Abstract
Purpose
To explore the association between baseline quality of life (QOL) scores and overall survival (OS) in ovarian cancer patients receiving adjuvant chemotherapy.
Methods
Patients with stage III ovarian cancer on Gynecologic Oncology Group protocol #172 completed the Functional Assessment of Cancer Therapy-General (FACT-G) and were then randomly assigned to either intravenous (IV) or intraperitoneal (IP) chemotherapy. The FACT scale includes physical, functional, social, and emotional well-being domains (PWB, FWB, SWB, EWB). The PWB item, lack of energy, was used to assess the presence of fatigue.
Results
After adjusting for patient age, treatment assignment, and the presence of gross disease, PWB was associated with OS. Patients who reported baseline PWB scores in the lowest 25% (PWB score < 15 points) relative to those who scored in the highest 25% (PWB score > 24 points) had decreased OS (HR: 1.81; 95% CI: 1.2~2.72; p=0.005). Patients experienced death rates 20% lower for every mean item point increase in PWB (Hazard Ratio [HR]: 0.80; 95% CI: 0.68 ~ 0.93; p=0.005). Patients complaining of fatigue did not have an increased risk of death compared with those not feeling fatigued (HR: 1.21; 95% CI: 0.91~1.61; p=0.19).
Conclusions
Poor physical well-being reported at baseline is associated with risk of death in patients undergoing adjuvant chemotherapy for advanced ovarian cancer. Identifying modifiable characteristics that are associated with survival offers the potential for providing support that may improve outcomes.
Keywords: ovarian cancer, quality of life, overall survival, physical well-being
INTRODUCTION
Quality of life (QOL) assessments are imperative in assessing cancer burden, treatment and prognosis. A recent meta-analysis using 30 randomized controlled trials from the European Organization for Research and Treatment of Cancer (EORTC) which included survival data for over 10,000 patients with 11 different cancer sites found that QOL is predictive of survival [1]. Wenzel et al., with the Gynecologic Oncology Group (GOG), examined QOL in ovarian cancer patients and established a predictive value of baseline QOL on survival [2].
QOL includes the physical, functional, social and emotional domains of an individual. Research from the GOG revealed that the domains most effected by chemotherapy are physical and functional well-being (PWB, FWB) [2,3]. Ancillary data analysis of domain item scores was conducted in women treated with intravenous (IV) chemotherapy. Large differences for lack of energy, and being bothered by side effects of treatment were observed in women whose overall QOL score was in the lowest quartile versus those in the higher three quartiles [4,5]. Therefore, patients whose total FACT score is in the lowest quartile are reporting problems in specific areas that may be applicable for clinical evaluation and clinical intervention.
These studies suggest there may be indicators within QOL that identify women at risk for reduced likelihood of overall survival (OS) which may be amenable to interventions. The primary objective of this study was to explore the association between baseline QOL domain scores and OS in ovarian cancer patients receiving IV and/or intraperitoneal (IP) adjuvant chemotherapy. The secondary objective was to assess the role of fatigue in physical well-being. We hypothesized that PWB and FWB would have the largest effect on OS and the contributions of specific line items within these domains would be substantial.
METHODS
Patients
GOG 172 measured QOL in a randomized study of IV paclitaxel and cisplatin versus IV paclitaxel, IP cisplatin and IP paclitaxel in optimally debulked stage III epithelial ovarian cancer patients. Participating institutions obtained institutional review board approval of the protocols before enrolling any patients; all patients provided written informed consent consistent with all federal, state, and local requirements before they received any protocol therapy. Questionnaires were administered before randomization, cycle 4, 3–6 weeks after treatment and 12 months after treatment and results were not a stratification variable [3]. Treatment information and outcome has been previously reported [3,6].
Methods
QOL was measured with the Functional Assessment of Cancer-Therapy-General (FACT-G) questionnaire. The FACT-G, version 4, is a 27-item core questionnaire evaluating the domains of physical, functional, family-social, and emotional well-being (PWB, FWB, SWB, EWB) [7]. Questions are answered on a 5-point Likert scale and items are summed to give scores for each domain. Reliability, validity and sensitivity to change of the FACT-G have been demonstrated in a variety of settings and relative scores can be compared to normative data.
Total FACT scores for all patients were calculated. Patients were divided into quartiles of scores according to their total FACT score (Q1-Q4). Patients in the lowest quartile (Q1) were compared to women in the upper 3 quartiles (Q2-4). This comparison was chosen to expand upon Wenzel et al's observation that baseline QOL was predictive for survival and was primarily attributed to the lowest-scoring quartile [2]. A Cox proportional hazards model [8] was fitted for the 4 subscale scores respectively to explore the association between the baseline FACT-G subscale scores and OS. The model was adjusted for patient age, treatment assignment, and the presence of gross disease.
To further explore the association between PWB and survival, the baseline PWB score was classified into 4 levels according to 25th, 50th, and 75th quartiles. In order to explore the effect of fatigue on PWB, patients who chose the worst 2 categories of lack of energy (that is ‘3= Quite a Bit’ or ‘4-very much’) were considered as having ‘worse fatigue’. The “lack of energy” question was used as a surrogate marker for fatigue as the FACT-F was not used in this study.
RESULTS
Between March 1998 and January 2001, 415 eligible patients in GOG 172 were randomly assigned to either the IV (n=210) or IP (n=205) treatment arm. Three hundred and ninety-nine (96%) eligible patients (201 patients in IV arm and 198 patients in IP arm) completed baseline QOL assessment. The majority of eligible patients were non-Hispanic white (>89%), between the ages of 41-70 (80%), with performance status of 0 or 1 (>92%) [3].
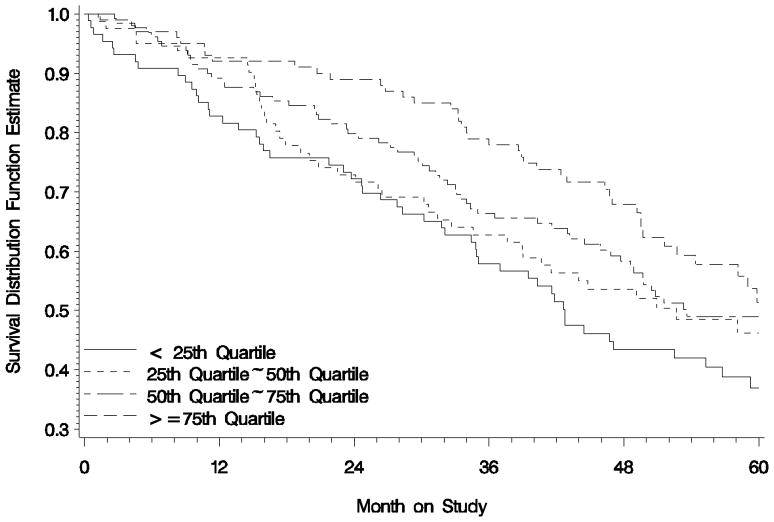
There were associations between baseline FACT-G subscales and OS. After adjusting for patient age, treatment assignment, and the presence of gross disease, none of the subscales as a continuous variable showed statistically significant association with OS except for the PWB score. These patients experienced death rates 20% lower for every mean item point (Hazard Ratio [HR]: 0.80; 95% CI: 0.68 ~ 0.93; p=0.005). Baseline PWB score was classified into 4 levels according to quartiles of 25th, 50th, and 75th which were 15, 19, and 24 respectively. The median duration of survival and relative risk of death for each PWB scoring levels are presented in Table 1 and Figure 1. The survival is attributed primarily to the patients who reported baseline PWB score in the lowest level (PWB score < 15 points) relative to those who scored in the highest level (PWB score > 24 points) (HR: 1.81; 95% CI: 1.2~2.72; p=0.005). This relationship was not observed in SWB, EWB and FWB.
Table 1.
Median overall survival and relative risk of death by physical well-being (PWB) quartiles at baseline
| PWB scoring level by quartiles | ||||
|---|---|---|---|---|
| <25th | 25th ~ 50th | 50th ~75th | >=75th | |
| No. at risk | 87 | 81 | 130 | 100 |
| No. of death | 53 | 42 | 63 | 43 |
| Median survival (months) | 42.7 | 52.7 | 53.6 | 60.4 |
| Relative Risk (95 % CI) relative to >75th quartile | 1.81 (1.20~2.72) P=0.005 |
1.37 (0.89~2.11) P=0.15 | 1.24 (0.85~1.83) P=0.27 | |
Note: a higher PWB score indicates better physical well-being.
Figure 1.
Overall Survival By Baseline PWB Score Quartiles
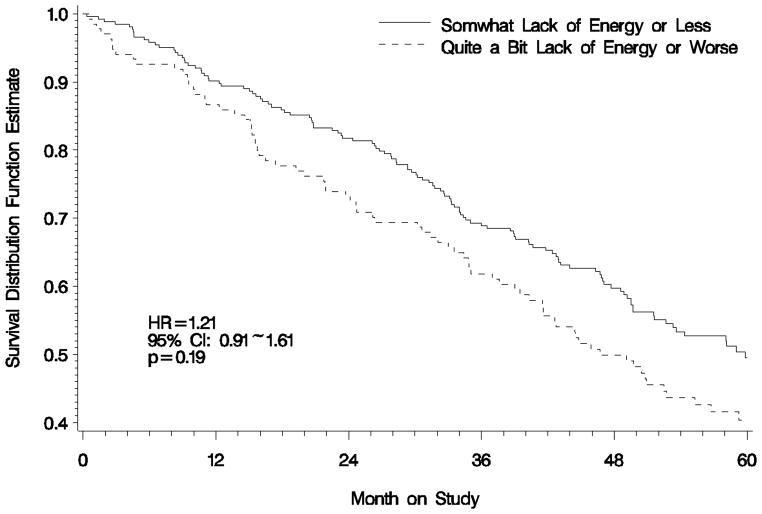
After adjusting for age and treatment assignment, the estimated death rate suggested that patients who complained of ‘worse fatigue’ did not experience a significantly greater risk of death compared with those not feeling ‘worse fatigue’ (HR: 1.21; 95% CI: 0.91~1.61; p=0.19) Figure 2.
Figure 2.
Overall Survival by Patient-Reported 'Lack of Energy' Score at Baseline
To explore QOL domain differences between IP and IV chemotherapy, subscales were analyzed. The FACT-G subscale scores of these patients are summarized in Table 2. During treatment, those who received IP therapy had statistically significant compromise in all domains compared to those who received IV, however, there was no difference in the pre-randomization, nor after treatment time points. In addition, PWB was compromised in the IP group during pre-randomization, PWB and FWB during treatment, and PWB 3-6 weeks after treatment.
Table 2.
FACT-G subscale scores between IV and IP arms
| IV Arm | IP Arm | IV-IP | |||||
|---|---|---|---|---|---|---|---|
| Assessment time point | Mean | SD | Mean | SD | Mean Difference | 95% CI | p |
| Pre-Randomization | N=187 | N=168 | |||||
| PWB | 19.8 | 5.7 | 18.3 | 6.1 | 1.5 | 0.3~2.7 | 0.016 |
| SWB | 23.5 | 4.0 | 23.0 | 4.1 | 0.5 | −0.3~1.4 | 0.227 |
| EWB | 16.7 | 4.7 | 16.6 | 4.9 | 0.1 | −0.9~1.1 | 0.907 |
| FWB | 16.1 | 6.4 | 14.8 | 5.9 | 1.3 | −0.0~2.5 | 0.051 |
| Pre-Cycle 4 | N=169 | N=143 | |||||
| PWB | 18.9 | 5.4 | 16.3 | 5.9 | 2.6 | 1.4~3.8 | <0.001 |
| SWB | 23.1 | 3.5 | 22.0 | 3.8 | 1.0 | 0.3~1.8 | 0.009 |
| EWB | 18.4 | 3.9 | 17.3 | 4.5 | 1.1 | 0.3~1.9 | 0.006 |
| FWB | 16.5 | 6.5 | 14.3 | 5.5 | 2.2 | 1.1~3.4 | <0.001 |
| 3~6 weeks after treatment | N=168 | N=154 | |||||
| PWB | 20.4 | 5.2 | 18.8 | 5.7 | 1.7 | 0.6~2.9 | 0.003 |
| SWB | 22.7 | 3.7 | 22.4 | 4.5 | 0.3 | −0.5~1.1 | 0.450 |
| EWB | 18.1 | 4.2 | 17.8 | 4.4 | 0.3 | −0.5~1.1 | 0.407 |
| FWB | 18.4 | 6.2 | 16.0 | 6.1 | 2.5 | 1.2~3.7 | <0.001 |
| 12 months after treatment | N=137 | N=135 | |||||
| PWB | 22.7 | 4.8 | 22.8 | 5.1 | -0.1 | −1.2~1.0 | 0.852 |
| SWB | 22.9 | 3.8 | 22.8 | 4.7 | 0.2 | −0.7~1.1 | 0.693 |
| EWB | 18.5 | 4.3 | 18.3 | 4.2 | 0.3 | −0.6~1.2 | 0.523 |
| FWB | 21.4 | 6.0 | 21.5 | 5.9 | −0.1 | −1.4~1.1 | 0.822 |
Functional Assessment of Cancer Therapy-General (FACT-G), PWB=physical well-being, SWB=social well-being, EWB=emotional well-being, FWB=functional well-being, SD=standard deviation, CI=confidence interval
The mean scores (mean ± S.E) in the follow-up assessments are the least squared means estimated from the fitted linear mixed model and adjusted for baseline scores and age.
DISCUSSION
QOL influences survivorship in cancer patients [1,2]. The purpose of this study was to assess what domains of QOL are affected in order to design interventions to improve QOL and OS. Physical well-being, remarkably, was the domain associated with OS. These patients experienced death rates 20% lower for every mean point item. If patients, with physician support, can improve even a point on the physical realm, patient outcomes may have significant improvements.
QOL dysfunction may be different and affect patients uniquely for each primary cancer site. The European Organization for Research and Treatment of Cancer (EORTC) selected 30 randomized controlled trials, which analyzed standard QOL measures with survival data for over 10,000 patients with 11 different cancer sites [1]. The QOL parameters of pain, appetite loss, and physical functioning were all significant for survival. Wenzel et al with the GOG examined QOL in ovarian cancer patients and established a predictive value of baseline QOL on survival, attributed primarily to the lowest-scoring quartile [2]. Specifically, the patients scoring in the lowest QOL quartile at mid-treatment baseline experienced a greater death rate.
Prior research from the GOG and others has revealed that the domains most affected by intravenous chemotherapy are PWB and FWB [3,9]. An ancillary data analysis of from GOG 152 and 172 in patients receiving adjuvant IV chemotherapy revealed large differences between women with QOL scores in the lowest quartile versus women in the higher 3 quartiles in lack of energy (p<0.001), being bothered by side effects of treatment and sleeping disturbance (p<0.001) [4]. Patients whose total FACT score is in the lowest quartile are reporting problems in specific areas that may be amenable to clinical evaluation and future interventions. Therefore, the identification of specific side effects in patients receiving IV or IP chemotherapy will aid in the development of interventions to improve QOL (such as symptom management) and may also aid in the identification of women with poor prognosis for survival.
Physical functioning and its relationship to cancer have become the focus of outcomes studies. Similar to ovarian cancer patients, adjuvant chemotherapy improves OS in breast cancer patients, yet causes unfavorable changes in QOL and physical functioning [10]. Furthermore, there is an inverse relationship between physical activity and mortality in breast cancer patients [11]. This supports the concept that the physical domain, in addition to physical activity is important in OS. Additionally, patients receiving IP chemotherapy for ovarian cancer have higher survival yet lower chemotherapy completion rates. Improving physical function and chemotherapy side effects may result in improving chemotherapy completion rates with the potential to improve OS.
Ovarian cancer patients commonly suffer from fatigue during chemotherapy. Research from a single institutional trial revealed that mean scores for fatigue domains decreased during adjuvant chemotherapy but increased to peri-operative levels following chemotherapy [8]. However, in this present study the line item for “lack of energy” may not be able to measure the global problem. Cella et al suggest that cancer-related fatigue is a multifaceted condition and specific tools have been developed to measure the problem [12]. This GOG study was initiated after development of the FACT-F.
Strengths of this study include an innovative approach to QOL analysis in a cooperative group setting, size of the study sample, and well-controlled data collection. The GOG collects limited demographic and clinical factors therefore we were unable to perform a more robust comparison of patient characteristics and develop clinical clusters for potential intervention. The GOG also does not collect co-morbidity measures and cause of death, which would be helpful in analyzing physical function. Additional study and potential line item analysis is needed to examine other factors which may account for additional variation of QOL in the patient population.
Poor PWB is associated with decreased OS. Results from this ancillary study will be central in forthcoming interventional trials focused on symptom management and reducing side effects from chemotherapy, and could help to improve stratification and the design of randomized controlled trials. Finally, understanding the significance of the physical domain may aid physicians in how to counsel patients receiving IV or IP chemotherapy. This type of research supports a patient-centered approach to cancer care.
RESEARCH HIGHLIGHTS.
Physical QOL is associated with OS in ovarian cancer patients receiving adjuvant chemotherapy.
Poor physical well-being at baseline is associated with risk of death in patients undergoing adjuvant chemotherapy for advanced ovarian cancer.
Identifying modifiable characteristics that are associated with survival offers the potential for providing support that may improve outcomes.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517). The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, University of Kentucky, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Columbus Cancer Council, University of Massachusetts Medical School, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Tacoma General Hospital, Thomas Jefferson University Hospital, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, North Shore University Hospital, Gynecologic Oncology Network, Ellis Fischel Cancer Center, and Fletcher Allen Health Care.
Footnotes
Presented at the American Society of Clinical Oncologists, Chicago, IL, June 4-8, 2010.
CONFLICT OF INTEREST
The co-authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–71. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel LB, Huang HQ, Monk BJ, Rose PG, Cella D. Quality-of life comparisons in a randomized trial of interval secondary cytoreduction in advanced ovarian carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2005;23:5605–12. doi: 10.1200/JCO.2005.08.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer; a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:437–43. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 4.von Gruenigen VE, Huang HQ, Gil KM, Gibbons HE, Monk BJ, Rose PG, et al. Assessment of factors that contribute to decreased quality of life in Gynecologic Oncology Group ovarian cancer trials. Cancer. 2009;115:4857–64. doi: 10.1002/cncr.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Gruenigen VE, Huang HQ, Gil K, Gibbons HE, Monk BJ, Rose PG, et al. A comparison of quality of life domains and clinical factors in ovarian cancer patients: a Gynecologic Oncology Group study. J Pain and Symptom Manage. 2010;39:839–46. doi: 10.1016/j.jpainsymman.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;54:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 7.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 8.Cox DR, Oaker D. Analysis of Survival Data. London: Chapman and Hall; 1984. [Google Scholar]
- 9.von Gruenigen VE, Gil K, Frasure HE, Jenison EL, Hopkins MP, Gil KM. Longitudinal assessment of quality of life and lifestyle in newly diagnosed ovarian cancer patients: the roles of surgery and chemotherapy. Gynecol Oncol. 2006;103:120–6. doi: 10.1016/j.ygyno.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 10.Kwan ML, Ergas IJ, Somkin CP, Quesenberry CP, Jr, Neugut AI, Hershman DL, et al. Quality of life among women recently diagnosed with invasive breast cancer: the Pathways Study. Breast Cancer Res Treat. 2010;123:507–24. doi: 10.1007/s10549-010-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2010;28:753–65. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 12.Cella D, Lai JS, Stone A. Self-reported fatigue: one dimension or more? Lessons from the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire Support Care. Cancer. 2011;19:1441–50. doi: 10.1007/s00520-010-0971-1. [DOI] [PubMed] [Google Scholar]