Abstract
Desensitization is a complex property of nicotinic acetylcholine receptors (nAChR). Several subtypes of nAChR have high sensitivity to nicotine and mediate effects of nicotine at concentrations found in blood of tobacco smokers. Desensitization of some of these receptor subtypes has been studied in model systems, however, other subtypes have been difficult to express heterologously in native forms. In addition, model systems may not have the same accessory molecules and post-translational modifications found in native populations. We have used wild-type and subunit null mutant mice to study desensitization properties of the high sensitivity α4β2-nAChRs including those that have α5 subunits at both GABAergic and dopaminergic nerve terminals. In addition, we have studied the desensitization of one subtype of α6β2-nAChRs at dopaminergic terminals using α4 subunit null mutant mice. Exposure to low nicotine concentrations, leads to rapid, but partial desensitization of activity mediated by these receptors. α4β2-nAChRs including α5 subunits show faster rates of recovery from desensitization than α4β2-nAChRs without α5. Inclusion of the α5 subunit significantly shifts the concentration response for desensitization to higher values, indicating that receptors with α5 subunits are less desensitized by a 10-min exposure to low concentrations of nicotine. Receptors with α6 subunits appear to desensitize to a lesser degree than those with α4 subunits, indicating that α6β2-nAChRs are somewhat resistant to desensitization by nicotine. These results highlight the importance of studying various receptor subtypes in native systems and how they may differentially respond to nicotine and to nicotinic drugs.
Keywords: nicotine, desensitization, chrna5 subunit, chrna6 subunit, dopamine, GABA
1. Introduction
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels known to desensitize with prolonged exposure to agonists (Changeux et al., 1984). A cyclical scheme to explain the process of desensitization of nAChRs at the neuromuscular junction was proposed in 1957 by Katz and Thesleff ((Katz and Thesleff, 1957). Since that time it has been recognized that all nAChRs are allosteric proteins that desensitize with varying kinetics (Changeux et al., 1984; Quick and Lester, 2002; Taly et al., 2009). Multiple states of desensitization and/or inactivation exist and many factors, including subunit composition, association with other proteins, lipids, ions, and/or accessory molecules (possibly unique to each cell type), as well as post-translational modifications, are likely to affect desensitization and inactivation (Ibanez-Tallon et al., 2002; Cheng et al., 2009; Neff et al., 2009; Taly et al., 2009).
The β2*-subtype is the most abundant nAChR in the CNS [* indicates presence of other subunits(Lukas et al., 1999)]. The propensity of the β2*-nAChR to desensitize with long-term exposure to even low concentrations of agonists, such as nicotine, is a complex property of these receptors (Lippiello et al., 1987; Fenster et al., 1999; Quick and Lester, 2002; Gentry et al., 2003; Guo and Lester, 2007; Taly et al., 2009; Papke et al., 2011). Desensitization by an agonist depends on the concentration and length of exposure, with long exposures resulting in deeper states of desensitization that recover more slowly (Quick and Lester, 2002).
The most common β2*-nAChR is the α4β2-nAChR which exists in both a high sensitivity (HS) form with the subunit stoichiometry (α4β2)2β2 and a low sensitivity (LS) form (α4β2)2α4 (Zwart and Vijverberg, 1998; Zhou et al., 2003; Marks et al., 2007; Gotti et al., 2008). The diversity of α4β2*-nAChR is further complicated by the α5 subunit, expressed in certain neurons that can assemble as (α4β2)2α5–nAChR, another HS form (Tapia et al., 2007; Bailey et al., 2010). The α5 subunit is an accessory subunit and not part of the ligand binding domain (Kuryatov et al., 2008), although recently a ligand binding site at the α4α4 interface has been described (Harpsoe et al., 2011; Mazzaferro et al., 2011), raising the possibility that α4α5 or α5β2 may be tertiary binding sites. The role of the α5 subunit in altering function of α4β2*-nAChRs has been studied in expression systems. In one study in oocytes, addition of the α5 subunit resulted in larger currents and more desensitization (Ramirez-Latorre et al., 1996). In another study using tandem constructs to better control populations of receptors, the (α4β2)2α5-nAChR showed equally high sensitivity as (α4β2)2β2, but with considerably increased Ca++ permeability (Tapia et al., 2007). The HS nature of the (α4β2)2α5-nAChR has also been demonstrated in mouse brain by 86Rb+ efflux experiments using synaptosomal preparations from striatum and thalamus (Brown et al., 2007) and in a transgenic mouse in cortico-thalamic terminals (Heath et al., 2010), as well as by [3H]-GABA release in cortex (McClure-Begley et al., 2009) and [3H]-DA release in striatum (Grady et al., 2010a). In layer VI cortical neurons, the α5 accessory subunit forms HS receptors with α4 and β2 subunits, and the presence of the α5 subunit protects against desensitization (Bailey et al., 2010).
Another important but less widely expressed β2*-nAChR contains the α6 subunit as a ligand binding subunit, and forms nAChRs of predominately the (α6β2)2β3 and (α6β2)(α4β2)β3 subtypes which are expressed in DAergic terminals [for review see (Quik et al., 2011)]. Both of these subtypes are HS forms, with the latter having the highest sensitivity for activation by nicotine of any reported subtype (Salminen et al., 2007).
All HS subtypes respond to low concentrations of nicotine [EC50 values of ~1 μM or lower and nM DC50 values (half-maximal effective concentration for desensitization)]. These subtypes will mediate effects of nicotine in vivo [with concentrations measured in venous blood of smokers reported to be in the range of 10-50 ng/ml equivalent to 60-310 nM (Jarvik et al., 2000; Brody, 2006).
Furthermore, genome wide association studies have implicated the α5 and α6 subunits in aspects of smoking dependence [for review see (Greenbaum and Lerer, 2009)]. Thus, information on how these subunits influence desensitization properties of the β2*-nAChR is of major importance in understanding the actions of nicotine as well as the actions of various therapeutic drugs (Picciotto et al., 2008; Andreasen et al., 2009; Buccafusco et al., 2009; Mansvelder et al., 2009; Taly et al., 2009).
This study was undertaken to assess the roles of α5 and α6 subunits on desensitization properties of β2*-nAChRs from native tissues which have intact the accessory molecules and post-translational modifications unique to neurons. Using α5 subunit null mutant mice (α5KO) as well as wild-type (WT) mice, desensitization of (α4β2)2β2 and (α4β2)2α5 was studied on GABA terminals. Similar methods with α5KO and α6KO mice and the selective antagonist α-conotoxin MII (α-CtxMII) were used to compare these same subtypes on DAergic terminals. In addition, using α4KO mice, the desensitization of α6β2*-nAChR on dopaminergic terminals was assessed.
2. Methods
2.1. Mice
Mice were bred and housed at the Institute for Behavioral Genetics, University of Colorado (Boulder, CO). All subunit null mutant mice were bred onto the C57BL/6 background for minimum of 10 generations. A 12 hour light/dark cycle was maintained (lights on 7AM to 7PM), at 22°C and mice had free access to food (Teklad Rodent Diet, Harlan, Madison WI) and water. Genotypes were assessed by PCR as previously described (Salminen et al, 2004, 2007). The α4 null mutant mice (α4KO) were originally obtained from Dr. John Drago (University of Melbourne, Victoria, Australia), the α5KO mice from Dr. Arthur Beaudet (Baylor College, Houston TX) and the α6KO mice from Dr. Uwe Maskos (Pasteur Institute, Paris, France). The guidelines for care and use of mice provided by the National Institutes of Health were followed and all protocols were approved by the Animal Care and Utilization Committee of the University of Colorado, Boulder. In addition, all efforts were made to minimize the number of animals used.
2.2. Materials
[3H]-γ-aminobutyric acid (GABA) (25-40 Ci/mmol), [3H]-dopamine (DA) (20-40 Ci/mmol) and Optiphase SuperMix scintillation cocktail were purchased from Perkin-Elmer Life and Analytical Sciences (Boston, MA). α-Conotoxin MII (α-CtxMII) was obtained from Dr. J. Michael McIntosh (University of Utah, Salt Lake City, UT). The following were products of Sigma-Aldrich (St. Louis, MO): bovine serum albumin (BSA), (−)-nicotine tartrate, 1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid hydrochloride (NO-711), atropine sulphate, pargyline, diisopropylfluorophosphate (DFP), aminooxyacetic acid and nomifensine. N-(2-hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid) (HEPES) and sodium salt were products of BDH Chemicals distributed by VWR (Denver, CO). All other chemicals were reagent grade.
2.3. Crude synaptosomal neurotransmitter release
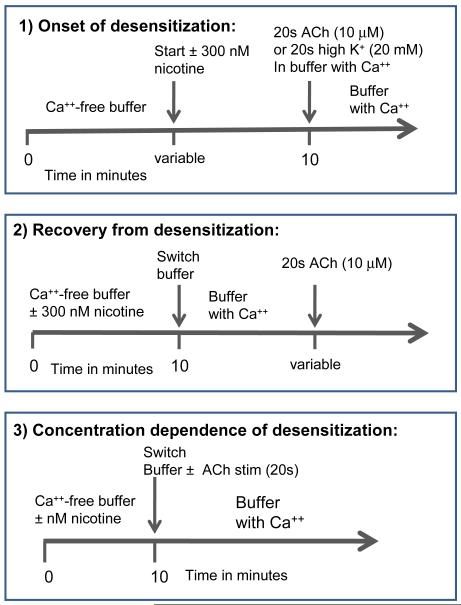
For [3H]-GABA release, the methods of (McClure-Begley et al., 2009) were followed. Briefly, mice were killed by cervical dislocation, the brain removed and dissected on ice. The regions of interest were homogenized in ice-cold isotonic sucrose buffered with HEPES (5 mM, pH 7.4) using 0.5 ml for thalamus and 1 ml for cortex in a glass-teflon homogenizer. After dilution (final volume 2 ml for thalamus; 3 ml for cortex), the crude synaptosomal preparation was divided into aliquots sufficient for 8 filters (1 ml thalamus; 0.25 ml cortex) and centrifuged at 4°C, 12,000g for 20 min. Pellets were resuspended in 0.8 ml of GABA uptake buffer (128 mM NaCl, 2.4 mM KCl, 3.2 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 10 mM glucose, 1.25 mM aminooxyacetic acid, 25 mM HEPES, pH 7.5). After incubation for 10 min at 37°C, [3H]-GABA (final concentration ~0.1 μM) and DFP (final concentration 10 μM) were added and the incubation continued for 10 min longer. At this point aliquots (80 μl) were pipetted onto individual filters for superfusion at 22°C at 0.7 ml/min with GABA perfusion buffer (uptake buffer without aminooxyacetic and with atropine (1 μM), NO-711 (100 nM) and 0.1% BSA added). For Ca++-free buffer, an equi-osmotic amount of Na+ was substituted. Protocols illustrated in Figure 1 and described below were followed. The perfusate of interest was collected into 96-well plates as 10-s fractions using a Gilson F204 fraction collector (Middleton WI). After addition of 0.15 ml of Optiphase SuperMix scintillation cocktail, radioactivity was determined in a 1450 MicroBeta Trilux counter (Perkin Elmer Life Sciences - Wallac Oy, Turku, Finland).
Figure 1.
Schematic presentation of experimental protocols. Timelines are shown for buffer superfusion for each of three types of experiments.
For [3H]-dopamine release, the methods of (Salminen et al., 2007) were used. The above procedure was used with the following modifications. Striatal tissue was dissected and homogenized in 0.5 ml ice-cold isotonic sucrose buffered with HEPES (5 mM, pH 7.4); diluted to 2 ml and 0.5 ml aliquots centrifuged. For DA uptake buffer, aminooxyacetic acid was omitted and ascorbic acid (1 mM) and pargyline (10 μM) were added. After the initial 10 min incubation at 37°C, [3H]-DA (~0.1 μM) and DFP (final concentration=10 μM) were added and the incubation continued for 5 min. DA superfusion buffer consisted of DA uptake buffer with atropine (1 μM), nomifensine (1 μM) and 0.1% BSA added. When α-CtxMII (50 nM) was used, it was included in the superfusion buffer for 5 min prior to the stimulation.
2.4. Experimental protocols
Using crude synaptosomal preparations and [3H]-neurotransmitter release as our functional assay, we measured desensitization by nicotine at several concentrations and recovery from desensitization for various nAChR subtypes. In order to avoid any influx of Ca++ that may lead to various intra-synaptosomal changes during the desensitization phase (Rusakov, 2006), we have conducted this portion of the experiment in Ca++-free buffer. This design necessitates a switch to buffer containing Ca++ for the functional measurements (see Figure 1 for a diagram of perfusion protocols). The use of Ca++-free buffer for superfusion results in a measurably lower baseline than the baseline with superfusion buffer containing Ca++ (see Figure 2 for representative superfusion data). Upon switching to Ca++-containing buffer, the baseline is elevated immediately and in approximately 20-30 seconds establishes a new level baseline, approximately twice the previous baseline. In the first 20-30s, there is a small rise higher than the new baseline (a “shift peak”). This shift peak is not affected by nicotine in the Ca++-free buffer. For example, in n = 6 experiments, the mean shift peak was 2.39 ± 0.22 units for the no nicotine condition and 2.54 ± 0.27 for 300 nM nicotine, both in the Ca++-free buffer for 10 min [the conditions shown in Figure 2 and where units are [(cpm released – baseline cpm)/(baseline cpm)]. Parallel samples were always used to determine this shift peak and it was subtracted from the release stimulated for 20s by either 10 μM ACh or 20 mM K+, when these were perfused simultaneously with the buffer switch (also shown in Figure 2). We have used 20 mM K+-induced release as a control for the effect of nicotine vs the effect of [Ca++] change (see Results). K+-induced release was not affected by the prior exposure of the samples to nicotine in the Ca++-free buffer.
Figure 2.
Representative superfusion scans illustrating the effect of the buffer switch protocol. Data shown is for [3H]-DA release from α6KO striatum. Units of release are net release as a percentage of baseline. The +Ca++ baseline is calculated as a single exponential decay from cpm collected in fractions 15-23. Open symbols represent only a buffer switch (□ with 300 nM nicotine in Ca++-free buffer, △ with no nicotine in Ca++-free buffer), a shift peak is seen which is not affected by the presence of nicotine in the Ca++-free buffer. The effect of nicotine treatment on stimulation by 10 μM ACh is shown by closed symbols (■ with nicotine exposure, and ▲ no nicotine exposure). As seen, nicotine treatment substantially decreases release promoted by ACh.
Using the α4KO, α5KO and α6KO mice as well as the selective α6β2* antagonist, α-CtxMII, to alter the receptor subtypes being measured, we have compared α4β2- and α4α5β2-nAChRs on both DAergic and GABAergic synaptosomes as well as α6β2β3-nAChRs on DAergic synaptosomes as follows:
WT mice for GABA release from cortex mediated by α4β2 plus α4α5β2-nAChR
α5KO mice for GABA release from cortex mediated by α4β2-nAChR only
WT mice for GABA release from thalamus mediated by α4β2-nAChR only
α6KO mice for DA release from striatum mediated by α4β2 plus α4α5β2-nAChR
α5KO mice with α-CtxMII block for DA release from striatum mediated by α4β2-nAChR only
α4KO mice for DA release from striatum mediated by α6β2β3-nAChR only
Regions assayed were chosen based on previous data indicating significant function mediated by receptors with α5 subunits (McClure-Begley et al., 2009). GABA release is ~50% lower in CX in the α5KO mouse than in WT ((McClure-Begley et al., 2009). DA release from striatum mediated by α4β2* is ~70% lower in the α5KO mouse than in WT (Grady et al, 2010a). In addition, thalamus was assayed from WT mice for GABA release as a region without significant α4β2α5-nAChR on GABA terminals ((McClure-Begley et al., 2009).
2.5. Data analysis
Analysis by non-linear curve-fitting was, in all cases, to all the individual data points using SigmaPlot and the following equations:
Onset
f = V * (e−kt) + Vr where V is the desensitizable amount of release, Vr is the remaining, undesensitized portion of release, t is the time of exposure to nicotine (300 nM), and k is the rate constant for onset of the effect of nicotine in min−1.
Recovery
Equation: f = V * (1−e−kt) + Vr where V is the desensitized but recoverable amount of release, Vr is the remaining, undesensitized portion of release, t is the time of recovery in min, k is the rate constant for recovery in min−1. The half-time for recovery (τ) = 0.693/k min. Constraints applied: for GABA: V + Vr = 100, and for DA: V + Vr = value at 30 min (87 or 86).
DC50
Equation: f = V / (1 + ([nic] / DC50))+Vr where V is the maximum desensitized but recoverable amount of release, Vr is the remaining undesensitized portion of release, [nic] is the concentration of nicotine used for desensitization (10 min, nM) and DC50 is the concentration of nicotine (nM) necessary for half-maximal effect.
For all curve fits, after all three variables were determined, V and Vr were fixed and k or DC50 recalculated for better accuracy. The half-time (τ) = 0.693/k min.
Statistics
One-way ANOVA with the Duncan post-hoc test was used to determine significant differences between variables determined by curve-fits.
3. Results
3.1 Measurement of desensitization
Neurotransmitter release assays measure an event that occurs several steps beyond the actual ion flux through the nAChR. Consequently, the potential for measuring desensitization of other steps in the chain of events leading to neurotransmitter release exists. However, in order to measure the effect of the α5 and α6 subunits, this approach may be our only way of isolating populations of nAChR. Therefore, we explored the use of Ca++-free buffer during the desensitizing exposure to see if we could prevent many of the downstream changes that might occur with ion flux via small amounts of channel opening with exposure to low concentrations of nicotine. Following the procedures illustrated in Figure 1, data of the type presented in Figure 2 were obtained. The baseline release, as shown previously, is lower in the absence of Ca++ indicating that some of the baseline is active vesicle-mediated release. When buffer is switched to Ca++-containing buffer, a new baseline is established with a small increase over the new baseline for about 30s (shift peak). This small increase was found to be independent of exposure to nicotine during the Ca++-free superfusion. In addition, release stimulated by exposure to high K+ (20 mM for 20s), was also independent of nicotine exposure. Furthermore, the shift peak and the K+-stimulated release are independent of nicotine exposure for both [3H]-GABA and [3H]-DA release as well as for WT and all null mutant mouse brain regions assayed. Therefore, these assays are likely able to distinguish desensitization properties of the different nAChR populations found on the selected nerve terminals.
3.2. Onset of desensitization
For measurements of onset of inhibition by 300 nM nicotine, protocol 1 illustrated in Figure 1, was followed. For this protocol, all samples were superfused with Ca++-free buffer for 10 min while time of exposure to 300nM nicotine varied between 0 and 10 min.
[3H]-GABA release from crude synaptosomal preparations of cortex was stimulated by 10 μM ACh (a concentration that activates only HS forms of nAChRs) to measure the onset of desensitization from GABA terminals. A significant fraction (~50%) of [3H]-GABA release from cortex of WT mice is mediated by (α4β2)2α5-nAChR ((McClure-Begley et al., 2009). In order to isolate the (α4β2)2β2-nAChR subtype, we assayed [3H]-GABA release from crude synaptosomal preparations of cortex from α5KO mice. We compared results with WT mice in which this assay measures a combination of the HS forms, (α4β2)2β2- and (α4β2)2α5-nAChR to data from a5KO mice which measures (α4β2)2β2-nAChR selectively. The results of these [3H]-GABA release experiments are shown in Figure 3a and Table 1. Onset of desensitization was quite rapid, and leveled off at a maximum 48-56% of control response (samples treated with Ca++-free buffer but without nicotine). For α5KO mice, half-time (τ) was 0.39 ± 0.04 min, and for WT mice, τ ≤ 0.17 ± 0.06 min (note that the onset of desensitization could actually be faster as the first time points measurable with this assay are longer than the calculated half-time). Figure 3a also shows a control response for the stimulation of [3H]-GABA release by K+ (20 mM) indicating that nicotine treatment for 10 min has no effect on this response and, therefore, the treatment is not affecting the release process in general, but only that mediated by nAChRs.
Figure 3.
Onset of desensitization to 300 nM nicotine. Panel a shows data for [3H]-GABA release from cortex for WT and α5KO mice stimulated by 10 μM ACh for 20s (WT, n=5 experiments; α5KO, n=6) or by 20 mM K+ for 20s (WT, n=4; α5KO n=3). Panel b presents data for [3H]-DA release from striatum for α6 KO mice and for α5KO mice in the presence of α-CtxMII (50 nM) using the same stimulations as for Panel a (all, n=3). Panel c shows data for [3H]-DA release from striatum for α6 KO mice using the same stimulations as for Panel a (n=8 for ACh; n=3 for K+).
Table 1.
Onset parameters for 300 nM nicotine desensitization
| Subtype assayed |
Genotype/ Antagonist |
Region | Assay | Half-time (τ) of onset (min)a | % desensitization by 300 nM nic |
|---|---|---|---|---|---|
| (α4β2)2β2 | α5KO | Cortex | GABA | 0.39 ± 0.04 | 56 ± 4 |
| (α4β2)2β2 | α5KO/αCtxMII | Striatum | DA | 0.32 ± 0.03 | 60 ± 4 |
| (α4β2)2β2 + (α4β2)2α5 |
WT | Cortex | GABA | 0.17 ± 0.06 | 48 ± 6 |
| (α4β2)2β2 + (α4β2)2α5 |
α6KO | Striatum | DA | 0.18 ± 0.05 | 48 ± 11 |
| (α6β2)2β3 | α4KO | Striatum | DA | 0.052 ± 0.008 | 25 ± 1 |
Half-times of onset should be considered estimates.
Similarly, we used the [3H]-DA release assay with crude synaptosomal preparations from striatum to assess desensitization of various nAChR subtypes. We have previously shown that significant (~70%) DA release activity in this preparation is mediated by the (α4β2)2α5-nAChR (Salminen et al, 2004; Grady et al, 2010a). Onset of desensitization was measured from crude synaptosomal preparations of striatum from α6KO mice (in which combination of (α4β2)2β2- and (α4β2)2α5-nAChRs are retained) and from α5KO mice using α-CtxMII to block all α6*-nAChRs (to measure only (α4β2)2β2-nAChR). This comparison is shown in Figure 3b and Table 1. The results found with this assay were similar to those for the [3H]-GABA release in cortex. For (α4β2)2β2-nAChR alone, τ = 0.32 ± 0.03 and for the combination of (α4β2)2β2- and (α4β2)2α5-nAChRs, τ ≤ 0.18 ± 0.05, with a maximum of 48-60% of control (note that the onset of desensitization could actually be faster as the first time points measurable with this assay are longer than the calculated half-time). Again the K+-stimulated release is not affected by treatment with nicotine.
We used the [3H]-DA release assay with crude synaptosomal preparations from α4 null mutant mice to measure the onset of desensitization of α6*-nAChR. This design isolates the (α6β2)2β3-nAChR. Unfortunately, we have no way of isolating the more complex, and likely important subtype, (α4β2)(α6β2)β3-nAChR, as there is no sufficiently selective inhibitor of α4β2*(non α6)-nAChRs, available. The results of these experiments are presented in Figure 3c and Table 1. These receptors rapidly desensitized (τ ≤ 0.052 ± 0.008) (Note that the onset of desensitization could actually be faster as the first time points measurable with this assay are longer than the calculated half-time), but to a lower extent (25%) than the α4β2* subtypes (48-60%). As for previous experiments, K+-stimulated release was not affected by treatment with nicotine.
Because of the rapid rates of desensitization, these measurements of half-times are underestimates as noted above and were not analyzed by ANOVA. Significant differences were found for percent desensitization by one-way ANOVA (F(4,10)=14.51, P<0.001 with only the α4KO by DA release ((α6β2)2β3-nAChR) differing from all other groups and showing a lower extent of desensitization.
3.3. Recovery from desensitization
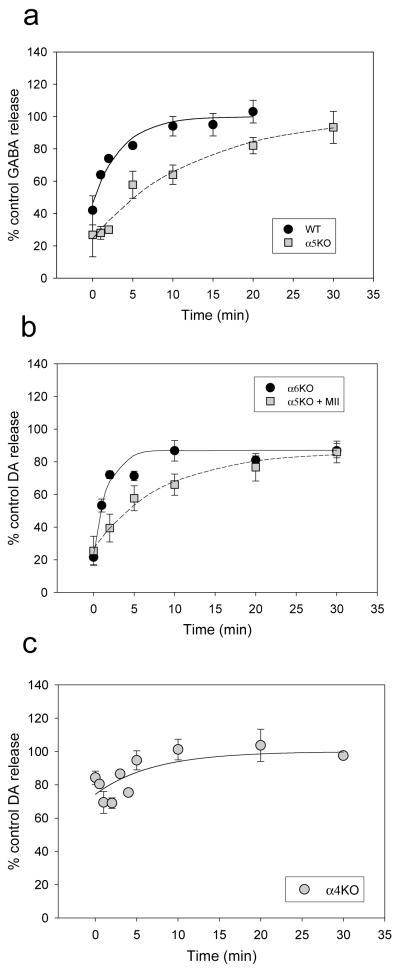
Using protocol 2 shown in Figure 1, we measured the recovery from desensitization induced by 10 min of exposure to 300 nM nicotine in Ca++-free buffer. Using the [3H]-GABA release assay and crude synaptosomal preparation from cortex of WT or α5KO mice, recovery was assessed by delaying the 20s test stimulation of 10 μM ACh for various lengths of time following cessation of the exposure to nicotine in Ca++ free medium. Ca++ was present during the recovery phase. Results of these experiments are presented in Figure 4a and Table 2. Recovery was essentially complete by 30 min and was faster in WT than in α5KO mice, indicating that (α4β2)2α5-nAChRs recover more quickly than (α4β2)2β2-nAChRs.
Figure 4.
Recovery from desensitization by exposure to 300 nM nicotine for 10 min. Panel a shows data for [3H] GABA release from cortex of WT mice (n=5) and α5KO mice (n=5). Panel b shows data for [3H]-DA release from striatum of α6KO mice (n=6) and α5KO mice (n=5) in the presence of α-CtxMII (50 nM). Panel c presents data for [3H]-DA release from striatum of α4KO mice (n=13).
Table 2.
Recovery parameters for 300 nM nicotine desensitization
| Subtype assayed |
Genotype/ Antagonist |
Region | Assay | Half-time (τ) of recovery (min) |
Starting % desensitization |
|---|---|---|---|---|---|
| (α4β2)2β2 | α5KO | Cortex | GABA | 8.8 ± 0.9 | 76 ± 10 |
| (α4β2)2β2 | α5KO/αCtxMII | Striatum | DA | 5.8 ± 0.5 | 59 ± 5 |
| (α4β2)2β2 + (α4β2)2α5 |
WT | Cortex | GABA | 2.4 ± 0.4 | 54 ± 6 |
| (α4β2)2β2 + (α4β2)2α5 |
α6KO | Striatum | DA | 1.1 ± 0.2 | 64 ± 8 |
| (α6β2)2β3 | α4KO | Striatum | DA | 6.8 ± 3.3 | 27 ± 16 |
Recovery was also assessed for these two subtypes using the [3H]-DA release assay and crude striatum synaptosomal preparations from α6KO mice (combination of (α4β2)2α5- and (α4β2)2β2-nAChRs) and in α5KO mice with α-CtxMII to block all α6*-nAChRs ((α4β2)2β2-nAChR only). These data are shown in Figure 4b and Table 2. Recovery was not quite complete but had leveled off by 30 min. As for the [3H]-GABA release assay, presence of the α5 subunit increased the rate of recovery.
The rate of recovery of the (α6β2)2β3-nAChRs was measured using the [3H]-DA release assay with crude synaptosomal preparations from striatum of α4KO mice. These data are shown in Figure 4c and Table 2. Complete recovery was seen with a rate similar to the (α4β2)2β2-nAChR subtype.
Analysis by one-way ANOVA for half-time of recovery for all 5 groups (Table 2) showed significant differences (F(4,10)=12.44, P=0.001). By Duncan’s post-hoc test (P<0.05), there were three subsets, group 1: WT by GABA release and α6KO by DA release (both assays for combination of (α4β2)2β2- and (α4β2)2α5-nAChRs), group 2: the α4KO by DA release [(α6β2)2β3-nAChR] and α5KO with α-CtxMII by DA release [(α4β2)2β2-nAChR only] and group 3: the α4KO by DA release [(α6β2)2β3-nAChR only] and α5KO by GABA release [(α4β2)2β2-nAChR only]. By this analysis, (α4β2)2α5 recovered significantly faster than the other subtypes. The (α4β2)2β2 in DA terminals subtype recovered faster than in GABA neurons, while the (α6β2)2β3 subtype did not significantly differ from either measure of (α4β2)2β2 in recovery half-time.
Significant differences were also found for % desensitization by one-way ANOVA (F(4,10)=10.28, P=0.001 with group 1: the α4KO by DA release ((α6β2)2β3-nAChR), group 2: WT by GABA release and α6KO by DA release (both assays for combination of (α4β2)2β2- and (α4β2)2α5-nAChRs) and α5KO with α-CtxMII by DA release [(α4β2)2β2-nAChR only] and group 3: the α5KO with α-CtxMII by DA release and α5KO by GABA release [both assays for (α4β2)2β2-nAChR only] plus the α6KO by DA release [[(α4β2)2β2-nAChR only]. Therefore, by this analysis, (α6β2)2β3 showed significantly less desensitization than the other subtypes after the 10 min exposure to nicotine replicating the data for onset.
3.4. Concentration dependence of desensitization
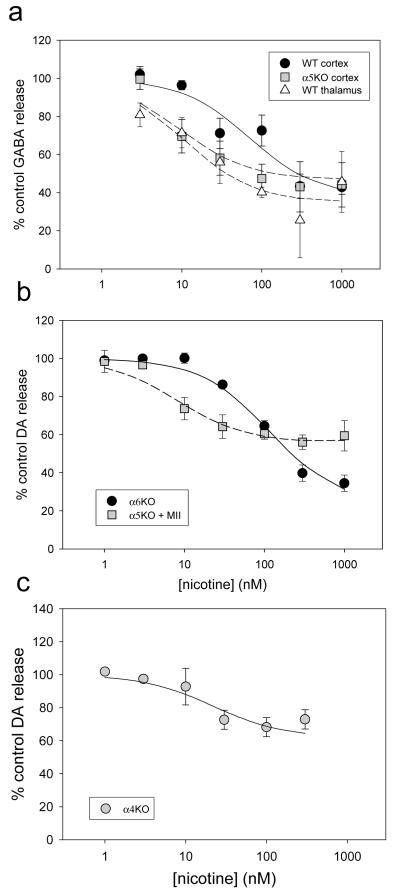
Desensitization by varying concentrations of nicotine exposure for 10 min in Ca++-free buffer was determined for various subtypes of nAChR using protocol 3 from Figure 1. The concentration dependence of this desensitization (as DC50) was assessed, using the [3H]-GABA release assay and crude cortex synaptosomal preparations from WT and α5 null mutant mice. Data are presented in Figure 5a and DC50 values in Table 3 where DC50 = 71 ± 17 nM for the WT [(α4β2)2β2-plus (α4β2)2α5-nAChRs] and in the a5KO, DC50 = 9 ± 4 nM [(α4β2)2β2-nAChR alone]. Somewhat surprisingly, the presence of the α5 subunit in the WT significantly shifted the concentration response curve to the right.
Figure 5.
Concentration response for desensitization by nicotine. Panel 5a shows data for [3H] GABA release from CX (n=10) and thalamus (n=6) of WT mice, as well as cortex of α5KO mice (n=7). Panel 5b shows data for [3H]-DA release from striatum of α6KO mice (n=4) and α5KO mice (n=5) in the presence of α-CtxMII (50 nM). Panel 5c presents data for [3H]-DA release from striatum of α4KO mice (n=5).
Table 3.
DC50 values for 10 min nicotine desensitization
| Subtype assayed |
Genotype/ Antagonist |
Region | Assay | nicotine DC50 (nM) |
Max % desensitization |
|---|---|---|---|---|---|
| (α4β2)2β2 | α5KO | Cortex | GABA | 9 ± 4 | 53 ± 9 |
| (α4β2)2β2 | WT | Thalamus | GABA | 11 ± 4 | 65 ± 9 |
| (α4β2)2β2 | α5KO + αCtxMII |
Striatum | DA | 8 ± 2 | 44 ± 4 |
| (α4β2)2β2 + (α4β2)2α5 |
WT | Cortex | GABA | 71 ± 17 | 63 ± 8 |
| (α4β2)2β2 + (α4β2)2α5 |
α6KO | Striatum | DA | 117 ± 12 | 77 ± 4 |
| (α6β2)2β3 | α4KO | Striatum | DA | 22 ± 11 | 38 ± 9 |
Because of the unexpected shift found with the presence of the α5 subunit, we assayed another region of WT mice, thalamus, that previous data indicated would not have α5 subunits assembled with α4β2-nAChRs on GABA terminals (McClure-Begley et al, 2009). We expected this region would have a concentration response curve similar to the α5 null mutant cortex. These results are also shown in Figure 5a and Table 3. This data is consistent with our hypothesis; the DC50 value seen for thalamus = 11 ± 4 nM was similar to the value determined from α5KO cortex (9 ± 4 nM).
This procedure was repeated using the [3H]-DA release assay with crude striatum synaptosomes from α6KO mice and from α5KO mice with addition of α-CtxMII to block all α6-mediated responses. Data are shown in Figure 5b and Table 3. Again, the presence of the α5 subunit in the α4β2-nAChR (WT) shifted the curve to the right; (DC50 value of 8 ± 2 in the α5KO and 117 ± 12 nM with the α5 subunit present in the WT).
Next, we measured the DC50 value for nicotine-induced desensitization of (α6β2)2β3-nAChR using the [3H-DA assay and crude striatum synaptosomal preparations from α4KO mice. These data are presented in Figure 5c and Table 3. Results indicate this subtype desensitizes to a maximum of about 38% with an intermediate DC50 value (22 ± 11 nM).
Analysis by one-way ANOVA for DC50 values for all 6 groups (Table 3) showed significant differences in DC50 values (F(5,12)=61.26, P<0.001). By Duncan’s post-hoc test (P<0.05), there were three subsets, group 1: α5KO in cortex and WT in thalamus by GABA release and α5KO with α-CtxMII by DA release (all assays for (α4β2)2β2-nAChR only) plus the α4KO by DA release (α6β2)2β3-nAChR), group 2: WT cortex by GABA release (α4β2)2β2- and (α4β2)2α5-nAChRs) and group 3: α6KO by DA release (α4β2)2β2- and (α4β2)2α5-nAChRs). By this analysis all assays for (α4β2)2β2 gave lower DC50 values than assays including (α4β2)2α5. The two assays for (α4β2)2β2 plus (α4β2)2α5-nAChRs were significantly different with the DA release assay having a higher DC50 value than the GABA release assay, possibly because a higher percentage of the measured function on DA neurons being mediated by (α4β2)2α5-nAChRs. The DC50 value for the (α6β2)2β3 subtype did not differ significantly from those measured for the (α4β2)2β2 subtype but was lower than assays including (α4β2)2α5.
Significant differences were also found for maximum extent of desensitization in 10 min by one-way ANOVA (F(5,12)=11.41, P=0.001. By Duncan’s post-hoc test (P<0.05), there were four subsets with overlap of groups among them. No group differed from all other groups; however, the (α6β2)2β3 subtype showed significantly less desensitization than (α4β2)2α5 on DA terminals.
4. Discussion
4.1. α4β2- and α4α5β2-nAChR
Experiments presented here utilized relatively short term (10 min) exposures to a nicotine concentration (300 nM) likely to be found in plasma of tobacco smokers (Matta et al., 2007) to study the desensitization of nAChRs mediating neurotransmitter release from mouse brain synaptosomes. The exposure to nicotine was conducted in the absence of external Ca++ to eliminate or minimize any Ca++-induced changes elicited by nicotine and any subsequent down-stream events. Under these conditions, α4β2-nAChRs are probably in an intermediate state of desensitization, and able to recover relatively rapidly (Fenster et al., 1999; Gentry et al., 2003). We chose these conditions as a simple model to investigate the effect of the addition of the α5 subunit to α4β2-nAChR in native nAChR populations. Our results indicate that, under these conditions, none of the nAChRs-mediated neurotransmitter release studied desensitized completely when exposed to nicotine for 10 min. However, the extent of functional desensitization in WT and α5 KO mice was similar indicating that inclusion of the α5 subunit did not affect steady-state transmitter release following a 10 min exposure to 300 nM nicotine. The data indicate that responses mediated by α4β2-nAChR which include the α5 subunit recover from desensitization more quickly than α4β2-nAChRs that do not include α5. This effect of the α5 subunit was seen for two neurotransmitter release assays ([3H]-GABA release from cortex and [3H]-DA release from striatum), indicating that the desensitization properties of α4β2*-nAChRs do not differ greatly in these two types of nerve terminals. It should be noted that responses of α4β2α5-nAChR were measured in WT mice that also include significant populations of α4β2-nAChR such that the effects of the α5 subunit are likely underestimated with these experiments as we are not able to isolate a pure population of (α4β2)2α5-nAChRs.
In addition, DC50 values for nicotine were considerably higher in WT mice that express both α4β2α5- and α4β2-nAChR than in α5 KO mice that express only α4β2-nAChR. The lower DC50 values in the absence of α5 are consistent with Ki values of 8-15 nM measured for nicotine binding (Marks et al., 1986). A larger difference in DC50 values between WT and α5KO mice was seen using [3H]-DA release from striatum (8 nM vs 117 nM) than for [3H]-GABA release from cortex (9 nM vs 71 nM), possibly because a greater percentage of the activity in DA terminals is mediated by receptors with the α5 subunit. This DC50 effect is not an artifact of use of the α5KO mice, as a lower DC50 value was also seen in WT mouse thalamus (11 nM) where GABA terminal α4β2-nAChR function is devoid of any effect of α5 subunit deletion (McClure-Begley et al., 2009). Thus, this regional difference in DC50 values for WT mice appears to be the result of the absence of the α5 subunit on GABA terminals in thalamus and its presence in cortex.
The effect of the α5 subunit expressed with α4 and β2 subunits on desensitization has been reported for studies using transfected cell lines and heterologously expressed receptors. In a study using HEKtsA201 cells expressing human α4β2-nAChRs, addition of the α5 subunit increased the rate and amount of acute desensitization when exposed to 20 μM ACh compared to a line with mostly (α4β2)2α4, a low sensitivity (LS) form of α4β2*-nAChR (Kuryatov et al., 2008). With long-term (6 hr) desensitization by lower concentrations of nicotine, the DC50 values were 6.1 ± 3.6 nM for (α4β2)2β2 and 8.9 ± 1.5 nM for (α4β2)2α5 (Kuryatov et al., 2008), an insignificant shift by addition of the α5 subunit. However in a study using concatamers to control subtypes and expression in Xenopus oocytes, a DC50 value of 87.2 ± 2.7 nM was reported for (α4β2)2α5 using a 1.5 hr exposure to 300 nM nicotine that resulted in ~70% desensitization (Kuryatov et al., 2011). Both this DC50 value and the extent of desensitization are comparable to data reported here for (α4β2)2α5-nAChR. It is not yet known whether a longer exposure to nicotine would decrease this DC50 value for concatamers or those we have measured for (α4β2)2α5-nAChR.
4.2. α6β2β3-nAChR
The α6 subunit is a ligand binding subunit when paired with β2, and forms nAChRs of predominately the (α6β2)2β3- and (α6β2)(α4β2)β3-nAChR subtypes in dopaminergic neurons [for review see (Quik et al., 2011)]. Both of these subtypes are HS forms, with the latter having the highest sensitivity to nicotine of any reported subtype (Salminen et al., 2007). These receptors have been difficult to express in oocytes or cell lines [for review see (Letchworth and Whiteaker, 2011)] and, therefore, little is known about their desensitization properties. Recent advances with expression of concatamers have facilitated study of the α6β2*-nAChR subtypes and desensitization has been shown to occur, but perhaps to a lesser extent than for α4β2 subtypes (Capelli et al., 2011; Kuryatov et al., 2011). Our results with the (α6β2)2β3-nAChR in striatum preparations from α4KO mice presented here indicate that this receptor subtype desensitizes rapidly with exposure to 300 nM nicotine, but to a quite limited degree (only ~25%). The rate of recovery from desensitization is similar to that of (α4β2)2β2-nAChR and slower than that of (α4β2)2α5-nAChR. The DC50 value (22 ± 11 nM) is similar to the Ki value for nicotine inhibition of α-CtxMII binding (22.6 ± 9.3 nM) (Salminen et al., 2005) or for nicotine inhibition of [125I]-epibatidine binding in α4KO tissue (44.9 ± 8.8 nM) (Grady et al., 2010b) both measures of the affinity of nicotine for the α6β2 site. The extent of desensitization is significantly less than that seen under similar conditions for α4(non α6)β2*-nAChRs as measured here.
4.3. Incomplete desensitization and shifts of DC50 values
With more classical methods, measurements of EC50 values for activation and Ki values for binding by a number of agonists indicates the ratio of these values for α6β2*-nAChR is ~5-10, considerably lower than the ratio for α4β2*-nAChR of ~100 (Salminen et al., 2005; Grady et al., 2010b). This observation implies that the ground state and desensitized state of α6β2*-nAChR may be almost equally stable (Quick and Lester, 2002). The prediction from this observation is that there may not be as much (or as long lasting) desensitization of α6β2*-nAChR (with ratio of ~5-10) as there is of α4β2*-nAChR where the ratio of EC50 value to binding Ki is ~100. The small amount of desensitization measured for α6β2β3-nAChR is consistent with this prediction.
The possibility of spare receptors cannot be ignored when using neurotransmitter release assays (Feuerstein and Limberger, 1999). If activation of only a percentage of receptors on each unit (synaptosome in this case) results in full measured activity (release) the apparent EC50 (or DC50) value will be shifted from values obtained in the absence of spare receptors. Spare receptor theory predicts measured EC50 value < Kd (or DC50 > Kd) when spare receptors are present, as well as deviations in slope of concentration response curves (Agneter et al., 1997). However, EC50 values also differ from Kd in allosteric models, because of different affinities for different conformational states (Changeux et al, 1984). In the absence of the α5 subunit, good correspondence of DC50 value with binding Ki was obtained for α4β2-nAChR, perhaps indicating a lack of spare receptors. This correspondence was found for the α6β2β3-nAChR measurement, also. To explain the large shift we measured for α4α5β2*-nAChR compared to α4β2-nAChR by spare receptor theory, a large change in spare receptors would need to be postulated.
4.4. Relevance to smoking levels of nicotine
These results suggest, at least with short term (10 min) exposure to nicotine, that functional responses mediated by nAChR including the α5 subunit would be more resistant to desensitization (owing to their relatively high DC50) than nAChR that do not include α5. Because the α5 subunit has limited distribution in the brain (Wada et al., 1990; Marks et al., 1992), tobacco smoking could then change relative activity of nicotinic receptors regionally, and/or by cell-type when assembled with α4 and β2 subunits. Since plasma levels of nicotine range from ~1nM for second-hand smoke exposure (Brody et al., 2011), and ~6 nM after overnight abstinence (Jarvik et al., 2000; Brody et al., 2006) to ~150-250 nM shortly after smoking (Jarvik et al., 2000), the shift in DC50 from ~10 nM for α4β2-nAChR to ~100 nM for α4β2α5-nAChR could have a significant effect on desensitization of these receptors following tobacco smoking. In addition, after exposure to 300 nM nicotine, the α6β2β3-nAChR desensitized to a maximum of only ~25%, suggesting that these receptors may retain significant activity in the presence of smoking levels of nicotine.
Serum levels of nicotine in smokers remain at mid-nM levels for considerably longer than the 10 min exposure time used for the experiments reported here. Prolonged exposures possibly lead to deeper desensitization states that may not recover easily and, in addition, may affect DC50 values. Study of long exposures to nicotine will require different experiments than those reported here. The level of nicotine likely modifies the amount of active receptor available, and our results suggest that different subtypes of HS nAChR, especially those with α5 or α6 subunits, may not fully desensitize thereby retaining some activity in smokers.
Highlights.
Desensitization of presynaptic nAChRs was assessed by neurotransmitter release.
α4α5β2-nAChR recovers from desensitization more quickly than α4β2-nAChR.
The desensitization concentration for α4α5β2-nAChR is higher than for α4β2-nAChR.
The extent of desensitization of α6β3β2-nAChR is less than α4β2- or α4α5β2-nAChR.
Acknowledgements
This work was supported by NIH grants: U19DA019375 and P30DA015663.
We thank J. Michael McIntosh for generously supplying α-conotoxin MII, William Van Morter for animal production and care, and Erin Meyers and Esteban Loetz for genotyping.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agneter E, Singer EA, Sauermann W, Feuerstein TJ. The slope parameter of concentration-response curves used as a touchstone for the existence of spare receptors. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:283–292. doi: 10.1007/pl00005052. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol. 2009;23:797–804. doi: 10.1177/0269881108091587. [DOI] [PubMed] [Google Scholar]
- Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006;40:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Khan A, Kozman D, Costello MR, Vellios EE, Archie MM, Bascom R, Mukhin AG. Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Arch Gen Psychiatry. 2011;68:953–960. doi: 10.1001/archgenpsychiatry.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, Whiteaker P. Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J Neurochem. 2007;103:204–215. doi: 10.1111/j.1471-4159.2007.04700.x. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV., Jr. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli AM, Castelletti L, Chen YH, Van der Keyl H, Pucci L, Oliosi B, Salvagno C, Bertani B, Gotti C, Powell A, Mugnaini M. Stable expression and functional characterization of a human nicotinic acetylcholine receptor with alpha6beta2 properties: discovery of selective antagonists. Br J Pharmacol. 2011;163:313–329. doi: 10.1111/j.1476-5381.2011.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Devillers-Thiery A, Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984;225:1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Amici SA, Ren XQ, McKay SB, Treuil MW, Lindstrom JM, Rao J, Anand R. Presynaptic targeting of alpha4beta 2 nicotinic acetylcholine receptors is regulated by neurexin-1beta. J Biol Chem. 2009;284:23251–23259. doi: 10.1074/jbc.M109.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick MW, Lester RA. Regulation of alpha4beta2 nicotinic receptor desensitization by calcium and protein kinase C. Mol Pharmacol. 1999;55:432–443. [PubMed] [Google Scholar]
- Feuerstein TJ, Limberger N. Mathematical analysis of the control of neurotransmitter release by presynaptic receptors as a supplement to experimental data. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:345–359. doi: 10.1007/pl00005361. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Wilkins LH, Jr., Lukas RJ. Effects of prolonged nicotinic ligand exposure on function of heterologously expressed, human alpha4beta2- and alpha4beta4-nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;304:206–216. doi: 10.1124/jpet.102.041756. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, Marks MJ. Partial deletion of the nicotinic cholinergic receptor alpha 4 or beta 2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of alpha 4 and beta 2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, McIntosh JM, Marks MJ, Collins AC. Mouse striatal dopamine nerve terminals express alpha4alpha5beta2 and two stoichiometric forms of alpha4beta2*-nicotinic acetylcholine receptors. J Mol Neurosci. 2010a;40:91–95. doi: 10.1007/s12031-009-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P, Bencherif M, Lester HA, Marks MJ. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010b;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum L, Lerer B. Differential contribution of genetic variation in multiple brain nicotinic cholinergic receptors to nicotine dependence: recent progress and emerging open questions. Mol Psychiatry. 2009;14:912–945. doi: 10.1038/mp.2009.59. [DOI] [PubMed] [Google Scholar]
- Guo X, Lester RA. Regulation of nicotinic acetylcholine receptor desensitization by Ca2+ J Neurophysiol. 2007;97:93–101. doi: 10.1152/jn.01047.2005. [DOI] [PubMed] [Google Scholar]
- Harpsoe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T. Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J Neurosci. 2011;31:10759–10766. doi: 10.1523/JNEUROSCI.1509-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, King SL, Gotti C, Marks MJ, Picciotto MR. Cortico-thalamic connectivity is vulnerable to nicotine exposure during early postnatal development through alpha4/beta2/alpha5 nicotinic acetylcholine receptors. Neuropsychopharmacology. 2010;35:2324–2338. doi: 10.1038/npp.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM, Heintz N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33:893–903. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Katz B, Thesleff S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Whiteaker P. Progress and challenges in the study of alpha6-containing nicotinic acetylcholine receptors. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiello PM, Sears SB, Fernandes KG. Kinetics and mechanism of L-[3H]nicotine binding to putative high affinity receptor sites in rat brain. Mol Pharmacol. 1987;31:392–400. [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Mansvelder HD, Mertz M, Role LW. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin Cell Dev Biol. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Meinerz NM, Drago J, Collins AC. Gene targeting demonstrates that alpha4 nicotinic acetylcholine receptor subunits contribute to expression of diverse [3H]epibatidine binding sites and components of biphasic 86Rb+ efflux with high and low sensitivity to stimulation by acetylcholine. Neuropharmacology. 2007;53:390–405. doi: 10.1016/j.neuropharm.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Romm E, Wehner JM, Collins AC. Nicotinic binding sites in rat and mouse brain: comparison of acetylcholine, nicotine, and alpha-bungarotoxin. Mol Pharmacol. 1986;30:427–436. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, Moroni M, Bermudez I. An additional ACh binding site at the {alpha}4/{alpha}4 interface of the ({alpha}4{beta}2)2{alpha}4 nicotinic receptor influences agonist sensitivity. J Biol Chem. 2011 doi: 10.1074/jbc.M111.262014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure-Begley TD, King NM, Collins AC, Stitzel JA, Wehner JM, Butt CM. Acetylcholine-stimulated [3H]GABA release from mouse brain synaptosomes is modulated by alpha4beta2 and alpha4alpha5beta2 nicotinic receptor subtypes. Mol Pharmacol. 2009;75:918–926. doi: 10.1124/mol.108.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff RA, 3rd, Gomez-Varela D, Fernandes CC, Berg DK. Postsynaptic scaffolds for nicotinic receptors on neurons. Acta Pharmacol Sin. 2009;30:694–701. doi: 10.1038/aps.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Trocme-Thibierge C, Guendisch D, Al Rubaiy SA, Bloom SA. Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. J Pharmacol Exp Ther. 2011;337:367–379. doi: 10.1124/jpet.110.177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Quik M, Perez XA, Grady SR. Role of alpha6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Rusakov DA. Ca2+-dependent mechanisms of presynaptic control at central synapses. Neuroscientist. 2006;12:317–326. doi: 10.1177/1073858405284672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-Conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48:696–705. doi: 10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (alpha 5) in the rat central nervous system. Brain Res. 1990;526:45–53. doi: 10.1016/0006-8993(90)90248-a. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]