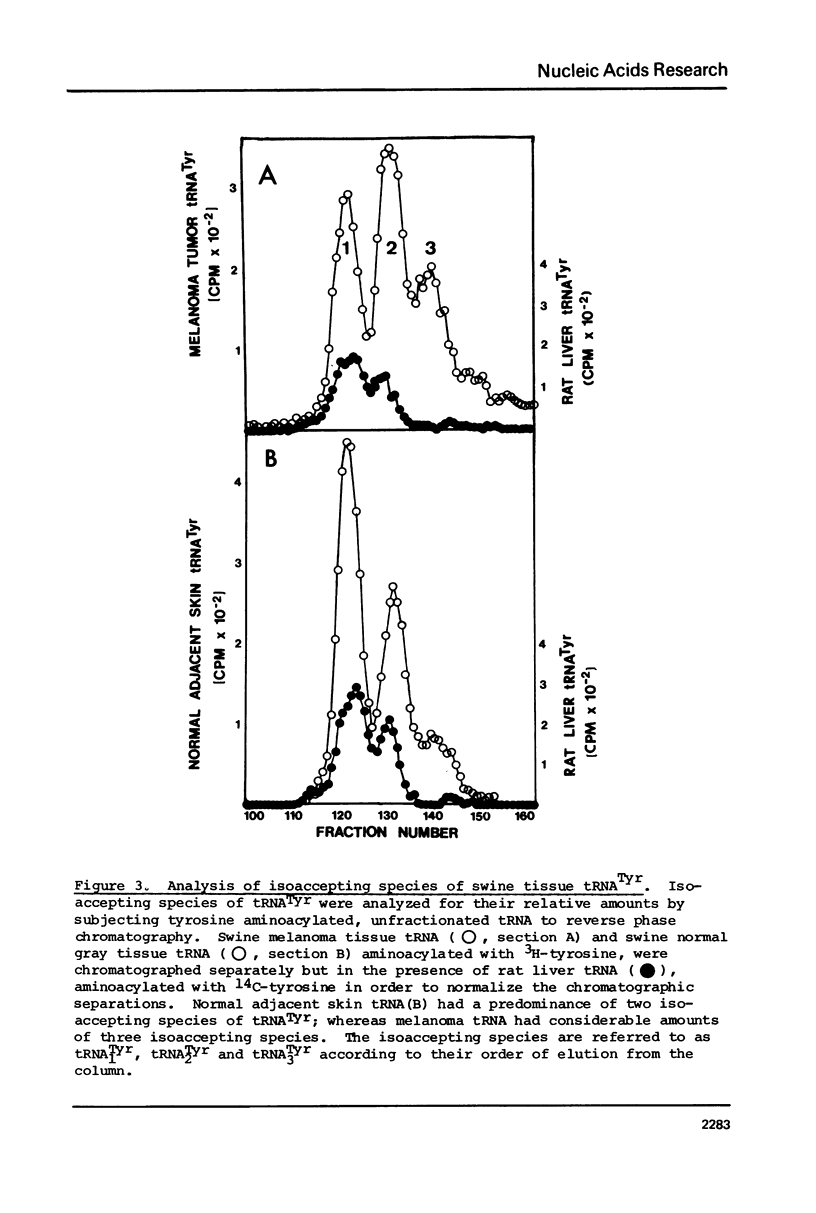
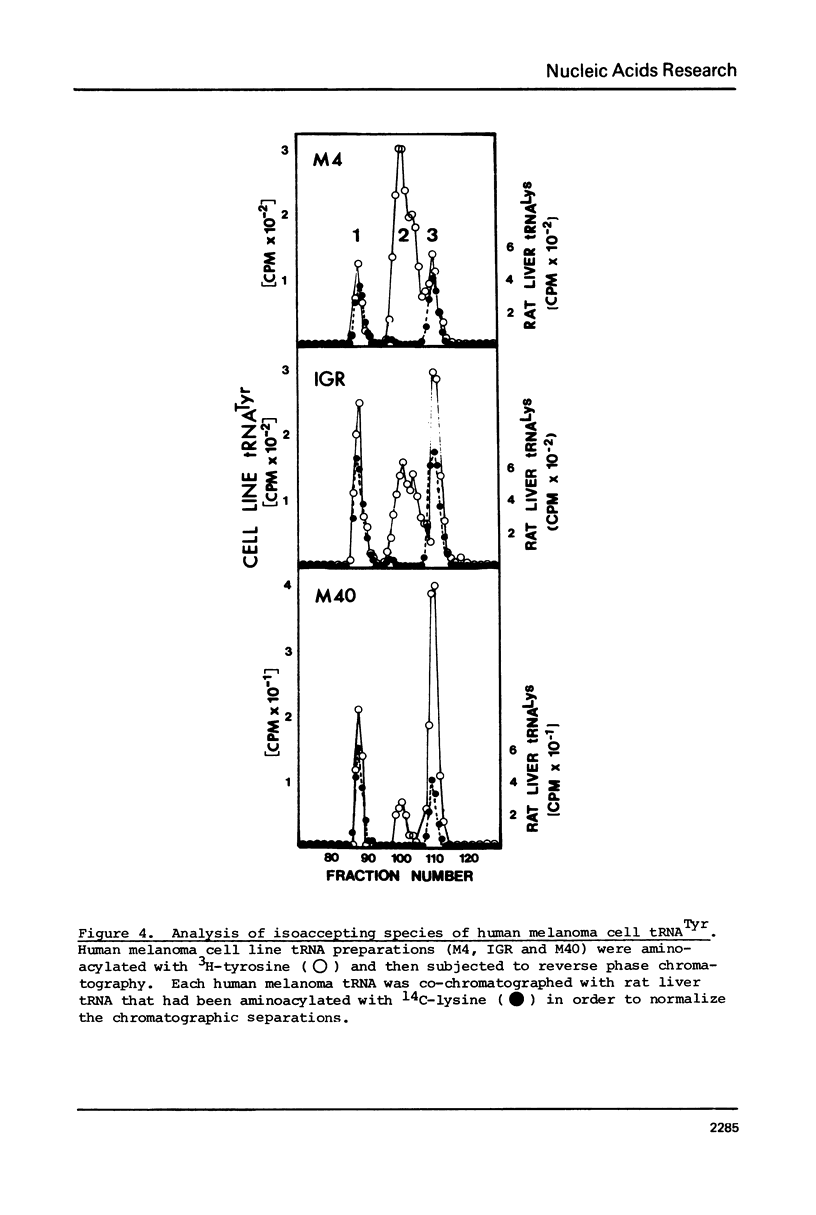
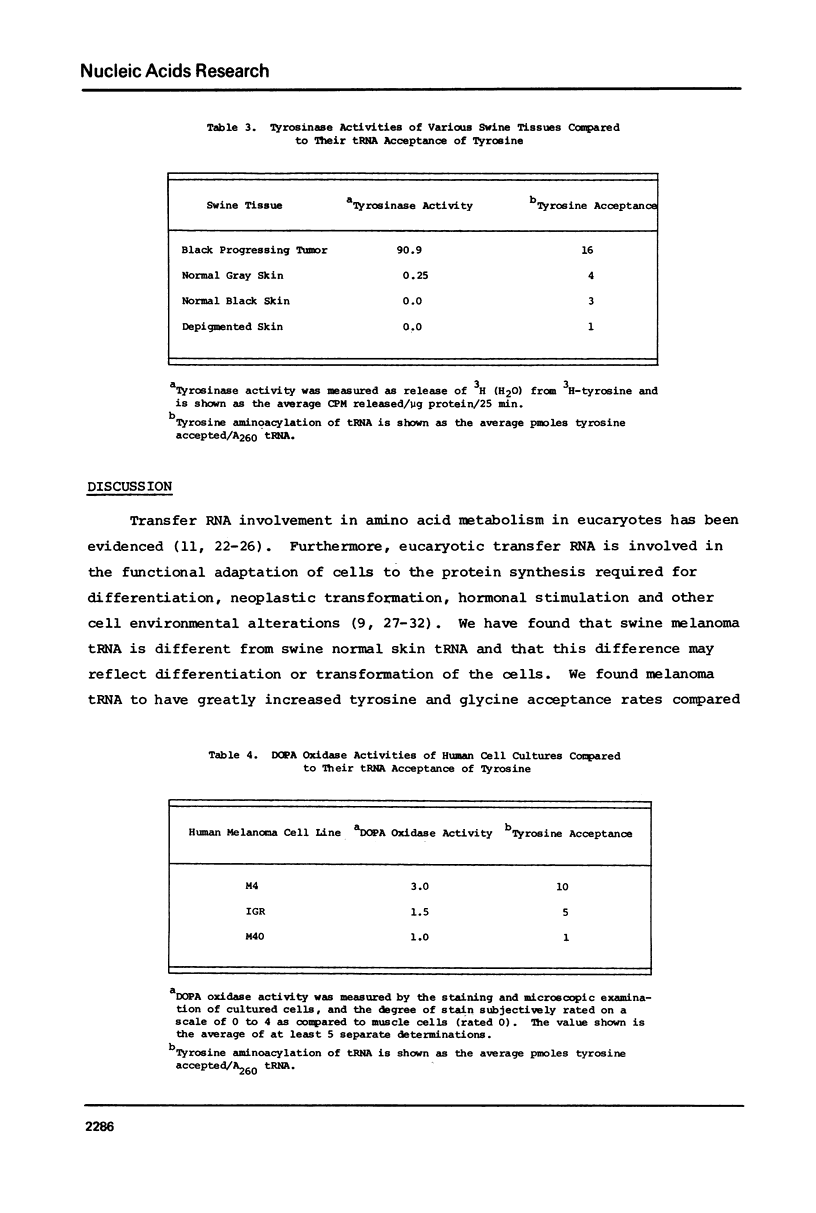
The tRNA present in swine melanoma tumor tissue and normal gray skin tissue were compared by aminoacylation of the unfractionated tRNA preparations. Of the seventeen amino acids studied, seven showed differences in rate of acceptance to tRNAs from normal and tumor tissues; the tRNAs of two amino acids, tyrosine and glycine, showed dramatic three fold increases in melanoma tumor. As melanin biosynthesis proceeds from tyrosine oxidation the investigations focused on the increase in tyrosine tRNA. Kinetic analysis of tyrosine aminoacylation to normal and melanoma tRNAs revealed no differences. Analysis of the isoaccepting species of tRNATyr from normal skin and melanoma tumor tissues identified three isoacceptors; tRNATyr, represented the predominant species in normal gray skin, while tRNA2Tyr predominated in melanoma tumor tissue. The tyrosine acceptances by tRNAs from three human melanoma cell lines were analyzed and found to be variable, but isoaccepting species analysis of the tRNATyr of these three cell lines still showed a correlation between the preponderance of tRNA2Tyr and extent of tyrosine acceptance. Additionally the enzymatic activity for the oxidation of tyrosine was found to be related to tyrosine acceptance and tRNA2Tyr predominance..
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F. Alterations of transfer RNA during erythroid differentiation of murine virus-induced leukemia cells. Arch Biochem Biophys. 1975 Sep;170(1):114–123. doi: 10.1016/0003-9861(75)90102-2. [DOI] [PubMed] [Google Scholar]
- Agris P. F. Nucleotide composition analysis of tRNA from leukemia patient cell samples and human cell lines. Nucleic Acids Res. 1975 Jul;2(7):1083–1091. doi: 10.1093/nar/2.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfin S. M., Simpson D. R., Chiang C. S., Andrulis I. L., Hatfield G. W. A role for asparaginyl-tRNA in the regulation of asparagine synthetase in a mammalian cell line. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2367–2369. doi: 10.1073/pnas.74.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe W. T., Syrewicz J. J., Marshall M. V., Griffin A. C. Regulation of an aspartyl-tRNA species in BHK cells in culture and in solid tumor form. Biochim Biophys Acta. 1975 Apr 2;383(4):441–445. doi: 10.1016/0005-2787(75)90314-7. [DOI] [PubMed] [Google Scholar]
- Burnett J. B., Seiler H. Multiple forms of tyrosinase from human melanoma. J Invest Dermatol. 1969 Feb;52(2):199–203. [PubMed] [Google Scholar]
- Calhoun D. H. Autoregulation of gene expression. Annu Rev Microbiol. 1975;29:275–299. doi: 10.1146/annurev.mi.29.100175.001423. [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Singh D. V. Tyrosinase activity and isozymes in B-16 mouse melanomas. Oncology. 1974;30(5):386–392. doi: 10.1159/000224979. [DOI] [PubMed] [Google Scholar]
- Garel J. P. Functional adaptation of tRNA population. J Theor Biol. 1974 Jan;43(1):211–225. doi: 10.1016/s0022-5193(74)80054-8. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B. Mechanism of suppression in Drosophila. VII. Correlation between disappearance of an isoacceptor of tyrosine tRNA and activation of the vermilion locus. Nucleic Acids Res. 1978 Jul;5(7):2391–2404. doi: 10.1093/nar/5.7.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. B. Role of an isoacceptor transfer ribonucleic acid as an enzyme inhibitor: effect on tryptophan pyrrolase of Drosophila. Nat New Biol. 1971 May 5;231(18):17–19. [PubMed] [Google Scholar]
- Körner A., Pawelek J. Activation of melanoma tyrosinase by a cyclic AMP-dependent protein kinase in a cell-free system. Nature. 1977 Jun 2;267(5610):444–447. doi: 10.1038/267444a0. [DOI] [PubMed] [Google Scholar]
- LERNER A. B., FITZPATRICK T. B. Biochemistry of melanin formation. Physiol Rev. 1950 Jan;30(1):91–126. doi: 10.1152/physrev.1950.30.1.91. [DOI] [PubMed] [Google Scholar]
- Messenguy F., Delforge J. Role of transfer ribonucleic acids in the regulation of several biosyntheses in Saccharomyces cerevisiae. Eur J Biochem. 1976 Aug 16;67(2):335–339. doi: 10.1111/j.1432-1033.1976.tb10696.x. [DOI] [PubMed] [Google Scholar]
- Meza L., Araya A., Leon G., Krauskopf M. Specific alanine-tRNA species associated with fibroin biosynthesis in the posterior sild-gland of Bombyx mori L. FEBS Lett. 1977 May 15;77(2):255–260. doi: 10.1016/0014-5793(77)80246-9. [DOI] [PubMed] [Google Scholar]
- Millikan L. E., Hook R. R., Manning P. J. Immunobiology of melanoma. Gross and ultrastructural studies in a new melanoma model: the Sinclair swine. Yale J Biol Med. 1973 Dec;46(5):631–645. [PMC free article] [PubMed] [Google Scholar]
- Mischke D., Kloetzel P., Schwochau M. Tryptophan pyrrolase activity regulation in Drosophila: role of an isoacceptor tRNA unsettled. Nature. 1975 May 1;255(5503):79–80. doi: 10.1038/255079a0. [DOI] [PubMed] [Google Scholar]
- Ortwerth B. J., Liu L. P. Correlation between a specific isoaccepting lysyl transfer ribonucleic acid and cell division in mammalian tissues. Biochemistry. 1973 Sep 25;12(20):3978–3984. doi: 10.1021/bi00744a030. [DOI] [PubMed] [Google Scholar]
- Ortwerth B. J., Yonuschot G. R., Carlson J. V. Properties of tRNALys4 from various tissues. Biochemistry. 1973 Sep 25;12(20):3985–3991. doi: 10.1021/bi00744a031. [DOI] [PubMed] [Google Scholar]
- Randerath E., Chia L. L., Morris H. P., Randerath K. Transfer RNA base composition studies in Morris hepatomas and rat liver. Cancer Res. 1974 Mar;34(3):643–653. [PubMed] [Google Scholar]
- Rodriguez H. A., Mcgavran M. H. A modified DOPA reaction for the diagnosis and investigation of pigment cells. Am J Clin Pathol. 1969 Aug;52(2):219–227. doi: 10.1093/ajcp/52.2.219. [DOI] [PubMed] [Google Scholar]
- SHEPPARD D. E. MUTANTS OF SALMONELLA TYPHIMURIUM RESISTANT TO FEEDBACK INHIBITION BY L-HISTIDINE. Genetics. 1964 Oct;50:611–623. doi: 10.1093/genetics/50.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma O. K., Beezley D. N., Borek E. Modulation of the synthesis in vitro of a hormone-induced protein by transfer RNA. Nature. 1976 Jul 1;262(5563):62–63. doi: 10.1038/262062a0. [DOI] [PubMed] [Google Scholar]


