Abstract
Purpose
Efflux transporters of the ATP-binding cassette (ABC) family are major determinants of chemoresistance in tumor cells. This study examined associations between functional variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes in patients with epithelial ovarian/primary peritoneal cancer (EOC/PPC) following platinum and taxane-based chemotherapy.
Methods
Sequenom iPLEXTMGOLD Assay and MALDI-TOF platform were used to genotype the non-synonymous G2677T/A (rs2032582; encoding Ala893Ser/Thr) and synonymous C3435T (rs1045642; encoding Ile1145Ile) variants in ABCB1, the non-synonymous G1249A variant in ABCC2 (rs2273697; encoding Val417Ile), and the non-synonymous C421A variant in ABCG2 (rs2231142; encoding Q141K, Gln141Lys) in normal DNA from up to 511 women in Gynecologic Oncology Group (GOG) phase III trials, GOG-172 or GOG-182. Progression-free survival (PFS) and overall survival (OS) were analyzed in relation to genetic polymorphisms using Kaplan-Meier and Cox proportional hazards model.
Results
The C421A variant (CA+AA versus CC) in ABCG2 was associated with a 6-month longer median PFS (22.7 versus 16.8 months, p=0.041). In multivariate analysis, patients with variant genotypes were at a reduced risk of disease progression (hazard ratio [HR]=0.75, 95% confidence interval [CI]=0.59–0.96, p=0.022). The association between C421A and OS was not statistically significant (HR=0.88, 95% CI=0.67–1.15, p=0.356). None of the other variants measured in either ABCB1 or ABCC2 was associated with PFS or OS.
Conclusion
The C421A variant in ABCG2, previously shown to be associated with enhanced protein degradation and drug sensitivity, was associated with longer PFS in advanced stage EOC/PPC patents treated with platinum+taxane-based chemotherapy. This finding requires further validation.
INTRODUCTION
Staging laparotomy with cytoreduction followed by platinum/taxane-based chemotherapy is the standard of care for women with advanced stage epithelial ovarian cancer (EOC). About 30% of advanced staged patients fail to respond initially to standard of care treatment, and a majority of the responders ultimately relapse over time [1–3]. While the mechanisms underlying inherent and acquired chemoresistance are not fully understood, there is growing evidence that ATP-binding cassette (ABC) multidrug efflux pumps play an important role. ABC transporters constitute a super-family of proteins with 48 members that transport a variety of endogenous or exogenous compounds across the cell membrane, using the energy provided by ATP hydrolysis [4–7]. The ABC transporters are subdivided into seven distinct subfamilies (ABCA through ABCG) with overlapping and/or unique selectivity for targets [7]. When properly functioning, these transporters effectively transport compounds out of cells.
Of the ABC transporters, ABCB1, ABCC1, ABCC2, ABCC3 and ABCG2 are most often associated with resistance of cancer cell to multiple drugs termed multidrug resistance (MDR) [7, 8]. ABCB1, previously known as multidrug resistance 1 (MDR1) gene, encodes p-glycoprotein (PgP). This was the first ABC transporter found to selectively confer MDR in cancer cell by directly pumping out a broad spectrum of anticancer drugs, including paclitaxel and doxorubicin [6,7]. Increased expression of ABCB1 in tumor cells has been shown to correlate with a poor response to paclitaxel [9,10]. ABCC1, ABCC2 and ABCC3 encode multidrug resistance-associated protein 1 (MRP1), 2 (MRP2) and 3 (MRP3), respectively, and transport drugs conjugated to glutathione including platinum agents [5,7,11,12] and taxanes [13,14]. Glutathione conjugation is a major cellular mechanism for inactivating exogenous compounds. Once conjugated, platinum agents are efficiently exported from the cell via ABCC transporters and cannot re-enter the cell. The ABCC2 gene is overexpressed in some platinum-resistant ovarian cancer cells [5,8,11,12]. ABCG2, also known as mitoxanthrone-resistance gene (MXR), breast cancer resistance protein (BCRP), or the ABC transporter in placenta (ABCP), encodes a broad-specificity transmembrane transporter [5,15] for a variety of compounds including platinum agents, paclitaxel, doxorubicin, and topotecan [16–18]. The list of ABCG2 substrates currently includes nucleoside analogues such as gemcitabine, tyrosine kinase inhibitors, anti-virals, HMG-CoA reductase inhibitors, and flavonoids [17,18].
Functional variants have been identified in a number of the MDR-associated ABC genes that appear to influence drug sensitivity/resistance through various mechanisms including expression level, stability, degradation, substrate-specificity and/or activity of these transporters [5,7,19–24]. The current study utilized germline DNA from two phase III Gynecologic Oncology Group (GOG) clinical trials, GOG-172 [25] and GOG-182 [26], to determine if functional variants in ABCB1, ABCC2, and ABCG2 genes in EOC patients were associated with progression-free survival (PFS) and/or overall survival (OS) following platinum+taxane-based chemotherapy. We evaluated variants of ABCB1, ABCC2 and ABCG2 because the substrates of these genes include the standard agents used to treat advanced, persistent or recurrent EOC, such as cisplatin, carboplatin, topotecan, paclitaxel, doxorubicin and gemcitabine. The four common variants were selected based on the previous publications in which these polymorphisms were extensively studied in ovarian cancer and other cancers, with variable results. We assessed their prognostic values by taking advantage of the clinical data from GOG phase III trials. Subset analyses were performed to explore if the relationship between functional variants in ABC transporter genes and clinical outcomes were influenced by protocol, tumor stage, residual disease, or treatment regimen.
METHODS
Study Population
This study focused on the subset of patients who participated in GOG-172 [25] or GOG-182 [26] with non-tumor DNA available for genotyping. All of the women gave written informed consent and provided a blood specimen for research consistent with all federal, state and local requirements. Details regarding eligibility criteria, treatment and clinical outcomes of these two protocols have been reported elsewhere [25,26]. In brief, GOG-172 was a randomized two-arm phase III clinical trial to compare intravenous with intraperitoneal cisplatin+paclitaxel chemotherapy in patients with optimally resected stage III EOC or primary peritoneal cancer (PPC). Treatment was given every three weeks for six cycles and the study results supported an improved prognosis for women with IP chemotherapy [25]. GOG-182 was a five-arm phase III trial using carboplatin+paclitaxel alone as the control arm plus one additional drug (gemcitabine, doxil, topotecan) in triplet or sequential doublet regimens. Patients with stage III or IV EOC or PPC, with either no (<0.1 cm), optimal (0.1–1 cm) or suboptimal (>1 cm) gross residual disease were randomized to five arms. It was shown that all four experimental arms had similar clinical outcomes relative to the control arm [26].
Genotyping
Blood was collected from patients at the time of enrollment, and genomic DNA was extracted from samples using either the Puregene DNA isolation kit (GentraSystems, Inc. Minneapolis, MN), or the ABI PRISM 6100 Nucleic Acid Prep Station (Applied Biosystems, Inc, Foster City, CA) as previously described [27]. Sequenom iPLEXTMGOLD Assay and MALDI-TOF platform were used to genotype the G2677T/A (rs2032582) and C3435T (rs1045642) polymorphisms in ABCB1, the G1249A polymorphism in ABCC2 (rs2273697), and the C421A polymorphism in ABCG2 (rs2231142) in normal DNA as described previously [28]. Ten percent blinded duplicates were included for quality control, and DNA from CEPH pedigrees was included to confirm genotypes.
Clinical End Points
PFS was calculated as the time in months from study enrollment to disease progression (surgical, clinical or serological evidence) or death from any cause, whichever came first. For women who were alive with no evidence of disease progression, PFS was the time until the date of last contact. OS was calculated as the time from enrollment to death from any cause or to the date of last contact for those who were still alive.
Statistical Analysis
The genotype data were tested for Hardy-Weinberg equilibrium (HWE) using exact permutation test, and the genotype distributions were also compared to published data to ensure the validation of genotyping. Associations between polymorphisms and clinical characteristics were assessed using Wilcoxon rank-sum (for age), Pearson-χ2 or Fisher exact test (for categorical variables). Kaplan-Meier method was used to estimate PFS and OS distributions, and the logrank test was used to compare the survival function by genotype. A Cox proportional hazards model was used to assess the associations between the polymorphisms and PFS or OS adjusted for cell type (clear cell/mucinous versus other histologic subtypes), an aggregate of stage and residual disease status (stage III with none or microscopic residual disease versus stage III with <1 cm residual disease versus stage III with ≥1 cm residual disease or stage IV) and treatment regimen. These covariates were chosen for adjustment based on a pre-evaluation and their documented prognostic relevance in previous GOG studies [29]. The adjusted hazard ratio (HR) for polymorphic versus common genotypes was estimated according to the codominant mode, followed by combining both heterozygous and homozygous variants. For analysis of the G2677T/A variant in ABCB1, the GT was combined with GA, and TT was combined with TA. For analysis of the C421A variant in ABCG2, AA was combined with CA. Subset analyses were conducted by protocol, the aggregate of stage and residual disease status, and treatment regimen. All statistical testing was two-sided and a p-value of <0.05 was considered statistically significant, except for the subset analyses which were exploratory to prioritize further testing.
RESULTS
This study included 511 eligible women with germline DNA available for genotyping. The patient characteristics of this cohort are provided in Table 1. The median age for the cohort was 58 years with 91% Caucasians, 94% with a 0 to 1 baseline GOG performance score, 78% had serous adenocarcinoma and 75% had optimal-resected stage III (none, microscopic or <1 cm residual) disease after surgical debulking. There were 232 patients from GOG-172 who were randomized to intraperitoneal or intravenous cisplatin+paclitaxel, and 279 patients from GOG-182 who were randomized to intravenous carboplatin+paclitaxel alone or in combination with gemcitabine, doxil or topotecan as a triplet or sequential doublet. At the time of analysis, 342 patients had died, 84 women were alive with no evidence of disease, and 85 were alive with documented recurrence. The median follow-up for those still alive was 72 months.
Table 1.
Patient Characteristics (n=511)
| GOG-0172 (n=232) No. patients (%) | GOG-0182 (n=279) No. patients (%) | All patients (n=511) No. patients (%) | |
|---|---|---|---|
| Age (years) | |||
| < 55 | 104 (44.8) | 101 (36.2) | 205 (40.1) |
| 55 – 64 | 63 (27.2) | 94 (33.7) | 157 (30.7) |
| ≥ 65 | 65 (28.0) | 84 (30.1) | 149 (29.2) |
| Median (range) | 57 (32–83) | 59(24–87) | 58 (24–87) |
| Race | |||
| White | 213 (91.8) | 251 (90.0) | 464 (90.8) |
| Black | 7 (3.0) | 18 (6.5) | 25 (4.9) |
| Other | 12 (5.2) | 10 (3.6) | 22 (4.3) |
| Performance Status | |||
| 0 | 101 (43.5) | 142 (50.9) | 243 (47.6) |
| 1 | 115 (49.6) | 124 (44.4) | 239 (46.8) |
| 2 | 16 (6.9) | 13 (4.7) | 29 (5.7) |
| Histology | |||
| Serous | 178 (76.7) | 219(78.5) | 397 (77.7) |
| Endometrioid | 15 (6.5) | 22 (7.9) | 37 (7.2) |
| Clear cell | 14 (6.0) | 9 (3.2) | 23 (4.5) |
| Mucinous | 1 (0.4) | 2 (0.7) | 3 (0.6) |
| Othersa | 24 (10.3) | 27 (9.7) | 51 (10.0) |
| Tumor Grade | |||
| 1 | 23 (9.9) | 23 (8.2) | 46 (9.0) |
| 2 | 92 (39.7) | 94 (33.7) | 186 (36.4) |
| 3 or clear cell | 117 (50.4) | 162 (58.1) | 279 (54.6) |
| Stage/debulking | |||
| Stage III-micro | 97 (41.8) | 55 (19.7) | 152 (29.8) |
| Stage III-optimal | 135 (58.2) | 98 (35.1) | 233 (45.6) |
| Stage III-suboptimal | - | 74 (26.5) | 74 (14.5) |
| Stage IV | - | 52 (18.6) | 52 (10.2) |
| Treatmentb | |||
| Cis+P (IV) | 105 (45.3) | - | 105 (20.5) |
| Cis+P (IP) | 127 (54.7) | - | 127 (24.9) |
| C+P (IV) | - | 55 (19.7) | 55 (10.8) |
| C+P+G (IV) | - | 55 (19.7) | 55 (10.8) |
| C+P+D (IV) | - | 54 (19.4) | 54 (10.6) |
| C+T→C+P (IV) | - | 66 (23.7) | 66 (12.9) |
| C+G→C+P (IV) | - | 49 (17.6) | 49 (9.6) |
Other histologic subtypes included: mixed epithelial, undifferentiated, transitional cell and adenocarcinoma, not otherwise specified
IP: intraperitoneal; IV: Intravenous; Cis: cisplatin; P: paclitaxel; C: carboplatin; G:gemcitabine; D: doxil ; T: topotecan
ABCB1 Polymorphisms
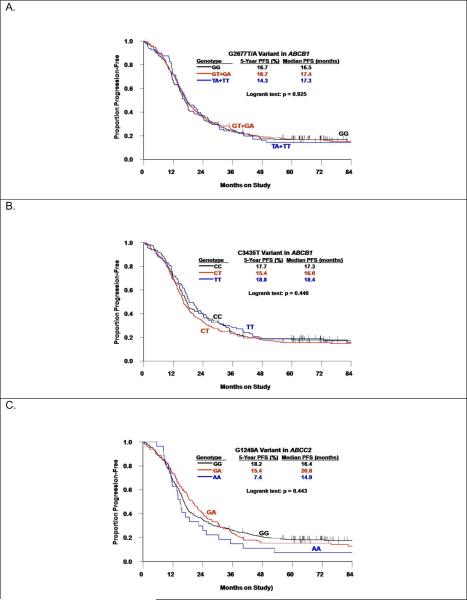
The G2677T/A and C3435T polymorphisms in ABCB1 were consistent with HWE (p>0.05) and demonstrated a strong linkage disequilibrium (p<0.001). Neither of these polymorphisms was associated with age, GOG performance score, tumor grade, histology, stage or residual disease status (data not shown), but both varied by race. A majority of African American women exhibited homozygous common allele genotypes for both of these polymorphisms in ABCB1, and rarely, if ever, displayed the homozygous variant(s). Neither polymorphism in ABCB1 appeared to be associated with PFS or OS in the full cohort (Table 2, Figure 1A and 1B) or the subset of non-African American patients (data not shown). In addition, exploratory subset analyses did not suggest any associations between these two polymorphisms in ABCB1 and outcomes in subgroups stratified by protocol, stage, residual disease status or treatment regimen.
Table 2.
Progression-free Survival (PFS) and Overall Survival (OS) by Polymorphisms in ABC Transporter Genes
| Genotype | No. (%) | HR | PFS 95% CI | P value | HR | OS 95% CI | P value |
|---|---|---|---|---|---|---|---|
| ABCB1 (G2677T/A) | |||||||
| GG | 165 (32.7) | Referent | Referent | ||||
| GT+GA | 259 (51.4) | 0.98 | 0.79–1.22 | 0.856 | 0.88 | 0.69–1.11 | 0.271 |
| TT+TA | 80 (15.9) | 1.02 | 0.76–1.38 | 0.877 | 0.93 | 0.67–1.29 | 0.663 |
| GT+GA+TT+TA | 339 (67.3) | 0.99 | 0.81–1.22 | 0.924 | 0.89 | 0.71–1.11 | 0.299 |
| ABCB1 (C3435T) | |||||||
| CC | 127 (25.8) | Referent | Referent | ||||
| CT | 266 (54.1) | 1.11 | 0.88–1.40 | 0.399 | 1.00 | 0.77–1.30 | 0.981 |
| TT | 99 (20.1) | 0.98 | 0.73–1.32 | 0.908 | 0.88 | 0.64–1.23 | 0.454 |
| CT+TT | 365 (74.2) | 1.07 | 0.86–1.34 | 0.550 | 0.97 | 0.76–1.24 | 0.803 |
| ABCC2 (G1249A) | |||||||
| GG | 313 (61.7) | Referent | Referent | ||||
| GA | 167 (32.9) | 0.98 | 0.80–1.20 | 0.839 | 0.89 | 0.71–1.12 | 0.318 |
| AA | 27 (5.3) | 1.62 | 1.06–2.48 | 0.025 | 0.86 | 0.52–1.44 | 0.572 |
| GA+AA | 194 (38.2) | 1.04 | 0.86–1.27 | 0.692 | 0.89 | 0.71–1.11 | 0.281 |
| ABCG2 (C421A) | |||||||
| CC | 404 (79.8) | Referent | Referent | ||||
| CA+AA | 102 (20.2) | 0.75 | 0.59–0.96 | 0.022 | 0.88 | 0.67–1.15 | 0.356 |
Hazard ratio (HR) with 95% confidence interval (CI) estimated from Cox model adjusted for cell type, stage/residual disease status, and treatment regimen.
Figure 1.
Kaplan-Meier estimates of progression-free survival (PFS) by the G2677T/A polymorphorism in ABCB1 (A), the C3435T polymorphorism in ABCB1 (B), or the G1249A polymorphorism in ABCC2 (C).
ABCC2 Polymorphism
The G1249A polymorphism in ABCC2 was not associated with patient characteristics including race (data not shown). Women with the AA vs the GA or GG genotype appeared to have worse PFS (Figure 1C) but this relationship did not achieve statistical significance (logrank test: p=0.443). When compared with women with the GG genotype, women with the AA genotype had an increased risk of disease progression (HR=1.62, 95% CI: 1.06–2.48, p=0.025) but not women with the GA genotype (HR=0.98, 95% CI: 0.80–1.20, p=0.839), or the GA+AA genotypes (HR=1.04, 95% CI: 0.86–1.27, p=0.692) (Table 2). The G1249A variant in ABCC2 was not associated with OS (Table 2). The exploratory subset analyses did not suggest that these results varied by protocol, stage, residual disease status or treatment regimen.
ABCG2 Polymorphism
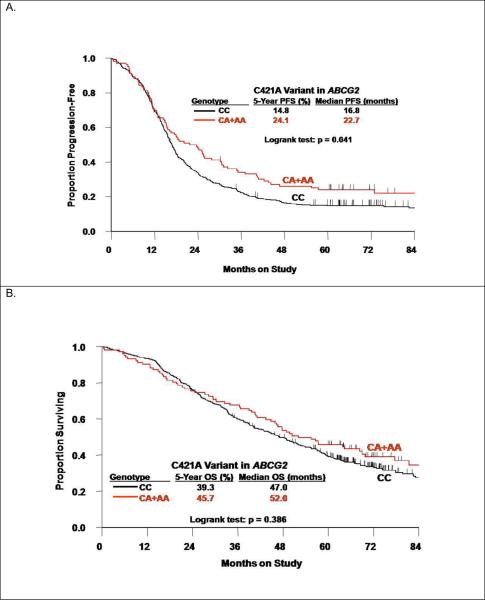
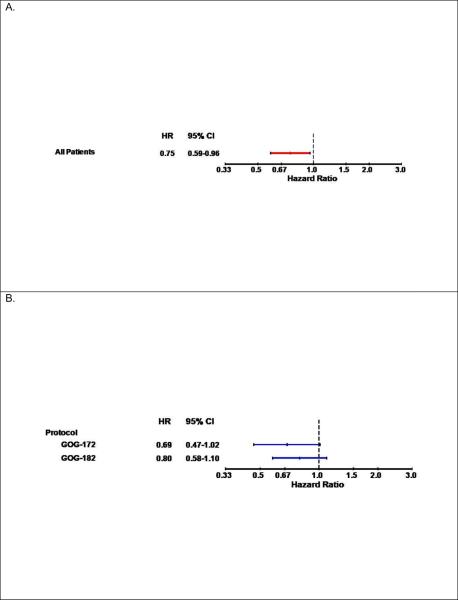
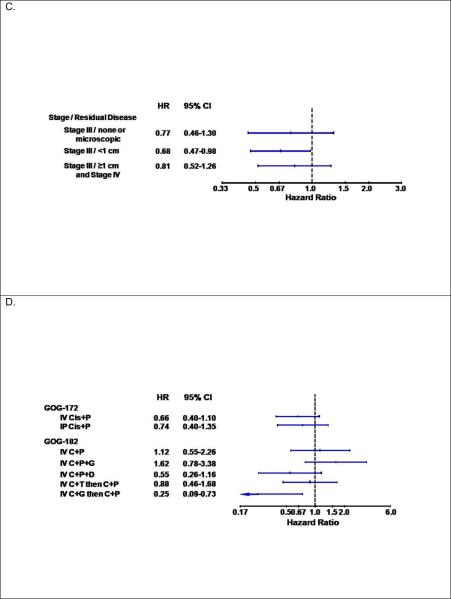
There were no associations between the C421A polymorphism in ABCG2 and patient characteristics including race (Table 3). Patients carrying at least one variant (A) allele in the ABCG2 C421A polymorphism appeared to have better PFS (logrank test: p=0.041, Figure 2A) and similar OS (Figure 2B, Table 3). Adjusted Cox modeling confirmed that women with the CA+AA genotypes compared with the CC genotype in the C421A polymorphism had a reduced risk of disease progression (HR=0.75, 95% CI: 0.59–0.96, p=0.022) and similar risk of death (Table 2). Figure 3 illustrates the results of the exploratory subset analyses for the ABCG2 C421A polymorphism and PFS. The strongest relationships appeared to be observed in the subset of patients with macroscopic stage III disease (Figure 3C) and those randomized to the sequential doublet with carboplatin+gemcitabine followed by carboplatin+paclitaxel (Figure 3D).
Table 3.
Association of ABCG2 Polymorphism with Clinical Characteristics
| CC No. (%) | CA+AA No. (%) | P value | |
|---|---|---|---|
| Age (years) | 0.453 | ||
| Median (range) | 58 (24–87) | 58 (30–83) | |
| Race | |||
| White | 368 (79.8) | 93 (20.2) | 0.912 |
| Black | 20 (87.0) | 3 (13.0) | |
| Other | 16 (72.7) | 6 (27.3) | |
| Performance Status | |||
| 0 | 194 (80.2) | 48 (19.8) | 0.232 |
| 1 | 191 (80.9) | 45 (19.1) | |
| 2 | 19 (67.9) | 9 (32.1) | |
| Stage/Debulking | 0.654 | ||
| Stage III – microscopic | 125 (82.2) | 27 (17.8) | |
| Stage III – optimal | 185 (79.7) | 47 (20.3) | |
| Stage III – suboptimal | 57 (79.2) | 15 (20.8) | |
| Stage IV | 37 (74.0) | 13 (26.0) | |
| Histology | |||
| Serous | 314 (79.9) | 79 (20.1) | 0.715 |
| Endometrioid | 27 (73.0) | 10 (27.0) | |
| Clear cell | 18 (78.3) | 5 (21.7) | |
| Mucinous | 3 (100) | 0 (0) | |
| Others | 42 (84.0) | 8 (16.0) | |
| Tumor Grade | |||
| 1 | 38 (82.6) | 8 (17.4) | 0.774 |
| 2 | 145 (78.4) | 40 (21.6) | |
| 3 | 221 (80.4) | 54 (19.6) |
Figure 2.
Kaplan-Meier estimates of progression-free survival (PFS) (A) and overall survival (OS) (B) by the C421A polymorphism in the ABCG2 gene.
Figure 3.
Plots with hazard ratios (HR) and 95% confidence intervals (CI) for disease progression for women with the CA+AA genotypes versus the CC genotype in the C421A variant in the ABCG2 gene in the full cohort (A) and by treatment protocol (B), the aggregate of stage and residual disease status (C) and treatment regimen (D). Cis: cisplatin; P: paclitaxel; C: carboplatin; G: gemcitabine; D: doxil; T: topotecan
DISCUSSION
This study focused on functional variants in ABCB1, ABCC2 and ABCG2 based on the role that these genes play in drug resistance [6–10], MDR [7, 8], and specifically in the efflux of cisplatin, carboplatin, paclitaxel, gemcitabine, doxorubicin and/or topotecan [5–7,11–14,16–18]. We found that the C421A variants in ABCG2 were associated with longer PFS in advanced stage EOC/PPC treated with platinum+paclitaxel-based chemotherapy. ABCG2 encodes BCRP/ABCP, a transporter for platinum agents, paclitaxel, doxorubicin, and topotecan that mediates drug absorption, distribution, elimination, MDR and appears to be associated with clinical outcome [5,15–18,30–39]. For example, Yoh et al [16] evaluated the expression of ABCB1, ABCC1, ABCC2, ABCC3 and ABCG2 in 72 patients with advanced non-small cell lung cancer (NSCLC) and reported an enhanced response to platinum (44% vs 24%) and improved PFS (p=0.0003) and OS (p=0.004) in ABCG2-negative patients compared with ABCG2-positive patients.
Although the functional effects of the ABCG2 C421A polymorphism remain inconclusive, some research suggests that the variant genotypes may result in altered ATPase activity [31,36] or reductions in the efflux activity of the transporter, thereby enhancing drug sensitivity [21–24,31,36–39]. The non-synonymous C421A polymorphism in ABCG2 results in an amino acid change, Gln141Lys. Sparreboom et al found that the CA heterozygote patients had a 1.34-fold increased oral bioavailability of topotecan compared with the common allele (42.0% vs 31.4%; p= 0.037) in the C421A polymorphism [39]. How variations in the functional activity of the transporter encoded by the ABCG2 gene influence chemotherapy response (directly or indirectly) in our study is not known. In the current investigation, we reported that patients with a variant allele (CA or AA genotype) had significantly longer PFS, but similar OS. The lack of an association between the ABCG2 C421A polymorphism and OS is thought to reflect, at least in part, the heterogeneity in salvage therapies employed following disease progression. In contrast to our findings, Marsh et al [40] showed that the C421A polymorphism in ABCG2 was not associated with PFS, CA125 response or clinical/radiologic response in the ovarian cancer Scottish Randomized Trial (SCOTROC1). Differences in the type of first-line chemotherapy, eligibility criteria, and end points as well as the statistical and laboratory methods employed in the GOG vs SCOTROC1 phase III trials, may explain, at least in part, the disparity in results observed between studies.
Our subset analyses suggest a stronger association between the ABCG2 C421A polymorphism and PFS in patients from GOG 172 as compared to GOG 182. GOG172 patients were treated with cisplatin+paclitaxel, while GOG 182 patients were treated with carboplatin+paclitaxel chemotherapy. Hence, it is likely that the difference in PFS and the C421A variant between the two trials is a result of the platinum used. There is evidence that the influx/efflux of carboplatin is slow, but persistent and less impacted by other metabolism factors as compared to cisplatin [6]. We speculate that the impact of this polymorphism on treatment effect is more pronounced in aggressive, toxic drugs, such as cisplatin. In addition, the exploratory subset analyses also identified the stronger associations between the C421A polymorphism and PFS in the subset of patients with macroscopic stage III disease and those randomized to the sequential doublet with carboplatin+gemcitabine followed by carboplatin+paclitaxel.
A number of studies suggest that expression of ABCB1 appears to be an indicator for efficacy of paclitaxel-based chemotherapy [10,41–43]. For example, expression of ABCB1 or p-glycoprotein was inversely associated with paclitaxel response and clinical outcome, and inhibition of ABCB1 expression by small interfering RNA was able to reverse the paclitaxel resistance in human ovarian cancer cells [10,41–43]. Of the more than 50 SNPs identified in the ABCB1 gene, G2677T/A is a non-synonymous polymorphism (encoding Ala893Ser/Thr), and C3435T is a synonymous polymorphism (encoding Ile1145Ile) associated with reduced ABCB1 mRNA expression [44]. To date, studies evaluating the relationship between ABCB1 polymorphisms and either gene function, pharmacokinetics, response or PFS have often yielded distinct results [40,45–48]. For example, our study examined ~500 EOC/PPC patients with stage III–IV disease treated with at least 6 cycles of cisplatin+paclitaxel or caboplatin+paclitaxel-based chemotherapy, and demonstrated that the either G2677T/A or C3435T polymorphism in ABCB1 was not correlated with PFS or OS, and no association was suggested in any subgroups stratified by stage, residual disease status or treatment regimen. In contrast, Gréen et al [47] demonstrated that the G2677T/A but not the C3435T polymorphism in ABCB1 was associated with categorized PFS (< 1 year vs ≥ 1 year) in 53 ovarian cancer patients treated with carboplatin+paclitaxel. Marsh et al. [40] studied stage I–IV ovarian cancer patients treated with either carboplatin+docetaxel or carboplatin+paclitaxel and showed that the G2677T/A but not the C3435T polymorphism in ABCB1 was associated with PFS (N<595) and that neither of these polymorphisms was associated with CA125 response (N<288) nor clinical/radiologic response (N<342). Johnatty et al [48] performed a population based study in 300 stage III–IV ovarian cancer patients in the Australian Ovarian Cancer study treated with at least 4 cycles of carboplatin+paclitaxel, and demonstrated that neither the G2677T/A or C3435T polymorphism was associated with PFS from multivariate analysis. They did identify an association of the G2677T/A polymorphism with PFS in the subset of patients optimally-resected (≤1 cm residual) disease but this finding was not validated in an independent set of patients [48]. The studies reported thus far are challenging to directly compare given methodologic limitations, inadequate power, confounding, multiple testing issues as well as differences in eligibility, end points, treatment regimens and analysis methods. Therefore, the prognostic or predictive role of the polymorphisms in ABCB1 in ovarian cancer therapy still remains to be investigated.
The final transporter gene we examined was ABCC2, which encodes MRP2. Expression of ABCC2 and MRP2 appears to be associated with the efflux of glutathione-conjugated drugs [6], MDR [8], cisplatin-resistance [11,12], paclitaxel-resistance [13] as well as the pharmacokinetics of paclitaxel [14]. In addition, knockdown of ABCC2 using anti-ABCC2 hammerhead ribozymes appears to restore platinum sensitivity in ovarian cancer cells [49]. Similar to other ABC transporter genes, there have been a number of reported SNPs in the ABCC2 gene. The G1249A polymorphism encodes Val417Ile, and one study reported that this polymorphism was associated with a higher activity of the intestinal transporter [50]. Our study did not demonstrate that the GA+AA genotypes vs GG genotype in the ABGCC2 G1249A polymorphism was associated with worse PFS or OS. There was weak evidence suggesting that women with the AA genotype had worse PFS relative to those with the GG or GA genotype, but this result should be interpreted with caution given the small number of patients with the AA genotype. The G1249A polymorphism was also studied in the ovarian cancer SCOTROC1 trial and found no association with PFS [47]. In addition, Han et al showed that this polymorphism was not associated with response in NSCLC patients treated with cisplatin+irinotecan [51,52].
In summary, the present study analyzed common polymorphisms in ABCB1 (G2677T/A; C3435T), ABCC2 (G1249A) and ABCG2 (C421A) in relation to PFS and OS. All patients had advanced stage EOC/PPC and were treated with first-line platinum+paclitaxel-based chemotherapy as part of their participation in the randomized phase III trial, GOG-172 or GOG-182. We found an association between ABCG2 C421A polymorphism and PFS in this population. Specifically, women with the CA+AA genotypes vs the CC genotype had improved PFS, and this association persisted after adjustment for clinical covariates. These findings may be particularly important given that this SNP occurs frequently in the population (~20% with a heterozygous or homozygous variant allele). Follow-up studies are being designed to validate the potential clinical utility of the C421A polymorphism in ABCG2 in independent GOG phase III trials of newly diagnosed EOC/PPC patients who undergo surgical staging with cytoreduction and are treated with platinum+taxane-based chemotherapy. Complimentary studies are also needed to determine the role that the C241A polymorphism plays, if any, in women with persistent or recurrent EOC/PPC patients. Studies such as this one provide direction for the design and execution of future studies. Ultimately clinical trials may employ genotype screening for treatment selection.
Supplementary Material
Research Highlights
Common variants in the ABC transporter genes are associated with survival in advanced stage EOC Patients
The C421A (CA+AA) variant in the ABCG2 gene shows improved PFS in patients with advanced stage EOC
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office and the GOG Tissue Bank (CA 27469), the GOG Statistical and Data Center (CA 37517) and to Roswell Park Cancer Institute (CA 016056-01) as well as grants from the Gynecologic Oncology Group/Ovarian Cancer Research Fund New Investigator Award (TK), The Jennie K. Scaife Foundation (JAD) and The Pittsburgh Foundation (JAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors meet the criteria for authorship, and each certifies that the manuscript represents valid work and has not been previously published nor is under consideration for publication elsewhere.
The following GOG member institutions participated in this translational research study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Emory University Clinic, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group, P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University Medical Center, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, SUNY Downstate Medical Center, University of Kentucky, Community Clinical Oncology Program, The Cleveland Clinic Foundation, Johns Hopkins Oncology Center, SUNY at Stony Brook, Eastern Pennsylvania GYN/ONC Center, PC, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, University of Massachusetts Medical Center, Fox Chase Cancer Center, Medical University of South Carolina, Women's Cancer Center, University of Oklahoma, University of Virginia, University of Chicago, Tacoma General Hospital, Thomas Jefferson University Hospital, Case Western Reserve University, Tampa Bay Cancer Consortium, North Shore University Hospital, Brookview Research, Inc.
CONFLICT OF INTEREST STATEMENT The authors wish to report that they have no conflicts of interest. However, Dr. Holly Gallion wishes to disclose that she is a stockowner of Precision Therapeutics and was an employee of Precision Therapeutics until 6/1/11.
REFERENCES
- 1.Cannistra SA. Cancer of the Ovary. N Engl J Med. 2005;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP. Current status of taxane and platinum-based chemotherapy in ovarian cancer. J Clin Oncol. 2003;21(10 Suppl):133s–135s. doi: 10.1200/JCO.2003.01.066. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33(2 Suppl 6):S3–11. doi: 10.1053/j.seminoncol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Modok S, Mellor HR, Callaghan R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr Opin Pharmacol. 2006;6:350–4. doi: 10.1016/j.coph.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–27. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 6.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 7.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2007;11:1156–66. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 8.Auner V, Sehouli J, Oskay-Oezcelik G, et al. ABC transporter gene expression in benign and malignant ovarian tissue. Gynecol Oncol. 2010;117:198–201. doi: 10.1016/j.ygyno.2009.10.077. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf RZ, Duan Z, Lamendola DE, et al. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets. 2003;3:1–19. doi: 10.2174/1568009033333754. [DOI] [PubMed] [Google Scholar]
- 10.Baekelandt MM, Holm R, Nesland JM, et al. P-glycoprotein expression is a marker for chemotherapy resistance and prognosis in advanced ovarian cancer. Anticancer Res. 2000;20:1061–7. [PubMed] [Google Scholar]
- 11.Guminski AD, Balleine RL, Chiew YE, et al. MRP2 (ABCC2) and cisplatin sensitivity in hepatocytes and human ovarian carcinoma. Gynecol Oncol. 2006;100:239–46. doi: 10.1016/j.ygyno.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 12.Surowiak P, Materna V, Kaplenko I, et al. ABCC2 (MRP2, cMOAT) can be localized in the nuclear membrane of ovarian carcinomas and correlates with resistance to cisplatin and clinical outcome. Clin Cancer Res. 2006;12:7149–58. doi: 10.1158/1078-0432.CCR-06-0564. [DOI] [PubMed] [Google Scholar]
- 13.Huisman MT, Chatter AA, van Tellingen O, et al. MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer. 2005;116:824–9. doi: 10.1002/ijc.21013. [DOI] [PubMed] [Google Scholar]
- 14.Lagas JS, Vlaming ML, van Tellingen O, et al. Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics. Clin Cancer Res. 2006;12(20 Pt 1):6125–32. doi: 10.1158/1078-0432.CCR-06-1352. [DOI] [PubMed] [Google Scholar]
- 15.Robey RW, Polgar O, Deeken J, et al. ABCG2: Determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 16.Yoh K, Ishii G, Yokose T, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–7. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 17.Robey RW, To KK, Polgar O, et al. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litman T, Brangi M, Hudson E, et al. The multidrug-resistant phenotype associated with over expression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2003;113(Pt 11):2011–21. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 19.Wada M. Single nucleotide polymorphisms in ABCC2 and ABCB1 genes and their clinical impact in physiology and drug response. Cancer Lett. 2006;234:40–50. doi: 10.1016/j.canlet.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 20.Cervenak J, Andrikovics H, Ozvegy-Laczka C, et al. The role of the human ABCG2 multidrug transporter and its variants in cancer therapy and toxicology. Cancer Lett. 2006;234:62–72. doi: 10.1016/j.canlet.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 21.Yanase K, Tsukahara S, Mitsuhashi J, et al. Functional SNPs of the breast cancer resistance protein-therapeutic effects and inhibitor development. Cancer Lett. 2006;234:73–80. doi: 10.1016/j.canlet.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Morisaki K, Robey RW, Ozvegy-Laczka C, et al. Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol. 2005;56:161–72. doi: 10.1007/s00280-004-0931-x. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa T, Wakabayashi K, Tamura A, et al. Major SNP (Q141K) variant of human ABC transporter ABCG2 undergoes lysosomal and proteasomal degradations. Pharm Res. 2009;26:469–79. doi: 10.1007/s11095-008-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollex EK, Anger G, Hutson J, et al. Breast cancer resistance protein (BCRP)-mediated glyburide transport: effect of the C421A/Q141K BCRP single-nucleotide polymorphism. Drug Metab Dispos. 2010;38:740–4. doi: 10.1124/dmd.109.030791. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 26.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of New Platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer InterGroup (GCIG) J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baysal BE, DeLoia JA, Willett-Brozick JE, et al. Analysis of CHEK2 gene for ovarian cancer susceptibility. Gynecol Oncol. 2004;95:62–9. doi: 10.1016/j.ygyno.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Weiss JR, Baer MR, Ambrosone CB, et al. Concordance of pharmacogenetic polymorphisms in tumor and germ line DNA in adult patients with acute myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 2007;16:1038–41. doi: 10.1158/1055-9965.EPI-06-0964. [DOI] [PubMed] [Google Scholar]
- 29.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 30.Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7:E118–33. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ota S, Ishii G, Goto K, et al. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer. 2009;64:98–104. doi: 10.1016/j.lungcan.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Kim YH, Ishii G, Goto K, et al. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer. 2009;65:105–11. doi: 10.1016/j.lungcan.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Brooks TA, Minderman H, O'Loughlin KL, et al. Taxane-based reversal agents modulate drug resistance mediated by P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Mol Cancer Ther. 2003;2:1195–205. [PubMed] [Google Scholar]
- 35.Maliepaard M, van Gastelen MA, de Jong LA, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–63. [PubMed] [Google Scholar]
- 36.Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int J Cancer. 2004;109:238–46. doi: 10.1002/ijc.11669. [DOI] [PubMed] [Google Scholar]
- 37.Imai Y, Nakane M, Kage K, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1:611–6. [PubMed] [Google Scholar]
- 38.Bram EE, Ifergan I, Grimberg M, et al. C421 allele-specific ABCG2 gene amplification confers resistance to the antitumor triazoloacridone C-1305 in human lung cancer cells. Biochem Pharmacol. 2007;74:41–53. doi: 10.1016/j.bcp.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Sparreboom A, Loos WJ, Burger H, et al. Effect of ABCG2 genotype on the oral bioavailability of topotecan. Cancer Biol Ther. 2005;4:650–8. doi: 10.4161/cbt.4.6.1731. [DOI] [PubMed] [Google Scholar]
- 40.Marsh S, Paul J, King CR, et al. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the Scottish Randomised Trial in Ovarian Cancer. J Clin Oncol. 2007;25:4528–35. doi: 10.1200/JCO.2006.10.4752. [DOI] [PubMed] [Google Scholar]
- 41.Hille S, Rein DT, Riffelmann M, et al. Anticancer drugs induce MDR1 gene expression in recurrent ovarian cancer. Anticancer Drugs. 2006;17:1041–4. doi: 10.1097/01.cad.0000231480.07654.b5. [DOI] [PubMed] [Google Scholar]
- 42.Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3:833–8. [PubMed] [Google Scholar]
- 43.Penson RT, Oliva E, Skates SJ, et al. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol Oncol. 2004;93:98–106. doi: 10.1016/j.ygyno.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 44.Hitzl M, Drescher S, van der Kuip H, Schäffeler E, et al. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics. 2001;11:293–8. doi: 10.1097/00008571-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Leschziner GD, Andrew T, Pirmohamed M, et al. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics. 2007;J 7:154–79. doi: 10.1038/sj.tpj.6500413. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima M, Fujiki Y, Kyo S, et al. Pharmacokinetics of paclitaxel in ovarian cancer patients and genetic polymorphisms of CYP2C8, CYP3A4, and MDR1. J Clin Pharmacol. 2005;45:674–82. doi: 10.1177/0091270005276204. [DOI] [PubMed] [Google Scholar]
- 47.Gréen H, Söderkvist P, Rosenberg P, et al. mdr-1 single nucleotide polymorphisms in ovarian cancer tissue: G2677T/A correlates with response to paclitaxel chemotherapy. Clin Cancer Res. 2006;12(3 Pt 1):854–9. doi: 10.1158/1078-0432.CCR-05-0950. [DOI] [PubMed] [Google Scholar]
- 48.Johnatty SE, Beesley J, Paul J, et al. ABCB1 (MDR 1) polymorphisms and progression-free survival among women with ovarian cancer following paclitaxel/carboplatin chemotherapy. Clin Cancer Res. 2008;14:5594–601. doi: 10.1158/1078-0432.CCR-08-0606. [DOI] [PubMed] [Google Scholar]
- 49.Materna V, Liedert B, Thomale J, et al. Protection of platinum-DNA adduct formation and reversal of cisplatin resistance by anti-MRP2 hammerhead ribozymes in human cancer cells. Int J Cancer. 2005;115:393–402. doi: 10.1002/ijc.20899. [DOI] [PubMed] [Google Scholar]
- 50.Haenisch S, May K, Wegner D, et al. Influence of genetic polymorphisms on intestinal expression and rifampicin-type induction of ABCC2 and on bioavailability of talinolol. Pharmacogenet Genomics. 2008;18:357–65. doi: 10.1097/FPC.0b013e3282f974b7. [DOI] [PubMed] [Google Scholar]
- 51.Han JY, Lim HS, Yoo YK, et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110:138–47. doi: 10.1002/cncr.22760. [DOI] [PubMed] [Google Scholar]
- 52.Han JY, Lim HS, Yoo YK, et al. Errata - Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2010;116(15):3749. doi: 10.1002/cncr.22760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.