Abstract
The ubiquitin proteasome system (UPS) is the main proteolytic system of cells. Recent evidence suggests that the UPS plays a regulatory role in regeneration processes. Here we explore the possibility that the UPS is involved during intestinal regeneration of the sea cucumber Holothuria glaberrima. These organisms can regenerate most of their digestive tract following a process of evisceration. Initially, we identified components of H. glaberrima UPS, including sequences for Rpn10, β3 and ubiquitin-RPL40. Predicted proteins from the mRNA sequences showed high degree of conservation that ranged from 60% (Rpn10) to 98% (Ub-RPL40). Microarrays and RT-PCR experiments showed that these genes were up-regulated during intestinal regeneration. In addition, we demonstrated expression of alpha 20S proteasome subunits and ubiquitinated proteins during intestinal regeneration and detected them in the epithelium and connective tissue of the regenerating intestine. Finally, the intestinal regeneration was altered in animals treated with MG132, a proteasome inhibitor. These findings support our contention that proteasomes are playing an important role during intestinal regeneration.
Keywords: Echinoderms, sea cucumber, regeneration, protein degradation, proteasome
INTRODUCTION
Every year thousands of people are afflicted by gastrointestinal diseases, such as inflammatory bowel disease, chronic colitis, ulcers and gastrointestinal cancers. Some of these diseases are due in part to a failure in the regenerative mechanisms of the digestive system (Thompson et al. 2000). The cellular interactions involved in gastrointestinal regeneration have received considerable attention; however, little is known about the molecular machinery that guides this process. The analysis of these molecular events deserves close attention, since it could provide new insights to regenerative medicine and its clinical applications.
Our laboratory has developed the use of the sea cucumber Holothuria glaberrima as an excellent model to study the digestive tract regenerative processes. This organism undergoes intestinal organogenesis following a process of evisceration. We have described the cellular events associated with intestinal regeneration (García-Arrarás et al. 1998, Quiñones et al. 2002, Murray and García-Arrarás 2004, Candelaria et al. 2006,) and are now interested in identifying and characterizing the molecules involved. Initial studies analyzing expressed sequence tags (ESTs) expression demonstrated that during intestinal regeneration there is a large differential expression of genes (Rojas-Cartagena et al. 2007). Of particular interest was the identification of ESTs associated with the ubiquitin proteasome system (UPS).
The UPS is the main cellular proteolytic system that uses ATP to degrade ubiquitinated proteins (Glickman and Ciechanover, 2002). This system is a multienzymatic complex, composed of a proteolytic core, termed 20S proteasome, and one or two regulatory particles (RP), known as PA700 or 19S that associate with the 20S proteasome to form the 26S proteasome. Proteolysis is accomplished by three protease activities: chymotrypsin-like, trypsin-like, and postglutamyl peptidyl hydrolases (PGPH) present in the β-subunits (Coux et al. 1996, Baumeister et al. 1998, Myung et al. 2001). Proteins to be degraded by the proteasome must be covalently linked to ubiquitin (Ub).
Several reports have indicated that the UPS may play an important role in embryonic development both in Drosophila (Lier and Paululat, 2002) and mammals (Mtango and Latham, 2007, El-Khodor et al. 2001, Morimoto et al. 2006). The UPS also appears to be involved in some regenerative processes, particularly those associated with bone regeneration (Garret et al. 2003, Mukherjee et al. 2008). Moreover, in echinoderms, ubiquitin conjugates have been shown to accumulate during arm regeneration (Patruno et al. 2001). In this work, we have used computational and biochemical approaches to analyze several holothurian UPS genes and to study their expression during intestinal regenerative organogenesis in H. glaberrima. Our results provide important information on the evolution and conservation of the proteasome subunit homologues in echinoderms. In addition, our data provide a view of their temporal expression pattern and suggest a possible regulatory role of the UPS during intestinal regenerative organogenesis.
RESULTS
Clones from cDNA libraries of H. glaberrima correspond to UPS components
To identify the holothurian putative UPS components, we isolated clones from H. glaberrima cDNA libraries that showed significant similarities to UPS components of other species. The clones were fully sequenced and when necessary RACE-PCR was done to obtain the missing upstream sequence. Their predicted protein sequence was obtained and compared using the BLAST algorithm against protein databases in NCBI and SwissProt.
Proteasome subunit Rpn10 (clone P3DP12H09)
One EST with similarity to the proteasome Rpn10 subunit was found in the 3 days post evisceration (dpe) cDNA library. The 1299 nucleotides sequence encoded a predicted protein of 394 amino acids (Supplementary, Fig. 1A). To determine the degree of conservation, we generated a multiple alignment that included Rpn10 sequences from vertebrate and invertebrate species (Fig. 1). The holothurian sequence showed an average 60–70% similarity to those of species used in the alignment. Thus, according to the nomenclature proposed by Finley (1998), we have called this protein HgRpn10.
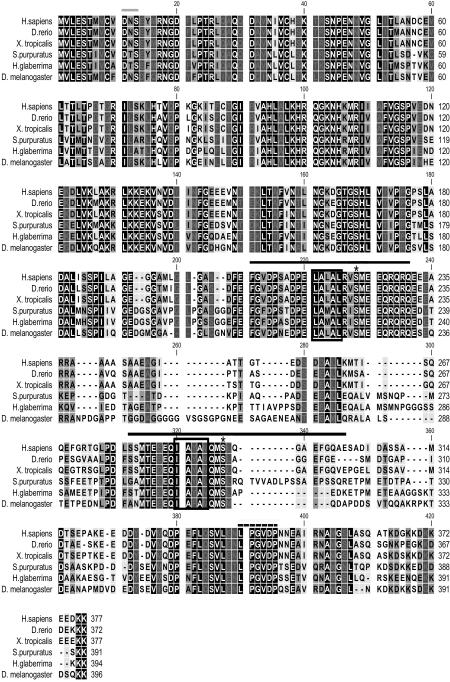
Figure 1.
Amino acid sequence alignment of H. glaberrima Rpn10 with Homo sapiens (gi:5292161), Danio rerio (gi:50344880), Xenopus tropicalis (gi:47497982) Strongylocentrotus purpuratus (gi:72168692) and Drosophila melanogaster (gi:28317298) homologues. BLAST results showed high similarity to the 26S proteasome non-ATPase subunit 4 transcript variant 1 (synonyms: Rpn10, S5a, pUB-R5) from a large number of species. The highest similarity was with the sea urchin Strongylocentrotus purpuratus Rpn10 with an e-value of 1e-143 (using BLASTP and the NCBI's non-redundant (nr) protein database) and 80% similarity. The black line shows the ubiquitin interacting motif 1 and 2 (UIM). The box shows the amino acids required to bind polyubiquitin chains and the asterisks show conserved serine residues. The characteristic motif common to all vWA domains DxS, is located at residues 11 (gray line). A conserved portion at the C-terminal end is marked by a dashed line. All the conserved sites are shown in white letters on black.
HgRpn10 has 17 additional residues than that of H. sapiens and X. tropicalis and 22 more than D. rerio. It was similar in size to those of S. purpuratus and D. melanogaster, which were 391 and 396 residues respectively. It showed all conserved domains and residues characteristic of Rpn10. First, the N-terminal region spans about 150 residues corresponding to a highly conserved von Willebrand factor A domain (vWA). Second, all vWA domains contain a conserved aspartate in a DXS motif (Hofmann and Falquet, 2001). Third, two ubiquitin interacting motifs (UIM 1 and 2) were located in the C-terminal half of the protein (Young et al. 1998). Fourth, the C-terminus end of all proteins contained an identical region of six residues L362PGVDP367 and a typical final portion enriched in glutamates (E) and lysines (K). In addition, we performed a phylogenetic analysis with 29 Rpn10 from a wide selection of organisms and 31 proteins representing 7 subfamilies of the vWA factor superfamily. The Maximum Likelihood tree shows the subfamilies clustered as expected, with the echinoderm sequences grouping together in the clade of invertebrate Rpn10 (Supplementary, Fig. 2).
Proteasome subunit β3 (PNLPO2E07)
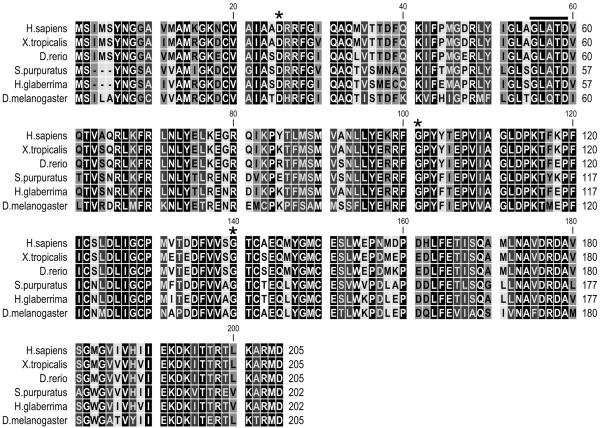
One 1058 nucleotide EST encoding a putative protein of 202 residues with strong similarity to the proteasome subunit β3 was found in the normal intestine cDNA library (Supplementary, Fig. 1B). Sequence comparisons showed around 90% similarity to proteasome subunit β3 of other animal species (Fig. 2).
Figure 2.
Amino acid sequence alignment of H. glaberrima β3 with Homo sapiens (gi:22538465), Xenopus tropicalis (gi:62858119), Danio rerio (gi:193788711), Strongylocentrotus purpuratus (gi:115927402) and Drosophila melanogaster (gi:21355629) homologues. The sequence had 94% similarity with the β3 subunit of the sea urchin (e-value = 1e−102). Searches of conserved domains in the holothurian sequence show highly significant to proteasome beta type 3 family, other proteasome beta types (1, 6, 7, 2, 4 and 5 respectively in significance) and the N-terminal nucleophile aminohydrolases (Ntn-hydrolase) superfamily. Residues conserved among all beta subunits D, G and G are indicated by asterisks. The characteristic motif GxxxD is marked by a black line. The conserved sites are shown in white letters on black.
The length of the H. glaberrima β3 sequence (202 amino acids) was similar to sequences of H. sapiens, X. tropicalis, D. rerio and D. melanogaster (205 amino acids each) and identical to that from S. purpuratus. The sequence similarity in all beta subunits is largely concentrated in the N-terminal portion. Examples of this similarity are the groups of well-conserved amino acids: Y3NG, A10M, G13K, C16V and R24RF. In addition, the holothurian sequence displayed the conserved residues and motifs previously identified not only for β3 homologues in other species but also conserved in all proteasome beta subunits. These include residues D22, G98 and G137 and the characteristic motif G52xxxD56 (numbers with reference to the H. glaberrima sequence) (Elenich et al. 1999). Phylogenetic analysis were performed using 58 sequences including proteasome subunit β3 homologues from a wide selection of animals and sequences from the closest related proteasome beta types 1, 6, 7, 2, 4 and 5 (Supplementary, Fig. 3). The holothurian sequence clustered with that of the sea urchin in the invertebrate β3cluster.
Ubiquitin-RPL40 (clone P7DP23A06)
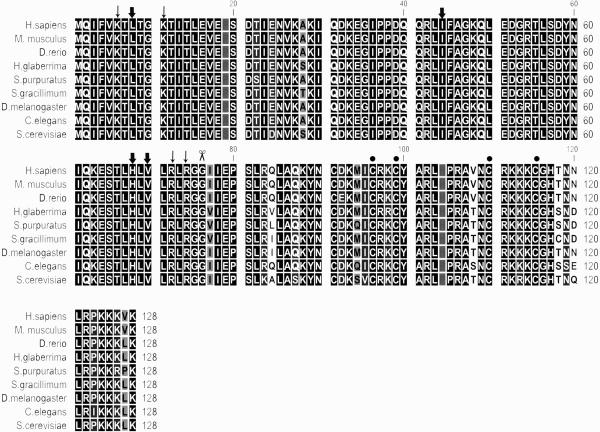
A contig consisting of seven ESTs that showed similarity to ubiquitin was found in the cDNA libraries of 3dpe (two ESTs), 7dpe (four ESTs) and normal (one ESTs). The contig had 595 nucleotides with a 384 residues ORF encoding a predicted protein of 128 amino acids with high similarity to ubiquitin fused to ribosomal protein L40 (Ub-RPL40) (also known as ubiquitin-60S ribosomal protein L40, UBA52 or ubiquitin-CEP52) (Supplementary, Fig. 1C). Multiple sequence alignments using sequences from various species showed that the holothurian ubiquitin-RPL40 was 98% similar in sequence and identical in size to all ubiquitin/ribosomal L40 proteins (Fig. 3). Phylogenetic analyses were performed with sub-families containing the Ubiquitin-associated domain (UBA) (Supplementary, Fig. 4). The Maximum Likelihood tree shows the high conservancy of the ubiquitin/ribosomal L40 branch. All subfamilies were clustered independently and the sea cucumber sequence clustered with the ubiquitin/ribosomal L40 fusion proteins.
Figure 3.
Multiple alignment of H. glaberrima Ubiquitin-RPL40 with Homo sapiens (gi:13569612), Mus musculus (gi:148708840), Danio rerio (gi:80751129), Strongylocentrotus purpuratus (gi:115928598), Scleronephthya gracillium (gi:55228559), Drosophila melanogaster (gi:24581598), Caenorhabditis elegans (gi:17554758) and Saccharomyces cerevisiae (gi:148708840) homologues. BLAST search showed very high similarity to ubuiquitin/ribosomal L40 fusion protein of several organisms. The highest e-value was with the cnidarian Scleronephthya gracillimum (3e-67) with which it shares 124 identical residues out of the 128. Scissor indicates the site of proteolytic cleavage required to generate free ubiquitin. Sites for interaction with UCH-L3 are shown by thin arrows. The hydrophobic patch (L8-I44-H68-V70) is shown by thick arrows. Cystein residues comprising a zinc finger motif in L40 protein are indicated by filled circles. The conserved sites are denoted in white letters on black.
The first 76 amino acids of the sequence corresponded to ubiquitin, while an extension of 52 amino acids following residue G76 corresponded to the ribosomal protein L40. The H. glaberrima homologue has the interaction sites for the C-terminal hydrolase L3 (UCH-L3), residues K6, K11, R72, and R74 (Wilkinson et al. 1999), the interaction site for conjugating enzyme E2 Ubc1 (G76) (Hamilton et al. 2001) and a putative cysteine-rich motif (C96, C99, C110 and C115) (Baker and Board, 1991) (Fig.3).
Together, the homology search, the conserved domains, the multiple sequence alignment and the phylogenetic analyses showed that these three genes, with their own modifications possibly associated with the echinoderm lineage, correspond to orthologues of the vertebrate Rpn10, β3 and Ub-RPL40. The holothurian sequences are now available in GenBank under the following accession numbers: HQ417117 (Rpn10), HQ417118 (β3) and HQ417119 (Ub-RPL40).
H. glaberrima UPS components are up-regulated during intestinal organogenesis mRNA studies
We next determined the expression levels of Rpn10, β3 and Ub-RPL40 during early intestinal regeneration (3-, 7- and 14-dpe) and compared them to those of normal intestines using gene expression microarrays. Statistical inferences including ANOVA and mean comparison between regeneration stages lead us to determine the expression profiles for these genes. Table 1 shows the replicate-averaged values of significance of gene expression changes in reference to the non-regeneration intestines.
Table 1.
Expression profile of UPS genes in the sea cucumber H. glaberrima during regeneration.
| GEO ID | Accssn | 3dpe p.val (F.C) | 7dpe p.val (F.C) | 14dpe p.val (F.C) | ||
|---|---|---|---|---|---|---|
| Rpn-10 | ||||||
| CUST_2176 | HQ417117 | 0.0023** (5.3) | ↑ | 0.0450* (0.3) | ← | 0.6611 (0.9) |
| CUST_3018 | HQ417117 | 0.0014** (5.3) | ↑ | 0.0109* (0.6) | ← | 0.6763 (0.9) |
| CUST_3019 | HQ417117 | 0.0023** (5.1) | ↑ | 0.0391* (0.6) | ← | 0.7903 (0.8) |
|
| ||||||
| β3 | ||||||
| CUST_10221 | HQ417118 | 0.0003** (9.2) | ↑ | 0.2317 (1.4) | 0.5088 (1.2) | |
| CUST_2247 | HQ417118 | 0.0001** (8.9) | ↑ | 0.1141 (1.3) | 0.3866 (1.3) | |
|
| ||||||
| UB-RPL40 | ||||||
| CUST_5951 | HQ417119 | 0.0036** (5.6) | ↑ | 0.0046** (3.8) | ↑ | 0.2747 (1.4) |
| CUST_9828 | HQ417119 | 0.0081** (5.4) | ↑ | 0.0075** (3.6) | ↑ | 0.2867 (1.4) |
GEO ID: Gene Expression Omnibus ID. Accssn: NCBI's accession number.
Arrows represent expression change.
p<0.01
p<0.05
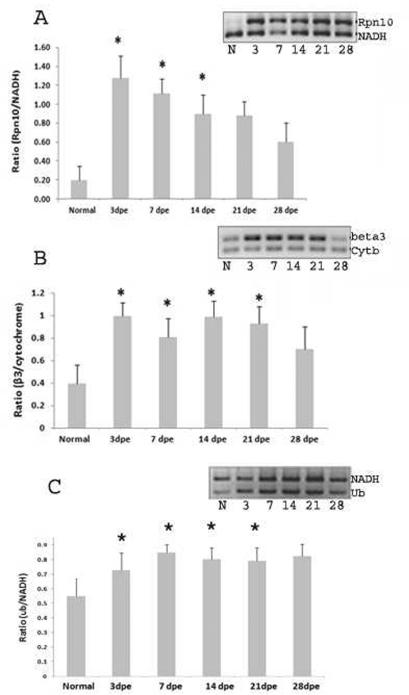
Microarray results were validated by semiquantitative methods such as RT-PCR. Expression profiles of Rpn10, β3, and Ub-RPL40 were analyzed using a set of specific primers (Supplementary, Table 1). Rpn10, β3, and Ub-RPL40 mRNAs were present at all stages of regeneration and in normal animals. Expression was significantly highest during the very early intestinal regeneration process; all sequences showing a highly significant increase at 3 dpe in comparison to non-regenerating animals (Fig. 4 A, B and C). At other time points, the mRNA profile expression was similar among the three components, but not identical. Rpn10 increased during the first two weeks of regeneration, while β3 and Ub-RPL40 remained at high levels until 28-dpe, the last stage analyzed (Fig. 4 A, B and C).
Figure 4.
Gene expression profile analysis of Rpn10 (A), β3 (B) and Ub-RPL40 (C) during intestinal regeneration. Analysis was performed by conventional RT-PCR and normalized against cytochrome b (Cyt b, 161 bp) or NADH (240 bp). Compared with normal animals, Rpn10, β3, Ub-RPL40 present a significant overexpression at 3 dpe. Rpn10 remained significantly high during the second week of regeneration while β3 and Ub-RPL40 remained high until the last stage analyzed. Bars represent means of the normalized optical density of the bands for each regeneration stage +/− the SE. n= 4 (Rpn10), n=3 (β3) and n=6 (Ub) animals were used for each stage. Significance level:*=p<0.05, t-test.
Expression and localization of ubiquitin and alpha 20S proteasome subunits
In view that the gene expression experiments showed an increase of holothurian UPS components mRNA during the process of intestinal regeneration, our next step was to identify UPS protein components. However, protein studies in our system are limited by the availability of antibodies that recognize the holothurian homologues. Nonetheless, we identified three different antibodies that appeared to recognize holothurian UPS proteins: A mouse monoclonal and a rabbit polyclonal anti-ubiquitin antibodies, produced against bovine and human ubiquitin respectively, and are known to detect ubiquitin, polyubiquitin and ubiquitinated proteins. In addition, the mouse monoclonal antibody PW8195, produced against the human proteasome, that recognizes the alpha subunits of the 20S proteasome in various species. We used the antibodies for Western blots and immunohistochemistry of regenerating (3-, 7-, and 14-dpe) and normal animals.
Western blots
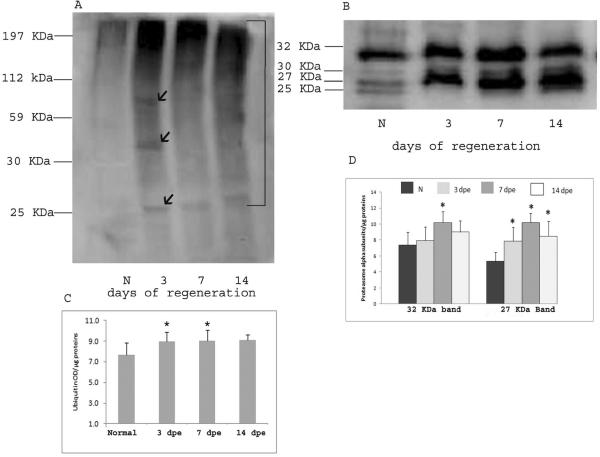
Conjugated ubiquitin was detected in regenerating (3-, 7-, 14-dpe) and normal animals using the monoclonal anti-ubiquitin antibody. The Western blot showed the typical smears of high molecular weight ubiquitin conjugates ranging from 112 to 197 KDa. An increase in the intensity of the bands was observed in protein extracts from regenerating animals. This increase is evident in some of low molecular weight proteins (see 3-dpe in bands in the 25 to 59 KDa range in the 3-dpe specimens) (Fig. 5A). Antibody PW8195 showed four bands of approximately 25, 27, 30 and 32 KDa in normal and regenerating animals, corresponding to alpha subunits of the proteasome. An increase in the intensity of these bands was also observed at all regeneration stages studied (Fig. 5B).
Figure 5.
Western blot analysis showing ubiquitin conjugates (A) and proteasome content (B) at different stages of intestinal regeneration in H. glaberrima. A. The monoclonal anti-ubiquitin antibody recognized ubiquitinated proteins in non-regenerating (N) and regenerating intestine at 3-7-14 days of regeneration. Compared with non-regenerating intestines an increase in the intensity of the labeling is observed at 3, 7 and 14 days. At 3 days, three individual bands of mean molecular weight 27, 32 and 60 KDa showed also a slight increase. (B) The antibody PW8195 recognized three bands corresponding to the alpha 20S proteasome subunits in non-regenerating animals (N). A marked increase in the intensity was observed from 3 to 14 days. (C–D) Bars represent mean ± SE of three experiments where the OD of the bands was divided by the μg of protein loaded in each experiment (20 μg) for ubiquitin (C) and proteasome (D). The right line in A represent the area used to make the OD measurements to ubiquitin conjugates. For proteasome, the bands of 27 and 30 KDa were used for the OD analysis (D). Significance level:*=p<0.05, t-test.
These findings demonstrate that UPS proteins are also being up-regulated during intestinal regeneration.
Immunohistochemistry
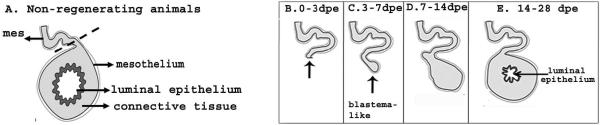
Our next step was to study the localization of the UPS components in the regenerating tissues. Since most readers might not be familiar with our model system we have included a figure (Fig. 6) that illustrates the process of intestinal regeneration in H. glaberrima, to facilitate the understanding of the results reported in this article.
Figure 6.
Drawings of a cross section of the intestine of H. glaberrima depicting the events that occur during intestinal regeneration. (A) In non-regenerating animals, the intestine is composed of three tissue layers. From outer to inner, a mesothelium that includes coelomic epithelium and muscle, an inner connective tissue layer and a pseudostratified luminal epithelium. During evisceration, the intestine detaches (dashed line) from the mesentery (mes) and is expelled through the cloaca. (B) After evisceration (0–3 dpe), a wound healing process takes place where the coelomic epithelia covers the tissue layers exposed at the rupture plane (arrow). (C) During the first week of regeneration (3–7 dpe), a thickening of the mesenterial edges forms the intestinal primordium (blastema-like structure). (D) This mesenterial thickening continues to increase in size during the second week of regeneration (7–14 dpe). (E) Formation of the luminal cavity and epithelial layer occurs from the second week forward (14 dpe) (Adapted from Cabrera-Serrano and Garcia-Arrarás, 2004).
Anti-ubiquitin antibody
To study ubiquitin distribution, we used both monoclonal and polyclonal anti-ubiquitin antibodies. Although both showed a similar labeling, the polyclonal antibody produced a more distinctive labeling and therefore, most of the immunolocalization experiments presented here were done with the polyclonal anti-ubiquitin antibody (Fig. 5 in the supplement shows the labeling using the monoclonal antibody).
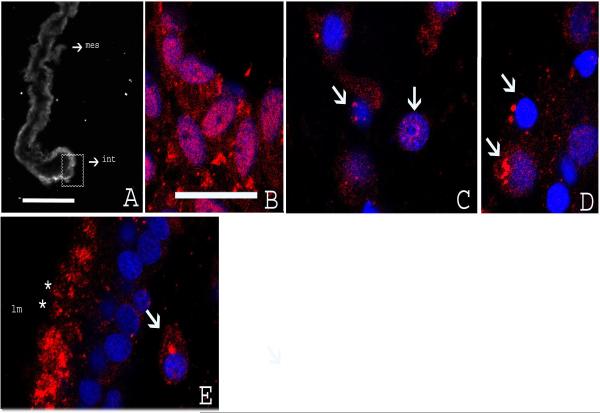
Strong immunofluorescence for ubiquitin was observed at 3 dpe in the mesothelial cells of the intestinal primordia. This labeling was also observed in the rest of the mesentery mesothelium, especially in the area proximate to the regenerating intestine (Fig. 7A). No labeling was noted in cells of the connective tissue of the primordium or mesentery. The labeling for ubiquitin was strong, uniform and concentrated mainly in the nuclei (Fig. 7B).
Figure 7.
Immunoreactivity to ubiquitin at different stage of intestinal regeneration in H. glaberrima. (A) Low magnification of cross section of the regenerating intestine at 3 dpe showing strong immunoreactivity in the intestinal primordium (int) and mesentery (mes). (B) Confocal micrograph of the section seen in A (square) showing nuclear staining in mesothelial cells of the intestinal primordium. (C–D) Micrograph at 7 dpe showing immunoreactivity to ubiquitin in cells of the connective tissue in perinuclear and nuclear areas (arrows). (E) Micrograph at 14 dpe showing cells of the luminal epithelium (lm). The immunoreactivity was localized in the apical domain (asterisks). In cells within the connective tissue, immunoreactivity remains primarily perinuclear. Red-immunofluorescense to Ub, blue-DAPI nuclear stain. Scale bar, A: 100 μm. B–D 18 μm.
At 7 dpe, some mesothelial cells of the regenerating intestine continue showing nuclear labeling while only a few cells showed some diffuse cytoplasmic labeling. In the connective tissue of the blastema and mesenteries we observed prominent dots corresponding to the ubiquitin labeling. In most cells they were associated with the perinuclear area and to a minor degree with the nuclear area (Fig. 7C–D). A few cells showed some weak cytoplasmic labeling.
At 14 dpe, in the connective tissue, the nuclear and perinuclear labeling to ubiquitin observed at 7 dpe was still present in some cells. Scarce labeling was observed in the mesothelial cells of the intestine and mesenteries. At this stage, the regenerating intestine presents a luminal epithelium. Strong immunoreactivity for ubiquitin was located in the apical domain of the luminal epithelial cells (Fig. 7E).
In the intestine of non-regenerating (normal) animals, ubiquitin labeling was found in cells of the coelomic epithelium and luminal epithelium. In these cells, a weak diffuse labeling associated with nuclei and cytoplasm was observed. Labeling in the luminal epithelium was similar to what was observed at 14 dpe, where labeling was associated with the apical domain of cells (data not shown). No labeling was observed in the negative controls.
PW8195 antibody
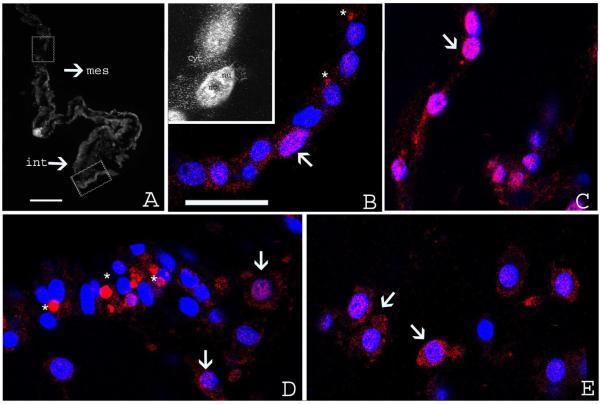
Similar to the immunoreactivity to ubiquitin, strong immunofluorescence was observed at 3 dpe in the mesothelial cells of the mesenterial thickening that will give rise to the intestinal primordium. The labeling was continuous along the mesothelial layer suggesting that most, if not all, of the epithelial cells were labeled (Fig. 8A). The labeling appeared to be evenly distributed throughout the cytoplasm and nuclei with unlabelled areas corresponding to nucleoli (Fig. 8B insert). The same staining was observed along the rest of the mesentery mesothelium (Fig. 8C). Additionally, in some cells of the intestinal primordium, fluorescence was observed as “aggregate-like” structures close to the cell nuclei (Fig. 8B asterisks). No labeling was observed in cells within the connective tissue at this stage.
Figure 8.
Immunoreactivity to antibody PW8195 at different stages of intestinal regeneration in H. glaberrima. (A) Low magnification of a cross section of the regenerating intestine at 3 dpe showing immunoreactivity in the intestinal primordia (int) and mesentery (mes). (B–C) Confocal micrographs of sections indicated in A (squares) showing labeling in the mesothelial cells of the regenerating intestine (B) and mesenteries (C). The insert in B shows the cytoplasmic (cyt) and nuclear (nu) localization of the immunoreactivity with unlabelled areas corresponding to nucleoli (no). In some of these cells the immunoreactivity was also observed as aggregates close to the nucleus (asterisks, B). (D) Confocal micrographs at 7 dpe. Immunoreactivity is uniformly distributed in the cytoplasm and nuclei of mesothelial cells of the regenerating intestine. A larger number of the aggregates (asterisks) are observed at this stage. (E) Micrographs at 7 dpe. In the connective tissue of the regenerating intestine the immunoreactivity is found uniformly distributed in the cytoplasm and nucleus of cells. Arrows identify some of the labeled cells. Red-immunofluorescense to PW8195, blue-DAPI nuclear stain. Scale bar: A: 100 μm. B–E 18 μm.
At 7 dpe, two clear and well-defined types of labeling were observed. First, the same “aggregate-like” structure observed at 3 dpe. Notably, a greater number of these structures were present at this stage (43% of the cells presented it versus 14% at 3 dpe) (Fig. 8D asterisks). Second, most cells continued presenting a labeling uniformly distributed in their cytoplasm and nucleus. This type of labeling was observed in most, if not all, coelomic epithelial cells of the mesenterial thickening. On the other hand, within the connective tissue of the intestinal primordium, labeling was found to be evenly distributed in the cytoplasm and nucleus of about 70% of cell (Fig. 8E).
At 14 dpe, the “aggregate-like” structures were observed in about half of the mesothelial cells of the primordium, however the number of cells presenting the uniform cytoplasmic and nuclear labeling had decreased. In contrast, in the connective tissue at this stage, most cells presented cytoplasmic and nuclear labeling. No labeling was observed in the luminal epithelium at 14 dpe (data not shown). No significant labeling was observed in the mesothelium, connective tissue or luminal epithelium of normal animals or in negative controls.
Function of the UPS during intestinal regeneration of H. glaberrima
In H. glaberrima the regenerated intestine forms at the tip of the mesentery where the intestine was attached prior to evisceration. The new intestine arises from a thickening of the mesentery that forms a blastema-like structure that eventually becomes the new intestine. Therefore, the size of this structure serves as an indicator of the ongoing regenerative process. In an initial attempt to determine a possible role of the UPS during intestinal regeneration, we carried out in vivo experiments by injecting the proteasome inhibitor, MG132 into regenerating animals. Our analyses showed no difference between animals injected with two MG132 concentrations (see methods), thus they were pooled together. However, our results did show significant differences in the size of the regenerating structure between animals injected with the proteasome inhibitor and those injected with the vehicle, the former showing a regenerating rudiment that although similar in structure to controls, had a smaller size (Fig. 9). These results provide an important step for a future analysis of the proteasome function in regeneration.
Figure 9.
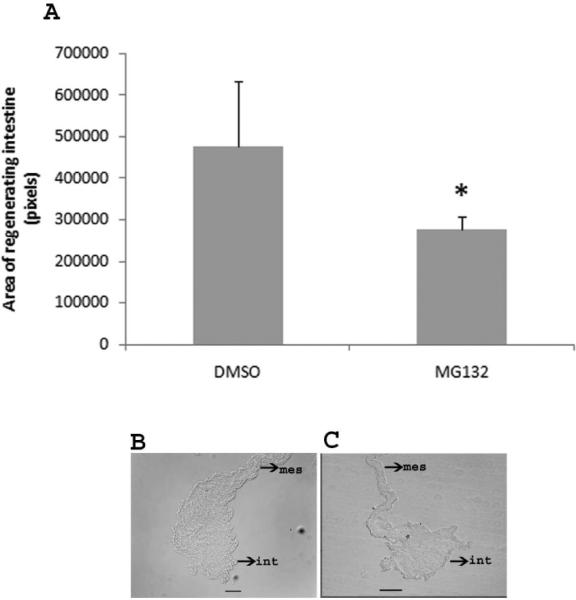
Effect of MG132 on size of the regenerating intestine. A. Injections of MG132 decrease the size of the regenerating intestinal primordia. The results for MG132 represent the summatory of two different concentrations (MG132 45 and 75 μM). *Different from DMSO (control). p<0.05, t-test. Results represent the mean ± SE, n=5 DMSO, n=12 MG132. B and C. Representative histological cross sections comparing the size of the regenerating intestine in control animals (DMSO) (B) and an animal treated with MG132 (C).
DISCUSSION
In recent years, interest in the molecular mechanisms of the UPS has increased considerably. Its function has been studied in different processes such as the cell cycle, stress response, apoptosis, cell differentiation, DNA repair, and metabolic regulation among others (Glickman and Ciechanover, 2002). Given the importance of UPS in regulating many biological processes, it is surprising that its role in regeneration has been hardly investigated. In this work we have characterized three UPS genes of the sea cucumber, Holothuria glaberrrima corresponding to Rpn10, β3 and Ub-RPL40 homologues. More importantly, we showed that the three components along with some alpha proteasome subunits and ubiquitinated proteins displayed significant expression changes during the first week of intestinal regeneration. Finally, we also explored the function of the UPS during the regeneration process using proteasome inhibitors in vivo. Our discussion will focus first on each gene separately and then on the overall context of UPS and regeneration.
H. glaberrima UPS homologues
Degradation of proteins by the UPS involves two main steps: 1) tagging of the substrate by ubiquitin molecules and 2) degradation of these by the 26S proteasome complex. In this process the ubiquitin chain attached to the protein substrate is recognized by specific receptors on the RP of the 26S proteasome. Different receptors to ubiquitin have been described, being Rpn10 one of them (Hamazaki et al. 2007).
Rpn10
The H. glaberrima Rpn10 is highly similar to other Rpn10 homologues described so far. It has a typical first portion defined as a von Willebrand Factor A domain (vWA) and the C-terminal extension is composed mainly by two ubiquitin interacting motifs (UIMs). Overall conservation of the UIMs is high in both vertebrates and invertebrates (Young et al. 1998, Kikukawa et al. 2002). The sequences of H. sapiens, X. tropicalis, D. rerio and D. melagnogaster contain, inside the first UIM, an identical hydrophobic patch that reads LALAL, followed by a conserved serine. Mutagenesis studies have demonstrated that the arrangement of the five residues and the nearby serine are essential for its association with a complementary hydrophobic patch of ubiquitin (Beal et al. 1996) (Fig.6). However, both H. glaberrima and S. purpuratus Rpn10s contain methionine (M) instead of lysine (L). This substitution supports the idea that in the course of echinoderm evolution, some amino acids have been replaced by others with similar properties in order to maintain the protein function.
β3 subunit
We described the full-length sequence of the β3 subunit, a structural non-proteolytic subunit located in the beta rings of the 20S core proteasome. Sequence analysis showed that it contains the residues characteristic of all β3 subunits described so far, confirming that it is the holothurian β3 subunit homologue. However, an interesting feature is that the sequences of H. glaberrima and S. purpuratus lack the N-terminal IMS sequence found in vertebrates suggesting that this is an echinoderm-specific characteristic.
Ub-RPL40
In the holothurian homologue the high level of conservation of ubiquitin and the extension of 52 amino acids corresponding to the ribosomal protein L40 is obvious when compared to sequences from other species. This should not be surprising because ubiquitin is one of the most phylogenetically conserved proteins in nature. All of the most important characteristics of Ub-RPL40 are present, including the residues that interact with Rpn10, with ubiquitin hydrolases, E2 conjugating enzyme (Wilkinson et al. 1999) and the presence of a conserved cysteine-rich motif (Baker and Board, 1991).
In summary, the high degree of conservation of Rpn10, β3 and Ub-RPL40 sequences indicated that these components are part of the holothurians UPS. Proteasomes had been previously studied in other echinoderms including sea urchin (Yokota and Sawada, 2007), starfish (Sawada et al. 2007) and sand dollar (Chiba et al. 1999) during the metabolic activation produced by fertilization, gamete interaction and early development. However, none of these previous works provided details on the nucleotide and amino acid sequences of the molecules. To the best of our knowledge, this work reports for first time the complete full-length codifying region of these UPS components specific to the holothurians.
UPS components expression during intestinal regeneration
Our gene expression results showed that Rpn10, β3 and Ub-RPL40 are up-regulated during intestinal regeneration. Differential expression of UPS components during development has been demonstrated in a large number of species, including C. elegans (Bowerman and Kurz, 2006), Manduca sexta (Dawson, 1995), Drosophila (Klein et al. 1990), Xenopus (Iijima et al. 2003), mouse (Wang et al. 2004) and monkey (Mtango et al. 2007). Similarly, the expression of UPS components has been demonstrated during organogenesis, for example, during somite formation in the mouse (Morimoto et al. 2006) and mesencephalon (El-Khodor et al. 2001) and lung in the rat (Weng et al. 2006).
On the other hand, in regenerative organogenesis, expression of UPS components has been described during lens regeneration in Xenopus (Malloch et al. 2009) and blastema formation in regenerating axolot limbs (Rao et al. 2009). Finally, in echinoderms, Patruno et al. (2001) demonstrated that during arm regeneration in sea stars, levels of conjugated ubiquitin increased during the early post amputation phase and later growth. The present study provides information on the expression of UPS components during regeneration of yet another species, the sea cucumber, H. glaberrima and another organ, the intestine.
During intestinal regeneration in H. glaberrima, we have also demonstrated differential expression of ESTs for some ribosomal genes at 3 and 7 dpe (Rojas-Cartagena et al. 2007), therefore we believe that the activation of Ub-RPL40 forms part of a finely regulated biological event in which this gene is activated in response to cells requiring ubiquitin for protein degradation by the 26S proteasome and ribosome biogenesis (and possibly other processes controlled by protein ubiquitination). In this respect it is important to highlight that in growing yeast, ubiquitin fused genes are the main source of free ubiquitin (Finley et al. 1987).
Gene-specific relative RT-PCRs validated the expression profiles identified in the microarrays experiments during intestinal regeneration at 3 dpe, thus confirming the robustness of the microarray (Table 1, Fig. 4). However, at other stages the correlation was not observed. This is probably due to differences in the sensitivity of the two techniques, where the PCR results provide a higher level of confidence. Moreover, while the microarray results at 3-dpe are highly significant, the level of significance declines at other stages. Finally, some of the differences between RT-PCR and microarray results might be due to small differences in the experimental procedures that were used to isolate and/or process the RNA.
In addition, we found overexpression of alpha 20S proteasome subunits. Differential expression of some proteasome subunits has been associated with alterations in proteosomal activities (Kapadia et al. 2009) and has been suggested as a mechanism to increase the production of 26S proteasomes units in response to biological stimulus. In this case, processes associated with intestinal regeneration could have a direct effect on production of new proteasomes.
Immunolocalization experiments
Proteasomes have been localized to the cell nucleus and cytoplasm (Brooks et al. 2000, Rockel et al. 2005, Girao et. al. 2005). Several findings in the literature indicate that it is uniformly distributed in the cytoplasm, usually associated with cytoskeleton elements. In the nucleus it can be associated with the chromosomes and spindle fibers during the cell cycle (Wojcik and DeMartino, 2003). Nuclear localization of ubiquitinated proteins have been described during lens differentiation in bovine (Girao et al. 2005), in human and rat brains (Adori et al. 2006) and in epithelial cells of the human prostate gland (Martin et al. 2000). In their work with regenerating arms of crinoids and asteroids, Patruno and colleagues (2001) demonstrated that ubiquitin conjugates were located in cells of the regenerating blastema and brachial nerve. In both cases, the labeling was localized to the cell nuclei. Our results are consistent with these observations. We showed that at distinct stages of regeneration the proteasome antigens were localized in the nuclei and cytoplasm of cells in the mesothelium and connective tissues of the regenerating intestine. Ubiquitin conjugates were preferentially present in nuclei.
In addition, at 3, 7 and 14 dpe we observed the fluorescence corresponding to the alpha proteasome subunits concentrated in aggregates close to the cell nuclei. These aggregates were observed only in the portion of the mesothelium of the regenerating intestine and their numbers increased considerably from 3 to 7 dpe, suggesting that they were being formed in response to regenerative events. In other systems, formation of cytoplasmic aggregates has been shown to occur when the capacity of the proteasome degradation pathway is exceeded, either by an increase in substrate expression or by a decrease in proteasome activity (Johnston et al. 1998; García-Mata et al. 1999). These aggregates have been called aggresomes (Johnston et al. 1998). Given the striking similitude between our aggregates and the aggresome, we do not exclude the possibility that the aggregate-like structures observed during the first week of regeneration can be aggresomes, formed in response to high proteolytic demand during the first week of intestinal regeneration. However, in order to be able to conclude that these are aggresomes we would need other markers, such as CFTR (cystic fibrosis transmembrane conductance regulator) or vimentin (Johnston et al. 1998), that at present are not available for the holothurians.
The fact that we have detected the presence of these components during intestinal regeneration, suggest that this system is functional and that its activity increases in response to a biological signal during intestinal regeneration in H. glaberrima.
Possible role of UPS during regeneration
The in vivo results suggest that the proteasomes are somehow regulating the biological events that allow for the new intestine to be formed. Previous cellular studies point that this effect could be at the level of cellular de-differentiation, cell proliferation, apoptosis or others processes that take part during intestinal regeneration in H. glaberrima. During the first week of regeneration, myocytes de-differentiate in a process that includes the loss of the contractile apparatus (Candelaria et al. 2006). This is of particular interest, since the UPS is known to degrade the major contractile skeletal muscle proteins and plays a major role in muscle wasting (Tisdale, 2005, Wang et al. 2010). On the other hand, changes in cell proliferation are important during intestinal regeneration of H. glaberrima as shown by the incorporation of Brdu (5'-bromo-2'deoxyuridine) (Garcia-Arrarás et al. 1998, 2001). The proteasome modulates cyclins and various inhibitors of cyclin-dependent kinases, such as p21WAF and p27 Kip (Clarke, 2002) and a large number of studies have shown that proteasome inhibition exerts an antiproliferative effect mainly by altering the levels of cell cycle regulatory proteins (Hershko 1997, Yin et al. 2005, Awasthi and Wagner 2006, Lu et al. 2010).
However, since the UPS functions in a wide array of cellular process, we do not exclude the possibility of additional or alternative functions during intestinal regeneration. For example, some studies have emphasized the idea that proteasomes can modulate events related with extracellular matrix remodeling (Wang et al. 2006; Koistinen et al. 2006). This is interesting since proteolytic activity during intestinal regeneration in H. glaberrima has been associated with extracellular matrix remodeling (Quiñones et al. 2002). Experiments in our laboratory, including enzymatic activities assay and in vivo treatments with other proteasome inhibitors, are in progress to study the precise function of the UPS during intestinal regeneration in H. glaberrima. The characterization of these components of the holothurian UPS and the demonstration that they are up-regulated during intestinal regeneration provide strong support for the ongoing experiments in order to show their role during regenerative processes in the echinoderms.
METHODS
Animals
Adult specimens of H. glaberrima were collected in the coast of Puerto Rico and kept in seawater aquaria at 20–24°C. Evisceration was induced by injecting 3–5 ml of 0.35M KCl. Non-eviscerated and regenerating animals were kept in aquaria for different days post-evisceration (dpe) (3, 7, 14, 21 and 28) and anesthetized by immersion in 1,1,1-trichloro-2-methyl-2-propanol hydrate 98% or ice water for 1 h before dissection.
Microarray
Microarray fabrication, hybridization and experimental design have been described previously (Ortiz-Pineda et al. 2009). Arrays containing 7209 ESTs from three intestinal cDNA libraries were custom made by Agilent (Santa Clara, CA). A design of 60-mer probes in a 15 k format was chosen, with 8 arrays per slide. Two 60-mer probes were designed from different regions of the β3 and Ub-RPL40 ESTs while for Rpn10 three 60-mer probes were printed on the array to account for technical replicates. Technical and biological controls were included in the microarrays. Hybridizations were carried out at 65°C for 17 hrs in a rotating oven (Agilent, Santa Clara, CA). Post-hybridization washes were conducted according to Agilent's two-color microarray-based gene expression analysis protocol (Version 5.5, February 2007). Slides were scanned with an Agilent microarray scanner and data was obtained through Agilent's feature extraction software (Version 9.5.3.1). The data was analyzed in R software with the Limma package from Bioconductor (http://www.R-project.org, www.bioconductor.org). Normalization was performed as previously described (Ortiz-Pineda et al. 2009). To estimate the average M-value for each gene and assess differential gene expression, a simple linear model was fitted to the data, and M-value averages and standard deviations for each gene were obtained. To find genes with significant expression changes between treatments, empirical Bayesian statistics were applied to the data by moderating the standard errors of the estimated M-values. P-values were obtained for both: 1) analysis of variance between regenerating stages and 2) t-test-statistic of comparison of means under the null hypothesis that there is no significant change in gene expression. The statistical significance threshold was set at p<0.05 (*) and p<0.01 (**). NCBI's microarray accession numbers (GEO): Series GSE16182; Platform GPL8574.
Isolation and sequencing of ubiquitin proteasome system components
ESTs for putative UPS components were obtained by mass excision and random sequencing from H. glaberrima cDNA libraries of 3, 7 dpe and normal animals. EST clones P3DP12H09, PNLPO2E07 and P7DP23A06 (contig) showed some similarities to the Rpn10 non-ATPase subunit of the 19S regulatory particle (Finley et al, 1998), β3 subunit of the 20S proteasome (Baumeister et al. 1998) and ubiquitin, respectively. Clones P3DP12H09 and PNLPO2E07 only contained partial sequences of the expected protein sequence, thus forward primers were used to complete the 3' end, and 5' RACE PCR to complete the 5' end (FirstChoice® RLM-RACE Kit, AMBION, Austin, TX, Cat. AM-1700). PCR products were used as templates for re-sequencing in a GENETIC ANALYZER (Applied Biosciences, Foster City, CA) to verify their identities.
Characterization of putative UPS components using bioinformatics tools
Sequences were analyzed with the chromatogram analysis program (Sequencher, Chromas) to assess their quality. CAP3 Sequence Assembly free Program was used to assemble the contiguous sequences. To identify the putative identities and homologies they were analyzed using Basic Local Alignment Search Tool (BlastX and BlastP) against the NCBI's nucleotide non-redundant and Refseq-protein databases respectively (Altschul et al. 1990). Alignments were performed using CLC free-workbench software with the progressive alignment algorithm (Feng and Doolitle, 1987). (v.3.2.1 by CLC bio A/S, 238 Denmark) and M-Coffee using pairwise and multiple methods (Notredame et al. 2000; Wallace et al. 2006). Phylogenetic trees were constructed with Maximum Likelihood in Uber-Geneious software (v.4.7.6) and edited for easy visualization using Dendroscope (v.2.7.4) (Huson et al. 2007).
cDNA synthesis and analysis by conventional RT-PCR
Total RNA was extracted from intestinal homogenates using Tri-Reagent (MRC, Cincinnati, OH) and the RNeasy Mini Kit from QUIAGEN (Valencia, CA) and quantified by spectrophotometry at 260-nm optical density in a NanoDrop (ND-1000) Spectrophotometer (NanoDrop Technologies, Rockland, DE). First-strand synthesis was performed with the M-MLV reverse transcriptase (PROMEGA, Madison WI). Primers for subunits Rpn10, β3 and ubiquitin were designed using the Biology workbench 3.2 and Net primer free software from PREMIER Biosoft International and are shown in Table 2. Cycling conditions for the amplified products were as follow: an initial denaturation step at 94°C for 2 min, then 35 cycles of denaturation at 94°C for 0.45 seconds, annealing at 55.6°C for 0.45 seconds and extension at 72°C for 10 min. Optical densitometric (OD) analysis of the PCR products were performed using Adobe Photoshop. Background values were substracted and then normalized against NADH-dehydrogenase unit 5 (NADH) or cytochrome b. PCR for Rpn10 and β3 were done under the same conditions. PCR for ubiquitin was done at 30 cycles. The exponential phase of amplification was used for analyses. Bands were visualized under UV light after staining with ethidium bromide.
Immunohistochemistry
Immunohistochemistry experiments were done as described previously (Murray and García-Arrarás 2004). Briefly, normal and regenerating (3, 7 and 14 days) intestines were fixed in 4% paraformaldehyde at 4°C overnight. Cryostat tissue sections were cut (20 μm), and mounted on Poly-L-lysine-coated slides. Sections were blocked with 10% serum, incubated with Triton X-100 (0.15%) and with 0.05 M HCl. The primary antibodies used were: mouse monoclonal antibody (1/50, Biomol International # PW8195) that reacts with α1, α2, α3, α5, α6 and α7 subunits of the 20S proteasome, rabbit polyclonal antibody anti-ubiquitin (1/800, Thermo Fisher Scientific # 1859660) or mouse monoclonal anti-ubiquitin (1/100, Cell Signaling # 3936). The secondary antibodies used were goat anti-mouse or anti-rabbit labeled with FITC (Invitrogen, Carlsbad, CA) or Cy3 (Jackson immune research, West Grove, PA). Controls were done by incubating only with the secondary antibody. Slides were mounted in glycerol-PBS containing 4', 6-diamidino-2-phenylindole (DAPI) to stain the cell nuclei and examined on a UV fluorescence microscope (Leitz Laborlux). Confocal images were obtained with a Carl Zeiss LSM 510 confocal microscope and analyzed with the software Zen 2008. Images were taken using 100× objective lens.
Western blot analysis
Homogenates were made from intestines at different stages of regeneration using lysis buffer (20 mM Tris base, pH 7.4; 150 mM NaCl; 1mM EDTA; 1mM EGTA; 1mM PMSF; 5 mM sodium pyrophosphate; 30 mM β-glycerophosphate; 30 mM sodium fluoride). Protein concentration was determined using the Comassie Plus Protein Assay (Thermo Scientific, Rockford, IL). Equal protein concentration samples were loaded on 12% SDS- PAGE gel (SDS–PAGE) and transferred to PVDF membrane for 1 h at 100 V at room temperature. Membranes were blocked in 5% non fat dry milk in TBS-T (Tris-buffered saline containing 0.1% Tween 20) for 1 h at room temperature. The blots were then probed overnight with the antibodies 3936 (1/1000) or PW8195 (1/1000). After three washes (15 min TBS-T) the membranes were incubated with the respective secondary horseradish peroxidase (HRP)-conjugated antibody for 1 hr at room temp. After washing, the membranes were incubated for 5 min in super Signal West Dura Chemiluminescent Substrate (Thermo Fisher Sicentific, Rockford, IL) and the signal was visualized using a Chemi System (BioRad Molecular Imager® GelDoc™ XR).
Positive controls were done for the anti-proteasome and anti-ubiquitin antibodies. Optical densitometric (OD) analyses of the bands were performed using Adobe Photoshop. Background values were substracted and then normalized by the amount of protein loaded in each experiment.
In vivo studies
H. glaberrima specimens were eviscerated (day 0) and placed in aquaria. Animals were injected intracoelomically with 400 μl of MG132 at concentrations of 0.9 and 1.5 mM. The volume of coelomic fluid is approximately 8 ml; therefore, the final concentration of the inhibitor in the coelom has been calculated to be approximately 45 and 75 μM. DMSO was injected in control animals at final concentrations of 0.25%, the vehicle concentration. Each animal was injected twice a day (one injection every 12 hours) on days 5 and 6 of regeneration. On the morning of day 7, animals were anesthetized and sacrificed. The intestines were obtained and processed as described above for immunohistochemistry. To determine if MG132 treatment affected the regenerating organ size, regenerating intestine sections were examined and photographed using the software SpotBasic 4.7. The contours of the regenerating structure were delineated using ImageJ, and the number of pixel measured in control and experimental groups.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jose Serrano from the Biological Imaging Group for assistance in the preparation of figures.
Grant sponsor: NIH-NIGMS
Grant number: 1SC1GM084770 and S06GM08102
LITERATURE CITED
- Adori C, Low P, Moszkovkin G, Bagdy G, László L, Kovács GG. Subcellular distribution of Components of the Ubiquitin–Proteasome System in Non-diseased Human and Rat Brain. J Histochem Cytochem. 2006;54:263–267. doi: 10.1369/jhc.5B6752.2005. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Awasthi N, Wagner BJ. Suppression of human lens epithelial cell proliferation by proteasome inhibition, a potential defense against posterior capsular opacification. Invest Ophthalmol Vis Sci. 2006;47:4482–9. doi: 10.1167/iovs.06-0139. [DOI] [PubMed] [Google Scholar]
- Baker RT, Board PG. The human ubiquitin-52 amino acid fusion protein gene shares several structural features with mammalian ribosomal protein genes. Nucleic Acids Res. 1991;19:1035–40. doi: 10.1093/nar/19.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister W, Walz J, Zühl F, Seemüller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–80. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- Beal R, Deveraux Q, Xia G, Rechsteiner M, Pickart C. Surface hydrophobic residues of multiubiquitin chains essential for proteolytic targeting. Proc Natl Acad Sci. 1996;93:861–866. doi: 10.1073/pnas.93.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman B, Kurz T. Degrade to create: developmental requirements for ubiquitin-mediated proteolysis during early C. elegans embryogenesis. Development. 2006;133:773–784. doi: 10.1242/dev.02276. [DOI] [PubMed] [Google Scholar]
- Brooks P, Fuertes G, Murray RZ, Bose S, Knecht E, Rechsteiner MC, Hendil KB, Tanaka K, Dyson J, Rivett J. Subcellular localization of proteasomes and their regulatory complexes in mammalian cells. Biochem J. 2000;346:155–61. [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Serrano A, García-Arrarás JE. RGD-containing peptides inhibit intestinal regeneration in the sea cucumber Holothuria glaberrima. Dev Dyn. 2004;231:171–178. doi: 10.1002/dvdy.20112. [DOI] [PubMed] [Google Scholar]
- Candelaria AG, Murray G, File SK, García-Arrarás JE. Contribution of mesenterial muscle dedifferentiation to intestine regeneration in the sea cucumber Holothuria glaberrima. Cell Tissue Res. 2006;325:55–65. doi: 10.1007/s00441-006-0170-z. [DOI] [PubMed] [Google Scholar]
- Chiba K, Alderton JM, Hoshi M, Steinhardt RA. Activation of the proteasomes of sand dollar eggs at fertilization depends on the intracellular pH rise. Dev Biol. 1999;209:52–9. doi: 10.1006/dbio.1999.9239. [DOI] [PubMed] [Google Scholar]
- Clarke DJ. Proteolysis and the cell cycle. Cell cycle. 2002;1:233–234. doi: 10.4161/cc.1.4.130. [DOI] [PubMed] [Google Scholar]
- Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–47. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- Dawson SP, Arnold JE, Mayer NJ, Reynolds SE, Billett MA, Gordon C, Colleaux L, Kloetzel PM, Tanaka K, Mayer RJ. Developmental changes of the 26S proteasome in abdominal intersegmental muscles of Manduca sexta during programmed cell death. J Biol Chem. 1995;270:1850–8. doi: 10.1074/jbc.270.4.1850. [DOI] [PubMed] [Google Scholar]
- Elenich LA, Nandi D, Kent AE, McCluskey TS, Cruz M, Iyer MN, Woodward EC, Conn CW, Ochoa AL, Ginsburg DB, Monaco JJ. The complete primary structure of mouse 20S proteasomes. Immunogenetics. 1999;10:835–42. doi: 10.1007/s002510050562. [DOI] [PubMed] [Google Scholar]
- El-Khodor BF, Kholodilov NG, Yarygina O, Burke RE. The expression of mRNAs for the proteasome complex is developmentally regulated in the rat mesencephalon. Brain Res Dev Brain Res. 2001;129:47–56. doi: 10.1016/s0165-3806(01)00181-x. [DOI] [PubMed] [Google Scholar]
- Feng DF, Doolittle RF. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25:351–60. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell. 1987;48:1035–46. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- Finley D, Tanaka K, Mann C, Feldmann H, Hochstrasser M, Vierstra R, Johnston S, Hampton R, Haber J, Mccusker J, Silver P, Frontali L, Thorsness P, Varshavsky A, Byers B, Madura K, Reed SI, Wolf D, Jentsch S, Sommer T, Baumeister W, Goldberg A, Fried V, Rubin DM, Toh-e A, et al. Unified nomenclature for subunits of the Saccharomyces cerevisiae proteasome regulatory particle. Trends Biochem Sci. 1998;23:244–5. doi: 10.1016/s0968-0004(98)01222-5. [DOI] [PubMed] [Google Scholar]
- García-Arrarás JE, Estrada-Rodgers L, Santiago R, Torres II, Díaz-Miranda L, Torres-Avillán I. Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea:Echinodermata) J Exp Zool. 1998;281:288–304. doi: 10.1002/(sici)1097-010x(19980701)281:4<288::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- García-Arrarás JE, Greenberg MJ. Visceral regeneration in holothurians. Microsc Res Tech. 2001;55:438–451. doi: 10.1002/jemt.1189. [DOI] [PubMed] [Google Scholar]
- García-Mata R, Bebök Z, Sorscher EJ, Sztul ES. Characterization and dynamics of aggresome formation by a cytosolic GFP chimera. J Cell Biol. 1999;146:1239–1244. doi: 10.1083/jcb.146.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, Harris SE, Gallwitz W, Kim KB, Hu S, Crews CM, Mundy GR. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–82. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girao H, Pereira P, Taylor A, Shang F. Subcellular Redistribution of Components of the Ubiquitin Proteasome Pathway during Lens Differentiation and Maturation. Invest Ophthalmol Vis Sci. 2005;46:1386–1392. doi: 10.1167/iovs.04-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman M, Ciechanover A. The Ubiquitin-Proteasome proteolytic Pathway: destruction for the sake of construction. Physiol Rev. 2002;83:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Hamazaki J, Sasaki K, Kawahara H, Hisanaga S, Tanaka K, Murata S. Rpn10-mediated degradation of ubiquitinated proteins is essential for mouse development. Mol Cell Biol. 2007;27:6629–38. doi: 10.1128/MCB.00509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KS, Ellison MJ, Barber KR, Williams RS, Huzil JT, McKenna S, Ptak C, Glover M, Shaw GS. Structure of a conjugating enzyme-ubiquitin thiolester intermediate reveals a novel role for the ubiquitin tail. Structure. 2001;10:897–904. doi: 10.1016/s0969-2126(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Hershko A. Roles of ubiquitin-mediated proteolysis in cell cycle control. Curr Opin Cell Biol. Biology. 1997;9:788–799. doi: 10.1016/s0955-0674(97)80079-8. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Falquet L. An ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci. 2001;26:347–50. doi: 10.1016/s0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R. Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics. 2007;8:460. doi: 10.1186/1471-2105-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima R, Homma KJ, Natori S. Participation of proteasomes in Xenopus embryogenesis. J Biochem (Tokio) 2003;134:467–71. doi: 10.1093/jb/mvg165. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia MR, Eng JW, Jiang Q, Stoyanovsky DA, Kibbe MR. Nitric oxide regulates the 26S proteasome in vascular smooth muscle cells. Nitric Oxide. 2009;20:279–88. doi: 10.1016/j.niox.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Kikukawa Y, Shimada M, Suzuki N, Tanaka K, Yokosawa H, Kawahara H. The 26S proteasome Rpn10 gene encoding splicing isoforms: evolutional conservation of the genomic organization in vertebrates. Biol Chem. 2002;383:1257–61. doi: 10.1515/BC.2002.139. [DOI] [PubMed] [Google Scholar]
- Klein U, Gernold M, Kloetzel P. Cell-specific accumulation of Drosophila Proteasomes (MCP) during early development. JBC. 1990;111:2275–2282. doi: 10.1083/jcb.111.6.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen H, Seppälä M, Knuutila S, Koistinen R. Extracellular matrix-induced changes in expression of cell cycle-related proteins and proteasome components in endometrial adenocarcinoma cells. Gynecol Oncol. 2006;102:546–51. doi: 10.1016/j.ygyno.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Lier S, Paululat A. The proteasome regulatory particle subunit Rpn6 is required for Drosophila development and interacts physically with signalosome subunit Alien/CSN2. Gene. 2002;298:109–19. doi: 10.1016/s0378-1119(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle. 2010;9:2342–2352. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloch EL, Perry KJ, Fukui L, Johnson VR, Wever J, Beck CW, King MW, Henry JJ. Gene expression profiles of lens regeneration and development in Xenopus laevis. Dev Dyn. 2009;238:2340–56. doi: 10.1002/dvdy.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R, Fraile B, Peinado F, Arenas MI, Elices M, Alonso L, Paniagua R, Martín JJ, Santamaría L. Immunohistochemical Localization of Protein Gene Product 9.5, Ubiquitin, and Neuropeptide Y Immunoreactivities in Epithelial and Neuroendocrine Cells from Normal and Hyperplastic Human Prostate. The J Histochem Cytochem. 2000;48:1121–1130. doi: 10.1177/002215540004800809. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Kiso M, Sasaki N, Saga Y. Cooperative Mesp activity is required for normal somitogenesis along the anterior-posterior axis. Dev Biol. 2006;300:687–98. doi: 10.1016/j.ydbio.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Mtango NR, Latham KE. Ubiquitin proteasome pathway gene expression varies in rhesus monkey oocytes and embryos of different developmental potential. Physiol Genomics. 2007;31:1–14. doi: 10.1152/physiolgenomics.00040.2007. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, Jones DC, Vallet S, Bouxsein ML, Pozzi S, Chhetri S, Seo YD, Aronson JP, Patel C, Fulciniti M, Purton LE, Glimcher LH, Lian JB, Stein G, Anderson KC, Scadden DT. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118:491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, García-Arrarás JE. Myogenesis during holothurian intestinal regeneration. Cell Tissue Res. 2004;318:515–24. doi: 10.1007/s00441-004-0978-3. [DOI] [PubMed] [Google Scholar]
- Myung J, Kim KB, Craig M. The ubiquitin-proteasome pathway and proteasome inhibitors. Medicinal Research Reviews. 2001;21:245–353. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–17. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Ortiz-Pineda PA, Ramirez-Gomez F, Pérez-Ortiz J, González-Díaz S, Santiago-De Jesús F, Hernández-Pasos J, Del Valle-Avila C, Rojas-Cartagena C, Suárez-Castillo EC, Tossas K, et al. Gene expression profiling of intestinal regeneration in the sea cucumber. BMC Genomics. 2009;10:262. doi: 10.1186/1471-2164-10-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patruno M, Thorndyke MC, Candia Carnevali MD, Bonasoro F, Beesley P. Changes in ubiquitin conjugates and Hsp72 levels during arm regeneration in echinoderms. Mar Biotechnol (NY) 2001;3:4–15. doi: 10.1007/s101260000018. [DOI] [PubMed] [Google Scholar]
- Quiñones JL, Rosa R, Ruiz DL, García-Arrarás JE. Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev Biol. 2002;250:181–197. doi: 10.1006/dbio.2002.0778. [DOI] [PubMed] [Google Scholar]
- Rao N, Jhamb D, Milner DJ, Li B, Song F, Wang M, Voss SR, Palakal M, King MW, Saranjami B, Nye HL, Cameron JA, Stocum. DL. Proteomic analysis of blastema formation in regenerating axolotl Limbs. BMC Biol. 2009;30:7–83. doi: 10.1186/1741-7007-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockel TD, Stuhlmann D, von Mikecz A. Proteasomes degrade proteins in focal subdomains of the human cell nucleus. J Cell Sci. 2005;118:5231–42. doi: 10.1242/jcs.02642. [DOI] [PubMed] [Google Scholar]
- Rojas-Cartagena C, Ortíz-Pineda P, Ramírez-Gómez F, Suárez-Castillo EC, Matos-Cruz V, Rodríguez C, Ortíz-Zuazaga H, García-Arrarás JE. Distinct profiles of expressed sequence tags during intestinal regeneration in the sea cucumber Holothuria glaberrima. Physiol Genomics. 2007;31:203–15. doi: 10.1152/physiolgenomics.00228.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada MT, Tamura T, Mitani Y, Kaya M, Ito G, Hashimoto H, Sawada H. Participation of proteasome-associating complex PC500 in starfish oocyte maturation as revealed by monoclonal antibodies. Biochem Biophys Res Commun. 2007;349:694–700. doi: 10.1016/j.bbrc.2006.08.080. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Saxena SK, Sharp JG. Regulation of intestinal regeneration: new insights. Microsc Res Tech. 2000;51:129–37. doi: 10.1002/1097-0029(20001015)51:2<129::AID-JEMT4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol. 2005;3:209–17. [PubMed] [Google Scholar]
- Wallace IM, O'Sullivan O, Higgins DG, Notredame C. M-Coffee: combining multiple sequence alignment methods with T-Coffee. Nucleic Acids Res. 2006;34:1692–9. doi: 10.1093/nar/gkl091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HM, Zhang X, Qian D, Lin HY, Li QL, Liu DL, Liu GY, Yu XD, Zhu C. Effect of ubiquitin-proteasome pathway on mouse blastocyst implantation and expression of matrix metalloproteinases-2 and -9. Biol Rep. 2004;70:481–487. doi: 10.1095/biolreprod.103.021634. [DOI] [PubMed] [Google Scholar]
- Wang HX, Wang HM, Lin HY, Yang Q, Zhang H, Tsang BK, Zhu C. Proteasome subunit LMP2 is required for matrix metalloproteinase-2 and -9 expression and activities in human invasive extravillous trophoblast cell line. J Cell Physiol. 2006;206:616–23. doi: 10.1002/jcp.20508. [DOI] [PubMed] [Google Scholar]
- Wang XH, Zhang L, Mitch WE, LeDoux JM, Hu J, Du J. Caspase-3 cleaves specific 19 S proteasome subunits in skeletal muscle stimulating proteasome activity. J Biol Chem. 2010;285:21249–57. doi: 10.1074/jbc.M109.041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T, Chen Z, Jin N, Gao L, Liu L. Gene expression profiling identifies regulatory pathways involved in the late stage of rat fetal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:1027–1037. doi: 10.1152/ajplung.00435.2005. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Laleli-Sahin E, Urbauer J, Larsen CN, Shih GH, Haas AL, Walsh ST, Wand AJ. The binding site for UCH-L3 on ubiquitin: mutagenesis and NMR studies on the complex between ubiquitin and UCH-L3. J Mol Biol. 1999;291:1067–1077. doi: 10.1006/jmbi.1999.3038. [DOI] [PubMed] [Google Scholar]
- Wójcik C, DeMartino GN. Intracellular localization of proteasomes. The International Journal of Biochemistry & Cell Biology. 2003;35:5579–589. doi: 10.1016/s1357-2725(02)00380-1. [DOI] [PubMed] [Google Scholar]
- Yin D, Zhou H, Kumagai T, Liu G, Ong JM, Black KL, Koeffler HP. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM) Oncogene. 2005;24:344–54. doi: 10.1038/sj.onc.1208225. [DOI] [PubMed] [Google Scholar]
- Yokota N, Sawada H. Sperm proteasomes are responsible for the acrosome reaction and sperm penetration of the vitelline envelope during fertilization of the sea urchin Pseudocentrotus depressus. Dev Biol. 2007;308:222–31. doi: 10.1016/j.ydbio.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Young P, Deveraux Q, Beal RE, Pickart CM, Rechsteiner M. Characterization of two polyubiquitin binding sites in the 26S protease subunit 5a. J Biol Chem. 1998;273:5461–7. doi: 10.1074/jbc.273.10.5461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.