Abstract
A new micro-computed tomography (μCT) image processing approach to estimate the loss of cement-bone interlock was developed using the concept that PMMA cement flows and cures around trabeculae during the total knee arthroplasty procedure. The initial mold shape of PMMA cement was used to estimate the amount of interdigitated bone at the time of implantation and following in vivo service using enbloc human postmortem retrievals. Laboratory prepared specimens, where there would be no biological bone resorption, were used as controls to validate the approach and estimate errors. The image processing technique consisted of identifying bone and cement from the μCT scan set, dilation of the cement to identify the cement cavity space, and Boolean operations to identify the different components of the interdigitated cement-bone regions. For laboratory prepared specimens, there were small errors in the estimated resorbed bone volume fraction (reBVfr = 0.11±0.09) and loss in contact area fraction (CAfr = 0.06±0.15). These values would be zero if there were no error in the method. For the postmortem specimens, the resorbed volume fraction (reBVfr = 0.85±0.16) was large, meaning that only 15% of the cement mold shape was still filled with bone. The loss of contact area fraction (CAfr = 0.84±0.17) was similarly large. This new approach provides a convenient method to visualize and quantify trabecular bone loss from interdigitated regions from postmortem retrievals. The technique also illustrates for the first time that there are dramatic changes in how bone is fixed to cement following in vivo service.
Introduction
Maintaining implant fixation during in vivo service of total joint replacements is critical to prevent aseptic loosening and need for revision. There has been longstanding interest in using postmortem retrievals to understand the in vivo function of joint replacements and to explore potential loosening mechanisms (Bishop et al., 2009; Charnley, 1979; Kwong et al., 1992; Maloney et al., 2002; Rao et al., 2010). For cemented fixation, identifying patterns of bone resorption at the cement-bone interface could improve our understanding of how implants loosen and also point to alternative designs or surgical techniques to improve long term fixation. Cemented knee arthroplasty utilizes doughy PMMA cement that infiltrates the bone bed and flows around trabecular bone prior to self-polymerization. The cement creates a mold around the individual trabeculae (Miller et al., 2010) and could serve as a means to identify the original state of the cement-bone interface prior to any biological changes during in vivo service.
In this work, we developed a new micro-computed tomography (μCT) image processing approach to estimate the state of cement-bone interlock at the time of implantation and following in vivo service using enbloc retrievals from the tibial components of knee replacements. Laboratory prepared specimens, where there would be no biological bone resorption, were used as controls to validate the approach and estimate errors. We asked two research questions. Can the amount of resorbed bone be estimated using the ‘mold shape’ created by viscous cement flowing and curing around trabeculae? Based on the initial mold shape, how much bone resorbs from postmortem retrievals?
Methods
Specimen Preparation
Laboratory-prepared samples were created from a fresh-frozen proximal tibia (male, age 51) that was prepared for a total knee replacement by performing a trans-axial cut at the subchondral level, followed by lavage with suction on the cut bone surface. The bone was warmed to 32 deg C and standard viscosity PMMA cement (Simplex, Stryker Orthopaedics, Mahwah, NJ) was applied to the bone surface in a doughy state using digital pressure and pressing motion with a spatula prior to placement of a flat surrogate metal ‘tibial tray’ (without stem). The amount of cement applied was chosen to approximate that used for the postmortem retrievals. Two enbloc, postmortem cemented tibial components were used to create samples for the ‘postmortem’ group. These were derived from: (1) an age 82 male after 6 years of service with a NexGen CoCr metal tray (Zimmer Inc., Warsaw, IN, USA), and (2) an age 78 male after 10 years of service with an AMK CoCr metal tray (Depuy Orthopaedics, Warsaw, IN, USA). The laboratory-prepared and postmortem constructs were sectioned through the tibial tray in the sagittal and frontal planes to create specimens with 8mm × 8mm cross sections containing cement, bone, and the interdigitated cement-bone interface. Cement-bone specimens were randomly selected from the underside of the tibial tray for the laboratory-prepared (n=10) and postmortem retrieval (n=20) groups. Donor bones were provided by the Anatomical Gift Programs at SUNY Upstate Medical University and Case Western Reserve University.
Image Processing
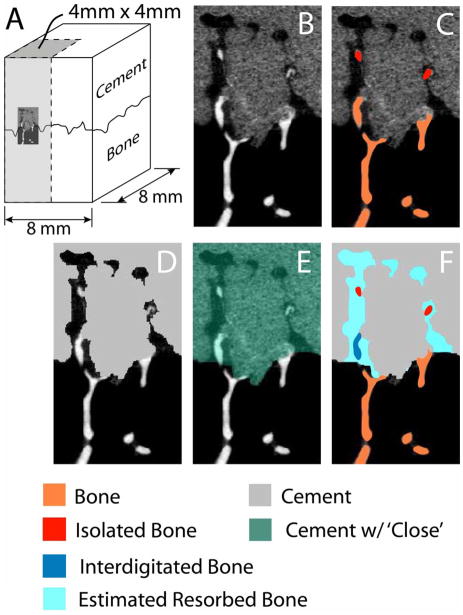
Micro-CT scans at 16 μm resolution (Scanco 40, Wayne, PA) were obtained for each specimen in air, and a sub region of 4mm×4mm cross-section were used for analysis (Figure 1A). Figure 1B shows a small 2-D representation of the cement-bone interface. MIMICS (Materialise, Leuven, Belgium) was used to identify bone, isolated bone (not connected to the supporting trabecular bone bed, Figure 1C), and cement (Figure 1D) using thresholding and region growing operations. Bone thresholding was performed using uniform thresholding, meaning that the same lower theshold level (480 mg/cc HA equivalent) was used for all bone images. An 8 voxel dilation followed by an 8 voxel erosion (MIMICS ‘close’ operation, Figure 1E) was used to identify the cement mold shape or ‘cement cavities’. This process filled cavities with a width up to 256 μm. Finally, Boolean operations (Figure 1F) were used to identify interdigitated bone and estimated resorbed bone.
Figure 1.
A series of Boolean operations was performed using micro-CT images of 4mm×4mm subregions (A, shown as shaded region) of the cement-bone interface. The micro-CT images (B) were converted to image masks for bone, isolated bone (C) and cement (D). A dilation followed by erosion operation (E) was used to identify the cement cavity (F), the interdigitated bone, and the estimated resorbed bone. Isolated bone was defined as bone that was not in contact with the bone below the cement-bone interface. In contrast, interdigitated bone was bone that is contiguous with the bone below the cement-bone interface, but also was in the space defined by the cement with close operation (E).
We developed several parameters to describe the local cement-bone morphology. Interdigitation depth described the initial extent of cement penetration into the bone (see Figure 2). Interdigitated (inBV), isolated (isBV), and resorbed bone volume (reBV) (see Figure 1) were normalized to the cross sectional area of the specimen. Resorbed BV fraction (reBVfr) was defined as reBV/(reBV+inBV+isBV). The estimated initial contact area fraction (esCAfr) between cement and bone was determined for the initial ‘mold state’ plus the adjacent support bone (orange in Figure 1). The current CAF (curCAfr) was determined using all post-resorption bone components. The esCAfr and curCAfr were normalized to cross sectional area. The loss in CAfr was calculated as: (esCAfr-curCAfr)/esCAfr. For the validation study using laboratory prepared specimens where there is no resorption, if there were no gaps between interdigiated cement and bone, then: reBV=0, reBVfr=0, and loss of CAfr=0.
Figure 2.
3-D reconstruction of the cement-bone interface from a laboratory prepared specimen illustrating the trabecular bone structure with interdigitated cement (A). The interdigitation depth for each sample was calculated as the average of measurements from four sampling quadrants as illustrated in panel A. The actual interdigitated bone (B) had a similar morphology when compared to the estimated interdigitated bone (C) calculated using the cement mold shape.
Two sample t-tests with Bonferroni correction for multiple sampling were used to determine if the morphology parameters were different for the laboratory prepared and postmortem specimens.
Results
For the validation study with laboratory prepared specimens (Figure 2), there were small errors in the estimated resorbed bone volume fraction (reBVfr=0.11±0.09) and loss in contact area fraction (CAfr=0.06±0.15) (Table 1). The estimated initial interdigation depth was very similar for the lab-prepared and postmortem groups, indicating that the initial state of cement infiltration was comparable. For the postmortem specimens, the amount of estimated resorbed bone was often quite dramatic (Figure 3, shown in blue). The initial estimated cement-bone contact area fraction (esCAfr) was also similar for the two groups. In contrast, the resorbed volume fraction (reBVfr) for the postmortem specimens was 0.85±0.16, meaning that only 15% of the cement mold shape was still filled with bone. In addition, the loss of CAfr was also extensive (0.84±0.17).
Table 1.
Morphology results of postmortem vs. lab-prepared specimens.
| Morphology Parameter | Lab Prepared (n=10) | Postmorte m (n=20) | p-value |
|---|---|---|---|
| Interdigitation depth (mm) | 2.67 (0.54) | 2.95 (0.65) | 0.49 |
|
| |||
| Interdigitated BV (inBV, mm3/mm2) | 0.20 (0.05) | 0.06 (0.11) | 0.0039 |
| Isolated BV (isBV, mm3/mm2) | NA | 0.003(0.005) | --- |
| Resorbed BV (reBV, mm3/mm2) | 0.02(0.02)* | 0.28 (0.15) | <0.0001 |
| Resorbed BV fraction (reBV fr) | 0.11(0.09)* | 0.85 (0.16) | <0.0001 |
| Estimated initial contact area fraction (esCAfr, mm2/mm2) | 4.78 (1.43) | 4.72 (2.14) | 0.396 |
| Current contact area fraction (curCAfr, mm2/mm2) | 4.36 (1.01) | 0.84 (1.29) | <0.0001 |
| Loss of CAfr | 0.06 (0.15)* | 0.84 (0.17) | <0.0001 |
Values indicated by a * would be zero if there were zero error with this method.
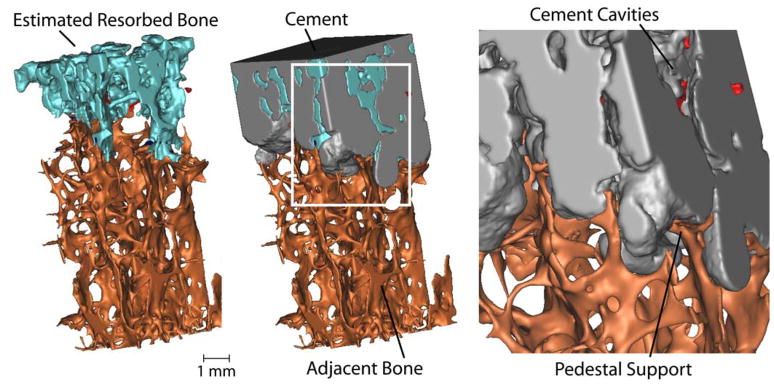
Figure 3.
3-D reconstruction showing estimated resorbed bone following 10 years in service. Cement cavities with small regions of isolated bone (red) are evident in the inset image (right). Following bony remodeling, there appears to be trabecular pedestal support of the cement layer. The specimen was taken from a region under the tibial tray with cross sectional area dimensions of 4 mm × 4mm.
It is interesting to note that the amount of bony resorption could vary widely in different regions from the same donor bone (Figure 4). This example shows three samples from the medial plateau region of the donor bone with six years in vivo service. Some interdigated regions were almost completely resorbed while others maintained robust interdigitation.
Figure 4.
Samples from same donor bone (6 yrs in service) reveal a wide range of bony resorption. Blue shows estimated resorbed bone volume (ReBV). The estimated resorbed bone volume fraction (ReBV fr) and current contact area fraction (CAfr) values are shown.
Discussion
The results of this study suggest that the mold shape left by cement that flows around trabeculae can be used to estimate the original trabecular volume in the interdigitated region. Errors with this method are on the order of 10% and are likely due to the fact that cement does not flow in perfect apposition with the bone and small gaps may be present after the cement cures. Further, the cement shrinks upon polymerization(Gilbert et al., 2000), and this could also cause small gaps at the interface. The results also show that bony resorption can be extensive following in vivo service with resorbed bone volume fractions of 85% and loss of cement-bone contact area fractions of 84%.
There have been a number of different approaches used to quantify the interlock between cement and bone. At the apparent level, Mann and coworkers (Mann et al., 1997) identified the quantity of interdigitated bone using the apparent density of the interlocked bone multiplied by the depth of interdigitation. Maher and McCormack (Maher and McCormack, 1999) defined a random undulating parameter to describe the interlock between cement and bone using two-dimensional images of sections from lab-prepared cemented hip replacements. Arola and coworkers (Arola et al., 2001) developed an estimate of the apparent volume of interdigitation using concepts from surface profilometry. The new approach presented here builds on these earlier concepts through use of 3D high-resolution micro-CT imaging and the ability to estimate the relative amounts of interdigitation in the initial postoperative condition and following bone remodeling with in vivo use.
There are several limitations to this study. Only two postmortem retrievals were used and it is possible that other retrievals will have different amounts of resorbed and residual interdigitated bone. We have sectioned (but not quantitatively analyzed) shorter and longer term implants and they all exhibit the characteristic loss of interdigitation shown here. Bone resorption from interdigitated regions with cemented hip replacements also has been reported by our group (Mann et al., 2010). A reasonable sample population with a wide range of years in service will be needed to answer important questions of temporal changes of bone resorption. The errors from our lab prepared specimens were not negligible, but were small relative to the magnitude of resorption in the retrievals. If errors were systematic (constant across all conditions), the average resorbed bone volume fraction would be 74% (85%-11% error) and the contact area fraction would be 78% (84%-6% error). However, it is difficult to know the systematic error for each specimen and the error for each will likely be different. For constructs with small amounts of resorption, care should be taken in interpreting results. It is possible that other forms of cement porosity at the trabeculae-cement interface could confound results. For example, trapped fat or fluid could form in the cement along the interface. In addition, if the original trabecular were very thick, the dilation and erosion procedure might not be sufficient to capture all of the morphology. Thresholding of the bone was conducted using a fixed gray scale value. It is possible that more complex thresholding algorithms could capture details of the bone morphology(Waarsing et al., 2004).
The finding that some regions can maintain good interlock, while others resorb within the same donor bone, suggests that resorption may not be a biocompatibility or thermal necrosis issue. For the example shown (Figure 3), the greatest residual interdigitated bone was located below the center of the medial condyle. It is possible that femoro-tibial contact stresses are transferred through this region, thereby maintaining trabecular stress at physiologic levels. Spatial and temporal mapping of the interdigitation patterns with a much larger series would be very useful to understand how fixation via cement-bone interdigitation changes following in vivo service.
Acknowledgments
This work was funded by NIH AR42017 and the SUNY Upstate Medical University Hendricks Foundation. The authors would like to thank Dr. Clare Rimnac from Case Western Reserve University for providing an enbloc retrieval used in this study.
Footnotes
Conflict of Interest Statement: The authors received funding from the Department of Health and Human Services, National Institutes of Health (AR42017) and the SUNY Upstate Medical University Hendricks Foundation for completion of this study. None of the authors received or will receive direct or indirect benefits from any third parties related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arola DD, Yang DT, Stoffel KA. The apparent volume of interdigitation: a new parameter for evaluating the influence of surface topographu on mechanical interlock. J Biomed Mat Res (Appl Biomater) 2001;58:519–524. doi: 10.1002/jbm.1049. [DOI] [PubMed] [Google Scholar]
- Bishop NE, Schoenwald M, Schultz P, Puschel K, Morlock MM. The condition of the cement mantle in femoral hip prosthesis implantations--a post mortem retrieval study. Hip Int. 2009;19:87–95. doi: 10.1177/112070000901900202. [DOI] [PubMed] [Google Scholar]
- Charnley J. Low friction arthroplasty of the hip. Springer-Verlag; New York: 1979. [Google Scholar]
- Gilbert JL, Hasenwinkel JM, Wixson RL, Lautenschlager EP. A theoretical and experimental analysis of polymerization shrinkage of bone cement: a potential major source of porosity. J Biomed Mat Res. 2000;52:210–218. doi: 10.1002/1097-4636(200010)52:1<210::aid-jbm27>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kwong LM, Jasty M, Mulroy RD, Maloney WJ, Bragdon C, Harris WH. The histology of the radiolucent line. J Bone Joint Surg Br. 1992;74:67–73. doi: 10.1302/0301-620X.74B1.1732269. [DOI] [PubMed] [Google Scholar]
- Maher SA, McCormack BAO. Quantification of interdigitation at bone cement/cancellous bone interfaces in cemented femoral reconstructions. Proc Instn Mech Engrs. 1999;213:347–354. doi: 10.1243/0954411991535176. [DOI] [PubMed] [Google Scholar]
- Maloney WJ, Schmalzried T, Harris WH. Analysis of long-term cemented total hip arthroplasty retrievals. Clin Orthop Relat Res. 2002:70–78. doi: 10.1097/00003086-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Mann KA, Ayers DC, Werner FW, Nicoletta RJ, Fortino MD. Tensile strength of the cement-bone interface depends on the amount of bone interdigitated with PMMA cement. J Biomech. 1997;30:339–346. doi: 10.1016/s0021-9290(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Mann KA, Miller MA, Verdonschot N, Izant TH, Race A. Functional interface micromechanics of 11 en-bloc retrieved cemented femoral hip replacements. Acta Orthop. 2010;81:308–317. doi: 10.3109/17453674.2010.480938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Eberhardt A, Cleary RJ, Verdonschot N, Mann KA. Micro-mechanics of post-mortem retrieved cement-bone interfaces. Journal of Orthopaedic Research. 2010;28:170–177. doi: 10.1002/jor.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AS, Engh JA, Engh GA, Parks NL. Mechanical stability of well-functioning tibial baseplates from postmortem-retrieved total knee arthroplasties. J Arthroplasty. 2010;25:481–485. doi: 10.1016/j.arth.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Waarsing JH, Day JS, Weinans H. An improved segmentation method for in vivo microCT imaging. J Bone Miner Res. 2004;19:1640–1650. doi: 10.1359/JBMR.040705. [DOI] [PubMed] [Google Scholar]