Abstract
Purpose
No reliable methods currently exist to predict patient response to intravesical immunotherapy with bacillus Calmette-Guérin (BCG), given after transurethral resection for high-risk non-muscle-invasive bladder cancer. We initiated a prospective clinical trial to determine whether fluorescence in situ hybridization (FISH) results during BCG immunotherapy can predict therapy failure.
Materials and Methods
Candidates for standard of care BCG were offered participation in a clinical trial. FISH was performed prior to BCG and at 6 weeks, 3 months, and 6 months during BCG therapy with maintenance. Cox proportional hazards regression was used to assess the relationship between FISH results and tumor recurrence or progression; the Kaplan-Meier product limit method was used to estimate recurrence- and progression-free survival.
Results
One hundred twenty-six patients participated. At a median follow-up of 24 months, 31% of patients had recurrent tumors and 14% experienced disease progression. Patients who had positive FISH results during BCG therapy were 3-5 times more likely than those who had negative FISH results to develop recurrent tumors and 5-13 times more likely to experience disease progression (p < 0.01). The timing of positive FISH results also affected outcome; for example, patients with a negative FISH result at baseline, 6 weeks, and 3 months demonstrated an 8.3% recurrence rate, compared to 48.1% in those with a positive FISH result at all three time points.
Conclusions
FISH results can identify patients who are at risk of tumor recurrence and progression during BCG immunotherapy. This information may be used to counsel patients about alternative treatment strategies.
Keywords: bladder cancer, BCG, FISH, response, prediction
Introduction
More than 70,000 new cases of bladder cancer are diagnosed yearly, with the majority presenting as NMIBC.1 Sixty percent to 70% of non-muscle invasive tumors recur and 10% to 20% progress to muscle-invasive disease.2 Immunotherapy with BCG is the most effective intravesical treatment utilized in patients with high-risk NMIBC,3 however a significant number of patients fail treatment and require more aggressive intervention such as radical cystectomy and/or chemotherapy. If patients do not respond to intravesical BCG, performing radical cystectomy within the first 24 months after diagnosis is believed to improve survival by at least at 20%.4 Thus, early identification of patients in whom BCG will fail would allow those patients to receive earlier curative radical cystectomy and improve their chances of survival.
Currently, the gold standard for post-TUR surveillance is cystoscopy and urine cytology at regular intervals; these diagnostics rely on detection of actual tumor recurrence and are poor predictors of therapy failure.5,6 Fluorescence in situ hybridization (FISH) analysis (UroVysion; Abbott/Vysis, Downers Grove, IL) of exfoliated urothelial cells is highly sensitive and specific for urothelial cancer, regardless of whether the patient is being treated with intravesical immunotherapy.7 Although FISH has been studied as a surveillance tool, there is limited data assessing the ability of FISH to predict bladder cancer recurrence or progression following initiation of intravesical BCG.8-11 The goal of this prospective clinical study was to determine whether molecular recurrence of NMIBC – as defined by the presence of cytogenetically abnormal cells on FISH analysis – during intravesical immunotherapy could predict the clinical outcomes of tumor recurrence and progression to muscle invasion.
Materials and Methods
Patients
All patients who were scheduled to undergo intravesical BCG immunotherapy at our center since July 2005 have been offered participation in this prospective, Institutional Review Board-approved clinical trial (National Clinical Trial #01007058). Patients were eligible if they had pathologically confirmed primary or recurrent NMIBC documented within 6 weeks of enrollment and normal upper urinary tract imaging. Pathologic inclusion criteria were similar to the European Organization for Research and Treatment of Cancer intermediate-/high-risk categories.12 Patients were excluded if they had a history of prior pelvic radiation, had variant histologic subtypes (squamous cell carcinoma, adenocarcinoma, micropapillary, or small cell), or were immunocompromised. All patients with high-grade tumors underwent re-resection between 4 and 6 weeks after the initial diagnosis in order to evaluate for occult muscle invasion. One immediate post-operative intravesical instillation of mitomycin C was administered when appropriate.
Intravesical Immunotherapy
Intravesical BCG was administered according to the protocol used in Southwest Oncology Group trial 8507.13 All patients received an induction course of BCG consisting of 6 weekly treatments, then maintenance consisting of 3 weekly treatments at 3 and 6 months and then every 6 months for a total of 36 months. Dose reductions were allowed at the discretion of the treating physician. As was reflective of our practice at the time of study initiation, augmentation of BCG with interferon-α-2b was allowed at the discretion of the treating physician, with the schedule of therapy similar to that outlined above.14
FISH Assays
Urine samples were collected for analysis at baseline (after TUR and prior to initiation of intravesical BCG), 6 weeks (right before sixth instillation of BCG), 3 months (at first maintenance immunotherapy and cystoscopic surveillance), and 6 months (at second maintenance immunotherapy and cystoscopic surveillance) after initiation of immunotherapy. FISH was performed according to instructions provided with the UroVysion Bladder Cancer Recurrence Kit. Freshly collected urine (volume ≥ 35 ml) was immediately fixed with alcohol and cytospin preparations were prepared. Following denaturation of DNA, fluorescent-labeled DNA probes comprising centromeric chromosome 3 (Spectrum Red), 7 (Spectrum Green), 17 (Spectrum Aqua), and locus specific identifier 9p21 (Yellow) were incubated with the specimen to hybridize with complementary DNA. Cells were counterstained with DAPI (4,6-diamidino-2-phenylindole). Interphase nuclei were analyzed using fluorescence microscopy to detect chromosomal copy number.15 Twenty-five morphologically atypical non-overlapping cells with distinct signals were scored. Atypical cytologic features include patchy and lighter DAPI staining, nuclear enlargement, and irregular nuclear contour. The assay was considered positive if at least four cells showed polysomy on at least two chromosomes (3, 7, or 17) and/or there were at least twelve cells with no signal (homozygous deletion) for 9p21.16 On the rare occasion when a result was uninterpretable (collection or processing error), the assays were repeated as allowed by the protocol.
Patient Monitoring
Patients were monitored during BCG treatment according to normal practices at our institution using cystoscopy and cytology at 3-month intervals for 2 years and 3- to 6-month intervals thereafter. Repeat TUR and other treatments were performed as necessary. Patient management was not mandated based on results of the FISH assay, but results were provided to the treating physician to be acted upon if so desired.
Statistical Analysis
Patient data were analyzed on an intent-to-treat basis. Descriptive statistics were used to summarize the study population characteristics. Recurrence was defined as any tumor found after the start of intravesical BCG, regardless of grade or stage. Progression was defined as an increase in stage to muscle-invasive disease. Logistic regression was used to assess relationships between patient and tumor characteristics and tumor recurrence or progression. Patient data were censored from time of recurrence, progression, or date of the last documented cystoscopy if recurrence/progression was not observed. Univariate Cox proportional hazards regression was used to model the association between FISH results and risk of recurrence or progression. Multivariate analysis was used to model the association of additional variables with risk of recurrence or progression. The Kaplan-Meier product limit method was used to estimate recurrence-free and progression-free survival. Statistical analyses were performed using STATA/SE version 10.1 statistical software (Stata Corp. LP, College Station, TX).
Results
Patient Characteristics
We have enrolled 126 patients into the study at the time of this report. The median age was 67.5 years; 96 (76%) were male and 79 (63%) had a history of smoking. The majority of patients (112 [89%]) had a history of previously treated bladder tumors, and 13 (10%) had been treated with intravesical BCG within the past 12 months. Ninety-one tumors (72%) were high-grade and 61 (48%) were clinical stage Ta or T1 each. Sixty-one patients (48%) had CIS as a secondary finding.
At a median follow up of 23.4 months, 39 patients (31%) had recurrent tumors and 18 (14%) underwent disease progression to muscle invasion. In our patient cohort, which was a rather homogeneous group in terms of disease characteristics, tumor grade was not related to the risk of progression (HR = 7.24, 95% CI = 0.95-54.51, p = 0.55).
FISH Results
A total of 442 FISH assays had been performed at the time of this report. Patients with a positive FISH result at any time point had a higher overall risk of recurrence and progression than patients who had a negative FISH result at the same time point (Figure 1 and 2). In particular, patients who had positive FISH results at 6 weeks, 3 months, or 6 months after the beginning of BCG therapy were 3-5 times more likely to develop recurrent tumors than those who had negative FISH results and 5-13 times more likely to experience disease progression (p < 0.01; Table 1). On multivariate analysis, the association of FISH results with tumor recurrence and progression persisted after accounting for other disease factors (stage, grade, history of prior tumors, concomitant CIS, use of post-operative mitomycin, etc.) at all time points.
Fig 1.
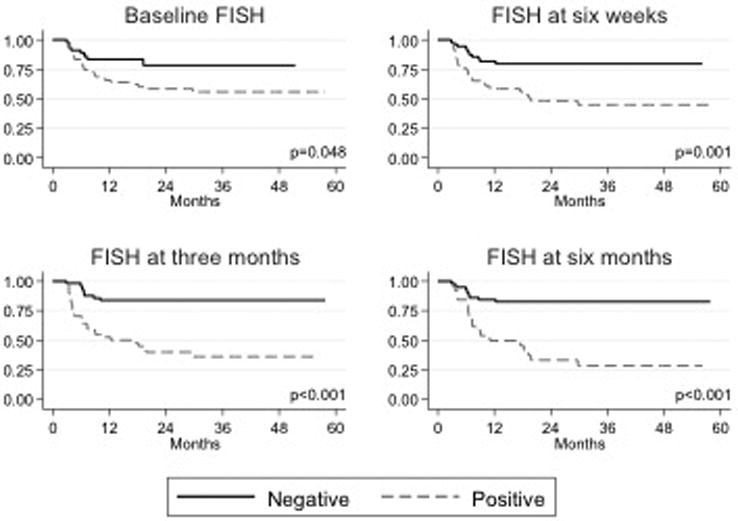
Kaplan-Meier estimated recurrence-free survival curves (represented as percentage) by FISH assay results. FISH assay time points include the following: baseline (before initiation of intravesical immunotherapy with BCG), six weeks after treatment initiation, three months after treatment initiation, and six months after treatment initiation.
Fig 2.
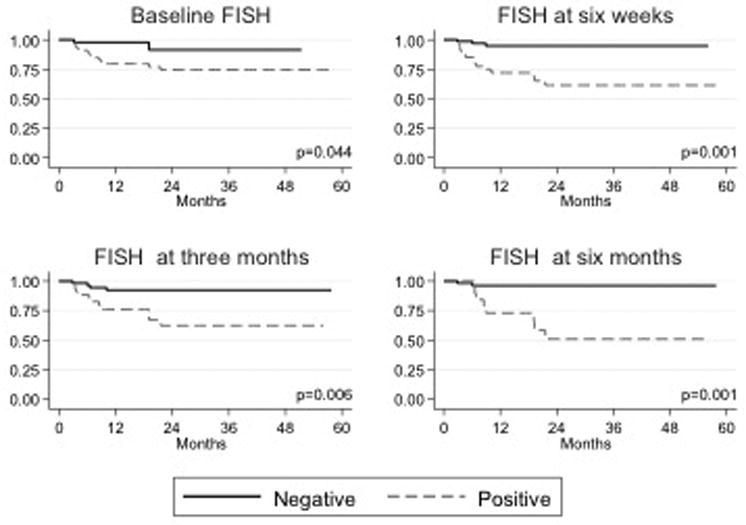
Kaplan-Meier estimated progression-free survival curves (represented as percentage) by FISH assay results. FISH assay time points include the following: baseline (before initiation of intravesical immunotherapy with BCG), six weeks after treatment initiation, three months after treatment initiation, and six months after treatment initiation.
Table 1.
Proportional hazards regression model showing risk of disease recurrence and progression by fluorescence in situ hybridization (FISH) assay results at various times*
| Time and assay result | No. at risk | No. with Recurrence/Progression | Hazard ratio | 95% confidence interval | p value |
|---|---|---|---|---|---|
| Risk of recurrence | |||||
| Baseline FISH | |||||
| Negative | 45 | 8 | |||
| Positive | 81 | 31 | 2.19 | (1.01-4.77) | 0.042 |
| FISH at 6 weeks | |||||
| Negative | 74 | 13 | |||
| Positive | 50 | 25 | 3.04 | (1.55-5.95) | 0.001 |
| FISH at 3 months | |||||
| Negative | 59 | 9 | |||
| Positive | 48 | 18 | 4.95 | (2.33-10.50) | <0.001 |
| FISH at 6 months | |||||
| Negative | 59 | 10 | |||
| Positive | 26 | 18 | 5.02 | (2.31-10.90) | <0.001 |
| Risk of progression | |||||
| Baseline FISH | |||||
| Negative | 45 | 2 | |||
| Positive | 81 | 16 | 4.53 | (1.04-19.70) | 0.027 |
| FISH at 6 weeks | |||||
| Negative | 74 | 3 | |||
| Positive | 50 | 15 | 8.00 | (2.32-27.67) | <0.001 |
| FISH at 3 months | |||||
| Negative | 59 | 4 | |||
| Positive | 48 | 8 | 4.94 | (1.59-15.37) | 0.002 |
| FISH at 6 months | |||||
| Negative | 59 | 2 | |||
| Positive | 26 | 8 | 12.63 | (2.67-59.78) | <0.001 |
Totals at each time include only those patients “at risk” at that time; hence n = 126 only for the baseline FISH data.
FISH results at baseline
Although a positive FISH result at baseline was not predictive of early tumor recurrence (during first surveillance at 3 months), it was predictive of overall recurrence. Patients who had a positive baseline FISH result had a significantly higher risk of tumor recurrence than patients who had a negative result (38.3% vs 17.8%, p = 0.020). Similarly, a positive FISH result at baseline was not predictive of early disease progression (during first surveillance at 3 months), but was predictive of disease progression overall. Patients who had a positive baseline FISH result had a significantly higher risk of experiencing disease progression than patients who had a negative result (19.8% vs 4.4%, p = 0.032).
FISH results at 6 weeks after treatment initiation
Patients who had a positive FISH result at 6 weeks had a significantly higher risk of overall tumor recurrence (34.0% vs 13.5%, p = 0.008) and tumor recurrence at first surveillance (3 months) than patients who had a negative result at 6 weeks (50.0% vs 17.6%, p < 0.001). Patients who had a positive FISH result at 6 weeks also had a significantly higher risk of experiencing overall disease progression (28.0% vs 12.2%, p = 0.030) and disease progression by the first surveillance (3 months) than patients who had a negative result at 6 weeks (30.0% vs 4.1%, p < 0.001).
FISH results at 3 months after treatment initiation
Because the 3-month FISH assay was performed at the time of first surveillance, our analysis of 3-month findings includes only results from patients without clinical evidence of recurrence or progression at that time; results were only analyzed for association with overall recurrence/progression rates. Patients with a positive FISH result at 3 months had a significantly higher risk of tumor recurrence than patients with a negative result (58.3% vs 15.3%, p < 0.001). Similarly, patients who had a positive FISH result at 3 months had a significantly higher risk of experiencing disease progression than patients with a negative result (25.0% vs 6.8%, p = 0.013).
FISH results at 6 months after treatment initiation
As in our analysis of FISH assay results at 3 months, our analysis of results at 6 months was restricted to patients without clinical evidence of recurrence or progression at that time; results were only analyzed for association with overall recurrence/progression rates. Patients with a positive FISH result at 6 months had a significantly higher risk of tumor recurrence than patients with a negative result (69.2% vs 16.9%, p <0.001). Similarly, patients with a positive FISH result at 6 months had a significantly higher risk of experiencing disease progression than patients with a negative result (69.2% vs 16.9%, p <0.001).
The total number of positive FISH results and persistence of positive FISH results correlate with an even higher risk of disease recurrence. Patients who had a positive FISH result at both 6 weeks and 3 months after treatment initiation were 24.59 times more likely to develop recurrent tumors than patients who had negative results at both times (95% CI = 4.08-148.17, p < 0.001). By contrast, patients who did not have a positive FISH result until 3 months after treatment initiation were only 6.29 times more likely to develop recurrent tumors than patients who had negative results at both times (95% CI = 1.05-37.66, p = 0.044). Recurrence rates based on timing of positive FISH assay are shown in Table 2. Similarly, Table 2 illustrates that risk of progression rises with an increase in number of positive FISH results for each patient.
Table 2.
Recurrence rates based on timing of first positive FISH assay.
| Time and assay result | Recurrence rate by 12 weeks, % | Recurrence rate overall, % | Progression rate overall, % |
|---|---|---|---|
| Negative FISH at Baseline, | |||
| Negative at 6 weeks | 5.1 | 12.8 | 0.0 |
| Positive at 6 weeks | 40.0 | 60.0 | 40.0 |
| Positive FISH at Baseline and Positive at 6 weeks | 13.3 | 48.9 | 28.9 |
| Negative FISH at Baseline and Negative at 6 weeks | |||
| Negative at 3 months | - | 8.3 | 8.3 |
| Positive at 3 months | - | 14.3 | 14.3 |
| Negative FISH at Baseline and Positive at 6 weeks and Positive at 3 months | - | 33.3 | 33.3 |
| Positive FISH at Baseline and Positive at 6 weeks and Positive at 3 months | - | 48.1 | 37.0 |
Using FISH results combined with standard cystoscopy and urine cytology, sensitivity and specificity for tumor detection was 75.7% and 71.4% respectively. Four of 14 patients (28.6%) with a positive FISH and normal cystoscopy/cytology were biopsied. All of those patients had cancer indicating no false positives with the FISH assay.
Discussion
FISH is known to detect recurrent bladder cancer before it is clinically evident by cystoscopy (i.e., molecular recurrence).17-19 Four prior studies have assessed the utility of FISH after intravesical BCG and found that a positive assay correlates with a higher risk of disease recurrence. However, the risk of progression has differed amongst these studies and remains in question.8-11
In this report, we build upon these previous findings and present results for 126 patients from our prospective clinical trial investigating the role of FISH in predicting response to intravesical BCG. We found that the results of FISH assays correlated with risk of tumor recurrence and progression. Notably, the risk of disease recurrence and progression increases with each additional positive FISH result, and the earlier a FISH result converts to positive from a negative baseline, the higher is the risk of recurrence and progression (a positive FISH result at 6 weeks indicated a 50% overall risk of recurrence and a 30% overall risk of disease progression). Our results suggest that FISH can be used as a surrogate marker to allow early identification of NMIBC patients who might be at high risk for tumor recurrence and progression so that alternative therapy may be offered while the potential for cure is high.20 FISH assays could potentially be used to develop a risk adapted and more efficient and cost-effective means of surveillance for patients with NMBIC.
Predictions can be made using not only the binary outcome of FISH analysis at a single time point but also the pattern of outcomes over time. For an individual patient, each additional positive FISH result correlates with an incremental increase in risk of recurrence and progression. Additionally, the earlier the conversion from a negative to positive FISH result, the higher the risk of disease recurrence. Thus, patients can be counseled with even greater accuracy based on their individual history of FISH results.
Our study has major strengths, the most important of which is that it is the largest cohort of patients recruited specifically to identify markers of response to intravesical BCG immunotherapy. In contrast to previous studies, we evaluated FISH assays at multiple time points throughout therapy and have the longest follow-up period reported to date. Our population includes the type of patients typical in BCG clinical trials – a large proportion had high-grade disease, and half had CIS as a secondary finding. Although we reported on the overall cohort because it is reflective of real-world patients treated with BCG, it is reassuring that the statistical power of FISH analysis to predict recurrence and progression persisted regardless of disease grade at study entry.
It is also important to point out that we incorporated what is still considered the standard of care today: patients with T1 and/or high-grade disease underwent a repeat TUR (to ensure there was no evidence of invasive disease), followed by induction and maintenance therapy with BCG. Even so, in the period under evaluation, 31% of patients developed recurrent disease and 14% experienced progression to muscle-invasive disease, which is reflective of the patients' high-risk status.
One limitation of our study, despite its large cohort population, is that it comes from a single center. Fortunately, external validation is ongoing in a multicenter prospective clinical trial that aims to recruit an estimated 134 patients. We recognize that it will be important to determine whether the predictive capability of FISH differs among sub-categories of tumors (Ta, T1, and Tis); such an analysis will be feasible only after a sufficient number of patients are pooled to avoid the small numbers that would otherwise result from sub-categorizing patients.
Conclusions
Patterns of FISH assay results can help identify patients at risk for tumor recurrence and progression during intravesical BCG immunotherapy. These data can be used in the design of future clinical trials as well as to counsel patients about alternative treatment strategies.
Supplementary Material
Acknowledgments
This research was supported by a grant to AMK from Flight Attendant Medical Research Institute (FAMRI). The University of Texas MD Anderson Cancer Center is supported in part by a Cancer Center Support Grant (CA016672) from the National Institutes of Health.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Heney NM, Ahmed S, Flanagan MJ, et al. Superficial bladder cancer: progression and recurrence. The Journal of urology. 1983;130:1083–6. doi: 10.1016/s0022-5347(17)51695-x. [DOI] [PubMed] [Google Scholar]
- 3.Kamat AM, Lamm DL. Immunotherapy for bladder cancer. Curr Urol Rep. 2001;2:62–9. doi: 10.1007/s11934-001-0027-7. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? The Journal of urology. 2001;166:1296–9. [PubMed] [Google Scholar]
- 5.Dalbagni G, Rechtschaffen T, Herr HW. Is transurethral biopsy of the bladder necessary after 3 months to evaluate response to bacillus Calmette-Guerin therapy? J Urol. 1999;162:708–9. doi: 10.1097/00005392-199909010-00020. [DOI] [PubMed] [Google Scholar]
- 6.Highshaw RA, Tanaka ST, Evans CP, deVere White RW. Is bladder biopsy necessary at three or six months post BCG therapy? Urol Oncol. 2003;21:207–9. doi: 10.1016/s1078-1439(02)00239-9. [DOI] [PubMed] [Google Scholar]
- 7.Bubendorf L, Grilli B. UroVysion multiprobe FISH in urinary cytology. Methods Mol Med. 2004;97:117–31. doi: 10.1385/1-59259-760-2:117. [DOI] [PubMed] [Google Scholar]
- 8.Kipp BR, Karnes RJ, Brankley SM, et al. Monitoring intravesical therapy for superficial bladder cancer using fluorescence in situ hybridization. J Urol. 2005;173:401–4. doi: 10.1097/01.ju.0000149825.83180.a4. [DOI] [PubMed] [Google Scholar]
- 9.Mengual L, Marín-Aguilera M, Ribal MJ, et al. Clinical utility of fluorescent in situ hybridization for the surveillance of bladder cancer patients treated with bacillus Calmette-Guérin therapy. Eur Urol. 2007;52:752–9. doi: 10.1016/j.eururo.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Whitson J, Berry A, Carroll P, Konety B. A multicolour fluorescence in situ hybridization test predicts recurrence in patients with high-risk superficial bladder tumours undergoing intravesical therapy. BJU International. 2009;104:336–9. doi: 10.1111/j.1464-410X.2009.08375.x. [DOI] [PubMed] [Google Scholar]
- 11.Savic S, Zlobec I, Thalmann GN, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guérin therapy. Int J Cancer. 2009;124:2899–904. doi: 10.1002/ijc.24258. [DOI] [PubMed] [Google Scholar]
- 12.Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology. 2006;49:466–5. doi: 10.1016/j.eururo.2005.12.031. discussion 75-7. [DOI] [PubMed] [Google Scholar]
- 13.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 14.O'Donnell MA, Lilli K, Leopold C. Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol. 2004;172:888–93. doi: 10.1097/01.ju.0000136446.37840.0a. [DOI] [PubMed] [Google Scholar]
- 15.Zellweger T, Benz G, Cathomas G, et al. Multi-target fluorescence in situ hybridization in bladder washings for prediction of recurrent bladder cancer. Int J Cancer. 2006;119:1660–5. doi: 10.1002/ijc.21704. [DOI] [PubMed] [Google Scholar]
- 16.Caraway NP, Khanna A, Fernandez RL, et al. Fluorescence in situ hybridization for detecting urothelial carcinoma: a clinicopathologic study. Cancer Cytopathol. 2010;118:259–68. doi: 10.1002/cncy.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halling KC, King W, Sokolova IA, et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol. 2000;164:1768–75. [PubMed] [Google Scholar]
- 18.Bubendorf L, Grilli B, Sauter G, Mihatsch MJ, Gasser TC, Dalquen P. Multiprobe FISH for enhanced detection of bladder cancer in voided urine specimens and bladder washings. Am J Clin Pathol. 2001;116:79–86. doi: 10.1309/K5P2-4Y8B-7L5A-FAA9. [DOI] [PubMed] [Google Scholar]
- 19.Sarosdy MF, Schellhammer P, Bokinsky G, et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. The Journal of urology. 2002;168:1950–4. doi: 10.1016/S0022-5347(05)64270-X. [DOI] [PubMed] [Google Scholar]
- 20.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


